Abstract
Background
The monoclonal antibody bevacizumab effectively inhibits angiogenesis in several types of cancers by blocking vascular endothelial growth factor. However, life‐threatening cardiovascular adverse effects could limit its use and may warrant specific follow‐up strategies.
Methods and Results
We systematically searched MEDLINE, Cochrane, EMBASE, and Web of Science for randomized controlled trials published until November 2016 that assessed patients with cancer treated with or without bevacizumab in addition to standard chemotherapy. A total of 20 050 patients with a broad range of cancer types from 22 studies were included in this analysis (10 394 in the bevacizumab group and 9656 in the control group). The risks of arterial and venous adverse events were higher in the bevacizumab groups (relative risk [RR], 1.37; 95% CI, 1.10–1.70 [P=0.004] and RR, 1.29; 95% CI, 1.12–1.47 [P<0.001], respectively), and more arterial adverse events occurred in patients taking high‐dose bevacizumab regimens. Bevacizumab treatment was associated with the highest risk of cardiac and cerebral ischemia in the high‐dose bevacizumab groups (RR, 4.4; 95% CI, 1.59–12.70 [P=0.004] and RR, 6.67; 95% CI, 2.17–20.66 [P=0.001], respectively). In addition, the risk of bleeding and arterial hypertension were higher in the bevacizumab groups (RR, 2.74; 95% CI, 2.38–3.15 [P<0.001] and RR, 4.73; 95% CI, 4.15–5.39 [P<0.00001], respectively), with higher values for patiens taking high‐dose regimens.
Conclusions
Treatment with bevacizumab increases the risk of arterial adverse events, particularly cardiac and cerebral ischemia, venous adverse events, bleeding, and arterial hypertension. This risk is additionally increased with high doses of bevacizumab. Further studies should determine the appropriate options for cardio‐oncology management.
Clinical Trial Registration
URL: https://www.crd.york.ac.uk. Unique identifier: PROSPERO(CRD42016054305).
Keywords: bevacizumab, cardio‐oncology, cardiovascular adverse events
Subject Categories: Coronary Artery Disease, Thrombosis, Meta Analysis
Clinical Perspective
What Is New?
The study combines data from 22 randomized controlled trials with 20 050 patients treated with bevacizumab on top of standard chemotherapy versus chemotherapy alone and makes an extensive assessment of the subtypes of arterial adverse events, subtypes of cancer, and impacts of different dosages and follow‐up times on outcomes.
What Are the Clinical Implications?
Because of the life‐threatening impact of severe cardiovascular adverse events, our findings are of substantial importance for the daily care of patients with cancer and could contribute to the advancement of treatment protocols, with particular emphasis on cardiovascular surveillance, prevention, and multidisciplinary decisions in cardio‐oncology teams.
Introduction
In the past few decades, substantial progress has been made in the treatment of patients with oncologic conditions, particularly in the field of targeted therapies using specific antibodies.1 However, despite the prolonged survival rates associated with therapy, concerns have been raised regarding the adverse effects of these novel drugs.2, 3, 4, 5, 6 Therefore, it is imperative to establish a comprehensive oncocardiologic management strategy for these patients.7
Among the most frequently prescribed novel antibodies is bevacizumab—a master regulator of tumor angiogenesis.8 Bevacizumab is a monoclonal antibody that binds to the vascular endothelial growth factor (VEGF) A ligand, which is thought to play a dominant role in regulating angiogenesis in cancerous cells.9 Currently, bevacizumab is approved by the European Medicines Agency for the treatment of colorectal carcinoma; breast cancer; non–small cell lung cancer; renal cell cancer; ovarian, fallopian tube, or primary peritoneal cancer; and carcinoma of the cervix.10 Furthermore, the US Food and Drug Administration has approved bevacizumab for the treatment of glioblastoma.11 For patients with metastatic colorectal cancer, it was estimated that bevacizumab was prescribed for 54% of patients as an initial first‐line treatment, for 58% of patients who needed a continued second‐line regimen, and for 50% of patients as third‐line therapy.12
There is a rapidly growing body of evidence demonstrating the efficacy of bevacizumab in prolonging survival by decreasing tumor growth and improving the delivery of cytotoxic drugs to neoplastic cells. However, randomized controlled trials (RCTs) have reported cardiovascular adverse events that are not fully characterized.13, 14, 15, 16, 17, 18 A complete analysis would include a precise evaluation of the type of adverse event (arterial/venous event, cardiac ischemia, or cerebral ischemia), determination of coexisting risk factors, assessment of dose dependency, and determination of whether a high‐dose bevacizumab regimen poses a higher relative risk than a low‐dose regimen.19, 20, 21, 22, 23 Furthermore, with the exception of RCTs from recent years, previous analyses focused primarily on colorectal cancer, included a broad range of tumors, and reported only the sum of adverse, and particularly arterial, events without a detailed focus on the type of cardiovascular damage.5, 6, 24
Given that the overall rate and risk of cardiac and cerebral ischemia, arterial and venous adverse events, and bleeding events are not known, we performed a meta‐analysis of published RCTs of patients treated with or without bevacizumab in addition to standard chemotherapy. It is hoped that this meta‐analysis will support the development of onco‐cardiological follow‐up and treatment strategies for these patients beyond the currently available standard oncologic care.
Methods
This meta‐analysis was performed in accordance with the Preferred Reporting of Items for Systematic Meta‐Analysis guidelines and the Cochrane Handbook for Systematic Reviews of Interventions.25, 26 The study was registered with PROSPERO (CRD42016054305).
Sources of Information and Search Strategies
A systematic search of studies published until November 21, 2016, was conducted using the MEDLINE, Cochrane, EMBASE, and Web of Science databases. We made our search specific and sensitive using MeSH terms and free text and considered studies in any language (Table S1).
Only those studies that complied with inclusion criteria as listed below were included:
Prospective RCTs involving patients with cancer.
Random assignment of patients to 2 groups: a bevacizumab group that included patients treated with bevacizumab along with standard chemotherapy and a control group that included patients treated with the same chemotherapy regimen without bevacizumab.
Reporting at least arterial and/or venous adverse events.
Sample size >100 patients.
The exclusion criteria were as follows:
Abstracts, reviews, animal studies, meta‐analyses, and case reports.
Studies with single‐arm bevacizumab treatment, treatment with bevacizumab in both groups described in the inclusion criteria, or treatment with other VEGF inhibitor.
Studies that did not report the selected outcomes or studies that reported the total (combined) number of events.
Subgroup population studies.
Radiotherapy.
After removing duplicates, R.I.M. and M.T. independently reviewed the abstracts. Any differences in results between the 2 investigators were resolved by discussion with T.R. When inclusion criteria appeared to be met, the entire text was reviewed. At the end of the review process, the full texts of the studies considered eligible were reviewed by all investigators.
Data Extraction and Quality Assessment
Two authors (R.I.M. and M.T.) independently performed the data extraction using a standard data extraction form that contained the following fields: publication details (name of the first author and year of publication); study design; characteristics of the study population (sample size, age, and sex distribution); type of cancer; chemotherapy regimen; dose of bevacizumab; mean follow‐up; and study end points.
The trial quality was assessed according to the Cochrane Handbook for Systematic Reviews of Interventions.25 Each study was assessed separately for the following biases: (1) random sequence generation (selection bias); (2) allocation concealment (selection bias); (3) blinding of participants and personnel (performance bias); (4) blinding of outcome assessment (detection bias); (5) incomplete outcome data; (6) selective reporting (reporting bias); and (7) other bias.
Study End Points
The study end points were arterial adverse events, with a focus on cardiac and cerebral ischemia, venous adverse events, risk of bleeding, and arterial hypertension. The end points were defined according to the National Cancer Institute's common terminology criteria for adverse events.27, 28 Arterial adverse events were defined as one of the following: myocardial ischemia or infarction, cerebral infarction, cerebrovascular accident, cerebral ischemia, ischemic stroke, and peripheral or visceral arterial thrombotic events. Cardiac ischemia was defined as stable angina, unstable angina, non–ST‐segment elevation myocardial infarction, or ST‐segment elevation myocardial infarction. Cerebral ischemia was defined as follows: asymptomatic, radiographic findings only or a transient ischemic event with neurological deficit shorter than 24 hours or a cerebral vascular accident with neurological deficit longer than 24 hours. Venous adverse events were defined as one of the following: deep vein thrombosis, pulmonary embolism, and mesenteric or any other vein thrombosis. Bleeding was defined as any type of bleeding. Arterial hypertension was defined as a new occurrence of arterial tension values >140/90 mm Hg.
Statistical Analysis
The meta‐analysis was conducted on eligible studies by dividing the patients into the following 2 groups: the bevacizumab group, which included patients with cancer treated with bevacizumab and standard chemotherapy regimens, and the control group, which included patients with cancer treated with standard chemotherapy without bevacizumab. The proportion of patients with adverse events receiving bevacizumab was compared with that of the control group in the same RCT. The data are expressed as the risk ratios (RRs) and 95% CIs for dichotomous outcomes.29 For the analysis, we used both fixed‐effects and random‐effects models. We performed a subgroup analysis of each type of cancer, and we explored the relationship between the bevacizumab dose and adverse events by separating bevacizumab treatments into low‐dose treatments (5 or 7.5 mg/kg per dose per schedule, which is equivalent to 2.5 mg/kg per week) and high‐dose treatments (10 or 15 mg/kg per dose per schedule, which is equivalent to 5 mg/kg per week). Heterogeneity between studies was assessed using the Q statistic, and inconsistencies were quantified using the I 2 statistic. Because this test has poor power when there are few studies, we considered both the presence of significant heterogeneity at the 10% level of significance and a value of I 2 ≥56% as an indicator of significant heterogeneity.30 The presence of publication bias was assessed using the funnel plot test (Egger test). Studies with high precision are plotted near the average and studies with low precision are spread evenly on both sides of the average, creating a roughly funnel‐shaped distribution. Deviation from this shape indicates publication bias.31 Use of the funnel plot test was not recommended when the analysis included <10 studies.25 All analyses were conducted using Review Manager version 5.3 (Revman, The Cochrane Collaboration).
Results
Study Selection
The study selection process is shown in Figure 1 as a Preferred Reporting of Items for Systematic Meta‐Analysis flowchart. A total of 1450 full‐text articles were assessed for eligibility and 22 studies were selected for the meta‐analysis.32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 The characteristics of the selected studies are shown in Table 1. The quality of the included studies was high, as analyzed according to the recommendations of the Cochrane handbook (Figure S1).25 Ten studies32, 33, 35, 38, 39, 40, 41, 42, 43, 52, 53 included 9443 patients (47.09% of all patients) with colorectal cancer, 4 studies19, 34, 37, 45 included 4421 patients (22.04% of all patients) with breast cancer, 2 studies48, 49 included 1858 patients (9.26% of all patients) with ovarian cancer, 2 studies36, 51 included 1350 patients (6.73% of all patients) with renal cell cancer, 2 studies46, 50 included 1161 patients (5.79% of all patients) with non–small lung cell cancer, 1 study43 included 1050 patients (5.23% of all patients) with prostate cancer, and 1 study47 included 767 patients (3.82% of all patients) with gastric cancer. Eleven studies were included in the low‐dose group32, 33, 34, 35, 39, 40, 41, 47, 48, 52, 53 (2.5 mg/kg per week), 7 studies were included in the high‐dose (5 mg/kg per week) group,36, 37, 43, 45, 46, 49, 51 and 3 studies42, 44, 50 had patients treated with both regimens that could be separated.
Figure 1.
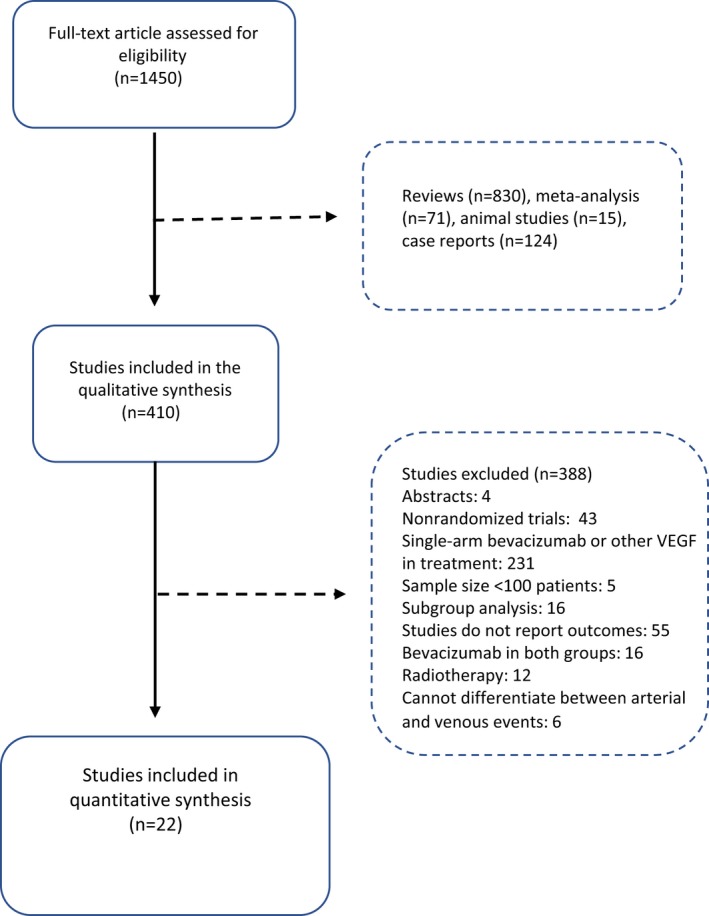
The Preferred Reporting of Items for Systematic Meta‐Analysis flowchart. VEGF indicates vascular endothelial growth factor
Table 1.
Studies Included in the Meta‐Analysis
| Study | Year | Type | Cancer Type | Treatment | Bevacizumab Dose, mg/kg per week | Mean Follow‐Up, mo | No. of Patients |
|---|---|---|---|---|---|---|---|
| Allegra32 | 2009 | RCT III | Stage II or III colon cancer | Bevacizumab+FOLFOX6 vs FOLFOX6 | 2.5 | 12 | 2647 |
| Bennouna33 | 2013 | RCT III | Metastatic colorectal cancer | Bevacizumab+oxaliplatin‐ or irinotecan‐based chemotherapy vs chemotherapy | 2.5 | 11 | 810 |
| Cameron34 | 2013 | RCT III | Triple‐negative breast cancer | Bevacizumab+chemotherapy (anthracycline, taxane, or both) vs chemotherapy | 5 | 32 | 2559 |
| de Gramont35 | 2012 | RCT III | Colon cancer | Bevacizumab+FOLFOX4 vs FOLFOX4 | 2.5 | 48 | 2271 |
| Escudier36 | 2007 | RCT III | Metastatic renal cell carcinoma | Bevacizumab+ interferon α‐2a vs placebo+interferon α‐2a | 5 | 13 | 641 |
| Gianni37 | 2013 | RCT III | HER2‐positive locally recurrent/metastatic breast cancer | Bevacizumab+docetaxel+trastuzumab vs docetaxel+trastuzumab | 5 | 26 | 421 |
| Giantonio38 | 2007 | RCT III | Metastatic colorectal cancer | Bevacizumab+FOLFOX4 vs placebo+FOLFOX 4 | 5 | 28 | 572 |
| Guan39 | 2011 | RCT III | Metastatic colorectal cancer | Bevacizumab+mIFL vs mIFL | 2.5 | 22 | 203 |
| Hurwitz40 | 2004 | RCT III | Metastatic colorectal cancer | Bevacizumab+IFL vs placebo+IFL | 2.5 | 21 | 790 |
| Hurwitz41 | 2005 | RCT III | Metastatic colorectal cancer | Bevacizumab+fluoruracil+leucovirin vs placebo+IFL | 2.5 | 30 | 207 |
| Kabbinavar42 | 2003 | RCT II | Metastatic colorectal cancer | Bevacizumab+fluorouracil and leucovorin vs placebo+fluorouracil and leucovorin | 2.5 or 5 | 21 | 102 |
| Kelly43 | 2012 | RCT III | Metastatic castration‐resistant prostate cancer | Bevacizumab+docetaxel+prednisone vs docetaxel+prednisone | 5 | 23 | 1050 |
| Miles44 | 2010 | RCT III | Human epidermal growth factor receptor 2–negative metastatic breast cancer | Bevacizumab+docetaxel vs placebo+docetaxel | 2.5 or 5 | 25 | 730 |
| Miller45 | 2007 | RCT III | Metastatic breast cancer | Bevacizumab+paclitaxel vs paclitaxel | 5 | 27 | 711 |
| Niho46 | 2012 | RCT II | Nonsquamous non–small cell lung cancer | Bevacizumab+carboplatin+paclitaxel vs carboplatin+paclitaxel | 5 | 23 | 175 |
| Ohtsu47 | 2011 | RCT III | Advanced gastric cancer | Bevacizumab+cisplatin vs placebo+cisplatin | 2.5 | 12 | 767 |
| Perren48 | 2011 | RCT III | Ovarian cancer | Bevacizumab+carboplatin+paclitaxel vs carboplatin+paclitaxel | 2.5 | 42 | 1498 |
| Pujade‐Lauraine49 | 2014 | RCT III | Platinum‐resistant recurrent ovarian cancer | Bevacizumab+chemotherapy (pegylated liposomal doxorubicin, paclitaxel, or topotecan) vs chemotherapy | 5 | 14 | 360 |
| Reck50 | 2009 | RCT III | Nonsquamous non–small cell lung cancer | Bevacizumab+cisplatin+gemcitabine vs cisplatin+gemcitabine | 2.5 or 5 | 13 | 986 |
| Rini51 | 2010 | RCT III | Metastatic renal cell carcinoma | Bevacizumab+ interferon α‐2a vs placebo+interferon α‐2a | 5 | 46 | 709 |
| Saltz52 | 2008 | RCT III | Metastatic colorectal cancer | Bevacizumab+FOLFOX4 or XELOX vs placebo+FOLFOX4 or XELOX | 2.5 | 28 | 1369 |
| Tebbutt53 | 2010 | RCT III | Metastatic colorectal cancer | Bevacizumab+capecitabine vs capecitabine | 2.5 | 31 | 472 |
FOLFOX, fluorouracil+folinate+oxaliplatin; IFL, irinotecan+leucovorin+fluorouracil; mIFL, modified irinotecan+leucovorin+fluorouracil; RCT, randomized controlled trial; XELOX, capecitabine+oxaliplatin.
Bevacizumab and Arterial Adverse Events
The patients treated with bevacizumab were at a higher risk of arterial adverse events compared with controls (RR, 1.37; 95% CI, 1.10–1.70 [P=0.004]). This result was obtained by pooling the data from 19 randomized studies32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 including 18 028 patients (Figure 2). The heterogeneity between the included studies was not significant. The risk of bias for reporting arterial adverse events was low based on the funnel plot test (Figure S2). The risk for arterial adverse events was higher in the high‐dose bevacizumab group (RR, 1.71; 95% CI, 1.06–2.77 [P=0.03]), as reported from 9 studies34, 36, 37, 38, 45, 46, 49, 50, 51 including 6671 patients, without significant heterogeneity. The risk for arterial adverse events was not significantly increased in the low‐dose bevacizumab group (RR, 1.22; 95% CI, 0.97–1.54 [P=0.09]). The analysis included 12 015 patients from 12 studies.1 Arterial adverse events were defined as one of myocardial ischemia or infarction, cerebral infarction, cerebrovascular accident, cerebral ischemia, ischemic stroke, and peripheral or visceral arterial thrombotic events, as defined by the National Cancer Institute's Common Toxicity Criteria.27, 28 To provide a more specific description of the subtypes of arterial events, we extracted from our data the RRs for cardiac and cerebral ischemic adverse events. Cardiac ischemia was defined as stable angina, unstable angina, non–ST‐segment elevation myocardial infarction, or ST‐segment elevation myocardial infarction. Cerebral ischemia was defined as asymptomatic, radiographic findings only or a transient ischemic event with neurological deficit shorter than 24 hours or a cerebral vascular accident with neurological deficit longer than 24 hours.
Figure 2.
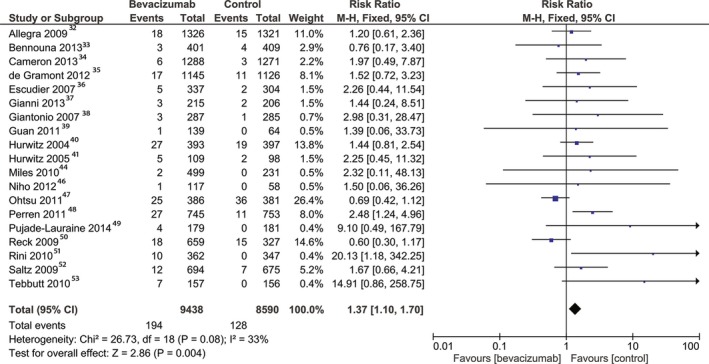
Overall estimate and estimates from each study of the risk ratio (RR) of arterial adverse events associated with bevacizumab treatment. The first author and the publication year were used for each study. The total number of events and the sample size are shown for each study. The weight of each study in the final analysis is indicated as a percentage. The RR for each study is shown numerically on the left and graphically on the right. Square boxes denote the risk ratio, horizontal lines represent 95% CIs, and the diamond plot represents the overall results of the included trials. Weights are from a fixed‐effects analysis. M‐H indicates Mantel‐Haenszel statistical method.
Bevacizumab and cardiac ischemia
The patients treated with bevacizumab were at higher risk of cardiac ischemia compared with the controls (RR, 2.47; 95% CI, 1.4–4.36 [P=0.002]). This result was obtained by pooling the data extracted from 5 studies32, 33, 38, 43, 51 that reported outcomes for a total of 5828 patients (Figure 3A). A total of 3457 patients had colorectal cancer, 1050 had prostate cancer, 709 had renal cancer, and 572 had breast cancer. The heterogeneity between the selected studies was not significant.
Figure 3.
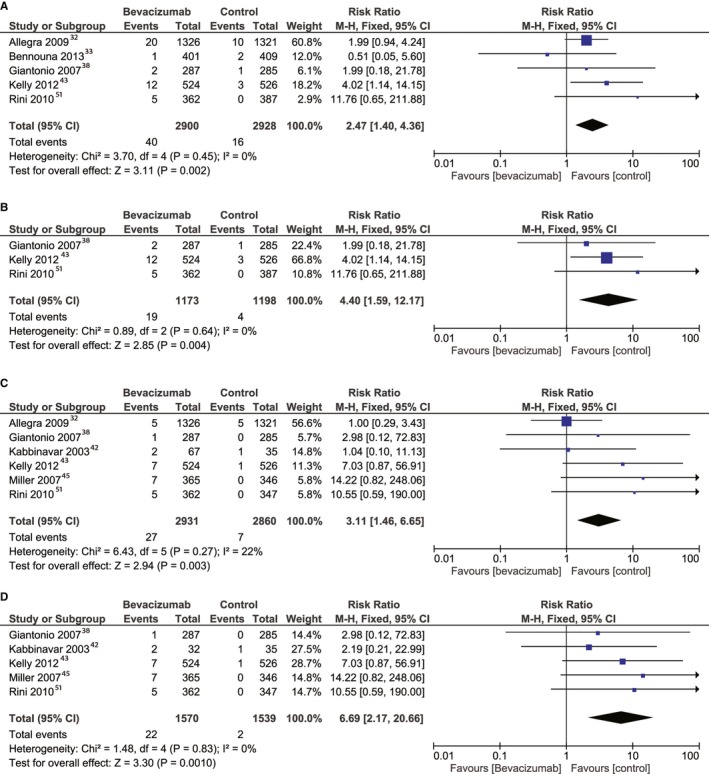
Overall estimate and estimates from each study of the risk ratio (RR) of cardiac ischemia associated with bevacizumab treatment (A), cardiac ischemia associated with high‐dose bevacizumab treatment (B), cerebral ischemia associated with bevacizumab treatment (C), and cerebral ischemia associated with a high‐dose bevacizumab regimen (D). The first author and the publication year were used for each study. The total number of events and sample size are shown for each study. The weight of each study in the final analysis is shown in percentages. The RR for each study is shown numerically on the left and graphically on the right. Square boxes denote the RR, horizontal lines represent 95% CIs, and the diamond plot represents overall results of the included trials. Weights are from fixed‐effects analysis. M‐H indicates Mantel‐Haenszel statistical method.
When pooling data from high‐dose bevacizumab from 3 studies38, 43, 51 that reported on cardiac ischemia, with a total number of 2371 patients, the RR was nearly doubled compared with that obtained by pooling data from all the patients taking bevacizumab, with a value of 4.4 (95% CI, 1.59–12.17; P=0.004), with statistically insignificant heterogeneity (Figure 3B). The low‐dose bevacizumab analysis included 3457 patients from 2 studies,32, 33 with an RR of 1.76 (95% CI, 0.86–3.59; P=0.12).
Bevacizumab and cerebral ischemia
An RR of 3.11 (95% CI, 1.46–6.65; P=0.003) indicated a higher risk of cerebral ischemia for patients treated with bevacizumab versus controls. The outcome was reported in 6 studies32, 38, 42, 43, 45, 51 for a total number of 5791 patients (Figure 3C). A total of 3321 patients had colorectal cancer, 1050 had prostate cancer, 711 had breast cancer, and 709 had renal cancer. The heterogeneity between the selected studies was statistically insignificant.
When pooling data from high‐dose bevacizumab from 5 studies19, 38, 42, 43, 54 that reported on cerebral ischemia, with a total of 3109 patients, the RR was 2‐fold higher than that obtained by pooling data from all the patients taking bevacizumab, with a value of 6.69 (95% CI, 2.17–20.66; P=0.001) and not significant heterogeneity (Figure 3D). The low‐dose bevacizumab analysis included 2717 patients from 2 studies,32, 42 with an RR for cerebral ischemia of 0.84 (95% CI, 0.27–2.63; P=0.77).
Bevacizumab and Venous Adverse Events
The patients treated with bevacizumab were at higher risk of venous adverse events compared with controls (RR, 1.29; 95% CI, 1.13–1.48 [P<0.001]). The result was obtained by pooling the data from 18 studies,32, 33, 34, 35, 36, 37, 40, 41, 42, 44, 45, 46, 47, 48, 49, 50, 52, 53 including a total of 17 339 patients (Figure 4). The heterogeneity was statistically insignificant among the studies. The risk of bias for reporting the venous adverse events was low (Figure S3). The analysis of high‐dose bevacizumab included 6068 patients from 9 studies34, 36, 37, 42, 44, 45, 46, 49, 50 and yielded an RR of 1.08 (95% CI, 0.79–1.47; P=0.63). The analysis of low‐dose bevacizumab included 11 564 patients from 12 studies2 and generated an RR of 1.36 (95% CI, 1.17–1.59; P<0.0001).
Figure 4.
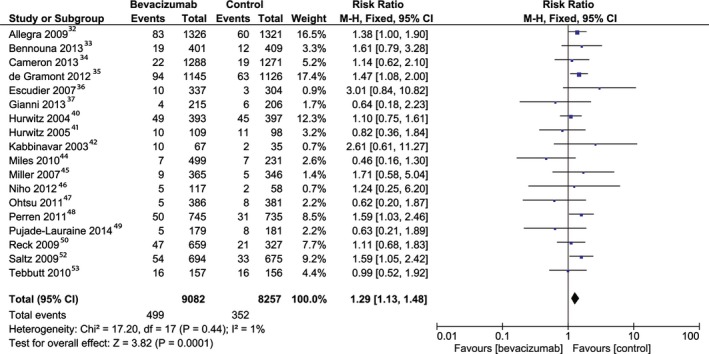
Overall estimate and estimates from each study of the risk ratio (RR) of venous adverse events associated with bevacizumab treatment. The first author and the publication year were used for each study. The total number of events and the sample size are shown for each study. The weight of each study in the final analysis is indicated as a percentage. The RR for each study is shown numerically on the left and graphically on the right. Square boxes denote the RR, horizontal lines represent 95% CIs, and the diamond plot represents the overall results of the included trials. Weights are from a fixed‐effects analysis.
Bevacizumab and Bleeding
The risk of bleeding was higher in the bevacizumab group (RR, 2.74; 95% CI, 2.38–3.15 [P<0.001]) (Figure 5A). The analysis included 19 studies32, 33, 35, 36, 37, 38, 39, 41, 42, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 consisting of 16 701 patients. The heterogeneity between the studies was significant and the risk of bias was low (Figure 5B).
Figure 5.
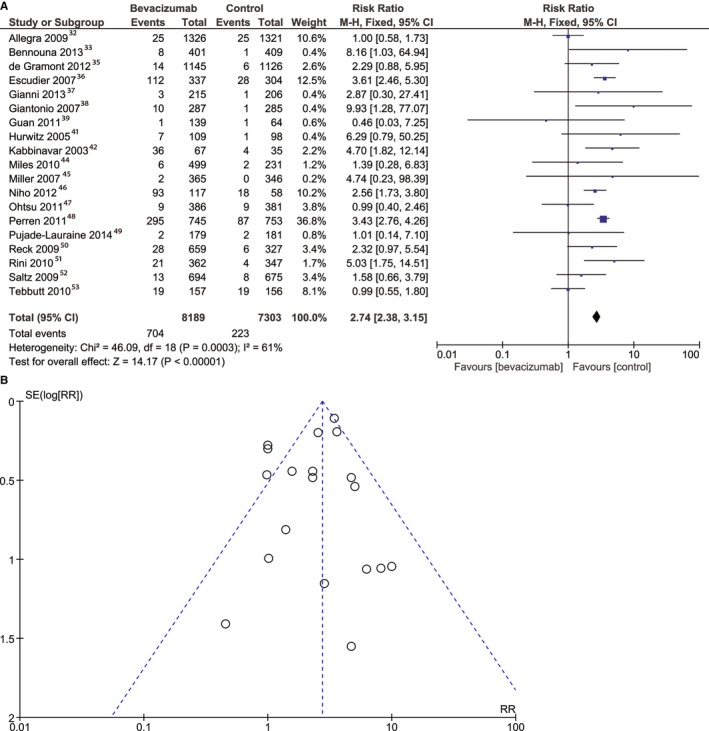
Overall estimate and estimates from each study of the risk ratio (RR) of bleeding (A) and risk of bias for bleeding (B) associated with bevacizumab treatment. The first author and the publication year were used for each study. The total number of events and the sample size are shown for each study. The weight of each study in the final analysis is indicated as a percentage. The relative risk for each study is shown numerically on the left and graphically on the right. Square boxes denote the RR, horizontal lines represent 95% CIs, and the diamond plot represents the overall results of the included trials. Weights are from a fixed‐effects analysis. Each dot represents one study included in the analysis of bleeding events. The SE (log RR) axis represents study precision, and the risk ratio (RR) axis shows the study results. M‐H indicates Mantel‐Haenszel statistical test.
The risk of bleeding was higher in the high‐dose bevacizumab group (RR, 3.32; 95% CI, 2.61–4.22 [P<0.001]); this analysis was based on data pooled from 10 studies36, 37, 38, 42, 44, 45, 46, 49, 50, 51 of a total of 4790 patients. The RR of bleeding between groups was 2.98 (95% CI, 2.47–3.61 [P<0.00001]), when the data were pooled from 12 studies of low‐dose bevacizumab with 11 295 patients.3
Bevacizumab and Arterial Hypertension
The risk of arterial hypertension was higher in the bevacizumab group (RR, 4.73; 95% CI, 4.15–5.39 [P<0.001]) (Figure 6A). The heterogeneity between studies was statistically significant. The risk of bias was low (Figure 6B). The risk for arterial hypertension was higher in the high‐dose bevacizumab group (RR, 7.11; 95% CI, 5.6–9.03 [P<0.001]), and it remained high in the low‐dose bevacizumab group with an RR of 5.07 (95% CI, 4.26–6.03 [P<0.00001]).
Figure 6.
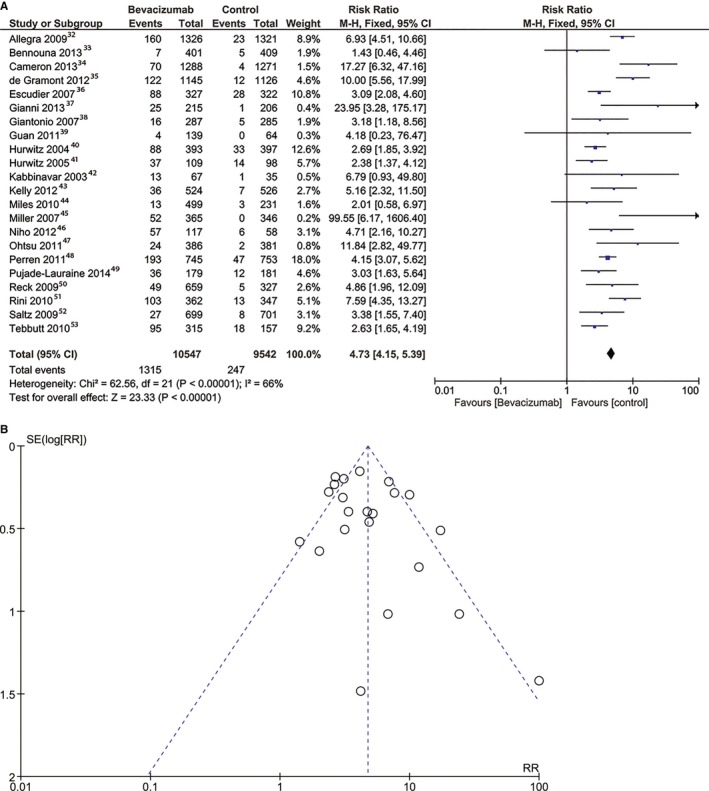
Overall estimate and estimates from each study of the risk ratio (RR) of arterial hypertension (A) and risk of bias for arterial hypertension (B) associated with bevacizumab treatment. The first author and the publication year were used for each study. The total number of events and the sample size are shown for each study. The weight of each study in the final analysis is indicated as a percentage. The relative risk for each study is shown numerically on the left and graphically on the right. The square boxes denote the RR, horizontal lines represent 95% CIs, and the diamond plot represents the overall results of the included trials. Weights are from a fixed‐effects analysis. Each dot represents one study included in the analysis of bleeding events. The SE (log risk ratio [RR]) axis represents study precision, and the RR axis shows the study results. M‐H indicates Mantel‐Haenszel statistical method.
Heterogeneity Between Studies, Inconsistency, and Publication Bias
There was no significant heterogeneity between studies, except for the bleeding and arterial hypertension analyses, as previously decribed (Figures 5A and 6A). The publication bias was not significant, as assessed using the Egger test.
Sensitivity Analysis
A sensitivity analysis was performed by excluding each study, in turn, from the analysis to address the relative importance of each study. Bevacizumab treatment remained a risk factor for the selected outcomes.
Subgroup Analysis
There was no significant difference in the age of the patients with different subtypes of cancer. The mean patient age in the bevacizumab group was 58±4 years compared with 58±4 years in the control group (P=0.9). The sex distribution was not different between the bevacizumab group and the control group. Taken together, based on the present data, the influence of sex and age on bevacizumab‐induced cardiovascular events cannot be determined.
Bevacizumab increased the risk of arterial adverse events in colorectal, renal, and ovarian cancer, with the highest RR observed for renal cancer, and increased the risk of cardiac ischemia in prostate cancer. The risk of venous adverse events was increased in colorectal cancer. For the other types of cancer, the risk of arterial and venous adverse events was similar between groups. The risk of bleeding was increased in colorectal, renal, ovarian, and lung cancer, with the highest RR for renal cancer. The risk of arterial hypertension was increased in all types of cancer, with the highest RR for breast cancer (Table 2).
Table 2.
Risk ratios for Adverse Events for Each Type of Cancer
| Cancer Type | Arterial Adverse Events | Cardiac Ischemia | Cerebral Ischemia | Venous Adverse Events | Bleeding | Arterial Hypertension |
|---|---|---|---|---|---|---|
| Colorectal cancer | 1.54 (1.12–2.12), P=0.008a | 1.77 (0.90–3.48), P=0.1 | 1.15 (0.41–3.20), P=0.79 | 1.34 (1.14–1.58), P=0.0003a | 1.78 (1.32–2.38), P=0.0001a | 3.68 (2.44–5.53), P<0.00001a |
| Renal cancer | 5.75 (1.53–21.58), P=0.01a | 11.76 (0.65–211.88), P=0.09 | 10.55 (0.59–190.00), P=0.11 | 3.01 (0.84–10.82), P=0.09 | 3.78 (2.63–5.43), P<0.00001a | 4.74 (1.94–11.61), P=0.0006a |
| Breast cancer | 1.82 (0.65–5.09), P=0.25 | Not estimable | 14.22 (0.82–248.06), P=0.07 | 0.94 (0.56–1.57), P=0.8 | 2.15 (0.67–6.88), P=0.2 | 13.45 (2.69–67.21), P=0.02a |
| Ovarian cancer | 2.77 (1.42–5.40), P=0.003a | Not estimable | Not estimable | 1.16 (0.49–2.74), P=0.74 | 3.37 (2.72–4.18), P<0.00001a | 3.91 (2.98–5.13), P=0.00001a |
| Lung cancer | 0.62 (0.32–1.20), P=0.16 | Not estimable | Not estimable | 1.12 (0.70–1.80), P=0.64 | 2.50 (1.73–3.60), P<0.00001a | 4.77 (2.64–8.63), P<0.00001a |
| Cancer type | Arterial adverse events | Cardiac ischemia | Cerebral ischemia | Venous adverse events | Bleeding | Arterial hypertension |
| Prostate cancer | Not estimable | 4.02 (1.14–14.15), P=0.03a | 7.03 (0.87–56.91), P=0.07 | Not estimable | Not estimable | 5.16 (2.32–11.50), P<0.0001a |
| Gastric cancer | 0.69 (0.42–1.12), P=0.13 | Not estimable | Not estimable | 0.62 (0.20–1.87), P=0.39 | 0.99 (0.40–2.46), P=0.98 | 11.84 (2.82–49.77), P=0.007a |
Data are expressed as risk ratio (95% CI), P value.
Statistically significant data.
We performed a subgroup analysis considering the follow‐up time of each study. We divided the studies into studies with 11 to 14 months of follow‐up,32, 33, 36, 47, 49, 50 21 to 24 months of follow‐up,37, 39, 40, 42, 43, 46 and more than 24 months of follow‐up.4 The RR for arterial adverse events, cerebral ischemia, and venous adverse events was significantly higher for the group with more than 24 months of follow‐up, without reaching significance for shorter follow‐up times. Cardiac ischemia was significantly higher for the group with 21 to 24 months of follow‐up, but this result was derived from a single study. Bleeding and arterial hypertension were significantly higher in the bevacizumab group for all 3 subgroups, irrespective of the follow‐up times (Table 3).
Table 3.
Risk ratios for Adverse Events for Different Follow‐Up Times
| Follow‐Up Time | 11–14 mo | 21–24 mo | >24 mo |
|---|---|---|---|
| Arterial adverse events | 0.86 (0.63–1.18), P=0.35 | 1.44 (0.85–2.44), P=0.18 | 2.40 (1.64–3.52), P<0.001a |
| Cardiac ischemia | 1.75 (0.86–3.54), P=0.12 | 4.02 (1.14–14.15), P=0.03a | 5.16 (0.91–29.33), P=0.06 |
| Cerebral ischemia | 1.00 (0.29–3.43), P=1 | 3.63 (0.85–15.45), P=0.08 | 12.39 (1.62–94.49), P=0.02a |
| Venous adverse events | 1.26 (0.95–1.67), P=0.12 | 1.06 (0.74–1.51), P=0.75 | 1.37 (1.11–1.68), P=0.03a |
| Bleeding | 2.26 (1.74–2.95), P<0.001a | 2.84 (1.98–4.06), P<0.001a | 2.96 (2.46–3.56), P<0.001a |
| Arterial hypertension | 4.06 (2.52–6.54), P<0.001a | 4.30 (2.59–7.14), P<0.001a | 4.81 (3.10–7.46), P=0.001a |
Data are expressed as risk ratio (95% CI), P value.
Statistically significant.
Discussion
We performed a comprehensive meta‐analysis of the cardiovascular complications in patients with cancer treated with bevacizumab compared with those treated with standard chemotherapy. The study pooled data from 22 studies, including more than 20 000 patients. The main findings are as follows: (1) patients treated with bevacizumab have a significantly higher risk of developing arterial adverse events compared with controls, with a higher risk of cardiac and cerebral ischemia; (2) patients treated with bevacizumab have a higher risk of venous adverse events compared with controls; (3) the risk of bleeding is significantly higher in patients with cancer treated with bevacizumab compared with controls; (4) the risk of developing arterial hypertension is significantly higher in the bevacizumab group; (5) patients treated with high‐dose bevacizumab have a higher risk of arterial adverse events, cardiac and cerebral ischemia, bleeding, and arterial hypertension, but the dosage had no effect on venous adverse events; and (6) the highest RR of arterial adverse events was observed for renal cancer, the highest RR of cardiac ischemia for prostate cancer, the higher RR of bleeding for renal cancer, and the highest RR of arterial hypertension for breast cancer. These findings are of substantial importance for the daily care of patients with cancer and could contribute to the advancement of treatment protocols, with emphasis on cardiovascular surveillance, prevention, and multidisciplinary decisions by cardio‐oncology teams.
Bevacizumab is the pioneer of all VEGF monoclonal antibodies and it has been extensively used since its first approval more than 1 decade ago. Consequently, it is mandatory to characterize the entire range of its potentially adverse effects. Notably, the underlying mechanisms through which bevacizumab produces a prothrombotic status have not yet been fully elucidated. It is well‐known that hypertension injures the endothelium, leading to a prothrombotic status.55 This effect may be caused and exacerbated by bevacizumab‐dependent inhibition of VEGF, leading to a decrease in NO generation by endothelial cells. NO, in turn, is a potent vasodilator whose absence contributes to platelet aggregation and adhesion.56, 57, 58, 59 Furthermore, VEGF blockade increases the expression of proinflammatory genes.56 In addition, these effects could accelerate in situ thrombus formation and explain the higher incidence of arterial and venous adverse events, including cardiac and cerebral ischemia. Conversely, the use of bevacizumab was associated with an increased risk of bleeding explained by the inhibition of VEGF, which diminishes the regenerative capacity of endothelial cells and causes endothelial defects that expose procoagulant phospholipids on the luminal plasma membrane or underlying matrix, leading to both thrombosis and hemorrhage.60, 61, 62, 63
Naturally, the majority of the current RCTs and meta‐analyses in the scope of VEGF inhibition have focused on either overall survival rates or event‐free survival, particularly in patients with colorectal cancer, without describing the complete range of adverse events in all types of malignancies for which bevacizumab is prescribed.64, 65, 66
A higher incidence of arterial adverse events in patients treated with bevacizumab has been previously described, concordant with our findings.6, 24, 67, 68 In contrast to prior analyses, here we have included several novel and important studies, assessed the differential impact of different cancer diagnoses on various types of arterial events, and approached the potential impact of dosage on risk. In addition, we have attempted to resolve contradictory reports on the risk of venous events, with respect to specific tumor types.17, 69, 70 When reporting the venous adverse events in all cancer types, the results were divergent, with patients treated with bevacizumab exhibiting similar or higher risk of venous adverse events compared with controls for both low‐dosage and high‐dosage subgroups.71, 72 These outcomes are in contradiction with our findings, suggesting that this issue should be further addressed in future RCTs, in order to precisely indicate the impact of tumor type, age, functional status, venous thromboembolism history, or the use of anticoagulants on developing venous adverse events.72
Cardiovascular adverse effects have been reported in colorectal cancer,5, 24, 73, 74, 75, 76 ovarian cancer,17 non–small cell lung cancer,70 breast cancer,69 and renal cancer77; however, as previously mentioned, these analyses do not include information regarding the complete spectrum of cardiovascular adverse events, the type of events, the impact of the dosage, or the cancer type.6, 71 The incidence of adverse events was shown to differ based on cancer type, and this result is concordant with our findings.78 Furthermore, the risk of arterial adverse events in different cancer subtypes is a controversial topic, with studies reporting an increased risk of arterial events in colorectal cancer and ovarian cancer,17 but not non–small cell lung cancer70 or breast cancer,69 which is partially concordant with our results. We reported the highest risk of arterial adverse events for patients with renal cancer, which could be explained by the higher incidence of arterial hypertension in this cancerous disease, with its subsequent endothelial damage and thrombosis.79 The different incidence of adverse events among specific cancer types could be partially explained by the variable expression of VEGF in different cancer types and subtypes.80 Additional explanations of this effect could be the concurrent comorbidities, different stages of the carcinoma, the different effect of co‐chemotherapies, and the lack of standardization in reporting the outcomes. Although we excluded patients who were treated with radiotherapy known to increase the risk of cardiovascular adverse events, the impact of chemotherapies such as 5‐fluorouracil or taxanes cannot be dissociated from the global outcomes.81 Taken together, randomized prospective studies are warranted regarding bevacizumab‐associated cardiovascular events.
There is less evidence regarding dose‐dependent increases in cardiovascular events. Here, we determined that higher‐dose bevacizumab regimens are associated with an increased risk of arterial adverse events, including cardiac and cerebral ischemia, bleeding events, and arterial hypertension, with no effect on the occurrence of venous adverse events. Moreover, the low‐dose regimens are not associated with a significantly higher incidence of arterial adverse effects, including cardiac and cerebral ischemia. These findings are similar to other analyses, but the small number of comparative studies, their small size, and the reporting modality make the comparison between dose regimens difficult.78, 82, 83 The only prior study comparing low‐dose and high‐dose regimens showed no differences in terms of safety between the 2 regimens, but it should be noted that in that study patients were selected for second‐line therapy, having been previously treated with bevacizumab, potentially limiting the generalizability of that result.84 It would be of importance to establish the ideal bevacizumab dose that would have antitumoral effects without causing cardiovascular adverse events. In vitro studies have shown that lower doses are sufficient to induce vascular normalization and that higher doses are necessary to obtain a direct cytotoxic effect.85 However, higher doses could generate additional unfavorable conditions, particularly hypoxia, that increase the incidence of adverse events.86 As a consequence, the actual data do not have sufficient power to indicate the ideal bevacizumab dosage or an algorithm of dose reduction in patients with cancer at risk for cardiovascular disease.19, 50, 82, 83
Bleeding events have been characterized as a major adverse event during therapy with bevacizumab. The risk of bleeding appears to be higher in patients treated with bevacizumab, concordant with our findings, but the risk of severe bleeding was not significantly increased in colorectal cancer.60, 61 The general risk of bleeding also includes minor bleeding events, such as epistaxis or gingival bleeding, and could be highly variable among subtypes of cancer, as shown in our report. In addition, the factors that increase the risk of hemorrhage could not be precisely identified, making the impact of bleeding on the management of these patients hard to estimate. The highest RR of bleeding in patients with renal cancer could be explained by the higher incidence of endothelial damage secondary to arterial hypertension.79 The risk of bleeding is different among cancer types and depends on the stages of carcinoma, the presence of thrombocytopenia, renal and hepatic function, the presence of comorbidities, and predisposal to bleeding of each patient.87 Further efforts are necessary to report indications that could be used as guidelines for clinical practice.60
The analysis of our data showed a low heterogeneity among studies for all outcomes, except for bleeding and arterial hypertension. In these 2 analyses, the heterogeneity could be explained by the fact that some studies report only high‐grade bleeding and hypertension and not events of all grades.
Because of the paramount impact of severe cardiovascular adverse events on survival, the use of an integrative cardio‐oncology approach has received increasing attention in the past years.88 Until now, the strategies to prevent cardiovascular adverse events in patients with cancer treated with VEGF inhibitors, such as baseline cardiovascular risk assessment, optimal control of arterial hypertension, and adjustment of chemotherapy dosage, have received the main attention, while the preventive administration of low‐molecular‐weight heparin in these patients is controversial.81, 89 Routine thromboprophylaxy with low‐molecular‐weight heparin is not recommended for ambulatory patients with cancer, but it may be considered for selected high‐risk patients. It is also indicated in the setting of major surgery and for the treatment of deep vein thrombosis or pulmonary embolism. The use of novel oral anticoagulants is not currently recommended for secondary prevention in patients with malignancy.90 Data regarding the value of aspirin prophylaxis for arterial thromboembolism in patients treated with bevacizumab raises unsolved controversies about the benefit‐risk balance.24, 91 The use of aspirin is limited at this moment to patients with multiple myeloma receiving antiangiogenesis agents with chemotherapy and/or dexamethasone, as an alternative to low‐molecular‐weight heparin.92 In addition, the favorable cardiomyocyte protective role of statins that arise from the anthracycline‐based studies could not be easily translated to antiangiogenic therapies because of different mechanisms of action and toxicity.93, 94 Moreover, the potential benefits of thromboprophylaxis would need to be carefully weighed against increased bleeding risk, and ideally in a prospective fashion in order to determine the optimal therapeutic attitude. As a consequence, there are still many unanswered questions regarding the efficacy of primary prevention or the effects of interrupting chemotherapy because of cardiovascular adverse events that need to be addressed in the future.95, 96 As derived from our subgroup analysis of the follow‐up time, arterial and venous adverse events tend to be significant and proportionally higher with more than 24 months of follow‐up, suggesting that these patients need long‐term cardiological follow‐up after treatment with bevacizumab.
Study Limitations
Our study has some limitations that need to be addressed. First, we analyzed different types of cancer treated with different chemotherapy regimens at different doses. Second, our study included all grades of adverse events, and some studies only reported high‐grade events. Third, the population included in the selected studies could have been selected using strict exclusion criteria, and the included patients could have been at low risk of cardiovascular events. Finally, in most of the studies, the vascular adverse events were secondary end points and were not always reported accurately.
Conclusions
Treatment with bevacizumab increases the risk of arterial adverse events, particularly cardiac and cerebral ischemia, venous adverse events, bleeding, and arterial hypertension. This risk is additionally increased with high doses of bevacizumab. Further studies should determine the appropriate cardio‐oncology management options.
Sources of Funding
Mincu was supported by a research grant from the European Society of Cardiology (R‐2016‐013). Rassaf was supported by a grant from the Medical Faculty of the University Duisburg‐Essen. The remaining authors have no disclosures to report.
Disclosures
None.
Supporting information
Table S1. Results of the MEDLINE Search Through November 21, 2016
Figure S1. The quality of the included studies as analyzed per recommendations from the Cochrane Handbook for Systematic Reviews of Interventions.
Figure S2. Risk of bias for arterial adverse events. Each dot represents one study included in the analysis of arterial adverse events. The SE (log risk ratio [RR]) axis represents study precision, and the RR axis shows the study results.
Figure S3. Risk of bias for venous adverse events. Each dot represents one study included in the analysis of venous adverse events. The SE (log RR) axis represents study precision, and the RR axis shows the study results.
(J Am Heart Assoc. 2017;6:e006278 DOI: 10.1161/JAHA.117.006278.)28862931
Notes
References
- 1. Brown C. Targeted therapy: an elusive cancer target. Nature. 2016;537:S106–S108. [DOI] [PubMed] [Google Scholar]
- 2. Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: a meta‐analysis. Lancet Oncol. 2009;10:559–568. [DOI] [PubMed] [Google Scholar]
- 3. Lin Z, Zhang Q, Luo W. Angiogenesis inhibitors as therapeutic agents in cancer: challenges and future directions. Eur J Pharmacol. 2016;793:76–81. [DOI] [PubMed] [Google Scholar]
- 4. Ahmadizar F, Onland‐Moret NC, de Boer A, Liu G, Maitland‐van der Zee AH. Efficacy and safety assessment of the addition of bevacizumab to adjuvant therapy agents in cancer patients: a systematic review and meta‐analysis of randomized controlled trials. PLoS One. 2015;10:e0136324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alahmari AK, Almalki ZS, Alahmari AK, Guo JJ. Thromboembolic events associated with bevacizumab plus chemotherapy for patients with colorectal cancer: a meta‐analysis of randomized controlled trials. Am Health Drug Benefits. 2016;9:221–232. [PMC free article] [PubMed] [Google Scholar]
- 6. Ranpura V, Hapani S, Chuang J, Wu S. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: a meta‐analysis of randomized controlled trials. Acta Oncol. 2010;49:287–297. [DOI] [PubMed] [Google Scholar]
- 7. Nazer B, Humphreys BD, Moslehi J. Effects of novel angiogenesis inhibitors for the treatment of cancer on the cardiovascular system: focus on hypertension. Circulation. 2011;124:1687–1691. [DOI] [PubMed] [Google Scholar]
- 8. Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti‐VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. [DOI] [PubMed] [Google Scholar]
- 9. Moens S, Goveia J, Stapor PC, Cantelmo AR, Carmeliet P. The multifaceted activity of VEGF in angiogenesis—implications for therapy responses. Cytokine Growth Factor Rev. 2014;25:473–482. [DOI] [PubMed] [Google Scholar]
- 10. European Medicines Agency . Science Medicines Health. Avastin. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000582/human_med_000663.jsp&mid=WC0b01ac058001d124. Accessed November 21, 2016.
- 11. National Cancer Institute . FDA Approval for bevacizumab. Available at: https://www.cancer.gov/about-cancer/treatment/drugs/fda-bevacizumab. Accessed November 21, 2016.
- 12. Hess GP, Wang PF, Quach D, Barber B, Zhao Z. Systemic therapy for metastatic colorectal cancer: patterns of chemotherapy and biologic therapy use in US medical oncology practice. J Oncol Pract. 2010;6:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Behera M, Pillai RN, Owonikoko TK, Kim S, Steuer C, Chen Z, Saba NF, Belani CP, Khuri FR, Ramalingam SS. Bevacizumab in combination with taxane versus non‐taxane containing regimens for advanced/metastatic nonsquamous non‐small‐cell lung cancer: a systematic review. J Thorac Oncol. 2015;10:1142–1147. [DOI] [PubMed] [Google Scholar]
- 14. Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65:671–680. [PubMed] [Google Scholar]
- 15. Golfinopoulos V, Salanti G, Pavlidis N, Ioannidis JP. Survival and disease‐progression benefits with treatment regimens for advanced colorectal cancer: a meta‐analysis. Lancet Oncol. 2007;8:898–911. [DOI] [PubMed] [Google Scholar]
- 16. Jubb AM, Harris AL. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol. 2010;11:1172–1183. [DOI] [PubMed] [Google Scholar]
- 17. Marchetti C, De Felice F, Palaia I, Musella A, Di Donato V, Gasparri ML, Musio D, Muzii L, Tombolini V, Panici PB. Efficacy and toxicity of bevacizumab in recurrent ovarian disease: an update meta‐analysis on phase III trials. Oncotarget. 2016;7:13221–13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu YS, Shui L, Shen D, Chen X. Bevacizumab combined with chemotherapy for ovarian cancer: an updated systematic review and meta‐analysis of randomized controlled trials. Oncotarget. 2016;8:10703–10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD, Sing AP, Langmuir V, Rugo HS. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. [DOI] [PubMed] [Google Scholar]
- 20. Passardi A, Nanni O, Tassinari D, Turci D, Cavanna L, Fontana A, Ruscelli S, Mucciarini C, Lorusso V, Ragazzini A, Frassineti GL, Amadori D. Effectiveness of bevacizumab added to standard chemotherapy in metastatic colorectal cancer: final results for first‐line treatment from the ITACa randomized clinical trial. Ann Oncol. 2015;26:1201–1207. [DOI] [PubMed] [Google Scholar]
- 21. Tewari KS, Sill MW, Long HJ III, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, Michael HE, Monk BJ. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. von Minckwitz G, Puglisi F, Cortes J, Vrdoljak E, Marschner N, Zielinski C, Villanueva C, Romieu G, Lang I, Ciruelos E, De Laurentiis M, Veyret C, de Ducla S, Freudensprung U, Srock S, Gligorov J. Bevacizumab plus chemotherapy versus chemotherapy alone as second‐line treatment for patients with HER2‐negative locally recurrent or metastatic breast cancer after first‐line treatment with bevacizumab plus chemotherapy (TANIA): an open‐label, randomised phase 3 trial. Lancet Oncol. 2014;15:1269–1278. [DOI] [PubMed] [Google Scholar]
- 23. Huang H, Zheng Y, Zhu J, Zhang J, Chen H, Chen X. An updated meta‐analysis of fatal adverse events caused by bevacizumab therapy in cancer patients. PLoS One. 2014;9:e89960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, Kabbinavar F, Bergsland E, Ngai J, Holmgren E, Wang J, Hurwitz H. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–1239. [DOI] [PubMed] [Google Scholar]
- 25. Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. Available at http://handbook.cochrane.org. [Google Scholar]
- 26. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. National Cancer Institute's Common Toxicity Criteria (version 3). 2006. Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed November 11, 2016.
- 28. National Cancer Institute's Common Toxicity Criteria (version 4). 2009. Available at: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed November 11, 2016.
- 29. Viera AJ. Odds ratios and risk ratios: what's the difference and why does it matter? South Med J. 2008;101:730–734. [DOI] [PubMed] [Google Scholar]
- 30. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 31. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Allegra CJ, Yothers G, O'Connell MJ, Sharif S, Colangelo LH, Lopa SH, Petrelli NJ, Goldberg RM, Atkins JN, Seay TE, Fehrenbacher L, O'Reilly S, Chu L, Azar CA, Wolmark N. Initial safety report of NSABP C‐08: a randomized phase III study of modified FOLFOX6 with or without bevacizumab for the adjuvant treatment of patients with stage II or III colon cancer. J Clin Oncol. 2009;27:3385–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E, von Moos R, Vieitez JM, Bouche O, Borg C, Steffens CC, Alonso‐Orduna V, Schlichting C, Reyes‐Rivera I, Bendahmane B, Andre T, Kubicka S. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29–37. [DOI] [PubMed] [Google Scholar]
- 34. Cameron D, Brown J, Dent R, Jackisch C, Mackey J, Pivot X, Steger GG, Suter TM, Toi M, Parmar M, Laeufle R, Im YH, Romieu G, Harvey V, Lipatov O, Pienkowski T, Cottu P, Chan A, Im SA, Hall PS, Bubuteishvili‐Pacaud L, Henschel V, Deurloo RJ, Pallaud C, Bell R. Adjuvant bevacizumab‐containing therapy in triple‐negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trial. Lancet Oncol. 2013;14:933–942. [DOI] [PubMed] [Google Scholar]
- 35. de Gramont A, Van Cutsem E, Schmoll HJ, Tabernero J, Clarke S, Moore MJ, Cunningham D, Cartwright TH, Hecht JR, Rivera F, Im SA, Bodoky G, Salazar R, Maindrault‐Goebel F, Shacham‐Shmueli E, Bajetta E, Makrutzki M, Shang A, Andre T, Hoff PM. Bevacizumab plus oxaliplatin‐based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol. 2012;13:1225–1233. [DOI] [PubMed] [Google Scholar]
- 36. Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar B, Bajetta E, Gorbunova V, Bay JO, Bodrogi I, Jagiello‐Gruszfeld A, Moore N. Bevacizumab plus interferon alfa‐2a for treatment of metastatic renal cell carcinoma: a randomised, double‐blind phase III trial. Lancet. 2007;370:2103–2111. [DOI] [PubMed] [Google Scholar]
- 37. Gianni L, Romieu GH, Lichinitser M, Serrano SV, Mansutti M, Pivot X, Mariani P, Andre F, Chan A, Lipatov O, Chan S, Wardley A, Greil R, Moore N, Prot S, Pallaud C, Semiglazov V. AVEREL: a randomized phase III trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first‐line therapy for HER2‐positive locally recurrent/metastatic breast cancer. J Clin Oncol. 2013;31:1719–1725. [DOI] [PubMed] [Google Scholar]
- 38. Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB III. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. [DOI] [PubMed] [Google Scholar]
- 39. Guan ZZ, Xu JM, Luo RC, Feng FY, Wang LW, Shen L, Yu SY, Ba Y, Liang J, Wang D, Qin SK, Wang JJ, He J, Qi C, Xu RH. Efficacy and safety of bevacizumab plus chemotherapy in Chinese patients with metastatic colorectal cancer: a randomized phase III ARTIST trial. Chin J Cancer. 2011;30:682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. [DOI] [PubMed] [Google Scholar]
- 41. Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E, Hambleton J, Novotny WF, Kabbinavar F. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first‐line metastatic colorectal cancer. J Clin Oncol. 2005;23:3502–3508. [DOI] [PubMed] [Google Scholar]
- 42. Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. [DOI] [PubMed] [Google Scholar]
- 43. Kelly WK, Halabi S, Carducci M, George D, Mahoney JF, Stadler WM, Morris M, Kantoff P, Monk JP, Kaplan E, Vogelzang NJ, Small EJ. Randomized, double‐blind, placebo‐controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration‐resistant prostate cancer: CALGB 90401. J Clin Oncol. 2012;30:1534–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, Harbeck N, Steger GG, Schneeweiss A, Wardley AM, Chlistalla A, Romieu G. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first‐line treatment of human epidermal growth factor receptor 2‐negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–3247. [DOI] [PubMed] [Google Scholar]
- 45. Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. [DOI] [PubMed] [Google Scholar]
- 46. Niho S, Kunitoh H, Nokihara H, Horai T, Ichinose Y, Hida T, Yamamoto N, Kawahara M, Shinkai T, Nakagawa K, Matsui K, Negoro S, Yokoyama A, Kudoh S, Kiura K, Mori K, Okamoto H, Sakai H, Takeda K, Yokota S, Saijo N, Fukuoka M. Randomized phase II study of first‐line carboplatin‐paclitaxel with or without bevacizumab in Japanese patients with advanced non‐squamous non‐small‐cell lung cancer. Lung Cancer. 2012;76:362–367. [DOI] [PubMed] [Google Scholar]
- 47. Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, Starnawski M, Kang YK. Bevacizumab in combination with chemotherapy as first‐line therapy in advanced gastric cancer: a randomized, double‐blind, placebo‐controlled phase III study. J Clin Oncol. 2011;29:3968–3976. [DOI] [PubMed] [Google Scholar]
- 48. Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade‐Lauraine E, Kristensen G, Carey MS, Beale P, Cervantes A, Kurzeder C, du Bois A, Sehouli J, Kimmig R, Stahle A, Collinson F, Essapen S, Gourley C, Lortholary A, Selle F, Mirza MR, Leminen A, Plante M, Stark D, Qian W, Parmar MK, Oza AM. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. [DOI] [PubMed] [Google Scholar]
- 49. Pujade‐Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P, Bamias A, Pereira D, Wimberger P, Oaknin A, Mirza MR, Follana P, Bollag D, Ray‐Coquard I. Bevacizumab combined with chemotherapy for platinum‐resistant recurrent ovarian cancer: the AURELIA open‐label randomized phase III trial. J Clin Oncol. 2014;32:1302–1308. [DOI] [PubMed] [Google Scholar]
- 50. Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, Manegold C. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first‐line therapy for nonsquamous non‐small‐cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. [DOI] [PubMed] [Google Scholar]
- 51. Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Archer L, Atkins JN, Picus J, Czaykowski P, Dutcher J, Small EJ. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol. 2010;28:2137–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Saltz LB, Clarke S, Diaz‐Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Cassidy J. Bevacizumab in combination with oxaliplatin‐based chemotherapy as first‐line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. [DOI] [PubMed] [Google Scholar]
- 53. Tebbutt NC, Wilson K, Gebski VJ, Cummins MM, Zannino D, van Hazel GA, Robinson B, Broad A, Ganju V, Ackland SP, Forgeson G, Cunningham D, Saunders MP, Stockler MR, Chua Y, Zalcberg JR, Simes RJ, Price TJ. Capecitabine, bevacizumab, and mitomycin in first‐line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol. 2010;28:3191–3198. [DOI] [PubMed] [Google Scholar]
- 54. Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME, Rusakov IG, Negrier S, Ou YC, Castellano D, Lim HY, Uemura H, Tarazi J, Cella D, Chen C, Rosbrook B, Kim S, Motzer RJ. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939. [DOI] [PubMed] [Google Scholar]
- 55. Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press. 2014;23:3–16. [DOI] [PubMed] [Google Scholar]
- 56. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. [DOI] [PubMed] [Google Scholar]
- 57. Rassaf T, Totzeck M, Hendgen‐Cotta UB, Shiva S, Heusch G, Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res. 2014;114:1601–1610. [DOI] [PubMed] [Google Scholar]
- 58. Rassaf T, Poll LW, Brouzos P, Lauer T, Totzeck M, Kleinbongard P, Gharini P, Andersen K, Schulz R, Heusch G, Modder U, Kelm M. Positive effects of nitric oxide on left ventricular function in humans. Eur Heart J. 2006;27:1699–1705. [DOI] [PubMed] [Google Scholar]
- 59. Totzeck M, Hendgen‐Cotta UB, Luedike P, Berenbrink M, Klare JP, Steinhoff HJ, Semmler D, Shiva S, Williams D, Kipar A, Gladwin MT, Schrader J, Kelm M, Cossins AR, Rassaf T. Nitrite regulates hypoxic vasodilation via myoglobin‐dependent nitric oxide generation. Circulation. 2012;126:325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hang XF, Xu WS, Wang JX, Wang L, Xin HG, Zhang RQ, Ni W. Risk of high‐grade bleeding in patients with cancer treated with bevacizumab: a meta‐analysis of randomized controlled trials. Eur J Clin Pharmacol. 2011;67:613–623. [DOI] [PubMed] [Google Scholar]
- 61. Hapani S, Sher A, Chu D, Wu S. Increased risk of serious hemorrhage with bevacizumab in cancer patients: a meta‐analysis. Oncology. 2010;79:27–38. [DOI] [PubMed] [Google Scholar]
- 62. Zhu X, Tian X, Yu C, Hong J, Fang J, Chen H. Increased risk of hemorrhage in metastatic colorectal cancer patients treated with bevacizumab: an updated meta‐analysis of 12 randomized controlled trials. Medicine (Baltimore). 2016;95:e4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kamba T, McDonald DM. Mechanisms of adverse effects of anti‐VEGF therapy for cancer. Br J Cancer. 2007;96:1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pinto C, Antonuzzo L, Porcu L, Aprile G, Maiello E, Masi G, Petrelli F, Scartozzi M, Torri V, Barni S. Efficacy and safety of bevacizumab combined with fluoropyrimidine monotherapy for unfit or older patients with metastatic colorectal cancer: a systematic review and meta‐analysis. Clin Colorectal Cancer. 2016;16:e61–e72. [DOI] [PubMed] [Google Scholar]
- 65. Botrel TE, Clark LG, Paladini L, Clark OA. Efficacy and safety of bevacizumab plus chemotherapy compared to chemotherapy alone in previously untreated advanced or metastatic colorectal cancer: a systematic review and meta‐analysis. BMC Cancer. 2016;16:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cao Y, Tan A, Gao F, Liu L, Liao C, Mo Z. A meta‐analysis of randomized controlled trials comparing chemotherapy plus bevacizumab with chemotherapy alone in metastatic colorectal cancer. Int J Colorectal Dis. 2009;24:677–685. [DOI] [PubMed] [Google Scholar]
- 67. Zuo PY, Chen XL, Liu YW, Xiao CL, Liu CY. Increased risk of cerebrovascular events in patients with cancer treated with bevacizumab: a meta‐analysis. PLoS One. 2014;9:e102484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Abdel‐Qadir H, Ethier JL, Lee DS, Thavendiranathan P, Amir E. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: a systematic review and meta‐analysis. Cancer Treat Rev. 2017;53:120–127. [DOI] [PubMed] [Google Scholar]
- 69. Cortes J, Calvo V, Ramirez‐Merino N, O'Shaughnessy J, Brufsky A, Robert N, Vidal M, Munoz E, Perez J, Dawood S, Saura C, Di Cosimo S, Gonzalez‐Martin A, Bellet M, Silva OE, Miles D, Llombart A, Baselga J. Adverse events risk associated with bevacizumab addition to breast cancer chemotherapy: a meta‐analysis. Ann Oncol. 2012;23:1130–1137. [DOI] [PubMed] [Google Scholar]
- 70. Lai XX, Xu RA, Yu‐Ping L, Yang H. Risk of adverse events with bevacizumab addition to therapy in advanced non‐small‐cell lung cancer: a meta‐analysis of randomized controlled trials. Onco Targets Ther. 2016;9:2421–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta‐analysis. JAMA. 2008;300:2277–2285. [DOI] [PubMed] [Google Scholar]
- 72. Hurwitz HI, Saltz LB, Van Cutsem E, Cassidy J, Wiedemann J, Sirzen F, Lyman GH, Rohr UP. Venous thromboembolic events with chemotherapy plus bevacizumab: a pooled analysis of patients in randomized phase II and III studies. J Clin Oncol. 2011;29:1757–1764. [DOI] [PubMed] [Google Scholar]
- 73. Ducreux M, Pignon JP. Bevacizumab versus anti‐epidermal growth factor receptor in first‐line metastatic colorectal cancer. A meta‐analysis: the last building block? Eur J Cancer. 2016;69:178–179. [DOI] [PubMed] [Google Scholar]
- 74. Heinemann V, Rivera F, O'Neil BH, Stintzing S, Koukakis R, Terwey JH, Douillard JY. A study‐level meta‐analysis of efficacy data from head‐to‐head first‐line trials of epidermal growth factor receptor inhibitors versus bevacizumab in patients with RAS wild‐type metastatic colorectal cancer. Eur J Cancer. 2016;67:11–20. [DOI] [PubMed] [Google Scholar]
- 75. Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta‐analysis of first‐line clinical trials. Eur J Cancer. 2016;70:87–98. [DOI] [PubMed] [Google Scholar]
- 76. Ilic I, Jankovic S, Ilic M. Bevacizumab combined with chemotherapy improves survival for patients with metastatic colorectal cancer: evidence from meta analysis. PLoS One. 2016;11:e0161912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang L, Ma L, Wang X, Li B, Guo S, Qiao Q. Therapeutic effects and associated adverse events of first‐line treatments of advanced renal cell carcinoma (RCC): a meta‐analysis. Int Urol Nephrol. 2015;47:617–624. [DOI] [PubMed] [Google Scholar]
- 78. Ranpura V, Pulipati B, Chu D, Zhu X, Wu S. Increased risk of high‐grade hypertension with bevacizumab in cancer patients: a meta‐analysis. Am J Hypertens. 2010;23:460–468. [DOI] [PubMed] [Google Scholar]
- 79. An MM, Zou Z, Shen H, Liu P, Chen ML, Cao YB, Jiang YY. Incidence and risk of significantly raised blood pressure in cancer patients treated with bevacizumab: an updated meta‐analysis. Eur J Clin Pharmacol. 2010;66:813–821. [DOI] [PubMed] [Google Scholar]
- 80. Berger DP, Herbstritt L, Dengler WA, Marme D, Mertelsmann R, Fiebig HH. Vascular endothelial growth factor (VEGF) mRNA expression in human tumor models of different histologies. Ann Oncol. 1995;6:817–825. [DOI] [PubMed] [Google Scholar]
- 81. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 82. Ranpura V, Hapani S, Wu S. Treatment‐related mortality with bevacizumab in cancer patients: a meta‐analysis. JAMA. 2011;305:487–494. [DOI] [PubMed] [Google Scholar]
- 83. Soria JC, Mauguen A, Reck M, Sandler AB, Saijo N, Johnson DH, Burcoveanu D, Fukuoka M, Besse B, Pignon JP. Systematic review and meta‐analysis of randomised, phase II/III trials adding bevacizumab to platinum‐based chemotherapy as first‐line treatment in patients with advanced non‐small‐cell lung cancer. Ann Oncol. 2013;24:20–30. [DOI] [PubMed] [Google Scholar]
- 84. Iwamoto S, Takahashi T, Tamagawa H, Nakamura M, Munemoto Y, Kato T, Hata T, Denda T, Morita Y, Inukai M, Kunieda K, Nagata N, Kurachi K, Ina K, Ooshiro M, Shimoyama T, Baba H, Oba K, Sakamoto J, Mishima H. FOLFIRI plus bevacizumab as second‐line therapy in patients with metastatic colorectal cancer after first‐line bevacizumab plus oxaliplatin‐based therapy: the randomized phase III EAGLE study. Ann Oncol. 2015;26:1427–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. von Baumgarten L, Brucker D, Tirniceru A, Kienast Y, Grau S, Burgold S, Herms J, Winkler F. Bevacizumab has differential and dose‐dependent effects on glioma blood vessels and tumor cells. Clin Cancer Res. 2011;17:6192–6205. [DOI] [PubMed] [Google Scholar]
- 86. Lu X, Kang Y. Hypoxia and hypoxia‐inducible factors: master regulators of metastasis. Clin Cancer Res. 2010;16:5928–5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kamphuisen PW, Beyer‐Westendorf J. Bleeding complications during anticoagulant treatment in patients with cancer. Thromb Res. 2014;133(suppl 2):S49–S55. [DOI] [PubMed] [Google Scholar]
- 88. Clarke E, Lenihan D. Cardio‐oncology: a new discipline in medicine to lead us into truly integrative care. Future Cardiol. 2015;11:359–361. [DOI] [PubMed] [Google Scholar]
- 89. Carrier M, Khorana AA, Moretto P, Le Gal G, Karp R, Zwicker JI. Lack of evidence to support thromboprophylaxis in hospitalized medical patients with cancer. Am J Med. 2014;127:82–86.e81. [DOI] [PubMed] [Google Scholar]
- 90. Lyman GH, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, Clarke JM, Flowers CR, Francis CW, Gates LE, Kakkar AK, Key NS, Levine MN, Liebman HA, Tempero MA, Wong SL, Prestrud AA, Falanga A. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2189–2204. [DOI] [PubMed] [Google Scholar]
- 91. Tebbutt NC, Murphy F, Zannino D, Wilson K, Cummins MM, Abdi E, Strickland AH, Lowenthal RM, Marx G, Karapetis C, Shannon J, Goldstein D, Nayagam SS, Blum R, Chantrill L, Simes RJ, Price TJ. Risk of arterial thromboembolic events in patients with advanced colorectal cancer receiving bevacizumab. Ann Oncol. 2011;22:1834–1838. [DOI] [PubMed] [Google Scholar]
- 92. Randomized trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS‐2.ISIS‐2 (Second International Study of Infarct Survival) Collaborative Group. J Am Coll Cardiol. 1988;12:3a–13a. [DOI] [PubMed] [Google Scholar]
- 93. Seicean S, Seicean A, Plana JC, Budd GT, Marwick TH. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. J Am Coll Cardiol. 2012;60:2384–2390. [DOI] [PubMed] [Google Scholar]
- 94. Chotenimitkhun R, D'Agostino R Jr, Lawrence JA, Hamilton CA, Jordan JH, Vasu S, Lash TL, Yeboah J, Herrington DM, Hundley WG. Chronic statin administration may attenuate early anthracycline‐associated declines in left ventricular ejection function. Can J Cardiol. 2015;31:302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer‐Crosbie M. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cardinale D, Colombo A, Torrisi R, Sandri MT, Civelli M, Salvatici M, Lamantia G, Colombo N, Cortinovis S, Dessanai MA, Nole F, Veglia F, Cipolla CM. Trastuzumab‐induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28:3910–3916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Results of the MEDLINE Search Through November 21, 2016
Figure S1. The quality of the included studies as analyzed per recommendations from the Cochrane Handbook for Systematic Reviews of Interventions.
Figure S2. Risk of bias for arterial adverse events. Each dot represents one study included in the analysis of arterial adverse events. The SE (log risk ratio [RR]) axis represents study precision, and the RR axis shows the study results.
Figure S3. Risk of bias for venous adverse events. Each dot represents one study included in the analysis of venous adverse events. The SE (log RR) axis represents study precision, and the RR axis shows the study results.


