Abstract
Background
Epicardial adipose tissue (EAT) is hypothesized to alter atherosclerotic plaque composition, with potential development of high‐risk plaque (HRP). EAT can be measured by volumetric assessment (EAT‐v) or linear thickness (EAT‐t). We performed a systematic review and random‐effects meta‐analysis to assess the association of EAT with HRP and whether this association is dependent on the measurement method used.
Methods and Results
Electronic databases were systematically searched up to October 2016. Studies reporting HRP by computed tomography or intracoronary imaging and studies measuring EAT‐v or EAT‐t were included. Odds ratios were extracted from multivariable models reporting the association of EAT with HRP and described as pooled estimates with 95% confidence intervals (CIs). Analysis was stratified by EAT measurement method. Nine studies (n=3772 patients) were included with 7 measuring EAT‐v and 2 measuring EAT‐t. Increasing EAT was significantly associated with the presence of HRP (odds ratio: 1.26 [95% CI, 1.11–1.43]; P<0.001). Patients with HRP had higher EAT‐v than those without (weighted mean difference: 28.3 mL [95% CI, 18.8–37.8 mL]; P<0.001). EAT‐v was associated with HRP (odds ratio: 1.19 [95% CI, 1.06–1.33]; P<0.001); however, EAT‐t was not (odds ratio: 3.09 [95% CI, 0.56–17]; P=0.2). Estimates remained significant when adjusted for small‐study effect bias (odds ratio: 1.13 [95% CI, 1.03–1.28]; P=0.04).
Conclusions
Increasing EAT is associated with the presence of HRP, and patients with HRP have higher quantified EAT‐v. The association of EAT‐v with HRP is significant compared with EAT‐t; however, a larger scale study is still required, and further evaluation is needed to assess whether EAT may be a potential therapeutic target for novel pharmaceutical agents.
Clinical Trial Registration
URL: https://www.crd.york.ac.uk/. Unique identifier: CRD42017055473.
Keywords: epicardial fat, high‐risk plaque, meta‐analysis, vulnerable plaque
Subject Categories: Computerized Tomography (CT), Echocardiography, Coronary Artery Disease
Clinical Perspective
What Is New?
Increasing epicardial adipose tissue (EAT) volume is associated with the presence of high‐risk coronary artery plaque characteristics.
Patients with high‐risk coronary plaque features have quantitatively higher EAT volumes.
EAT should ideally be measured by complete volumetric analysis rather than by linear thickness measurements.
What Are the Clinical Implications?
Incorporation of EAT measurement with routinely performed cardiac computed tomography may assist in improved risk stratification for patients.
EAT may represent an important cardiovascular therapeutic target.
Introduction
Epicardial adipose tissue (EAT) is a metabolically active fat depot, abundant in proinflammatory cytokines, and has been correlated with the extent and severity of coronary artery disease (CAD).1 EAT shares the same embryologic origin of omental and mesenteric fat2, 3 and encases the coronary arteries with no fascial barrier.4 Consequently, it has been postulated that EAT may display vasocrine or paracrine effects on the adjacent arterial wall to influence atherosclerotic plaque composition, resulting in the development of high‐risk plaque (HRP).5, 6, 7, 8, 9 The presence of HRP has shown association with future adverse prognosis,10, 11 but the management of these patients remains uncertain. HRP may be visualized invasively by several methods including intravascular ultrasound and optical coherence tomography and noninvasively by computed tomography (CT) coronary angiography with good diagnostic agreement between techniques.12, 13, 14 EAT may be measured either volumetrically by CT coronary angiography or noncontrast CT (EAT‐v) or by a linear thickness measurement on echocardiography (EAT‐t). Both thickness and volume measures have been associated with incident CAD1; however, linear thickness may underrepresent the totality of EAT.
The objective of this systematic review and meta‐analysis was to explore the association between EAT and the presence of HRP. The secondary aims were to evaluate whether increasing EAT volume is associated with HRP presence and the strength of association with presence of HRP by EAT measurement method.
Materials and Methods
Data Sources and Search Strategy
The search was conducted using the Medline, Embase, and PubMed databases with no start date up to October 2016. Keywords using Medical Subject Headings included epicardial adipose tissue, epicardial fat, pericardial adipose tissue, pericardial fat, vulnerable plaque, high‐risk plaque, low‐attenuation plaque, napkin ring, positive remodeling, spotty calcification, coronary artery disease, plaque characteristics, plaque composition, plaque vulnerability, thin‐cap fibroatheroma, intravascular ultrasound, optical coherence tomography, and angioscopy. The reference lists of eligible articles were hand‐searched for additional articles. Searches were restricted to human studies. We conducted this systematic review in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) statement, and the trial was registered with PROSPERO (registration no. CRD42017055473). A flow chart describing the study search is presented in Figure 1, and an example of the search term strategy is shown in Table S1.
Figure 1.
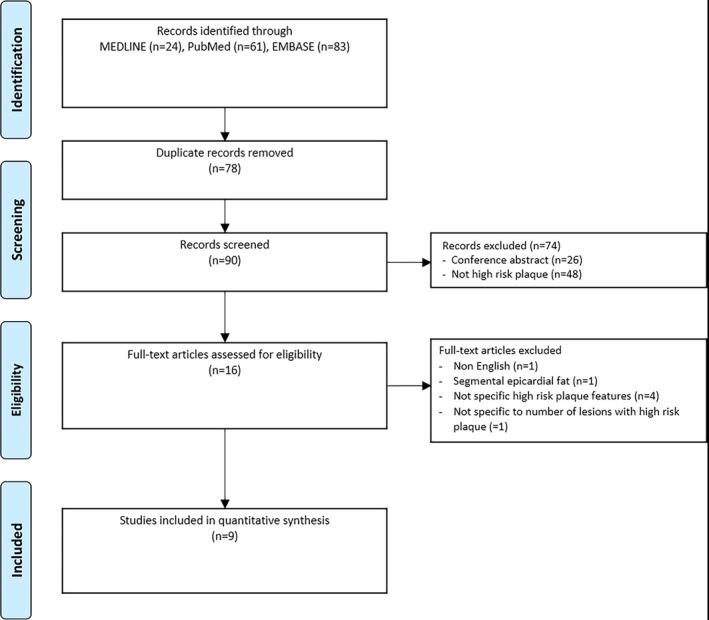
Search strategy.
Study Selection
The following inclusion criteria were used for the study: patients undergoing either intracoronary imaging or CT coronary angiography evaluation with reported HRP features, noninvasive measurement of EAT by either CT‐derived volume (on contrast or noncontrast CT) or linear thickness (by CT or echocardiography), and reports fully published in peer‐reviewed journals. For intracoronary imaging studies, HRP was defined as the presence of thin‐cap fibroatheroma. For CT studies, HRP included plaques with ≥1 of the following features: low‐attenuation plaque, positive remodeling, spotty calcification, and the napkin ring sign. Study specific definitions of HRP are reported in Table S2.
Data Extraction
Odds ratios (ORs) and their respective 95% confidence intervals (95% CIs) for association of EAT with HRP were extracted. If possible, ORs from multivariable models that adjusted for other CAD risk factors were used, and covariates within the model were recorded. Mean and standard deviation of EAT volume between groups with and without HRP were entered. Studies reporting medians with interquartile ranges were converted to means, as recommended previously.15
End Points
The primary end point was the pooled association of EAT with the presence of HRP. Secondary end points included the pooled quantitative difference of EAT‐v in patients with and without HRP and the association of EAT with HRP stratified by EAT measurement method (EAT‐v or EAT‐t).
Statistical Analysis
Statistical analysis was performed using StataMP 14.0 (StataCorp). ORs were examined on the log scale and transformed for graphical presentation with 95% CI reported. If multiple outcomes were reported (ie, by individual plaque feature or by grouped features), the analyzed estimate was the association of EAT with any HRP if specified. Random‐effects modeling was used with the method of DerSimonian and Laird.16 The weighted mean difference for EAT between groups with and without HRP was calculated. Statistical heterogeneity was evaluated by the I2 statistic and quantified as low (<25%), moderate (25–75%), or high (>75%).17 Sensitivity analysis was performed by EAT measurement method (EAT‐v or EAT‐t, by pooled estimates of similarly defined EAT covariate parameters; ie, when EAT was included as a continuous variable or assessed in 10‐mL increments and for individual plaque features, if possible). Additional sensitivity analysis using random effects with the Hartung–Knapp–Sidik–Jonkman (HKSJ) approach was used to explore effect sizes when 2 studies were grouped.18, 19 Exploratory metaregression was performed to assess the influence of independent variables (mean study ages, mean EAT volume, mean study body mass index, and proportion of HRP). Publication bias was assessed by the Egger and Begg test. In addition, the Duval and Tweedie trim‐and‐fill method was used to investigate publication bias, and systematic exclusion of individual studies was used to assess changes in the pooled estimate. A 2‐sided P value of <0.05 was considered significant.
Results
A total of 90 publications were reviewed with 9 studies included for final analysis (3772 participants; Figure 1). One study was excluded because it presented the association of EAT with plaque lipid percentage rather than specified numbers of patients with HRP.20 Seven studies reported CT assessment of HRP21, 22, 23, 24, 25, 26, 27 (n=3573) and 2 studies reported invasive assessment of HRP29, 30 (n=199). Seven studies measured EAT‐v21, 22, 23, 24, 25, 26, 29, 30 (n=3284), and 2 studies measured EAT‐t27, 28 (n=488). All study designs were cross‐sectional. All patients were from cohorts with suspected CAD, with 2 studies evaluating patients with suspected acute coronary syndrome.21, 22 Study characteristics are presented in Tables 1 and 2, and regression modeling outcomes and model covariates are presented in Table 3. Individual‐study EAT measurement characteristics and HRP definitions are presented in Table S2.
Table 1.
Demographic, EAT, and HRP Parameters of Included Studies
| Study | EAT Method | Population | N | EAT Value | HRP Proportions |
|---|---|---|---|---|---|
| Lu et al21 | EAT‐v (CACS) | Suspected ACS | 467 |
Median EAT: 108.5 cm3 (IQR: 76.4–140.6 cm3) With HRP: 123 cm3 (IQR: 93–156 cm3) Without HRP: 98 cm3 (IQR: 68–127 cm3) |
HRP in 167 (36%) patients; NRS in 15%; PR in 32.3%; LAP in 23.4%; SpC: in 91% |
| Schlett et al22 | EAT‐v (CTCA) | Suspected ACS | 358 |
Median EAT: 95.2 cm3 (IQR: 66–130.1) With HRP: 151.9 cm3 (IQR: 109.0–179.4) Without HRP: 110 cm3 (IQR: 81.5–137.4) |
Any HRP in 13 (4%) patients |
| Rajani et al24 | EAT‐v (CACS) | Suspected CAD | 402 |
Mean EAT: 103±51 cm3
With any HRP: 116±53 cm3 Without HRP: 99±57 cm3 |
Any HRP in 113 (59%) patients; LAP in 67 (35%); PR in 93 (48%) |
| Oka et al23 | EAT‐v (CACS) | Suspected CAD | 357 | Mean EAT: 125±44 mL; EAT analysis threshold of 100 mL |
87 (24%) with all 3 HRPs LAP: EAT <100 mL: 52%; EAT ≥100 mL: 27% PR: EAT <100 mL: 58%; EAT ≥100 mL: 37% LAP with or without PR: EAT <100 mL: 46%; EAT ≥100 mL: 25% |
| Ito et al25 | EAT‐v (CACS) | Suspected CAD (symptomatic) with CACS 0 | 1308 |
Mean EAT: 98.1±41.3 cm3
With HRP: 133±40.2 cm3 Without HRP: 95.1±40.3 cm3 |
Any HRP in 63 (5%) patients |
| Nakanishi et al26 | EAT‐v (CTCA) | Suspected CAD in patients with CKD | 275 |
Mean EAT: CKD: 111±41 mL (n=110) No CKD: 81±29 mL (n=165) |
Any HRP in 44 (16%) patients |
| Ito et al29 | EAT‐v (CTCA) | Scheduled for PCI and underwent CT in addition to OCT | 117 (244 plaques) |
EAT‐v Tertiles: T1: <104.1 cm3 (n=39) T2: 104.1 to 130.7 cm3 (n=39) T3: >130.7 cm3 (n=39) |
Total TCFA: 51 (21%) plaques T1: Single TCFA n=6 (15%); Multiple TCFA n=1 (3%) T2: Single TCFA n=7 (18%); Multiple TCFA n=3 (8%) T3: Single TCFA n=12 (31%); Multiple TCFA n=8 (21%) Minimum fibrous cap thickness: T1: 102.7±69.2 μm; T2: 102.5±56.5 μm; T3: 78.2±43.9 μm Maximal lipid arc: T1: >2 quadrants, 13 (33%); T2: >2 quadrants, 14 (36%); T3: >2 quadrants, 25 (64%) CT characteristics: T1: LAP, 4 (10%); PR, 8 (21%) T2: LAP, 14 (36%); PR, 13 (33%) T3: LAP, 16 (41%); PR, 21 (54%) |
| Park et al28 | EAT‐t (Echo) | Angiographically significant CAD undergoing PCI with or without IVUS | 82 |
Mean EAT‐t: 3.4±2.2 mm EAT‐t 3.5 mm threshold: EAT <3.5 mm (n=21); EAT ≥3.5 mm (n=39) |
TCFA (n): EAT <3.5 mm: 3.3±2.2; EAT ≥3.5 mm: 2.1±1.6 Mean volume index necrotic core (mm3/mm): EAT <3.5 mm: 0.3±0.2; EAT ≥3.5 mm: 0.6±0.4 Plaque volume (mm3): EAT <3.5 mm: 1360.1±492.1; EAT ≥3.5 mm: 1048.5±398.2 |
| Tachibana et al27 | EAT‐t (Echo) | Suspected CAD | 406 | EAT‐t 5.8 mm threshold: EAT ≥5.8 mm (n=238); EAT <5.8 mm (n=168) |
HRP in 45 (11%) patients LAP: EAT <5.8 mm: 4%; EAT ≥5.8 mm: 24% PR: EAT <5.8 mm: 39%; EAT ≥5.8 mm: 60% LAP+PR: EAT <5.8 mm: 3%; EAT ≥5.8 mm: 17% |
ACS indicates acute coronary syndrome; CACS, coronary artery calcium score (noncontrast computed tomography); CAD, coronary artery disease; CKD, chronic kidney disease; CT, computed tomography; CTCA, computed tomography coronary angiography; EAT, epicardial adipose tissue; EAT‐t, epicardial adipose tissue thickness; EAT‐v, epicardial adipose tissue volume; HRP, high‐risk plaque; IQR, interquartile range; IVUS, intravascular ultrasound; LAP, low‐attenuation plaque; NRS, napkin ring sign; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; PR, positive remodeling; SpC, spotty calcification; T, tertile; TCFA, thin‐cap fibroatheroma.
Table 2.
Study Demographic Data
| Study | Diabetes Mellitus(%) | Hypertension (%) | Hyperlipidemia (%) | BMI | Ethnicity | Age, y | Sex (%) |
|---|---|---|---|---|---|---|---|
| Lu et al21 | 17 | 53 | 45 | 29±5 | Not specified | 54±8 | 53 |
| Schlett et al22 | 10 | 39 | 37 | 28 (25–32) | Not specified | 51 (45–59) | 62 |
| Rajani et al24 | 14 | 54 | 63 | 27±4 | Not specified | 66 (23–92) | 56 |
| Oka et al23 | 31 | 68 | 50 | 24±5 | Japanese institution | 66±11 | 63 |
| Ito et al25 | 8 | 33 | 26 | 23±4 | Japanese institution | 59±12 | 46 |
| Nakanishi et al26 | 38 | 65 | 59 | 24±4 | Japanese institution | 65±10 | 66 |
| Park et al28 | 29 | 61 | 20 | 25±3 | Korean Institution | 59±11 | 54 |
| Ito et al29 | 24 | 61 | 44 | 24±3 | Japanese institution | 66±9 | 82 |
| Tachibana et al27 | 27 | 58 | 31 | 23±4 | Japanese institution | 68±13 | 57 |
Values are expressed as total study cohort proportions (%), mean±SD, or median (interquartile range). BMI indicates body mass index.
Table 3.
EAT Modeling Outcomes and Model Covariates
| Study | EAT Modeling | Regression Outcomes | Covariates in Multivariable Model | Threshold/ROC AUC Values |
|---|---|---|---|---|
| Lu et al21 | Indexed and absolute EAT |
Any HRP with indexed EAT‐v: OR: 1.04 (95% CI, 1–1.08; P=0.04) Any HRP with absolute EAT‐v: OR: 1.02 (95% CI, 1–1.03; P=0.046) |
Age, sex, number of cardiovascular risk factors, log CACS, >50% stenosis | Optimal threshold 62.3 cm3/m2 with sensitivity 48.5%, specificity 72.7%; no ROC AUC specified |
| Schlett et al22 | EAT per SD (49.8 mL) | Presence of HRP: OR: 1.79 (95% CI, 1.13–2.76; P=0.008) | Not specified | Not reported |
| Rajani et al24 | Log EAT‐v |
Any HRP: OR: 1.7 (95% CI, 0.9–3.4; P=0.038) LAP: OR: 2.4 (95% CI, 1.1–5.1; P=0.02) PR: OR: 1.8 (95% CI, 1.0–3.4; P=0.07) Both HRP features: OR: 2.6 (95% CI, 1.1–6.2; P=0.03) |
Age, BMI, diabetes mellitus, hypercholesterolemia, smoking, family history, hypertension | ROC AUC of 0.756 for any HRP presence with sensitivity 62%, specificity 84%; optimal threshold of EAT <74.07 cm3 excluded any HRP |
| Oka et al23 | High vs low‐EAT‐v (100 mL threshold) |
LAP: OR: 3.08 (95% CI, 1.66–5.83; P<0.001) PR: OR: 2.08 (95% CI, 1.12–3.88; P=0.02) SpC: OR: 1.11 (95% CI, 0.61–2.04; P=0.73) LAP+PR: OR: 2.56 (95% CI, 1.38–4.85; P=0.003) All 3 features: OR: 1.65 (95% CI 0.81–3.44; P=0.17) |
Age, sex, hypertension, diabetes mellitus, smoking, BMI, VAT area, CACS | Using a threshold of 100 mL, sensitivity for LAP+PR was 80%, specificity was 41% |
| Ito et al25 | EAT‐v per 10 cm3 | Any HRP: OR: 1.19 (95% CI, 1.12–1.27; P<0.01) | Male, diabetes mellitus, hypertension, BMI | ROC AUC of 0.75 for any HRP presence at optimal threshold 127.1 cm3 with sensitivity 64%, specificity 81% |
| Nakanishi et al26 | EAT‐v per 10 mL | Presence of HRP: OR: 1.15 (95% CI, 1.05–1.26; P=0.003) | Age per 10 y, sex, hypertension, diabetes mellitus, hyperlipidemia, smoking, BMI | … |
| Ito et al29 | Highest tertile of EAT |
Presence of TCFA: OR: 2.92 (95% CI, 1.13–7.55; P=0.027) Correlation of EAT with fibrous cap thickness: r=−0.400, P<0.01 |
ACS, BMI | ROC AUC of 0.721 for detection of TCFA with optimal threshold 126.7 cm3, sensitivity 69% specificity 71% |
| Park et al28 | High vs low‐EAT‐t (3.5 mm threshold) | Total TCFAs in symptom‐related vessel: β=0.106 (95% CI, 0.004–0.208; P=0.043) | BMI, diabetes mellitus, dyslipidemia, metabolic syndrome | Not specified |
| Tachibana et al27 | High vs low‐EAT‐t (5.8 mm threshold) | Presence of HRP: OR: 7.98 (95% CI, 2.77–22.98; P<0.01) | Age, sex, BMI, VAT, hypertension, dyslipidemia, diabetes mellitus, smoker, CACS >100, stenotic vessel number, renal insufficiency, statins | ROC AUC of 0.77 for HRP (combination of LAP+PR) at threshold of 5.8 mm with sensitivity 83%, specificity 64% |
ACS indicates acute coronary syndrome; BMI, body mass index; CACS, coronary artery calcium score (noncontrast computed tomography); CI, confidence interval; EAT, epicardial adipose tissue; EAT‐t, EAT thickness; EAT‐v, volumetric EAT; HRP, high‐risk plaque; LAP, low‐attenuation plaque; OR, odds ratio; PR, positive remodeling; ROC AUC, receiver operating characteristic area under the curve; SpC, spotty calcification; TCFA, thin‐cap fibroatheroma; VAT, visceral adipose tissue.
The prevalence of HRP ranged widely, from 4% to 59% at a per‐patient level (Table 1). The primary end point demonstrated a significant association of increasing EAT with the presence of HRP (pooled OR: 1.26 [95% CI, 1.11–1.43]; P<0.001, I2=81%; Figure 2).
Figure 2.
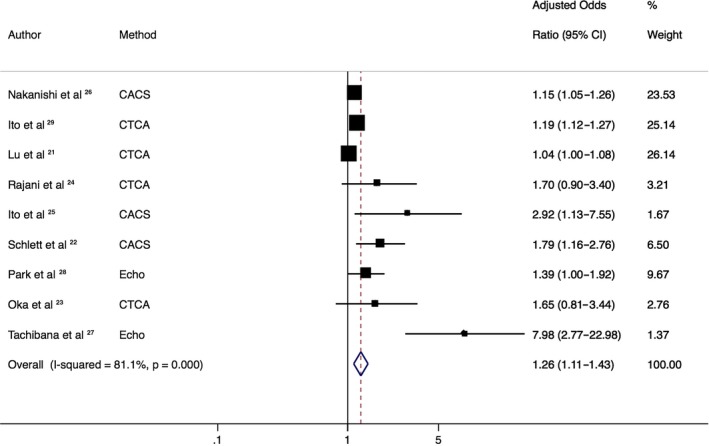
Association of epicardial adipose tissue (EAT) with presence of high‐risk plaque (HRP). Forest plot displays summary odds ratios and 95% confidence intervals (CIs) for the increasing association of EAT with HRP. Method represents the radiologic method of calculating EAT. This demonstrates a significant association of increasing EAT with HRP. CACS indicates coronary artery calcium score (noncontrast computed tomography); CTCA, computed tomography coronary angiography; Echo, echocardiography.
Analysis to assess quantitative differences in EAT between patients with and without HRP demonstrated a weighted mean difference of +28.3 mL in those patients with HRP (95% CI, 18.8–37.8 mL]; P<0.001; I2: 58%) based on 4 studies (Figure 3).
Figure 3.
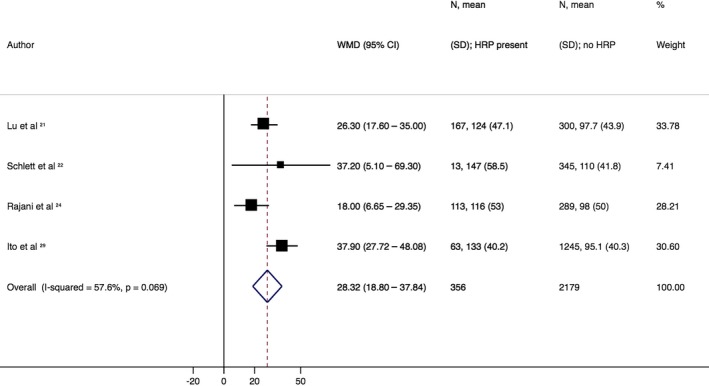
Difference in quantitative epicardial adipose tissue (EAT). Forest plot displays weighted mean differences (WMDs) and 95% confidence intervals (CIs) for differences between patients with and without high‐risk plaque (HRP). This indicates that patients with HRP have a significantly higher volume of EAT (WMD: 28.3 mL [95% CI, 18.8–37.8 mL]) compared with those patients without HRP.
When stratified by EAT measurement method, in the 7 studies measuring EAT‐v, the pooled OR was significantly associated with HRP presence (OR: 1.19 [95% CI, 1.06–1.33]; P<0.001, I2: 78%). However, no significant association was observed with the 2 EAT‐t studies and presence of HRP (OR: 3.09 [95% CI, 0.56–17]; P=0.20; I2: 90%; Figure 4). This remained statistically nonsignificant on sensitivity analysis with the HKSJ method (Table 4).
Figure 4.
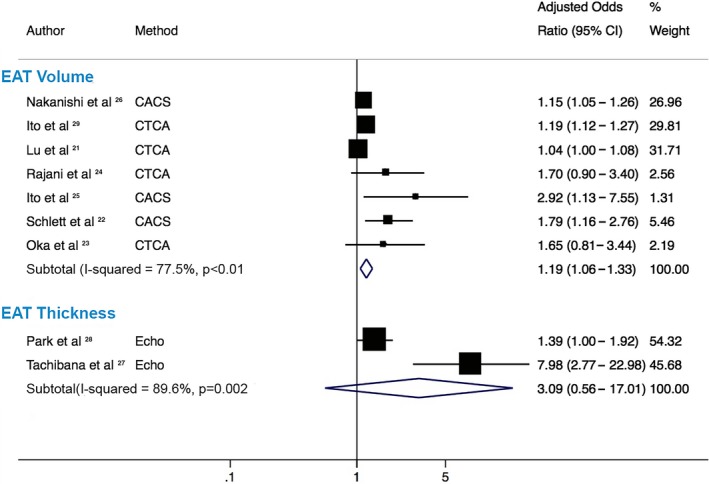
Pooled estimates by epicardial adipose tissue (EAT) measurement method. Forest plot displays odds ratios and 95% confidence intervals (CIs) for the association of increasing EAT with high‐risk plaque (HRP) stratified by measurement method of EAT measurement, either by volume or thickness. This demonstrates that increasing EAT volume has a significant association with HRP; however, increasing EAT thickness is not significantly associated with HRP and has a markedly wide CI crossing the line of unity. CACS indicates coronary artery calcium score (noncontrast computed tomography); CTCA, computed tomography coronary angiography; Echo, echocardiography.
Table 4.
Sensitivity Analysis of Random‐Effects Meta‐Analysis With Alternative Methods When Pooled Estimates Were From Combination of 2 Studies
| Variable | Random‐Effects Method | Pooled OR | 95% CI | P Value |
|---|---|---|---|---|
| EAT measurement method | ||||
| EAT thickness27, 28 | DL | 3.09 | 0.56–17.01 | 0.20 |
| HKSJ | 3.09 | 0–19 | 0.49 | |
| Covariate modeling method | ||||
| EAT continuous21, 24 | DL | 1.18 | 0.77–1.81 | 0.44 |
| HKSJ | 1.18 | 0.08–18.5 | 0.58 | |
| EAT per 10 mL25, 26 | DL | 1.18 | 1.12–1.24 | <0.01 |
| HKSJ | 1.18 | 0.96–1.45 | 0.06a | |
| HRP subtype | ||||
| LAP23, 24 | DL | 2.79 | 1.71–4.53 | <0.01 |
| HKSJ | 2.79 | 0.59–13.2 | 0.08a | |
| PR23, 24 | DL | 1.93 | 1.25–2.99 | 0.003 |
| HKSJ | 1.93 | 0.77–4.84 | 0.07a | |
| Both LAP and PR23, 24 | DL | 2.58 | 1.55–4.28 | <0.01 |
| HKSJ | 2.58 | 2.34–2.83 | 0.005 | |
References indicate studies that were pooled. ORs are presented using DL and HKSJ methods. CI indicates confidence interval; DL, DerSimonian and Laird; EAT, epicardial adipose tissue; HKSJ, Hartung–Knapp–Sidik–Jonkman; HRP, high‐risk plaque; LAP, low attenuation plaque; OR, odds ratio; PR, positive remodeling.
Signifies when there was a change in P value resulting in statistical nonsignificance (P>0.05) after applying the HKSJ method.
Sensitivity analysis was performed to assess pooled estimates of studies using EAT as a similarly measured covariate. Two studies analyzed EAT‐v in 10‐mL increments and demonstrated a pooled OR of 1.18 (95% CI, 1.12–1.24; P<0.001; I2: 0%) that became nonsignificant when analyzed with the HKSJ method (OR: 1.18 [95% CI, 0.84–1.64]; P=0.10). In the 2 studies that analyzed EAT‐v as a continuous variable, the pooled OR was 1.18 (95% CI, 0.77–1.81; P=0.44; I2: 52%), which remained nonsignificant after analysis with HKSJ (Table 4). EAT measured in the remaining studies were modeled as per standard deviation or by a dichotomous threshold level and not formally pooled.
Further sensitivity analysis was performed to assess the association between specific HRP subtypes with information obtainable from 2 studies. An association was demonstrated between increasing EAT and low‐attenuation plaque (OR: 2.79 [95% CI, 1.71–4.53]; P<0.001; I2: 0%), positive remodeling, (OR: 1.93 [95% CI, 1.25–2.99]; P=0.003; I2: 0%), and the presence of both features (OR: 2.58 [95% CI, 1.55–4.28]; P=0.001; I2: 0%). The results for both low‐attenuation plaque and positive remodeling became statistically nonsignificant after application of the HKSJ method, but the presence of both features remained significantly associated with increasing EAT (Table 4).
Exploratory metaregression demonstrated no significant influence of varying study‐level predictors on the overall effect size; these included mean BMI (OR: 0.95 [95% CI, 0.79–1.14]; P=0.55), mean age (OR: 1.03 [95% CI, 0.96–1.10]; P=0.38), population proportion of HRP (OR: 0.99 [95% CI, 0.98–1.00]; P=0.42), and mean EAT volume (OR: 1.00 [95% CI, 0.97–1.03]; P=0.99).
There was evidence of publication bias by calculation of the Egger test for small‐study effects (P=0.005). Using the trim‐and‐fill method, the overall estimate remained significant for the association of EAT and HRP (pooled estimate OR: 1.13 [95% CI, 1.03–1.28]; P=0.04; I2: 81%; Figure S1). Analysis to assess the influence of single studies on the effect estimate demonstrated a persistent significant association of increasing EAT with HRP. The lowest pooled estimate OR of 1.16 (95% CI, 1.06–1.27; P=0.001; I2: 74%) occurred with the exclusion of Tachibana et al, and the highest pooled estimate OR of 1.27 (95% CI, 1.12–1.45; P<0.001; I2: 70%) occurred with the exclusion of Lu et al (Table S3).
Discussion
The results from this meta‐analysis of 9 observational studies demonstrate 3 important findings. First, increasing EAT is significantly associated with the presence of HRP features. Second, patients with HRP have a significantly increased volume of EAT compared with those without HRP. Third, EAT is associated with HRP presence ideally when measured by complete volumetric analysis rather than EAT linear thickness measurements.
EAT is a visceral adipose tissue depot rich in proinflammatory and proatherogenic cytokines including monocyte chemoattractant protein 1, IL‐6 (interleukin 6). IL‐1β, IL‐6sR, and tumor necrosis factor α.31 Because of EAT's anatomic proximity to the adjacent myocardium and lack of fascial barrier with the epicardial coronary arteries, there may be paracrine or vasocrine signaling of cytokines between the surrounding fat and the underlying arterial wall.2 This suggested pathophysiology is analogous to the visceral intra‐abdominal adipose tissue surrounding the portal circulation that is purported to influence the development of hepatic steatosis.32 It has been demonstrated that increased EAT volume is related to both the extent and the lesion severity of coronary stenosis33 and that EAT contains a greater amount of inflammatory cytokines than serum circulating levels and subcutaneous adipose stores.34 The apposition of EAT with the arterial adventitia suggests the “outside‐in” hypothesis of atherosclerosis, whereby the inflammatory milieu of EAT leads to vascular inflammation of the adventitia progressing inward to the intima, leading to plaque formation. Consequently, it is possible that cellular cross‐talk may lead to the development of plaque characteristics considered to be “high risk” given their association with major adverse cardiovascular events. It has also been reported that high EAT levels are associated with mortality, although it remains unclear whether these levels are specifically related to preceding cardiovascular events.35 Our results indicate a uniform association of increasing EAT with HRP, but further study is needed to establish the influence and interaction of these parameters with prognosis. Importantly, we aimed to use risk estimates from multivariable models, which suggests an incremental effect of EAT with HRP presence beyond traditional cardiovascular risk factors.
Because there is no guideline‐advocated technique for EAT quantification, individual studies are subject to authors’ discretion and experience. The interobserver variability for EAT‐t has shown mixed results,36 and a measure of linear thickness by 2‐dimensional assessment may under‐ or overrepresent total EAT volume due to changes in probe angulation. It has been suggested that a threshold of 7 mm confers elevated EAT‐t; this is a significantly higher threshold than our included studies and may also influence interpretation. Only 1 previous study evaluated EAT‐t versus EAT‐v, in 71 patients, and reported a modest correlation (r=0.595).37 EAT‐v, however, also has limitations, with differing values measurable with the use of contrast media38 and possible differences related to vendor‐specific software algorithms. In our analysis of EAT‐v versus EAT‐t, we demonstrated that EAT‐t had a decidedly wide CI for the association with HRP and failed to reach statistical significance, although this is based on only 2 studies with a total of 488 patients. On the contrary, EAT‐v displayed a significant association with HRP with more precise confidence limits. We attempted to explore the association further by analyzing the modeling method of EAT, which demonstrated uncertainty in estimates for differing techniques and highlighted the need for a standardized and consistent approach when incorporating EAT into models to assess disease outcomes.
In our subgroup analysis of EAT association with HRP subtype, we noted a strong association individually with low‐attenuation plaque and positive remodeling as well as with the presence of both features after adjustment for conventional cardiovascular risk factors. Association with individual plaque feature types diminished due to imprecision in 95% CIs but remained for the presence of both high‐risk features. The largest study to date, of 3158 patients by Motoyama et al, reported that these HRP characteristics, defined as the presence of either feature or both, are strongly associated with future acute coronary syndrome development (adjusted hazard ratio: 8.24 [95% CI, 5.26–12.96]; P<0.001).10 EAT was not measured in this study, and its contribution to prognosis remains unclear.
It is notable that some observational studies have demonstrated a lack of relationship between EAT and significant CAD39, 40—similar to our included studies, all of which are observational and prone to significant bias. Biases include selection and ascertainment bias and variable use of predictors in regression modeling that may alter reported estimates and contribute to between‐study heterogeneity. To assess study quality, we evaluated the Grading of Recommendations Assessment, Development and Evaluation (GRADE) classification41, 42, 43 (Tables S4 and S5), which apportions an overall study‐quality assessment. Because none of the trials are, by definition, of high quality, given that they are not randomized controlled trials, the overall information quality is regarded as low and should be interpreted as such without drawing firm conclusions that may alter clinical decision making. Despite the inconsistency of CAD association, given the association of HRP with cardiac prognosis, it remains plausible that EAT may influence plaque composition that may not be diagnosed as functionally or anatomically significant. Rigorous prospective study to assess the role of EAT in atherogenesis is still warranted.
The management of HRP features is uncertain. EAT is currently measured only for research purposes; however, the importance of assessing EAT and its association with HRP relates to a potential target for therapeutic intervention. EAT has demonstrated temporal changes in plaque and cardiovascular risk. In a study of nonobese patients undergoing serial CT over 4 years, an increase in EAT volume was associated with HRP as well as future acute coronary syndrome despite optimal management of cardiovascular risk factors.44 Calorie restriction and bariatric surgery rather than exercise have shown promise as methods for EAT reduction, as explored recently in a meta‐analysis by Rabkin and Campbell,45 and animal data have demonstrated that selective surgical excision of EAT slows the progression of atherosclerosis.46 It remains to be seen whether targeted EAT reduction may improve dynamic atherosclerosis in human participants, and randomized controlled trial data are lacking.
Study Limitations
Our analysis is limited by the observational nature of included studies and by a lack of access to patient‐level data to allow adjustment for other covariates that may influence EAT including sex differences and stratification and assessment by other population features such as traditional cardiovascular risk factors of hypertension, hyperlipidemia and diabetes mellitus. We attempted to account for this by using model estimates that adjusted for several of these variables. The majority of studies were also performed in Japanese centers, which may limit the generalizability of our findings to other ethnic populations. Another important limitation is the inclusion of only 2 studies evaluating EAT thickness and other subgroup parameters. The interpretation of results is limited by this methodology because of the potential lack of power and the inability to draw firm conclusions. Importantly, we noted that when more robust statistical methods were applied when few studies were pooled, statistical significance was reversed, highlighting the need for more data in these areas. We noted a significant degree of heterogeneity, a limitation that has been demonstrated in other published EAT meta‐analyses that report I2 values >90%.1, 47 This is probably in part representative of variable EAT quantification methods and differing measures of EAT as a covariate in regression analyses. We attempted to adjust for this heterogeneity by systematic exclusion of studies that did not significantly attenuate the summary estimates from statistical significance and by sensitivity analysis by subgroup analysis and exploratory metaregression.
Conclusion
Increasing EAT is associated with the presence of HRP, ideally, when measured by complete volumetric analysis. Further investigation is still required to establish the role of EAT‐t in evaluating HRP and consistent methods for modeling EAT as a variable for disease outcomes and the effect of EAT on individual HRP features. Incorporating the measurement of EAT into clinically performed CT coronary angiography has the potential to improve patient risk stratification. Further prospective studies are needed to confirm this finding, which holds potential as a novel therapeutic target for atherosclerotic treatment.
Disclosures
None.
Funding
Dr. Nerlekar is supported by the National Health and Medical Research Council of Australia and National Heart Foundation Scholarship. Dr Brown is supported through a Monash University Early Career Practitioner Fellowship.
Supporting information
Table S1. Example Search Strategy (Embase)
Table S2. Study Epicardial Adipose Tissue Measurement Parameters and High‐Risk Plaque Definitions
Table S3. Sensitivity Analysis Displaying Pooled Odds Ratios and 95% Confidence Intervals With Systematic Exclusion of Individual Studies
Table S4. Newcastle–Ottawa Scale Evaluation of Study Quality
Table S5. GRADE Quality Assessment
Figure S1. Funnel plot.
(J Am Heart Assoc. 2017;6:e006379 DOI: 10.1161/JAHA.117.006379.)28838916
References
- 1. Xu Y, Cheng X, Hong K, Huang C, Wan L. How to interpret epicardial adipose tissue as a cause of coronary artery disease: a meta‐analysis. Coron Artery Dis. 2012;23:227–233. [DOI] [PubMed] [Google Scholar]
- 2. Talman AH, Psaltis PJ, Cameron JD, Meredith IT, Seneviratne SK, Wong DT. Epicardial adipose tissue: far more than a fat depot. Cardiovasc Diagn Ther. 2014;4:416–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–917. [DOI] [PubMed] [Google Scholar]
- 4. Fitzgibbons TP, Czech MP. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J Am Heart Assoc. 2014;3:e000582 DOI: 10.1161/JAHA.113.000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Psaltis PJ, Talman AH, Munnur K, Cameron JD, Ko BS, Meredith IT, Seneviratne SK, Wong DT. Relationship between epicardial fat and quantitative coronary artery plaque progression: insights from computer tomography coronary angiography. Int J Cardiovasc Imaging. 2016;32:317–328. [DOI] [PubMed] [Google Scholar]
- 6. Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O'Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community‐based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. [DOI] [PubMed] [Google Scholar]
- 7. Shimabukuro M, Hirata Y, Tabata M, Dagvasumberel M, Sato H, Kurobe H, Fukuda D, Soeki T, Kitagawa T, Takanashi S, Sata M. Epicardial adipose tissue volume and adipocytokine imbalance are strongly linked to human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33:1077–1084. [DOI] [PubMed] [Google Scholar]
- 8. Ding J, Hsu FC, Harris TB, Liu Y, Kritchevsky SB, Szklo M, Ouyang P, Espeland MA, Lohman KK, Criqui MH, Allison M, Bluemke DA, Carr JJ. The association of pericardial fat with incident coronary heart disease: the Multi‐Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2009;90:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prati F. Eccentric atherosclerotic plaques with positive remodelling have a pericardial distribution: a permissive role of epicardial fat? A three‐dimensional intravascular ultrasound study of left anterior descending artery lesions. Eur Heart J. 2003;24:329–336. [DOI] [PubMed] [Google Scholar]
- 10. Motoyama S, Ito H, Sarai M, Kondo T, Kawai H, Nagahara Y, Harigaya H, Kan S, Anno H, Takahashi H, Naruse H, Ishii J, Hecht H, Shaw LJ, Ozaki Y, Narula J. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid‐term follow‐up. J Am Coll Cardiol. 2015;66:337–346. [DOI] [PubMed] [Google Scholar]
- 11. Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW; Investigators P . A prospective natural‐history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. [DOI] [PubMed] [Google Scholar]
- 12. Nakazato R, Otake H, Konishi A, Iwasaki M, Koo BK, Fukuya H, Shinke T, Hirata K, Leipsic J, Berman DS, Min JK. Atherosclerotic plaque characterization by ct angiography for identification of high‐risk coronary artery lesions: a comparison to optical coherence tomography. Eur Heart J Cardiovasc Imaging. 2015;16:373–379. [DOI] [PubMed] [Google Scholar]
- 13. Marwan M, Taher MA, El Meniawy K, Awadallah H, Pflederer T, Schuhback A, Ropers D, Daniel WG, Achenbach S. In vivo CT detection of lipid‐rich coronary artery atherosclerotic plaques using quantitative histogram analysis: a head to head comparison with IVUS. Atherosclerosis. 2011;215:110–115. [DOI] [PubMed] [Google Scholar]
- 14. Gauss S, Achenbach S, Pflederer T, Schuhback A, Daniel WG, Marwan M. Assessment of coronary artery remodelling by dual‐source CT: a head‐to‐head comparison with intravascular ultrasound. Heart. 2011;97:991–997. [DOI] [PubMed] [Google Scholar]
- 15. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. IntHout J, Ioannidis JP, Borm GF. The Hartung‐Knapp‐Sidik‐Jonkman method for random effects meta‐analysis is straightforward and considerably outperforms the standard DerSimonian‐Laird method. BMC Med Res Methodol. 2014;14:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rover C, Knapp G, Friede T. Hartung‐Knapp‐Sidik‐Jonkman approach and its modification for random‐effects meta‐analysis with few studies. BMC Med Res Methodol. 2015;15:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harada K, Harada K, Uetani T, Kataoka T, Takeshita M, Kunimura A, Takayama Y, Shinoda N, Kato B, Kato M, Marui N, Ishii H, Matsubara T, Amano T, Murohara T. The different association of epicardial fat with coronary plaque in patients with acute coronary syndrome and patients with stable angina pectoris: analysis using integrated backscatter intravascular ultrasound. Atherosclerosis. 2014;236:301–306. [DOI] [PubMed] [Google Scholar]
- 21. Lu MT, Park J, Ghemigian K, Mayrhofer T, Puchner SB, Liu T, Fleg JL, Udelson JE, Truong QA, Ferencik M, Hoffmann U. Epicardial and paracardial adipose tissue volume and attenuation—association with high‐risk coronary plaque on computed tomographic angiography in the ROMICAT II trial. Atherosclerosis. 2016;251:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schlett CL, Ferencik M, Kriegel MF, Bamberg F, Ghoshhajra BB, Joshi SB, Nagurney JT, Fox CS, Truong QA, Hoffmann U. Association of pericardial fat and coronary high‐risk lesions as determined by cardiac CT. Atherosclerosis. 2012;222:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oka T, Yamamoto H, Ohashi N, Kitagawa T, Kunita E, Utsunomiya H, Yamazato R, Urabe Y, Horiguchi J, Awai K, Kihara Y. Association between epicardial adipose tissue volume and characteristics of non‐calcified plaques assessed by coronary computed tomographic angiography. Int J Cardiol. 2012;161:45–49. [DOI] [PubMed] [Google Scholar]
- 24. Rajani R, Shmilovich H, Nakazato R, Nakanishi R, Otaki Y, Cheng VY, Hayes SW, Thomson LE, Friedman JD, Slomka PJ, Min JK, Berman DS, Dey D. Relationship of epicardial fat volume to coronary plaque, severe coronary stenosis, and high‐risk coronary plaque features assessed by coronary CT angiography. J Cardiovasc Comput Tomogr. 2013;7:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ito T, Suzuki Y, Ehara M, Matsuo H, Teramoto T, Terashima M, Nasu K, Kinoshita Y, Tsuchikane E, Suzuki T, Kimura G. Impact of epicardial fat volume on coronary artery disease in symptomatic patients with a zero calcium score. Int J Cardiol. 2013;167:2852–2858. [DOI] [PubMed] [Google Scholar]
- 26. Nakanishi K, Fukuda S, Tanaka A, Otsuka K, Taguchi H, Yoshikawa J, Shimada K. Epicardial adipose tissue accumulation is associated with renal dysfunction and coronary plaque morphology on multidetector computed tomography. Circ J. 2016;80:196–201. [DOI] [PubMed] [Google Scholar]
- 27. Tachibana M, Miyoshi T, Osawa K, Toh N, Oe H, Nakamura K, Naito T, Sato S, Kanazawa S, Ito H. Measurement of epicardial fat thickness by transthoracic echocardiography for predicting high‐risk coronary artery plaques. Heart Vessels. 2016;31:1758–1766. [DOI] [PubMed] [Google Scholar]
- 28. Park JS, Choi SY, Zheng M, Yang HM, Lim HS, Choi BJ, Yoon MH, Hwang GS, Tahk SJ, Shin JH. Epicardial adipose tissue thickness is a predictor for plaque vulnerability in patients with significant coronary artery disease. Atherosclerosis. 2013;226:134–139. [DOI] [PubMed] [Google Scholar]
- 29. Ito T, Nasu K, Terashima M, Ehara M, Kinoshita Y, Ito T, Kimura M, Tanaka N, Habara M, Tsuchikane E, Suzuki T. The impact of epicardial fat volume on coronary plaque vulnerability: insight from optical coherence tomography analysis. Eur Heart J Cardiovasc Imaging. 2012;13:408–415. [DOI] [PubMed] [Google Scholar]
- 30. Yamashita K, Yamamoto MH, Ebara S, Okabe T, Saito S, Hoshimoto K, Yakushiji T, Isomura N, Araki H, Obara C, Ochiai M. Association between increased epicardial adipose tissue volume and coronary plaque composition. Heart Vessels. 2014;29:569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol. 2015;11:363–371. [DOI] [PubMed] [Google Scholar]
- 32. Nov O, Shapiro H, Ovadia H, Tarnovscki T, Dvir I, Shemesh E, Kovsan J, Shelef I, Carmi Y, Voronov E, Apte RN, Lewis E, Haim Y, Konrad D, Bashan N, Rudich A. Interleukin‐1beta regulates fat‐liver crosstalk in obesity by auto‐paracrine modulation of adipose tissue inflammation and expandability. PLoS One. 2013;8:e53626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bo X, Ma L, Fan J, Jiang Z, Zhou Y, Zhang L, Li W. Epicardial fat volume is correlated with coronary lesion and its severity. Int J Clin Exp Med. 2015;8:4328–4334. [PMC free article] [PubMed] [Google Scholar]
- 34. Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov‐Blat L, O'Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. [DOI] [PubMed] [Google Scholar]
- 35. Larsen BA, Laughlin GA, Saad SD, Barrett‐Connor E, Allison MA, Wassel CL. Pericardial fat is associated with all‐cause mortality but not incident CVD: the Rancho Bernardo Study. Atherosclerosis. 2015;239:470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saura DO, Oliva MJ, Rodriguez D, Pascual‐Figal DA, Hurtado JA, Pinar E, de la Morena G, Valdes M. Reproducibility of echocardiographic measurements of epicardial fat thickness. Int J Cardiol. 2010;141:311–312. [DOI] [PubMed] [Google Scholar]
- 37. Hirata Y, Yamada H, Kusunose K, Iwase T, Nishio S, Hayashi S, Bando M, Amano R, Yamaguchi K, Soeki T, Wakatsuki T, Sata M. Clinical utility of measuring epicardial adipose tissue thickness with echocardiography using a high‐frequency linear probe in patients with coronary artery disease. J Am Soc Echocardiogr. 2015;28:1240–1246.e1241. [DOI] [PubMed] [Google Scholar]
- 38. Bucher AM, Joseph Schoepf U, Krazinski AW, Silverman J, Spearman JV, De Cecco CN, Meinel FG, Vogl TJ, Geyer LL. Influence of technical parameters on epicardial fat volume quantification at cardiac CT. Eur J Radiol. 2015;84:1062–1067. [DOI] [PubMed] [Google Scholar]
- 39. Tanami Y, Jinzaki M, Kishi S, Matheson M, Vavere AL, Rochitte CE, Dewey M, Chen MY, Clouse ME, Cox C, Kuribayashi S, Lima JA, Arbab‐Zadeh A. Lack of association between epicardial fat volume and extent of coronary artery calcification, severity of coronary artery disease, or presence of myocardial perfusion abnormalities in a diverse, symptomatic patient population: results from the CORE320 multicenter study. Circ Cardiovasc Imaging. 2015;8:e002676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Muthalaly RG, Nerlekar N, Wong DT, Cameron JD, Seneviratne SK, Ko BS. Epicardial adipose tissue and myocardial ischemia assessed by computed tomography perfusion imaging and invasive fractional flow reserve. J Cardiovasc Comput Tomogr. 2017;11:46–53. [DOI] [PubMed] [Google Scholar]
- 41. Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, Devereaux PJ, Montori VM, Freyschuss B, Vist G, Jaeschke R, Williams JW Jr, Murad MH, Sinclair D, Falck‐Ytter Y, Meerpohl J, Whittington C, Thorlund K, Andrews J, Schunemann HJ. Grade guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64:1283–1293. [DOI] [PubMed] [Google Scholar]
- 42. Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso‐Coello P, Glasziou P, Jaeschke R, Akl EA, Norris S, Vist G, Dahm P, Shukla VK, Higgins J, Falck‐Ytter Y, Schunemann HJ. Grade guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294–1302. [DOI] [PubMed] [Google Scholar]
- 43. Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, Montori V, Akl EA, Djulbegovic B, Falck‐Ytter Y, Norris SL, Williams JW Jr, Atkins D, Meerpohl J, Schunemann HJ. Grade guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011;64:407–415. [DOI] [PubMed] [Google Scholar]
- 44. Nakanishi K, Fukuda S, Tanaka A, Otsuka K, Jissho S, Taguchi H, Yoshikawa J, Shimada K. Persistent epicardial adipose tissue accumulation is associated with coronary plaque vulnerability and future acute coronary syndrome in non‐obese subjects with coronary artery disease. Atherosclerosis. 2014;237:353–360. [DOI] [PubMed] [Google Scholar]
- 45. Rabkin SW, Campbell H. Comparison of reducing epicardial fat by exercise, diet or bariatric surgery weight loss strategies: a systematic review and meta‐analysis. Obes Rev. 2015;16:406–415. [DOI] [PubMed] [Google Scholar]
- 46. McKenney ML, Schultz KA, Boyd JH, Byrd JP, Alloosh M, Teague SD, Arce‐Esquivel AA, Fain JN, Laughlin MH, Sacks HS, Sturek M. Epicardial adipose excision slows the progression of porcine coronary atherosclerosis. J Cardiothorac Surg. 2014;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rabkin SW. The relationship between epicardial fat and indices of obesity and the metabolic syndrome: a systematic review and meta‐analysis. Metab Syndr Relat Disord. 2014;12:31–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Example Search Strategy (Embase)
Table S2. Study Epicardial Adipose Tissue Measurement Parameters and High‐Risk Plaque Definitions
Table S3. Sensitivity Analysis Displaying Pooled Odds Ratios and 95% Confidence Intervals With Systematic Exclusion of Individual Studies
Table S4. Newcastle–Ottawa Scale Evaluation of Study Quality
Table S5. GRADE Quality Assessment
Figure S1. Funnel plot.


