Abstract
Background
Different pathways likely underlie the association between early weight gain and cardiovascular disease risk. We examined whether birth weight for length relationship and weight gain up to 2 years of age are associated with lipid profiles and blood pressure (BP) in early adolescence and determined whether childhood adiposity mediates these associations.
Methods and Results
Data from QUALITY (Quebec Adipose and Lifestyle Investigation in Youth), a cohort of white children with parental history of obesity, were analyzed (n=395). Sex‐specific weight for length z scores from birth to 2 years were computed. Rate of postnatal weight gain was estimated using individual slopes of weight for length z‐score measurements. Percentage of body fat was measured at 8 to 10 years. Fasting lipids and BP were measured at 10 to 12 years. Using path analysis, we found indirect effects of postnatal weight gain, through childhood adiposity, on all outcomes: Rate of postnatal weight for length gain was positively associated with childhood adiposity, which in turn was associated with unfavorable lipid and BP levels in early adolescence. In contrast, small beneficial direct effects on diastolic BP z scores, independent of weight at other time points, were found for birth weight for length (β=−0.05, 95% CI, −0.09 to −0.002) and for postnatal weight gain (β=−0.02, 95% CI, −0.03 to −0.002).
Conclusions
Among children with at least 1 obese parent, faster postnatal weight gain leads to cardiovascular risk factors in early adolescence through its effect on childhood adiposity. Although heavier newborns may have lower BP in early adolescence, this protective direct effect could be offset by a deleterious indirect effect linking birth weight to later adiposity.
Keywords: birth weight, blood pressure, lipid profile, obesity, postnatal weight gain
Subject Categories: Cardiovascular Disease, Obesity, Pediatrics, Epidemiology
Clinical Perspective
What Is New?
Children born at term who had at least 1 obese parent and who experienced faster weight gain in the first 2 years of life had higher adiposity at ages 8 to 10 years, which in turn predicted worse fasting lipid and blood pressure levels 2 years later.
Our findings do not support direct associations of birth weight for length and postnatal weight gain up to 2 years of age on fasting lipid or blood pressure levels, independent of childhood adiposity, with the exception of diastolic blood pressure.
What Are the Clinical Implications?
Promoting healthy patterns of weight gain throughout the life course, including prenatally, during the first 2 years of life and throughout childhood may reduce the occurrence of obesity and its deleterious consequences for cardiovascular health.
Introduction
Cardiovascular diseases are leading causes of death worldwide,1 and associated morbidities result in major economic burdens.2 There is mounting evidence that cardiovascular diseases originate early in life3 and that associated risk factors in childhood are predictive of later diseases.4, 5 Among Canadian children and youth, it is estimated that 4% have high or borderline blood pressure (BP)6 and 35% have unfavorable levels of total cholesterol.7 Timely primary prevention is crucial, yet our understanding of the earliest predictors of cardiovascular risk factors remains incomplete.
Growth patterns throughout infancy, in particular small birth size for gestational age and rapid postnatal weight gain have been associated with cardiovascular risk factors in both children and young adults, including abnormal lipid levels and elevated BP.8, 9, 10, 11, 12, 13 Similarly, being born large for gestational age has been linked with adverse cardiovascular consequences.10, 14, 15 However, in studies carried out on representative population‐based samples, mixed findings have been reported for associations of birth weight and postnatal weight gain with lipid profiles16, 17, 18, 19 and with BP16, 20, 21, 22, 23 in children. Substantial heterogeneity between studies, namely, with regard to the inclusion of participants with birth weights at the lower and upper extremes of the distribution, may in part explain contrasting findings. In addition, most of the literature has focused on birth weight and postnatal weight gain without accounting for growth in length. Weight for length during infancy may be a better predictor of later overweight/obese status than weight alone.24, 25
Childhood weight status may be an important mediator in associations between early weight gain and cardiovascular risk factors.26 Birth weight and rate of postnatal weight gain are positively associated with adiposity in childhood,27, 28 and adiposity is an important predictor of cardiovascular risk factors.29 In analyses estimating direct effects of birth weight and postnatal weight gain on cardiovascular risk factors, it has been common practice to statistically adjust for adiposity measured contemporaneously with cardiovascular risk factors16, 17, 18; however, collider‐stratification bias may result in biased direct effect estimates.30 To better understand prospective associations among early weigh gain, childhood adiposity, and cardiovascular risk factors in early adolescence, we examined the associations between birth weight for length and rate of postnatal gain in weight for length up to 2 years of age with lipid profiles and BP at ages 10 to 12 years, and we assessed whether these associations were mediated by childhood adiposity.
Methods
Participants were drawn from the QUALITY (Quebec Adipose and Lifestyle Investigation in Youth) cohort, an ongoing longitudinal study of the natural history of obesity and cardiovascular risk factors in white youth. Children were recruited through elementary schools located within 3 major urban centers in Quebec, Canada. Eligibility criteria required participants to be white, aged 8 to 10 years at recruitment, with both biological parents available to participate in baseline data collection and at least 1 parent being obese (ie, body mass index ≥30 and/or waist circumference >102 cm in men and >88 cm in women). At baseline, 630 families participated in a clinic visit during which biological and physiological measurements were obtained, and questionnaires were completed between 2005 and 2008. A similar assessment was conducted 2 years later, when participants were aged 10 to 12 years, between 2008 and 2011 (n=564). Written informed consent was obtained from parents, and children provided assent. The ethics review boards of the CHU Sainte Justine and the Quebec Heart and Lung Institute approved the study. A detailed description of the study design and data collection methods is available.31
For the current analysis, we used a subsample of QUALITY participants (n=395) who were born at term (ie, 37 to <42 weeks of gestation), who had complete anthropometric data up to 2 years of age, and who completed a follow‐up visit at age 10 to 12 years. Participants included in the analysis differed from those excluded in that they had slightly higher triglycerides (P=0.049), higher diastolic BP (P=0.021), and lower likelihood of having been exposed to tobacco in utero (P=0.010; Table S1).
Measures at Birth and Up to 2 Years
Health booklets are issued to all Quebec parents of newborn children; growth and other health‐related information is subsequently entered by health professionals (nurses or physicians) during routine medical visits. From these booklets, birth weight and length, gestational age, and measurements of weight and length collected at different times up to 24 months of age were obtained. For the current analysis, participants were required to have a minimum of 3 measurement times over the first 24 months of life (ie, at birth, at least once between 1 and 12 months, and at least once between 12.1 and 24 months; median: 6 entries [range: 3–10 entries]). Measures of weight and length were transformed to sex‐specific weight‐for‐length z scores using the World Health Organization growth standards.32 Individual slopes (rate of increase or decrease) for weight for length z scores from birth to 2 years were used to estimate postnatal weight gain. To do so, simple linear regressions were fitted to measurements of each participant, with age in months as an independent variable and weight‐for‐length z score as a dependent variable. The slope thus represents the estimated rate of postnatal growth in weight‐for‐length z score per month during the first 2 years of life. To facilitate its interpretation, we rescaled the variable such that 1 U of the slope variable corresponds to a change of z=0.02 per month, which is equivalent to ≈0.5 SD (z=0.48) over a 24‐month period. The latter also corresponds to biologically plausible growth in weight for length.
Size at birth for gestational age was based on Canadian reference values for gestational age and sex‐specific percentile: small for gestational age was defined as a birth weight <10th percentile, large size for gestational age as a birth weight >90th percentile, and appropriate for gestational age (AGA) otherwise.33 Parent‐completed questionnaires assessed history of gestational diabetes mellitus, smoking, and hypertension during the pregnancy of the child participating in the study and whether the child was breastfed (never, <3 months, 3–6 months, or >6 months).
Measures in Childhood (8–10 Years)
Body composition was measured by dual‐energy x‐ray absorptiometry when participants were aged 8 to 10 years. Childhood adiposity was estimated using the percentage of body fat calculated as total fat mass/total body mass×100. Parental educational attainment was assessed by questionnaire (2 parents with secondary school or less versus at least 1 parent with a technical/vocational/trade or university degree). Maternal body mass index was computed from weight and height measured using standard protocols when children were aged 8 to 10 years.31 Pubertal development stage was assessed by trained nurses using the 5‐stage Tanner scales34, 35 and was dichotomized as prepubertal (Tanner 1) versus puberty initiated (Tanner >1).
Measures in Early Adolescence (10–12 Years)
Blood samples were obtained by venipuncture after a 10‐hour overnight fast. Blood samples were centrifuged, aliquoted, and stored at −80°C until analyzed. Lipid profiles (total cholesterol, high density lipoprotein [HDL] cholesterol, and triglycerides) were measured on a Synchron LX20 analyzer, with Beckman Instruments reagents, by the Department of Clinical Biochemistry at CHU Sainte‐Justine, according to the recommendations of the International Federation of Clinical Chemistry. Low‐density lipoprotein cholesterol was calculated based on the Friedewald equation.36 BP was measured on the right arm, with the child in a sitting position and at rest for at least 5 minutes, using an oscillometric instrument (Dinamap XL Vital Signs Monitors, model 9300; Johnson & Johnson Medical Inc). Five consecutive measures were obtained at 1‐minute intervals, and the average of the last 3 (of 5) measures of systolic and diastolic BP was used in the analyses. These were then transformed to age‐, sex‐ and height‐specific z scores.37 Last, pubertal development was assessed at age 8 to 10 years.
Statistical Analyses
Means and standard deviations or proportions were used to describe participants. Multivariable linear regression analyses were used to examine the association of weight for length at birth and rate of postnatal weight gain with each outcome. Variables from the lipid profile were transformed (100×natural logarithm of variable) to normalize their distribution. The β coefficients for lipid variables thus represent the percentage of change in the dependent variable for a 1‐U increase in the independent variable.38 In addition to birth weight for length and postnatal weight gain, all models included sex; age; parental education; maternal history of gestational diabetes mellitus, smoking, or hypertension during pregnancy; gestational age; and breastfeeding duration. Pubertal development is likely not a confounder and could be in the causal pathway for associations of interest (eg, if children who grow more rapidly experience earlier puberty). However, given that puberty was not associated with birth weight or postnatal weight gain in our data and that its inclusion in models did not change the magnitude of β coefficients associated with early weight gain, models were not adjusted for pubertal development. Regression models were also adjusted for the suspected mediator (ie, childhood percentage of body fat mass) to estimate direct effects of birth weight for length and subsequent postnatal weight gain on cardiovascular risk factors. The linearity assumption for associations between independent variables and outcomes was tested using nonparametric smoothing splines and was found not to be violated. Indirect effects of birth weight for length and of postnatal weight gain on cardiovascular risk factors, via childhood adiposity, were estimated using path analysis (SAS proc calis; Figure).
Figure 1.
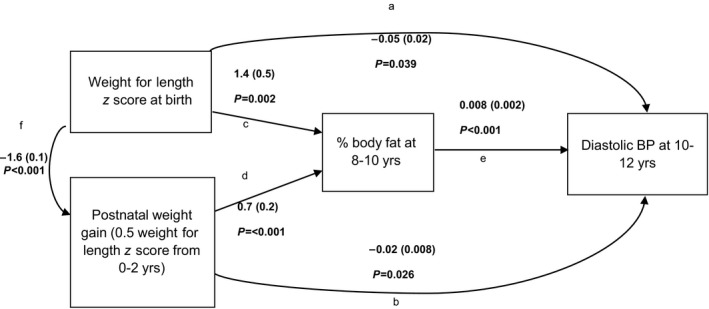
Path diagrams showing relationships between birth weight for length z score, postnatal weight gain, adiposity at 8 to 10 years of age, and diastolic blood pressure (BP) at 10 to 12 years of age among 395 children from the QUALITY cohort. The β coefficients (standard errors) and P values are presented for diastolic BP. All associations are adjusted for child's age, sex, parental education, gestational age, breastfeeding, and in utero exposure to gestational diabetes mellitus, maternal hypertension, and maternal smoking. The direct effect of birth weight for length (not mediated by adiposity) on the outcome corresponds to coefficient (a). The direct effect of postnatal weight gain (not mediated by adiposity) on the outcome corresponds to coefficient (b). The indirect effect of birth weight for length on the outcome (mediated by postnatal growth and by adiposity) corresponds to (f×b)+(f×d×e)+(c×e). The indirect effect of postnatal weight gain on the outcome (mediated by adiposity) corresponds to (d×e).
In sensitivity analyses, we tested the presence of interactions between sex and birth weight for length and between sex and postnatal weight gain using interaction terms included one at a time in covariate‐adjusted models. Statistically significant interactions were found for triglycerides and for HDL cholesterol; sex‐stratified analyses for these 2 outcomes are presented in Tables S3–S4. Last, given that mechanisms linking early growth and later cardiovascular risk factors may be different for participants born with extreme birth weights, we repeated all analyses in a sample restricted to children born AGA (n=318), for which results are also presented in Tables S5–S7. All analyses were conducted with SAS version 9.4.
Results
Participants included in this analysis were born between August 1994 and September 2000. Mean birth weight and birth length were 3.6 kg (SD: 0.5) and 51.4 cm (SD: 2.3). The correlation between birth weight for length and postnatal weight gain was −0.54. At 8 to 10 years of age, 54% of children were normal weight, whereas 22% were overweight and 24% were obese. Lipid profiles and BP were measured when children were, on average, aged 11.6 years (SD: 0.9). Characteristics of participants are shown in Table 1. Correlations between measurements at ages 8 to 10 and 10 to 12 years for adiposity, lipid profiles, and BP are shown in Table S2.
Table 1.
Characteristics of Study Population for 395 Children of the QUALITY Cohort, Quebec, Canada, 2005–2011
| Characteristics | All (n=395) | Boys (n=225) | Girls (n=170) |
|---|---|---|---|
| Birth to 2 years of age | |||
| Birth weight, kg, mean, SD | 3.6 (0.5) | 3.6 (0.5) | 3.5 (0.5) |
| Birth length, cm, mean, SD | 51.4 (2.3) | 51.8 (2.2) | 51.0 (2.2) |
| Birth weight for length z score, mean, SD | −0.3 (1.3) | −0.3 (1.3) | −0.3 (1.3) |
| Postnatal weight gaina, z score per month, mean, SD | 0.06 (0.08) | 0.06 (0.08) | 0.06 (0.07) |
| Gestational age, mean, SD | 39.6 (1.1) | 39.5 (1.1) | 39.6 (1.1) |
| Size at birth for gestational age, % (n) | |||
| SGA | 6.6 (26) | 5.3 (12) | 8.2 (14) |
| AGA | 80.5 (318) | 81.3 (183) | 79.4 (135) |
| LGA | 12.9 (51) | 13.3 (30) | 12.4 (21) |
| Breastfeeding duration, % (n) | |||
| Never breastfed | 19.5 (76) | 20.6 (45) | 18.2 (31) |
| Breastfed <3 mo | 26.7 (104) | 28.8 (63) | 24.1 (41) |
| Breastfed 3–6 mo | 24.4 (95) | 23.3 (51) | 25.9 (44) |
| Breastfed >6 mo | 29.3 (114) | 27.4 (60) | 31.8 (54) |
| In utero exposure to gestational diabetes mellitus, % (n) | 17.2 (68) | 20.4 (46) | 12.9 (22) |
| In utero exposure to maternal hypertension, % (n) | 9.9 (39) | 11.6 (26) | 7.7 (13) |
| In utero exposure to maternal smoking, % (n) | 12.4 (49) | 13.4 (30) | 11.2 (19) |
| Childhood (8–10 y) | |||
| Total body fat mass, %, mean, SD | 25.9 (10.7) | 23.6 (10.7) | 28.9 (9.9) |
| BMI categoryb, % (n) | |||
| Normal weight | 54.2 (214) | 52.9 (119) | 55.9 (95) |
| Overweight | 21.5 (85) | 22.2 (50) | 20.6 (35) |
| Obese | 24.3 (96) | 24.9 (56) | 23.5 (40) |
| Puberty (Tanner stage >1), % (n) | 21.3 (84) | 8.9 (20) | 37.7 (64) |
| Early adolescence (10–12 y) | |||
| Child's age, y, mean, SD | 11.6 (0.9) | 11.7 (0.9) | 11.6 (0.9) |
| Puberty (Tanner stage >1), % (n) | 66.8 (264) | 52.4 (118) | 85.9 (146) |
| Lipids | |||
| Total cholesterol, mmol/L, mean, SD | 3.7 (0.7) | 3.8 (0.6) | 3.7 (0.7) |
| LDL cholesterol, mmol/L, mean, SD | 2.2 (0.6) | 2.2 (0.6) | 2.2 (0.6) |
| HDL cholesterol, mmol/L, mean, SD | 1.2 (0.2) | 1.2 (0.3) | 1.1 (0.2) |
| Triglycerides, mmol/L, mean, SD | 0.8 (0.4) | 0.8 (0.5) | 0.8 (0.4) |
| Blood pressure | |||
| Systolic, mm Hg, mean, SD | 97.7 (8.9) | 98.6 (8.6) | 96.5 (9.1) |
| Diastolic, mm Hg, mean, SD | 50.7 (5.3) | 50.7 (5.2) | 50.8 (5.5) |
| Parent characteristics | |||
| Parental education, % (n) | |||
| 2 parents with high school degree or less | 6.6 (26) | 4.9 (11) | 8.8 (15) |
| 1 or 2 parents with technical or university degree | 93.4 (369) | 95.1 (214) | 91.2 (155) |
| Maternal age at conception of child, y, mean, SD | 30.1 (4.6) | 30.1 (4.6) | 30.0 (4.7) |
| Maternal BMI, kg/m2, mean, SD | 29.2 (6.5) | 29.3 (6.4) | 29.0 (6.7) |
AGA indicates appropriate birth size for gestational age; BMI, body mass index; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LGA, large birth size for gestational age; QUALITY, Quebec Adipose and Lifestyle Investigation in Youth; SGA, small birth size for gestational age.
Postnatal weight gain is estimated using the slope for weight for length z scores from birth to 24 months of age.
BMI categories are based on World Health Organization cutoffs for sex‐ and age‐adjusted BMI z scores.
Before adding childhood adiposity to models for the lipid profile (Table 2), postnatal weight gain was associated with HDL cholesterol: Every additional z=0.5 in weight for length over the first 2 years of life was associated with a 0.7% decrease in HDL cholesterol at 10 to 12 years (95% confidence interval, −1.4 to −0.007). In sex‐specific analysis, this association was found only in boys (Table S3). Following adjustment for adiposity, neither birth weight for length nor postnatal weight gain had direct effects on lipid levels. Childhood adiposity was strongly associated with lipid levels in early adolescence.
Table 2.
Associationsa of Birth Weight for Length, Postnatal Weight Gain, and Childhood Adiposity With Plasma Lipids Measured at Ages 10–12 Years in 395 Children From the QUALITY Cohort, Quebec, Canada, 2005–2011
| Total Cholesterol | LDL Cholesterol | Triglycerides | HDL Cholesterol | |||||
|---|---|---|---|---|---|---|---|---|
| Model 1: adjusted for covariates | ||||||||
| Birth weight for length z score | −0.1 | (−1.8 to 1.6) | 0.2 | (−2.3 to 2.6) | −0.6 | (−5.2 to 3.9) | −0.6 | (−2.6 to 1.4) |
| Postnatal weight gain (0.5 weight‐for‐length z score from 0 to 2 y) | −0.3 | (−0.8 to 0.3) | −0.3 | (−1.1 to 0.5) | 1.4 | (−0.2 to 2.9) | −0.7 | (−1.4 to −0.007) |
| Model 2: adjusted for covariates and percentage of body fat | ||||||||
| Birth weight for length z score | −0.4 | (−2.1 to 1.3) | −0.4 | (−2.9 to 2.0) | −3.1 | (−7.5 to 1.3) | 0.4 | (−1.5 to 2.3) |
| Postnatal weight gain (0.5 weight‐for‐length z score from 0 to 2 y) | −0.4 | (−1.0 to 0.2) | −0.6 | (−1.5 to 0.2) | 0.1 | (−1.3 to 1.6) | −0.2 | (−0.8 to 0.5) |
| Percentage of total body fat (at ages 8–10 y) | 0.2 | (0.03 to 0.4) | 0.4 | (0.1 to 0.7) | 1.7 | (1.2 to 2.1) | −0.7 | (−0.9 to −0.5) |
CI indicates confidence interval; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; QUALITY, Quebec Adipose and Lifestyle Investigation in Youth.
Birth weight for length and postnatal growth in weight for length are entered simultaneously in model 1, and models are adjusted for child's age, sex, parental education, gestational age, breastfeeding, and in utero exposure to gestational diabetes mellitus, maternal hypertension, and maternal smoking. Model 2 is the same as model 1 but is further adjusted for percentage of total body fat mass. The β coefficients (95% CI) represent the percentage increase or decrease in the outcome for a 1‐U increase in the corresponding independent variable.
Prior to adjustment for childhood adiposity, no associations were found between birth weight for length or postnatal weight gain and BP (Table 3). However, adjusting for adiposity resulted in small negative associations between birth weight for length z score and diastolic BP (β=−0.05, 95% confidence interval, −0.09 to −0.002) and between postnatal weight gain and diastolic BP (β=−0.02, 95% confidence interval, −0.03 to −0.002). These correspond to decreases in diastolic BP of ≈0.6 mm Hg for every additional weight for length z score at birth and 0.2 mm Hg for every additional z=0.5 increase in weight for length over the first 2 years of life.
Table 3.
Associationsa of Birth Weight for Length, Postnatal Weight Gain, and Childhood Adiposity With BP Measured at Ages 10–12 Years in 395 Children From the QUALITY Cohort, Quebec, Canada, 2005–2011
| Systolic BP | Diastolic BP | |||
|---|---|---|---|---|
| Model 1: adjusted for covariates | ||||
| Birth weight for length z score | −0.01 | (−0.09 to 0.06) | −0.03 | (−0.08 to 0.01) |
| Postnatal weight gain (0.5 weight for length z score from 0 to 2 y) | 0.01 | (−0.01 to 0.04) | −0.01 | (−0.03 to 0.004) |
| Model 2: adjusted for covariates and percentage of body fat | ||||
| Birth weight for length z score | −0.04 | (−0.1 to 0.04) | −0.05 | (−0.09 to −0.002) |
| Postnatal weight gain (0.5 weight for length z score from 0 to 2 y) | 0.0004 | (−0.02 to 0.03) | −0.02 | (−0.03 to −0.002) |
| Percentage of total body fat (at 8–10 y) | 0.02 | (0.009 to 0.03) | 0.008 | (0.003 to 0.01) |
BP indicates blood pressure; CI, confidence interval; QUALITY, Quebec Adipose and Lifestyle Investigation in Youth.
Birth weight for length and postnatal growth in weight for length were entered simultaneously in model 1, and models were adjusted for child's age, sex, parental education, gestational age, breastfeeding, and in utero exposure to gestational diabetes mellitus, maternal hypertension and maternal smoking. Model 2 is the same as model 1 but is further adjusted for percentage of total body fat mass. β coefficients (95% CI) for BP z scores based on values from the National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents.
Path analysis showed indirect effects of postnatal weight gain on all outcomes: Rate of postnatal weight gain was positively associated with childhood adiposity, with the latter predicting unfavorable lipid and BP levels in early adolescence (Table 4 and Figure). For triglycerides and HDL cholesterol, these indirect effects were slightly stronger in girls compared with boys but still present in both sexes (Table S4). Birth weight for length had an indirect effect (ie, mediated by postnatal weight gain and childhood adiposity) on diastolic BP only. Indirect effects of birth weight for length and postnatal weight gain on diastolic BP were in opposite directions of respective direct effects.
Table 4.
Coefficientsa for Total, Direct, and Indirect Effects (Mediated by Percentage of Body Fat at Ages 8–10 Years) of Birth Weight for Length and Postnatal Weight Gain on Cardiovascular Risk at Ages 10–12 Years Among 395 Children From the QUALITY Cohort, Quebec, Canada, 2005–2011
| Effect | Total Cholesterol | LDL Cholesterol | Triglycerides | HDL Cholesterol | Systolic BP, z (95% CI) | Diastolic BP, z (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Birth weight for length | ||||||||||||
| Total | 0.3 | (−1.1 to 1.7) | 0.7 | (−1.3 to 2.7) | −2.8 | (−6.6 to 1.0) | 0.5 | (−1.2 to 2.1) | −0.03 | (−0.09 to 0.03) | −0.01 | (−0.05 to 0.02) |
| Direct | −0.4 | (−2.1 to 1.2) | −0.4 | (−2.8 to 2.0) | −3.1 | (−7.4 to 1.2) | 0.4 | (−1.5 to 2.3) | −0.04 | (−0.1 to 0.03) | −0.05 | (−0.09 to −0.002) |
| Indirect | 0.7 | (−0.2 to 1.7) | 1.1 | (−0.3 to 2.5) | 0.3 | (−2.5 to 3.0) | 0.08 | (−1.1 to 1.3) | 0.004 | (−0.04 to 0.05) | 0.03 | (0.004 to 0.05) |
| Postnatal weight gain | ||||||||||||
| Total | −0.3 | (−0.8 to 0.3) | −0.3 | (−1.1 to 0.5) | 1.4 | (−0.2 to 2.9) | −0.7 | (−1.4 to −0.2) | 0.01 | (−0.01 to 0.04) | −0.01 | (−0.03 to 0.004) |
| Direct | −0.4 | (−1.0 to 0.2) | −0.6 | (−1.4 to 0.2) | 0.1 | (−1.3 to 1.6) | −0.2 | (−0.8 to 0.5) | 0.0004 | (−0.02 to 0.03) | −0.02 | (−0.03 to −0.002) |
| Indirect | 0.2 | (0.007 to 0.3) | 0.3 | (0.07–0.5) | 1.2 | (0.6–1.8) | −0.5 | (−0.8 to −0.2) | 0.01 | (0.005–0.02) | 0.006 | (0.002–0.01) |
BP indicates blood pressure; CI, confidence interval; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; QUALITY, Quebec Adipose and Lifestyle Investigation in Youth.
Path analysis is applied separately for each outcome variable, models include the 2 main exposure variables (birth weight for length and postnatal weight gain), the suspected mediator (percentage of body fat at ages 8–10 years) and covariates (child's age, sex, parental education, gestational age, breastfeeding, and in utero exposure to gestational diabetes mellitus, maternal hypertension, and maternal smoking). The β coefficients (95% CI) associated with lipid outcomes represent the percentage increase or decrease in the outcome for a 1‐U increase in the corresponding independent variable. The β coefficients (95% CI) associated with BP outcomes represent an increase or decrease in BP z score for a 1‐U increase in the corresponding independent variable.
Overall, results were similar when restricting the sample to children born AGA (Tables S5–S7). However, in the restricted sample, after adjustment for childhood adiposity, postnatal weight gain was no longer associated with diastolic BP.
Discussion
Our study elucidates the distinct links among birth weight adjusted for length, postnatal weight gain during the first 2 years of life, childhood adiposity, and cardiovascular risk factors in young adolescents who were born at term and who have at least 1 obese parent. With the exception of diastolic BP, we found no evidence of direct effects of early growth (at birth or in the first 2 years of life) on cardiovascular risk factors in early adolescence once childhood adiposity was accounted for. Rate of postnatal weight gain, however, was positively associated with childhood adiposity, which in turn predicted unfavorable lipid and BP outcomes in early adolescence.
The absence of a direct association between birth weight for length and plasma lipid levels is consistent with findings reported elsewhere.17, 39, 40, 41, 42 A systematic review by Huxley et al called into question previous reports of associations between birth weight and cholesterol levels, stating that these associations are likely largely a result of publication bias or, if present, that associations are not substantial enough to affect later cardiovascular diseases.43 We also did not find any direct effect of postnatal weight gain on lipid levels. Some studies that have reported such associations oversampled participants born small for gestational age.17, 44 The physiological response in the lipid profile of faster postnatal weight gain following in utero growth restriction may differ from that of faster postnatal weight gain in largely normal‐weight newborns. Our sample included few children born small for gestational age (7%) and thus had insufficient power to test for interactions between size at birth for gestational age and postnatal weight gain. In a study of Chilean adolescent participants with birth weights ≥3 kg, a positive association was reported between faster weight gain from 0 to 3 months and a metabolic syndrome score, including higher triglycerides and lower HDL cholesterol; however, it is not known whether this association is independent of weight in childhood.45 Weight gain during infancy is likely less predictive of an adverse lipid profile in comparison to weight gain in subsequent years.16, 40, 46
Our findings are also consistent with the documented inverse association of diastolic BP with birth weight.26, 47 As in other studies, this association became apparent only once childhood adiposity was accounted for.26, 48 Similarly, postnatal weight gain became inversely associated with diastolic BP only after adjustment for childhood adiposity. Collider‐stratification bias, which may occur when conditioning on a variable that is in the causal pathway between exposure and outcome (ie, adiposity), has been evoked to explain previously observed direct effects of birth weight on BP49 and is a possible explanation for our results. Restricting the sample to children born AGA attenuated these direct associations, suggesting that, if present, the beneficial effects of higher birth weight for length and faster postnatal weight gain are driven largely by children whose birth weight was at the extremes of the distribution. More important, the magnitude of the direct effects of birth weight for length and postnatal weight gain on diastolic BP measured in early adolescence was relatively small, suggesting that interventions targeting increased prenatal and postnatal weight gain may have small direct effects on diastolic BP only in early adolescence or even contrary to expected effects via positive associations between early weight gain and childhood adiposity.50
Although our study does not support direct effects of birth weight for length and postnatal weight gain on lipid profiles or systolic BP, it distinctly shows that rate of postnatal weight gain progresses into childhood adiposity, which ultimately adversely affects cardiovascular disease risk. Other studies have reported a relationship between postnatal weight gain and BP with associations strengthening for weight gain more proximal to when BP was measured.51, 52, 53, 54 Promoting healthy patterns of weight gain early on could affect cardiovascular disease risk factors through the former's influence on adiposity.
Findings for indirect effects of birth weight for length were less consistent across outcomes: A positive indirect effect was found only for diastolic BP. The indirect effect of birth weight is mediated not only by childhood adiposity but also by postnatal weight gain. In the presence of multiple mediators, the indirect effect coefficient is calculated as the sum of the product of coefficients within each path (Figure). The coefficient for the path linking birth weight for length, childhood adiposity, and cardiovascular risk was systematically positive (negative for HDL cholesterol); however, the coefficient for the path linking birth weight, postnatal growth, childhood adiposity, and cardiovascular risk was systematically negative (positive for HDL cholesterol). When not specifying a path between birth weight for length and postnatal growth (Figure, path f), so as to examine the indirect effect of each variable mediated only by adiposity, we found adverse indirect effects for both higher birth weight and faster postnatal weight gain on all outcomes (data not shown). This suggests that a higher birth weight could indirectly lead to the development of cardiovascular risk factors in early adolescence, specifically when high birth weight is not followed by slower postnatal weight gain.
Our study includes white children with relatively high standards of living and education from the QUALITY cohort who were born at term. In sensitivity analyses, restricting the sample to participants born AGA yielded similar results, particularly with respect to the detrimental indirect effect of faster postnatal weight gain. To date, few studies have applied statistical methods designed to assess mediation in studies examining early weight gain and cardiovascular risk factors.21, 55 Using path analysis, we were able to estimate direct effects (ie, independent of childhood adiposity) and indirect effects (ie, mediated by postnatal weight gain and childhood adiposity). In addition to our analytical approach, strengths of this study include the availability of repeated measures of weight and length from birth to 2 years of age and adiposity measures from dual‐energy x‐ray absorptiometry scans obtained 2 years before the measurement of cardiovascular risk factors. Our study has limitations. First, weight and length data were obtained from health booklets and were not collected for research purposes, so variability in measurement precision is likely. Growth charts plotted for each participant were examined visually by 2 pediatricians, and a total of 209 (7%) measurements of weight or length were removed (eg, physiologically implausible measurements). Second, other than breastfeeding duration, we did not have nutritional data for the infancy period (eg, age at introduction of solid foods); confounding by early nutrition on associations of interest could not be investigated. Third, prepregnancy maternal weight status or weight gain during pregnancy were not available. In sensitivity analyses, however, estimated coefficients did not change following adjustment for maternal body mass index measured when participants were aged 8 to 10 years.
In sum, our findings suggest that it is not birth weight or postnatal weight gain during the first 2 years of life per se that predict cardiovascular risk factors in children; rather, gaining weight more rapidly up to 2 years of age sets the stage for the development of increased adiposity in childhood that then adversely affects lipid and BP levels in early adolescence. Efforts to promote healthy patterns of weight gain are likely needed throughout the life course, including prenatally, postnatally, and throughout childhood. These efforts may contribute to the prevention of childhood obesity and, ultimately, its deleterious consequences for cardiovascular health.
Sources of Funding
The QUALITY cohort is funded by the Canadian Institutes of Health Research (#OHF‐69442, #NMD‐94067, #MOP‐97853, #MOP‐119512), the Heart and Stroke Foundation of Canada (#PG‐040291) and the Fonds de la Recherche du Québec—Santé. Andraea Van Hulst holds a Canadian Institutes of Health Research Postdoctoral Fellowship and a Fellowship in Preventive Cardiology (4th ICPC/HSFC/CCS). Mélanie Henderson holds a Diabetes Junior Investigator Award from the Canadian Society of Endocrinology and Metabolism—AstraZeneca and a Fonds de Recherche du Québec—Santé Junior 1 salary awards and Tracie A. Barnett holds a Senior salary award from the latter institution. Funders played no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the article; and decision to submit the article for publication.
Disclosures
None.
Supporting information
Table S1. Comparison of QUALITY Study Participants Included and of Those Excluded From the Analytic Sample
Table S2. Comparison of Participants at Ages 8–10 and 10–12 Years in a Subsample (n=395) of the QUALITY Cohort
Table S3. Associations* of Birth Weight for Length, Postnatal Weight Gain, and Childhood Adiposity With Triglycerides and High‐Density Lipoprotein Cholesterol Measured at Ages 10–12 Years, Stratified by Sex, Among 395 Children of the QUALITY Cohort, Quebec, Canada, 2005–2011
Table S4. The β Coefficients* for Total, Direct, and Indirect Effects (Mediated by Percentage of Body Fat at Ages 8–10 Years) of Birth Weight for Length and Postnatal Weight Gain on Triglycerides and High‐Density Lipoprotein Cholesterol at Ages 10–12 Years, Stratified by Sex Among 395 Children of the QUALITY Cohort, Quebec, Canada, 2005–2011
Table S5. Associations* of Birth Weight for Length, Postnatal Weight Gain, and Childhood Adiposity With Plasma Lipids Measured at Ages 10–12 Years Among Children From the QUALITY Cohort Born With an Appropriate Size for Gestational Age (n=318)
Table S6. Associations* of Birth Weight for Length, Postnatal Weight Gain, and Childhood Adiposity With Blood Pressure Measured at Ages 10–12 Years Among Children From the QUALITY Cohort Born With an Appropriate Size for Gestational Age (n=318)
Table S7. Coefficients* for Total, Direct, and Indirect Effects (Mediated by Percentage of Body Fat at Ages 8–10 Years) of Birth Weight for Length and Postnatal Weight Gain on Cardiovascular Risk at Ages 10–12 Years Among Children From the QUALITY Cohort Born With an Appropriate Size for Gestational Age (n=318)
Acknowledgments
The authors wish to thank Katherine Gray‐Donald PhD and Arnaud Chiolero MD, PhD (IUMSP, Lausanne University Hospital, Switzerland & Department of Epidemiology, McGill University, Montréal) for their contributions. Dr Marie Lambert (July 1952–February 2012), pediatric geneticist and researcher, initiated the QUALITY cohort. Her leadership and devotion to QUALITY will always be remembered and appreciated. The cohort integrates members of TEAM PRODIGY, an inter‐university research team including Université de Montréal, Concordia University, INRS‐Institute‐Armand Frappier, Université Laval, and McGill University. The research team is grateful to all the children and their families who took part in this study, as well as the technicians, research assistants and coordinators involved in the QUALITY cohort project.
(J Am Heart Assoc. 2017;6:e006302 DOI: 10.1161/JAHA.117.006302.)28778942
References
- 1. World Health Organisation . Global atlas on cardiovascular disease prevention and control. 2011.
- 2. Bloom DE, Cafiero ET, Jané‐Llopis E, Abrahams‐Gessel S, Bloom LR, Fathima S, Feigl AB, Gaziano T, Mowafi M, Pandya A, Prettner K, Rosenberg L, Seligman B, Stein AZ, Weinstein C. The Global Economic Burden of Non‐Communicable Diseases. World Economic Forum: Geneva; 2011. [Google Scholar]
- 3. Kelishadi R, Poursafa P. A review on the genetic, environmental, and lifestyle aspects of the early‐life origins of cardiovascular disease. Curr Probl Pediatr Adolesc Health Care. 2014;44:54–72. [DOI] [PubMed] [Google Scholar]
- 4. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta‐regression analysis. Circulation. 2008;117:3171–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berenson GS. Childhood risk factors predict adult risk associated with subclinical cardiovascular disease. The Bogalusa Heart Study. Am J Cardiol. 2002;90:3L–7L. [DOI] [PubMed] [Google Scholar]
- 6. Statistics Canada . Blood Pressure of Canadian Children and Youth, 2009 to 2011. Statistics Canada: Ottawa; 2016. [Google Scholar]
- 7. Kakinami L, Paradis G, O'Loughlin J, Seguin L, Delvin EE, Lambert M. Is the obesity epidemic worsening the cardiovascular risk factor profile of children? Evidence from two Quebec samples measured 10 years apart. Ann Hum Biol. 2012;39:322–326. [DOI] [PubMed] [Google Scholar]
- 8. Levy‐Marchal C, Czernichow P. Small for gestational age and the metabolic syndrome: which mechanism is suggested by epidemiological and clinical studies? Horm Res. 2006;65(suppl 3):123–130. [DOI] [PubMed] [Google Scholar]
- 9. Kerkhof GF, Leunissen RW, Hokken‐Koelega AC. Early origins of the metabolic syndrome: role of small size at birth, early postnatal weight gain, and adult IGF‐I. J Clin Endocrinol Metab. 2012;97:2637–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chiavaroli V, Marcovecchio ML, de Giorgis T, Diesse L, Chiarelli F, Mohn A. Progression of cardio‐metabolic risk factors in subjects born small and large for gestational age. PLoS One. 2014;9:e104278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fabricius‐Bjerre S, Jensen RB, Faerch K, Larsen T, Molgaard C, Michaelsen KF, Vaag A, Greisen G. Impact of birth weight and early infant weight gain on insulin resistance and associated cardiovascular risk factors in adolescence. PLoS One. 2011;6:e20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Law CM, Shiell AW, Newsome CA, Syddall HE, Shinebourne EA, Fayers PM, Martyn CN, de Swiet M. Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation. 2002;105:1088–1092. [DOI] [PubMed] [Google Scholar]
- 13. Taine M, Stengel B, Forhan A, Carles S, Botton J, Charles MA, Heude B. Rapid early growth may modulate the association between birth weight and blood pressure at 5 years in the EDEN Cohort study. Hypertension. 2016;68:859–865. [DOI] [PubMed] [Google Scholar]
- 14. Evagelidou EN, Giapros VI, Challa AS, Cholevas VK, Vartholomatos GA, Siomou EC, Kolaitis NI, Bairaktari ET, Andronikou SK. Prothrombotic state, cardiovascular, and metabolic syndrome risk factors in prepubertal children born large for gestational age. Diabetes Care. 2010;33:2468–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dyck RF, Klomp H, Tan L. From, “thrifty genotype” to “hefty fetal phenotype”: the relationship between high birthweight and diabetes in Saskatchewan Registered Indians. Can J Public Health. 2001;92:340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fujita Y, Kouda K, Nakamura H, Iki M. Association of rapid weight gain during early childhood with cardiovascular risk factors in Japanese adolescents. J Epidemiol. 2013;23:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horta BL, Victora CG, Lima RC, Post P. Weight gain in childhood and blood lipids in adolescence. Acta Paediatr. 2009;98:1024–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gardner DS, Hosking J, Metcalf BS, Jeffery AN, Voss LD, Wilkin TJ. Contribution of early weight gain to childhood overweight and metabolic health: a longitudinal study (EarlyBird 36). Pediatrics. 2009;123:e67–e73. [DOI] [PubMed] [Google Scholar]
- 19. Huang RC, Burke V, Newnham JP, Stanley FJ, Kendall GE, Landau LI, Oddy WH, Blake KV, Palmer LJ, Beilin LJ. Perinatal and childhood origins of cardiovascular disease. Int J Obes (Lond). 2007;31:236–244. [DOI] [PubMed] [Google Scholar]
- 20. Howe LD, Chaturvedi N, Lawlor DA, Ferreira DL, Fraser A, Davey Smith G, Tilling K, Hughes AD. Rapid increases in infant adiposity and overweight/obesity in childhood are associated with higher central and brachial blood pressure in early adulthood. J Hypertens. 2014;32:1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tilling K, Davies N, Windmeijer F, Kramer MS, Bogdanovich N, Matush L, Patel R, Smith GD, Ben‐Shlomo Y, Martin RM. Is infant weight associated with childhood blood pressure? Analysis of the Promotion of Breastfeeding Intervention Trial (PROBIT) cohort. Int J Epidemiol. 2011;40:1227–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kwok MK, Au Yeung SL, Leung GM, Schooling CM. Birth weight, infant growth, and adolescent blood pressure using twin status as an instrumental variable in a Chinese birth cohort: “Children of 1997”. Ann Epidemiol. 2014;24:509–515. [DOI] [PubMed] [Google Scholar]
- 23. Derraik JG, Rowe DL, Cutfield WS, Hofman PL. Decreasing birth weight is associated with adverse metabolic profile and lower stature in childhood and adolescence. PLoS One. 2015;10:e0119433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benn RT. Some mathematical properties of weight‐for‐height indices used as measures of adiposity. Br J Prev Soc Med. 1971;25:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wells JC. A critique of the expression of paediatric body composition data. Arch Dis Child. 2001;85:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Edvardsson VO, Steinthorsdottir SD, Eliasdottir SB, Indridason OS, Palsson R. Birth weight and childhood blood pressure. Curr Hypertens Rep. 2012;14:596–602. [DOI] [PubMed] [Google Scholar]
- 27. Schellong K, Schulz S, Harder T, Plagemann A. Birth weight and long‐term overweight risk: systematic review and a meta‐analysis including 643 902 persons from 66 studies and 26 countries globally. PLoS One. 2012;7:e47776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331:929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Raitakari OT, Juonala M, Viikari JS. Obesity in childhood and vascular changes in adulthood: insights into the Cardiovascular Risk in Young Finns Study. Int J Obes (Lond). 2005;29 S101–S104. [DOI] [PubMed] [Google Scholar]
- 30. Chiolero A, Kaufman JS, Paradis G. Why adjustment for current weight can bias the estimate of the effect of birth weight on blood pressure: shedding light using causal graphs. J Hypertens. 2012;30:1042–1045. [DOI] [PubMed] [Google Scholar]
- 31. Lambert M, Van Hulst A, O'Loughlin J, Tremblay A, Barnett TA, Charron H, Drapeau V, Dubois J, Gray‐Donald K, Henderson M, Lagace G, Low NC, Mark S, Mathieu ME, Maximova K, McGrath JJ, Nicolau B, Pelletier C, Poirier P, Sabiston C, Paradis G. Cohort profile: the Quebec adipose and lifestyle investigation in youth cohort. Int J Epidemiol. 2012;41:1533–1544. [DOI] [PubMed] [Google Scholar]
- 32. WHO child growth standards based on length/height, weight and age . Acta Paediatr Suppl. 2006;450:76–85. [DOI] [PubMed] [Google Scholar]
- 33. Kramer MS, Platt RW, Wen SW, Joseph KS, Allen A, Abrahamowicz M, Blondel B, Breart G. A new and improved population‐based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108:E35. [DOI] [PubMed] [Google Scholar]
- 34. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 37. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents . The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 38. Cole TJ. Sympercents: symmetric percentage differences on the 100 log(e) scale simplify the presentation of log transformed data. Stat Med. 2000;19:3109–3125. [DOI] [PubMed] [Google Scholar]
- 39. Haas GM, Liepold E, Schwandt P. Low birth weight as a predictor of cardiovascular risk factors in childhood and adolescence? The PEP Family Heart Study. Int J Prev Med. 2015;6:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Owen CG, Whincup PH, Odoki K, Gilg JA, Cook DG. Birth weight and blood cholesterol level: a study in adolescents and systematic review. Pediatrics. 2003;111:1081–1089. [DOI] [PubMed] [Google Scholar]
- 41. Lawlor DA, Riddoch CJ, Page AS, Anderssen SA, Froberg K, Harro M, Stansbie D, Smith GD. The association of birthweight and contemporary size with insulin resistance among children from Estonia and Denmark: findings from the European Youth Heart Study. Diabet Med. 2005;22:921–930. [DOI] [PubMed] [Google Scholar]
- 42. Zhang Z, Kris‐Etherton PM, Hartman TJ. Birth weight and risk factors for cardiovascular disease and type 2 diabetes in US children and adolescents: 10 year results from NHANES. Matern Child Health J. 2014;18:1423–1432. [DOI] [PubMed] [Google Scholar]
- 43. Huxley R, Owen CG, Whincup PH, Cook DG, Colman S, Collins R. Birth weight and subsequent cholesterol levels: exploration of the “fetal origins” hypothesis. JAMA. 2004;292:2755–2764. [DOI] [PubMed] [Google Scholar]
- 44. Leunissen RW, Kerkhof GF, Stijnen T, Hokken‐Koelega A. Timing and tempo of first‐year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA. 2009;301:2234–2242. [DOI] [PubMed] [Google Scholar]
- 45. Khuc K, Blanco E, Burrows R, Reyes M, Castillo M, Lozoff B, Gahagan S. Adolescent metabolic syndrome risk is increased with higher infancy weight gain and decreased with longer breast feeding. Int J Pediatr. 2012;2012:478610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bekkers MB, Brunekreef B, Smit HA, Kerkhof M, Koppelman GH, Oldenwening M, Wijga AH. Early‐life determinants of total and HDL cholesterol concentrations in 8‐year‐old children; the PIAMA birth cohort study. PLoS One. 2011;6:e25533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch‐up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18:815–831. [DOI] [PubMed] [Google Scholar]
- 48. Pereira JA, Rondo PH, Lemos JO, Pacheco de Souza JM, Dias RS. The influence of birthweight on arterial blood pressure of children. Clin Nutr. 2010;29:337–340. [DOI] [PubMed] [Google Scholar]
- 49. Chiolero A, Paradis G, Kaufman JS. Assessing the possible direct effect of birth weight on childhood blood pressure: a sensitivity analysis. Am J Epidemiol. 2014;179:4–11. [DOI] [PubMed] [Google Scholar]
- 50. Huxley R, Neil A, Collins R. Unravelling the fetal origins hypothesis: is there really an inverse association between birthweight and subsequent blood pressure? Lancet. 2002;360:659–665. [DOI] [PubMed] [Google Scholar]
- 51. Hemachandra AH, Howards PP, Furth SL, Klebanoff MA. Birth weight, postnatal growth, and risk for high blood pressure at 7 years of age: results from the Collaborative Perinatal Project. Pediatrics. 2007;119:e1264–e1270. [DOI] [PubMed] [Google Scholar]
- 52. Chiolero A, Paradis G, Madeleine G, Hanley JA, Paccaud F, Bovet P. Birth weight, weight change, and blood pressure during childhood and adolescence: a school‐based multiple cohort study. J Hypertens. 2011;29:1871–1879. [DOI] [PubMed] [Google Scholar]
- 53. Perng W, Rifas‐Shiman SL, Kramer MS, Haugaard LK, Oken E, Gillman MW, Belfort MB. Early weight gain, linear growth, and mid‐childhood blood pressure: a prospective study in project viva. Hypertension. 2016;67:301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lurbe E, Garcia‐Vicent C, Torro MI, Aguilar F, Redon J. Associations of birth weight and postnatal weight gain with cardiometabolic risk parameters at 5 years of age. Hypertension. 2014;63:1326–1332. [DOI] [PubMed] [Google Scholar]
- 55. Mann KD, Pearce MS, Sayers SM, Singh GR. Pathways between birth weight and later body size in predicting blood pressure: Australian Aboriginal Cohort Study 1987–2007. J Hypertens. 2015;33:933–939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of QUALITY Study Participants Included and of Those Excluded From the Analytic Sample
Table S2. Comparison of Participants at Ages 8–10 and 10–12 Years in a Subsample (n=395) of the QUALITY Cohort
Table S3. Associations* of Birth Weight for Length, Postnatal Weight Gain, and Childhood Adiposity With Triglycerides and High‐Density Lipoprotein Cholesterol Measured at Ages 10–12 Years, Stratified by Sex, Among 395 Children of the QUALITY Cohort, Quebec, Canada, 2005–2011
Table S4. The β Coefficients* for Total, Direct, and Indirect Effects (Mediated by Percentage of Body Fat at Ages 8–10 Years) of Birth Weight for Length and Postnatal Weight Gain on Triglycerides and High‐Density Lipoprotein Cholesterol at Ages 10–12 Years, Stratified by Sex Among 395 Children of the QUALITY Cohort, Quebec, Canada, 2005–2011
Table S5. Associations* of Birth Weight for Length, Postnatal Weight Gain, and Childhood Adiposity With Plasma Lipids Measured at Ages 10–12 Years Among Children From the QUALITY Cohort Born With an Appropriate Size for Gestational Age (n=318)
Table S6. Associations* of Birth Weight for Length, Postnatal Weight Gain, and Childhood Adiposity With Blood Pressure Measured at Ages 10–12 Years Among Children From the QUALITY Cohort Born With an Appropriate Size for Gestational Age (n=318)
Table S7. Coefficients* for Total, Direct, and Indirect Effects (Mediated by Percentage of Body Fat at Ages 8–10 Years) of Birth Weight for Length and Postnatal Weight Gain on Cardiovascular Risk at Ages 10–12 Years Among Children From the QUALITY Cohort Born With an Appropriate Size for Gestational Age (n=318)


