Abstract
Background
Sex‐specific effectiveness of rivaroxaban (RIVA), dabigatran (DABI), and warfarin in reducing myocardial infarction (MI), heart failure (HF), and all‐cause mortality among patients with atrial fibrillation are not known. We assessed sex‐specific associations of RIVA, DABI, or warfarin use with the risk of MI, HF, and all‐cause mortality among patients with atrial fibrillation.
Methods and Results
Medicare beneficiaries (men: 65 734 [44.8%], women: 81 135 [55.2%]) with atrial fibrillation who initiated oral anticoagulants formed the study cohort. Inpatient admissions for MI, HF, and all‐cause mortality were compared between the 3 drugs separately for men and women using 3‐way propensity‐matched samples. In men, RIVA use was associated with a reduced risk of MI admissions compared with warfarin use (hazard ratio [95% confidence interval (CI): 0.59 [0.38–0.91]), with a trend towards reduced risk compared with DABI use (0.67 [0.44–1.01]). In women, there were no significant differences in the risk of MI admissions across all 3 anticoagulants. In both sexes, RIVA use and DABI use were associated with reduced risk of HF admissions (men: RIVA; 0.75 [0.63–0.89], DABI; 0.81 [0.69–0.96]) (women: RIVA; 0.64 [0.56–0.74], DABI; 0.73 [0.63–0.83]) and all‐cause mortality (men: RIVA; 0.66 [0.53–0.81], DABI; 0.75 [0.61–0.93]) (women: RIVA; 0.76 [0.63–0.91], DABI; 0.77 [0.64–0.93]) compared with warfarin use.
Conclusions
RIVA use and DABI use when compared with warfarin use was associated with a reduced risk of HF admissions and all‐cause mortality in both sexes. However, reduced risk of MI admissions noted with RIVA use appears to be limited to men.
Keywords: atrial fibrillation, heart failure, mortality, myocardial infarction, sex
Subject Categories: Quality and Outcomes, Arrhythmias, Women
Clinical Perspective
What Is New?
Women with atrial fibrillation have a greater incidence of myocardial infarction, heart failure, and all‐cause mortality compared with men.
Rivaroxaban and dabigatran use were associated with reduced heart failure admissions, and all‐cause mortality in atrial fibrillation patients from both sexes.
Rivaroxaban's association with a reduced myocardial infarction risk seems to be limited to men.
What Are the Clinical Implications?
Association of rivaroxaban use with reduced myocardial infarction risk in men may guide clinician decision making regarding the choice of anticoagulant in men.
Future studies should explore newer anticoagulants with superior outcome profile specific to women.
Introduction
Acute myocardial infarction (MI) and heart failure (HF) account for >70% of deaths in patients with nonvalvular atrial fibrillation (AF).1 Patients with AF have a 2 to 3 times higher risk of incident HF1, 2, 3 and incident MI3, 4 compared with patients without AF. Hence, there is a need for effective strategies to help reduce these cardiovascular events in patients with AF.5 Furthermore, there appears to be a sex difference in the incidence of these cardiovascular events.2 Women with AF have nearly 2 times higher risk for these cardiovascular events when compared with their male counterparts.2 It is important to assess sex‐specific effectiveness of treatment strategies that show promise with reducing MI, HF, and all‐cause mortality in patients with AF.
Direct oral anticoagulants (DOAC) (rivaroxaban [RIVA], dabigatran [DABI]), and warfarin are associated with a reduction in all‐cause mortality and vascular mortality including those related to HF hospitalizations in patients with AF.6 Warfarin7 and RIVA8 use are associated with a reduced risk of MI in this patient population, while evidence regarding the association between DABI and MI risk is inconsistent.9, 10 Data assessing sex‐specific associations of DOACs with the risk of MI, HF, and all‐cause mortality are lacking in the literature.
In order to bridge this literature gap, we used a nationally representative cohort of elderly Medicare beneficiaries with newly diagnosed AF to compare outcomes pertaining to cardiovascular disease (MI and congestive heart failure) and all‐cause mortality in patients taking DOACs (RIVA and DABI) or warfarin. Relative outcomes for each drug were investigated separately for men and women.
Methods
The study was approved by the University of Iowa Institutional review board. Since this was a retrospective analysis of claims data, the need for informed consent was waived.
A description of the methods is mentioned in our prior work.11 We used the Centers for Medicare and Medicaid Services patient records and linked data sources including (1) Beneficiary Summary File Base and Chronic Conditions segments, (2) Inpatient (Part A) and Carrier (Part B) Standard Analytic Files for 2011 through 2013, and (3) Pharmacy Drug Event (Part D) files for 2011–2013. We identified 213 705 Medicare beneficiaries who were enrolled in Centers for Medicare and Medicaid Services Part D prescription drug coverage plan, were newly diagnosed with AF between November 1, 2011 and October 31, 2013, and initiated DABI 150 mg twice daily, RIVA 20 mg once daily, or warfarin within 90 days after AF diagnosis. New AF was defined based on previously published algorithms (ie, 1 inpatient claim or 2 outpatient claims within 90 days with International Statistical Classification, 9 th Revision, Clinical Modification (ICD‐9‐CM) code 427.31 as primary or first secondary diagnosis).12 Medicare Part D benefit plan is a prescription drug plan via which beneficiaries procure prescription drugs in ambulatory settings. We note that, for some patients, anticoagulants may be initiated during hospitalization if AF is diagnosed during an inpatient stay. These drugs are generally not reflected in Part D claims, but would be reflected in subsequent medication fills after discharge. Thus, the 213 705 Medicare beneficiaries with AF who initiated on 1 of the 3 anticoagulants reflect outpatient prescriptions for long‐term stroke prevention.
From a total of 213 705 Medicare beneficiaries, 5698 were excluded because of incomplete claims data. Another 46 266 patients who were already on oral anticoagulants before the first AF diagnosis claims date were excluded. Furthermore, 7931 patients who underwent open heart surgeries and 6270 patients who underwent joint replacement surgeries or were hospitalized for pulmonary thromboembolism or deep vein thrombosis treatment were excluded. Another 669 patients with mechanical heart valves or on dialysis were excluded. Our final study cohort (n=146 871 patients) included 101 715 patients receiving warfarin (69.4%), 23 177 patients receiving RIVA (15.7%), and 21 979 patients receiving DABI (14.9%).
Outcomes: Inpatient admissions for incident MI, HF, and all‐cause mortality were the outcomes assessed in this study. MI and HF were based on the primary ICD‐9‐CM diagnosis on inpatient standard analytical file claims for acute care stays (inpatient admissions) occurring after initiating oral anticoagulation. All‐cause mortality was defined using the validated date of death on the Medicare beneficiary enrollment file.
Patient Characteristics were derived from Medicare enrollment data and inpatient and carrier claims. Age, sex, and race were identified from Medicare enrollment data. Comorbid diseases defined by Elixhauser et al13 were identified by ICD‐9‐CM diagnoses in inpatient and outpatient claims during the 12 months preceding AF diagnosis. We identified additional comorbidities of importance to AF outcomes, including: other dysrhythmias (ICD‐9‐CM codes 427.X, excluding 427.3), cardiomyopathy (ICD9 codes 425.X), cardiac conduction disorder (eg, bundle branch block; ICD9 codes 426.X), and previous implantable cardiac device (eg, pacemaker; ICD9 codes V45.0, V53.3). The CHA2DS2‐VASc stroke risk score was calculated using standard protocol.14 The HAS‐BLED (Hypertension, Abnormal renal and liver functions, Stroke, Bleeding, Labile INR, Elderly, Drugs or alcohol) score was used to represent bleeding risk,15 which may impact anticoagulant choice. The comorbidity score defined by Gagne et al16 was calculated to assess disease burden. Finally, we identified the setting of the original AF diagnosis (inpatient or ambulatory setting).
Statistical Analysis
We divided the total cohort into male and female cohorts. We used χ2 test or 1‐way ANOVA, as appropriate, to compare demographic variables, comorbid conditions, AF diagnosis setting, medication use, CHA2DS2‐VASc score, HAS‐BLED score, and Gagne score between the 3 treatment groups (participants initiated on DABI 150 mg twice a day [DABI group], participants initiated on RIVA 20 mg daily [RIVA group], and participants who were initiated on warfarin [warfarin group]). Comparisons were conducted separately in men and women. We then used the 3‐way propensity matching method described by Rassen et al17 to create groups of patients receiving DABI, RIVA, or warfarin that were balanced with respect to patient covariates and also had clinical equipoise—that is, patients included in the matched samples were plausible candidates for all 3 anticoagulants under study. Propensity matching was conducted separately for men and women. We assessed the success of the matching algorithm by comparing standardized differences in demographic variables, comorbid diseases, AF diagnosis setting, medication use, CHA2DS2‐VASc score, HAS‐BLED score, and the Gagne score between each drug in the matched samples. As recommended by Austin18 we evaluated the success of the matching algorithm using standardized differences rather than P values, as P values depend on sample sizes and may therefore not adequately reflect meaningful differences. Standardized differences <10% (ie, 0.10 times the SD of the difference) suggest adequate balance.18
We used the propensity matched samples to calculate event rates/100 patient years of follow‐up for each outcome for the 3 anticoagulant groups in men and women separately. We then plotted Kaplan–Meier curves for each anticoagulant for each study outcome in males and females separately. Log‐rank test was performed to compare the curves for the 3 anticoagulants. Finally, we used multivariable Cox proportional hazards regression on the matched samples to further control for possible differences between treatment groups within sex. In these models, the dependent variables were time (in days) from anticoagulant initiation to a given event (eg, admission for MI or censoring), while candidate independent variables included patient demographics, comorbid conditions, concurrent medication use, and prior health services utilization as described previously. Censoring events included end of observation (December 31, 2013), cessation of the initial anticoagulant (defined as the date of the last fill plus days supplied), or death. Variables were selected for inclusion in Cox models based on relationship to the outcome, using a statistical criterion of 0.05. Covariates adjusted in the Cox models for each of the outcomes are detailed in Table S1. Models also included indicators for the type of anticoagulant used (DABI versus warfarin [reference], RIVA versus warfarin [reference], and RIVA versus DABI [reference]). Since propensity matching created dependencies in the data, we used robust standard errors for the Cox regression models. Results of the regression analyses were reported as hazard ratios and 95% confidence intervals for DABI versus warfarin, RIVA versus warfarin, and RIVA versus DABI. Data set creation and propensity matching were conducted using SAS; all other analysis was performed using STATA 11 software.
Results
Overall, the final study cohort included 23 177, 21 979, and 101 715 patients who initiated RIVA, DABI, and warfarin, respectively. Of the 65 734 men (44.7%) in the study, 11 606 initiated RIVA, 10 740 initiated DABI, and 43 388 initiated warfarin. Of the 81 137 women (55.3%), 11 571 initiated RIVA, 11 239 initiated DABI, and 58 327 initiated warfarin. Prior to propensity matching, there were significant differences in the baseline characteristics across the 3 anticoagulant groups in men as well as women (Table 1). After propensity matching (Tables 2, 3 through 4), there were 22 827 men (7609 taking each drug), and 33 111 women (11 037 taking each drug). After propensity matching, there were no significant differences in the baseline characteristics between the 3 anticoagulant groups in men (Table 4). Also, in men, all standardized differences between the 3 anticoagulant groups were lower than 10%, suggesting a good covariate match. In women, after propensity matching, statistically significant differences remained for some comorbid conditions (eg, heart failure, prior stroke). However, all standardized differences between the 3 anticoagulant groups were substantially lower than 10%, suggesting good covariate balance.
Table 1.
Characteristics of Study Patients Taking Dabigatran (150 mg Twice Daily), Rivaroxaban (20 mg Once Daily), or Warfarin (Before Propensity Matching)
| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
| Dabigatran | Rivaroxaban | Warfarin | P Value | Dabigatran | Rivaroxaban | Warfarin | P Value | |
| Number of patients | 11 239 | 11 571 | 58 327 | 10 740 | 11 606 | 43 388 | ||
| Mean age, y (SD) | 76.9 (6.6) | 76.6 (6.6) | 79.6 (7.2) | <0.001 | 74.7 (5.9) | 74.9 (6.0) | 76.9 (6.8) | <0.001 |
| Number (%) >85 y | 1599 (14.2%) | 1565 (13.5%) | 16 048 (27.5%) | <0.001 | 749 (6.9%) | 906 (7.8%) | 6779 (15.6%) | <0.001 |
| Race | ||||||||
| White, % | 9874 (87.9%) | 10 342 (89.4%) | 50 357 (86.3%) | <0.001 | 9849 (91.7%) | 10 677 (92.0%) | 38 187 (88.0%) | <0.001 |
| Black, % | 495 (4.4%) | 460 (3.9%) | 3525 (6.04%) | 273 (2.5%) | 284 (2.4%) | 1895 (4.4%) | ||
| Hispanic, % | 496 (4.4%) | 492 (4.3%) | 2839 (4.9%) | 328 (3.1%) | 359 (3.1%) | 1850 (4.3%) | ||
| Other, % | 374 (3.3%) | 277 (2.4%) | 1606 (2.8%) | 290 (2.7%) | 286 (2.5%) | 1456 (3.4%) | ||
| Comorbid conditions | ||||||||
| Heart failure | 2777 (24.7%) | 2682 (23.2%) | 21 753 (37.3%) | <0.001 | 2533 (23.6%) | 2723 (23.5%) | 15 945 (36.8%) | <0.001 |
| Cardiomyopathy | 570 (5.1%) | 636 (5.5%) | 4536 (7.8%) | <0.001 | 898 (8.4%) | 1037 (8.9%) | 5216 (12.0%) | <0.001 |
| Other dysrhythmia | 3676 (32.7%) | 3940 (34.1%) | 20 690 (35.5%) | <0.001 | 3457 (32.2%) | 3798 (32.7%) | 15 799 (36.4%) | <0.001 |
| Implantable device | 415 (3.7%) | 468 (4.0%) | 2874 (4.9%) | <0.001 | 621 (5.8%) | 814 (7.0%) | 3868 (8.9%) | <0.001 |
| Peripheral vascular disease | 2042 (18.2%) | 2088 (18.1%) | 14 262 (24.5%) | <0.001 | 2014 (18.8%) | 2247 (19.4%) | 11 245 (25.9%) | <0.001 |
| Hypertension | 9614 (85.5%) | 9945 (85.9%) | 51 394 (88.1%) | <0.001 | 8785 (81.8%) | 9603 (82.7%) | 36 058 (83.1%) | 0.005 |
| Diabetes mellitus | 3554 (31.6%) | 3521 (30.4%) | 20 892 (35.8%) | <0.001 | 3640 (33.9%) | 3947 (34.0%) | 17 148 (39.5%) | <0.001 |
| Renal disease | 945 (8.4%) | 868 (7.5%) | 12 116 (20.8%) | <0.001 | 1032 (9.6%) | 1000 (8.6%) | 10 361 (23.8%) | <0.001 |
| Liver disease | 462 (4.1%) | 489 (4.2%) | 2751 (4.7%) | 0.003 | 399 (3.7%) | 458 (3.9%) | 2105 (4.8%) | <0.001 |
| Stroke or transient ischemic attack | 1523 (13.6%) | 1443 (12.5%) | 10 364 (17.8%) | <0.001 | 1115 (10.4%) | 1236 (10.7%) | 6609 (15.2%) | <0.001 |
| Previous major bleeding | ||||||||
| Intracranial | 56 (0.5%) | 56 (0.5%) | 511 (0.88%) | <0.001 | 45 (0.42%) | 46 (0.40%) | 368 (0.85%) | <0.001 |
| Gastrointestinal | 3125 (27.8%) | 3378 (29.2%) | 18 336 (31.4%) | <0.001 | 2452 (22.8%) | 2621 (22.6%) | 11 006 (25.4%) | <0.001 |
| Comorbidity Scores | ||||||||
| Gagne Score | 3.1 (2.2) | 3.0 (2.2) | 4.2 (2.8) | <0.001 | 2.9 (2.2) | 2.9 (2.2) | 4.2 (2.9) | <0.001 |
| CHADS2‐VascScore | 4.9 (1.5) | 4.8 (1.5) | 5.4 (1.6) | <0.001 | 3.7 (1.6) | 3.8 (1.6) | 4.3 (1.7) | <0.001 |
| HAS‐BLED Score | 1.7 | 1.6 | 1.8 | <0.001 | 1.6 | 1.6 | 1.9 | <0.001 |
| Medications in prior 90 days | ||||||||
| Statin | 4804 (42.7%) | 4844 (41.9%) | 24 436 (41.9%) | 0.234 | 5038 (46.9%) | 5555 (47.9%) | 19 564 (45.1%) | <0.001 |
| Antiplatelet | 523 (4.7%) | 486 (4.2%) | 3295 (5.7%) | <0.001 | 551 (5.1%) | 650 (5.6%) | 3157 (7.3%) | <0.001 |
| Proton pump inhibitors | 2482 (22.1%) | 2593 (22.4%) | 14 072 (24.1%) | <0.001 | 1884 (17.5%) | 2093 (18.0%) | 8045 (18.5%) | 0.041 |
| NSAID | 1741 (15.5%) | 1691 (14.6%) | 7416 (12.7%) | <0.001 | 1192 (11.1%) | 1279 (11.0%) | 4251 (9.8%) | <0.001 |
| Prior health services utilization | ||||||||
| Inpatient hospital days | 2.7 | 2.7 | 5.3 | <0.001 | 2.0 | 2.1 | 4.5 | <0.001 |
| Number of prescriptions | 9.6 | 9.6 | 10.3 | <0.001 | 8.3 | 8.3 | 9.0 | <0.001 |
| Skilled nursing facility | 577 (5.1%) | 562 (4.9%) | 6534 (11.2%) | <0.001 | 269 (2.3%) | 278 (2.6%) | 2920 (6.7%) | <0.001 |
| AF diagnosed in inpatient setting | 5147 (45.8%) | 5004 (43.3%) | 31 444 (53.9%) | <0.001 | 5015 (46.7%) | 4886 (42.1%) | 23 559 (54.3%) | <0.001 |
AF indicates atrial fibrillation; CHA2DS2‐VASc, 1 point each for congestive heart failure diagnosis, female sex, hypertension diagnosis, diabetes mellitus diagnosis, age 65 to 75 years, and vascular disease diagnosis; 2 points each for age >75 years and prior stroke or transient ischemic attack; HAS‐BLED, 1 point each for hypertension diagnosis, renal disease, liver disease, stroke history, prior major bleeding, labile international normalized ratio, age >65 years, medication use predisposing to bleeding and alcohol or drug use history; NSAID, nonsteroidal anti‐inflammatory drugs.
Table 2.
Standardized Differences (%) Before and After Propensity Matching in Women
| Dabigatran vs Rivaroxaban | Dabigatran vs Warfarin | Rivaroxaban vs Warfarin | ||||
|---|---|---|---|---|---|---|
| Pre‐Matching | Post‐Matching | Pre‐Matching | Post‐Matching | Pre‐Matching | Post‐Matching | |
| Demographics | ||||||
| Age | 4.82% | 0.32% | −38.27% | −0.60% | −42.82% | −0.90% |
| Race | ||||||
| White | −4.80% | −1.97% | 4.53% | −3.19% | 9.33% | −1.36% |
| Black | 2.14% | 0.77% | −7.37% | 1.02% | −9.49% | 0.33% |
| Other | 4.24% | 1.82% | 0.45% | 3.16% | −3.79% | 1.44% |
| Comorbid conditions | ||||||
| Heart failure | 3.59% | 0.87% | −27.47% | 3.48% | −31.10% | 2.70% |
| Cardiomyopathy | −1.90% | −1.86% | −11.05% | 0.78% | −9.17% | 2.44% |
| Peripheral vascular disease | 0.32% | −1.18% | −15.39% | −0.13% | −15.71% | 0.98% |
| Hypertension | −0.59% | −0.46% | −8.93% | 1.21% | −8.34% | 1.69% |
| Diabetes mellitus | 2.85% | 0.72% | −9.18% | 1.81% | −12.03% | 1.11% |
| Renal disease | 3.35% | 0.70% | −35.57% | 0.18% | −38.80% | −0.37% |
| Liver disease | −0.58% | −0.01% | −2.95% | 2.25% | −2.37% | 2.24% |
| Previous stroke or transient ischemic attack | 3.21% | 1.35% | −11.62% | 3.60% | −14.83% | 2.38% |
| Previous myocardial infarction | −1.60% | −1.98% | −16.86% | 1.60% | −15.29% | 3.32% |
| Other arrhythmia | −2.85% | −1.44% | −5.84% | 2.64% | −2.99% | 4.05% |
| Cardiac device | −1.83% | −2.26% | −6.08% | −0.85% | −4.27% | 1.27% |
| Previous major bleeding | ||||||
| Intracranial hemorrhage | 0.20% | 0.52% | −4.57% | 0.55% | −4.77% | 0.11% |
| Gastrointestinal hemorrhage | −3.08% | −1.97% | −7.96% | 1.71% | −4.88% | 3.63% |
| Comorbidity scores | ||||||
| Gagne Score | 3.36% | −0.35% | −43.58% | 1.95% | −46.84% | 2.27% |
| CHA2DS2‐Vasc Score | 3.35% | −0.58% | −33.65% | 4.23% | −36.99% | 4.80% |
| HAS‐BLED Score | 2.56% | 0.29% | −23.14% | 4.10% | −25.70% | 3.85% |
| Previous healthcare services | ||||||
| Inpatient hospital days | 0.84% | −1.09% | −35.76% | 0.12% | −35.48% | 0.94% |
| AF diagnosed inpatient | 5.11 | 1.79 | −16.3% | 5.64% | −21.45 | 3.87 |
| Number unique prescription ingredients | 1.22% | 0.40% | −10.48% | 0.79% | −11.75% | 0.40% |
| Prior extended care or skilled nursing stay | 1.27% | 0.25% | −22.29% | −0.23% | −23.51% | −0.44% |
| Medications in prior 90 days | ||||||
| Statin | 1.78% | 1.27% | 1.72% | 1.03% | −0.06% | −0.24% |
| Prescription antiplatelet | 2.20% | 0.26% | −4.51% | 0.57% | −6.70% | 0.34% |
| Proton pump inhibitors | −0.78% | −0.98% | −4.85% | 1.18% | −4.06% | 2.14% |
| NSAID | 2.45% | 1.52% | 7.98% | 2.14% | 5.53% | 0.58% |
AF indicates atrial fibrillation; CHA2DS2‐VASc, 1 point each for congestive heart failure diagnosis, female sex, hypertension diagnosis, diabetes mellitus diagnosis, age 65 to 75 years, and vascular disease diagnosis; 2 points each for age >75 years and prior stroke or transient ischemic attack; HAS‐BLED, 1 point each for hypertension diagnosis, renal disease, liver disease, stroke history, prior major bleeding, labile international normalized ratio, age >65 years, medication use predisposing to bleeding and alcohol or drug use history; NSAID, nonsteroidal anti‐inflammatory drugs.
Table 3.
Standardized Differences (%) Before and After Propensity Matching in Men
| Dabigatran vs Rivaroxaban | Dabigatran vs Warfarin | Rivaroxaban vs Warfarin | ||||
|---|---|---|---|---|---|---|
| Pre‐Matching | Post‐Matching | Pre‐Matching | Post‐Matching | Pre‐Matching | Post‐Matching | |
| Demographics | ||||||
| Age | −3.28% | −2.56% | −34.02% | 2.02% | −30.71% | 4.38% |
| Race | ||||||
| White | −1.07% | −1.59% | 12.25% | −2.53% | 13.31% | −1.10% |
| Black | 0.61% | 0.08% | −10.01% | 0.50% | −10.60% | 0.44% |
| Other | 0.85% | 1.82% | −7.47% | 2.69% | −8.32% | −0.11% |
| Comorbid conditions | ||||||
| Heart failure | 0.29% | 0.81% | −28.98% | 0.12% | −29.28% | −0.64% |
| Cardiomyopathy | −2.04% | −1.22% | −12.12% | 0.78% | −10.09% | 1.89% |
| Peripheral vascular disease | −1.55% | −0.20% | −17.27% | 0.76% | −15.72% | 0.94% |
| Hypertension | −2.48% | 0.46% | −5.28% | −0.43% | −2.81% | −0.91% |
| Diabetes mellitus | −0.37% | 0.58% | −12.30% | −0.05% | −11.94% | −0.62% |
| Renal disease | 3.45% | −1.74% | −38.94% | −0.61% | −42.29% | 0.76% |
| Liver disease | −1.20% | 2.74% | −5.61% | 1.49% | −4.42% | −1.09% |
| Previous stroke or transient ischemic attack | −0.87% | −1.33% | −14.55% | −3.79% | −13.68% | −2.55% |
| Previous myocardial infarction | −0.91% | −0.80% | −18.30% | −1.59% | −17.39% | −0.87% |
| Other arrhythmia | −1.15% | −0.59% | −8.91% | −0.50% | −7.76% | 0.08% |
| Cardiac device | −5.03% | −1.56% | −12.03% | 0.40% | −7.03% | 1.80% |
| Previous major bleeding | ||||||
| Intracranial hemorrhage | 0.36% | −1.03% | −5.41% | 0.01% | −5.75% | 0.84% |
| Gastrointestinal hemorrhage | 0.59% | −0.50% | −5.93% | −0.68% | −6.52% | −0.18% |
| Comorbidity scores | ||||||
| Gagne Score | −0.80% | 0.29% | −47.13% | −0.71% | −46.17% | −0.95% |
| CHA2DS2‐Vasc Score | −3.88% | −1.25% | −34.89% | −2.63% | −31.15% | −1.42% |
| HAS‐BLED Score | −0.10% | 0.62% | −30.32% | −0.17% | −30.25% | −0.74% |
| Previous healthcare services | ||||||
| Inpatient hospital days | 0.36% | 0.56% | −36.13% | −1.94% | −36.09% | −2.31% |
| Number unique prescription ingredients | −0.76% | 2.91% | −12.45% | 1.69% | −11.75% | −1.10% |
| Previous extended care or skilled nursing stay | 1.75% | −0.17% | −19.75% | −0.25% | −21.35% | −0.13% |
| Medications in the prior 90 days | ||||||
| Statin | −1.91% | 1.82% | 3.65% | −0.66% | 5.56% | −2.48% |
| Prescription antiplatelet | −2.09% | 0.93% | −8.90% | 1.36% | −6.83% | 0.48% |
| Proton pump inhibitors | −1.29% | 0.72% | −2.60% | −0.48% | −1.31% | −1.19% |
| NSAID | 0.25% | 1.34% | 4.25% | 1.46% | 4.00% | 0.09% |
CHA2DS2‐VASc indicates 1 point each for congestive heart failure diagnosis, female sex, hypertension diagnosis, diabetes mellitus diagnosis, age 65 to 75 years, and vascular disease diagnosis; 2 points each for age >75 years and prior stroke or transient ischemic attack; HAS‐BLED, 1 point each for hypertension diagnosis, renal disease, liver disease, stroke history, prior major bleeding, labile international normalized ratio, age >65 years, medication use predisposing to bleeding and alcohol or drug use history; NSAID, nonsteroidal anti‐inflammatory drugs.
Table 4.
Characteristics of Study Patients Taking Dabigatran (150 mg Twice Daily), Rivaroxaban (20 mg Once Daily), or Warfarin (After Propensity Matching)
| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
| Dabigatran | Rivaroxaban | Warfarin | P Value | Dabigatran | Rivaroxaban | Warfarin | P Value | |
| No. of patients | 11 037 | 11 037 | 11 037 | 7609 | 7609 | 7609 | ||
| Mean age, y (SD) | 76.8 (6.2) | 76.8 (6.1) | 76.9 (6.4) | 0.51 | 74.9 (6.1) | 75.1 (6.2) | 74.8 (6.1) | 0.24 |
| Number (%) >85 y | 1522 (13.8) | 1565 (14.2) | 1598 (14.5) | 0.338 | 626 (8.2) | 655 (8.6) | 615 (8.1) | 0.479 |
| Race | ||||||||
| White, % | 9756 (88.4) | 9825 (89.0) | 9874 (89.5) | 0.08 | 6930 (91.2) | 6963 (91.5) | 6988 (91.8) | 0.81 |
| Black, % | 471 (4.3) | 454 (4.1) | 446 (4.0) | 205 (2.7) | 204 (2.7) | 198 (2.6) | ||
| Hispanic, % | 484 (4.4) | 481 (4.4) | 449 (4.1) | 259 (3.4) | 242 (3.3) | 235 (3.0) | ||
| Other, % | 333 (3.0) | 277 (2.5) | 282 (2.6) | 215 (2.8) | 191 (2.5) | 208 (2.7) | ||
| Comorbid conditions | ||||||||
| Heart failure | 2661 (24.1%) | 2620 (23.7%) | 2485 (22.5%) | 0.014 | 1898 (24.9%) | 1872 (24.6%) | 1894 (24.9%) | 0.87 |
| Previous myocardial infarction | 760 (6.9%) | 816 (7.4%) | 709 (6.4%) | 0.02 | 792 (10.4%) | 810 (10.7%) | 832 (10.9%) | 0.57 |
| Cardiomyopathy | 560 (5.1%) | 606 (5.5%) | 539 (4.9%) | 0.11 | 668 (8.8%) | 694 (9.1%) | 650 (8.5%) | 0.45 |
| Other dysrhythmia | 3632 (32.9%) | 3707 (33.6%) | 3494 (31.7%) | 0.01 | 2478 (32.6%) | 2499 (32.8%) | 2496 (32.8%) | 0.926 |
| Implantable device | 408 (3.7%) | 456 (4.1%) | 427 (3.9%) | 0.24 | 491 (6.5%) | 520 (6.8%) | 483 (6.4%) | 0.44 |
| Peripheral vascular disease | 1979 (17.9%) | 2029 (18.4%) | 1985 (18.0%) | 0.63 | 1518 (20.0%) | 1524 (20.0%) | 1494 (19.6%) | 0.42 |
| Hypertension | 9565 (86.7%) | 9582 (86.8%) | 9522 (86.3%) | 0.47 | 6389 (84.0%) | 6376 (83.8%) | 6401 (84.1%) | 0.86 |
| Diabetes mellitus | 3512 (31.8%) | 3475 (31.5%) | 3417 (31.0%) | 0.38 | 2732 (35.9%) | 2711 (35.6%) | 2734 (35.9%) | 0.91 |
| Renal disease | 889 (8.1%) | 868 (7.9%) | 882 (8.0%) | 0.87 | 800 (10.5%) | 838 (11.0%) | 817 (10.7%) | 0.61 |
| Liver disease | 451 (4.1%) | 451 (4.1%) | 400 (3.6%) | 0.13 | 325 (4.3%) | 285 (3.8%) | 302 (4.0%) | 0.25 |
| Stroke or transient ischemic attack | 1461 (13.2%) | 1411 (12.8%) | 1317 (11.9%) | 0.02 | 826 (10.9%) | 857 (11.3%) | 922 (12.1%) | 0.054 |
| Previous major bleeding | ||||||||
| Intracranial | 54 (0.49%) | 50 (0.45%) | 49 (0.44%) | 0.87 | 32 (0.42%) | 37 (0.49%) | 32 (0.42%) | 0.78 |
| Gastrointestinal | 3072 (27.8%) | 3170 (28.7%) | 2986 (27.1%) | 0.03 | 1742 (22.9%) | 1758 (23.1%) | 1764 (23.2%) | 0.91 |
| Comorbidity Scores | ||||||||
| Gagne Score | 3.0 | 3.1 | 3.0 | 0.14 | 3.1 | 3.1 | 3.1 | 0.644 |
| CHA2DS2‐VASc Score | 4.8 | 4.8 | 4.8 | 0.711 | 3.8 | 3.8 | 3.8 | 0.68 |
| HAS‐BLED Score | 1.6 | 1.6 | 1.6 | 0.091 | 1.7 | 1.7 | 1.7 | 0.12 |
| Medications in prior 90 days | ||||||||
| Statin | 4700 (42.6%) | 4631 (42.0%) | 4644 (42.1%) | 0.61 | 3573 (47.0%) | 3504 (46.1%) | 3598 (47.3%) | 0.29 |
| Antiplatelet | 488 (4.4%) | 482 (4.4%) | 474 (4.3%) | 0.90 | 432 (5.7%) | 416 (5.5%) | 407 (5.4%) | 0.67 |
| Proton pump inhibitors | 2435 (22.1%) | 2480 (22.5%) | 2380 (21.6%) | 0.27 | 1378 (18.1%) | 1357 (17.8%) | 1392 (18.3%) | 0.76 |
| NSAID | 1690 (15.3%) | 1630 (14.8%) | 1608 (14.6%) | 0.28 | 843 (11.1%) | 811 (10.7%) | 809 (10.6%) | 0.61 |
| Prior health services utilization | ||||||||
| Hospital days | 2.7 | 2.7 | 2.7 | 0.891 | 2.3 | 2.3 | 2.4 | 0.24 |
| Prescriptions (n) | 9.6 | 9.6 | 9.5 | 0.60 | 8.5 | 8.3 | 8.4 | 0.771 |
| Prior extended care | 559 (5.1%) | 553 (5.0%) | 566 (5.1%) | 0.92 | 226 (3.0%) | 228 (3.0%) | 230 (3.0%) | 0.98 |
| AF diagnosed in inpatient setting | 4987 (45.2%) | 4889 (44.3%) | 4677 (42.4%) | 0.001 | 2726 (35.8%) | 2625 (34.5%) | 2725 (35.8%) | 0.14 |
AF indicates atrial fibrillation; CHA2DS2‐VASc, 1 point each for congestive heart failure diagnosis, female sex, hypertension diagnosis, diabetes mellitus diagnosis, age 65 to 75 years, and vascular disease diagnosis; 2 points each for age >75 years and prior stroke or transient ischemic attack; HAS‐BLED, 1 point each for hypertension diagnosis, renal disease, liver disease, stroke history, prior major bleeding, labile international normalized ratio, age >65 years, medication use predisposing to bleeding and alcohol or drug use history; NSAID, nonsteroidal anti‐inflammatory drugs.
Outcomes
Sex‐specific outcome rates in the propensity‐matched cohorts are detailed in Table 5. Overall, 150 inpatient hospitalizations for MIs and 751 inpatient hospitalizations for HF were noted in men, and 507 men died by the end of follow‐up. In women, 166 inpatient hospitalizations for MIs and 1295 inpatient hospitalizations for HF were noted and 659 died by the end of follow‐up.
Table 5.
Sex‐Specific Outcomes in Propensity‐Matched Samples Reported as Number of Events (%), Rates/100 Patient‐Year of Follow‐Up
| Women | Men | |||||
|---|---|---|---|---|---|---|
| Dabigatran | Rivaroxaban | Warfarin | Dabigatran | Rivaroxaban | Warfarin | |
| Number of patients | 11 037 | 11 037 | 11 037 | 7609 | 7609 | 7609 |
| Myocardial infarction | 56 (0.51%) 0.75 | 51 (0.46%) 0.80 | 59 (0.53%) 0.80 | 54 (0.7%) 1.3 | 38 (0.5%) 0.7 | 58 (0.8%) 1.2 |
| Heart failure | 408 (3.7%) 5.6 | 345 (3.1%) 5.5 | 542 (4.9%) 7.6 | 236 (3.1%) 5.3 | 227 (3.0%) 4.7 | 288 (3.8%) 6.1 |
| All‐cause mortality | 201 (1.8%) 2.6 | 198 (1.8%) 3.1 | 260 (2.4%) 3.5 | 154 (2.0%) 3.4 | 147 (1.9%) 3.0 | 206 (2.7%) 4.3 |
In men, RIVA use was associated with a reduced risk of MI admissions compared with warfarin use (hazard ratios [95% confidence intervals], 0.64 [0.43–0.97]), with a trend toward low risk compared with DABI use (0.67 [0.44–1.01]; P=0.06) (Table 6). The risk of MI admissions was similar with DABI use compared with warfarin use. Furthermore, in men, RIVA use and DABI use were associated with a reduced risk of HF admissions (RIVA: 0.75 [0.63–0.89]), (DABI: 0.81 [0.69–0.96]) and all‐cause mortality (RIVA: 0.66 [0.53–0.81]), (DABI: 0.75 [0.61–0.93]) compared with warfarin use. The risk of HF admissions and all‐cause mortality were similar with RIVA use compared with DABI use in men.
Table 6.
Hazard of Outcomes in Matched Cohorts of Men and Women
| Women | Men | |||
|---|---|---|---|---|
| Adjusted Hazard Ratio (95% Confidence Interval) | P Value | Adjusted Hazard Ratio (95% Confidence Interval) | P Value | |
| Myocardial infarction | ||||
| Rivaroxaban vs warfarin | 0.94 (0.65–1.37) | 0.76 | 0.64 (0.43–0.97) | 0.03 |
| Dabigatran vs warfarin | 0.96 (0.67–1.39) | 0.84 | 0.96 (0.66–1.39) | 0.83 |
| Rivaroxaban vs dabigatran | 0.98 (0.67–1.43) | 0.92 | 0.67 (0.44–1.01) | 0.06 |
| Heart failure | ||||
| Rivaroxaban vs warfarin | 0.64 (0.56–0.74) | <0.001 | 0.75 (0.63–0.89) | 0.001 |
| Dabigatran vs warfarin | 0.73 (0.63–0.83) | <0.001 | 0.81 (0.69–0.96) | 0.02 |
| Rivaroxaban vs dabigatran | 0.88 (0.77–1.02) | 0.09 | 0.92 (0.77–1.10) | 0.39 |
| All‐cause mortality | ||||
| Rivaroxaban vs warfarin | 0.76 (0.63–0.91) | 0.004 | 0.66 (0.53–0.81) | <0.001 |
| Dabigatran vs warfarin | 0.77 (0.64–0.93) | 0.006 | 0.75 (0.61–0.93) | 0.008 |
| Rivaroxaban vs dabigatran | 0.98 (0.81–1.20) | 0.86 | 0.81 (0.70–1.10) | 0.25 |
In women, the risk of MI admissions was similar across all 3 anticoagulants (Table 6). The risk of HF admissions was lower with RIVA use and DABI use compared with warfarin use in women (RIVA: 0.64 [0.56–0.74], DABI: 0.73 [0.63–0.83]), as was all‐cause mortality (RIVA: 0.76 [0.63–0.91], DABI: 0.77 [0.64–0.93]). HF admissions and all‐cause mortality did not differ between RIVA and DABI in women. Figures 1A, 1B, 2A, 2B, 3A and 3B show the associated survival curves (with embedded graphs showing log‐transformed survival rates to provide visual separation between curves).
Figure 1.
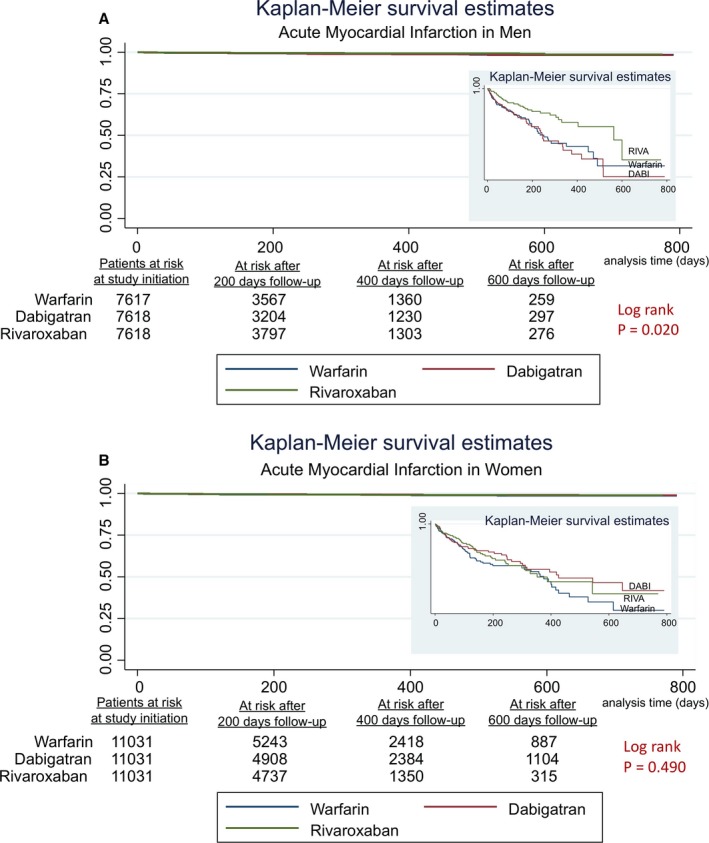
A, Acute myocardial infarction in men. Survival curves for acute myocardial infarction comparing the 3 anticoagulants in men with atrial fibrillation. On the right‐hand side corners are the curve separation figures, which are based on log‐transformed survival rates. B, Acute myocardial infarction in women. Survival curves for acute myocardial infarction comparing the 3 anticoagulants in women with atrial fibrillation. On the right‐hand side corners are the curve separation figures, which are based on log‐transformed survival rates.
Figure 2.
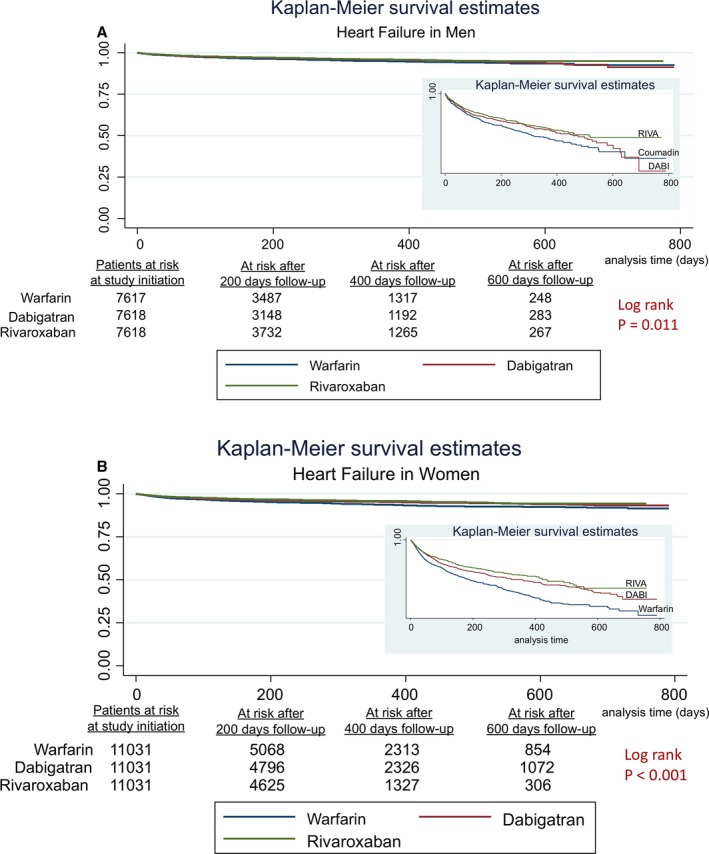
A, Heart failure in men. Survival curves for heart failure comparing the 3 anticoagulants in men with atrial fibrillation. On the right‐hand side corner is the curve separation figure, which is based on log‐transformed survival rates. B, Heart failure in women. Survival curves for heart failure comparing the 3 anticoagulants in women with atrial fibrillation. On the right‐hand side corner is the curve separation figure, which is based on log‐transformed survival rates.
Figure 3.
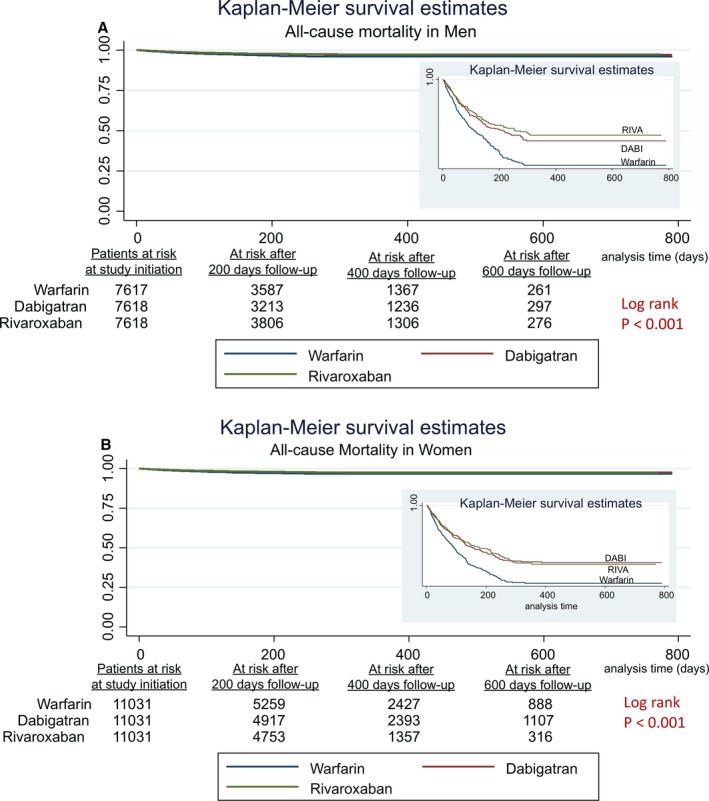
A, All‐cause mortality in men. Survival curves for all‐cause mortality comparing the 3 anticoagulants in men with atrial fibrillation. On the right‐hand side corner is the curve separation figure, which is based on log‐transformed survival rates. B, All‐cause mortality in women. Survival curves for all‐cause mortality comparing the 3 anticoagulants in women with atrial fibrillation. On the right‐hand side corner is the curve separation figure, which is based on log‐transformed survival rates.
Discussion
In our analysis of Medicare claims data for elderly patients with newly diagnosed AF in the United States, we observed significant differences in cardiovascular outcomes and all‐cause mortality by anticoagulant type within sex. In men, RIVA use was associated with a lower risk of MI compared with either DABI use or warfarin use, while the risk of MI was similar across all 3 anticoagulants in women. In both sexes, RIVA use and DABI use were both associated with lower risk of HF admissions and all‐cause mortality compared with warfarin use.
A reduced risk of MI with RIVA use in patients with AF has been documented previously.8 The Rivaroxaban–Once‐daily, oral, direct Factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation (ROCKET‐AF) trial19 that compared RIVA daily to warfarin reported a lower rate of incident MI in the RIVA arm (0.91/100 patient years) compared with the warfarin arm (1.12/100 patient years). However, sex‐specific MI rates were not reported. The Anti‐Xa Therapy to Lower Cardiovascular Events in Addition to Standard Therapy in Subjects With Acute Coronary Syndrome 2 (ATLAS‐ACS 2) trial20 compared low‐dose RIVA (2.5 or 5 mg twice daily) to placebo in patients with a recent acute coronary syndrome, and found RIVA to reduce risk of death from cardiovascular causes, MI, and stroke. The relative impact of RIVA on these outcomes combined did not differ significantly by sex. However, direct comparisons to our analysis cannot be drawn because of differences in the patient populations (patients with recent acute coronary syndrome versus patients with AF in our study), different RIVA dose and timing (RIVA 2.5 or 5 mg twice daily versus RIVA 20 mg daily in our study), and different comparators (placebo versus warfarin and DABI in our study).
Inconsistencies exist with regard to the association between DABI use and risk of MI in patients with AF. Randomized trials report an increased MI risk with DABI use compared with warfarin use10 and platelet activation potential of DABI is suspected to be the etiology of this increased MI risk.10 In contrast, observational data suggest a reduced MI risk with DABI use when compared with warfarin use.9, 21 Sex‐specific comparisons of DABI use compared with warfarin use and the associated risk of MI were not reported in these studies.
While previous studies have evaluated the risk of MI between RIVA and warfarin, or between DABI and warfarin, few studies have compared RIVA use to DABI use. One meta‐analysis found that RIVA use was associated with a reduced MI risk compared with DABI use in patients with AF,22 while another observational analysis found similar risk of MI in patients with AF using RIVA or DABI.23 These reports did not mention sex‐specific comparisons of RIVA to DABI and associated MI risk.
Compared with MI prevention, the use of anticoagulants for HF has received little attention in the literature. RIVA is thought to have the potential to prevent HF episodes. Specifically, RIVA inhibits Factor Xa, an enzyme necessary for thrombin generation. Recent molecular studies have identified thrombin‐related pathways that simulate myocyte injury, enhance myocardial inflammation, promote endothelial dysfunction, and increase microvascular thrombosis.24, 25 This milieu is suspected to orchestrate HF pathogenesis. By inhibiting steps preceding thrombin generation, RIVA is suspected to inactivate the abovementioned thrombin cascade.24, 25 With this underlying hypothesis, the Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction or Stroke in Participants With Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure (COMMANDER‐HF) trial26 is assessing the efficacy of RIVA in reducing HF readmissions as a secondary end point among patients with HF and coronary artery disease. DABI, a direct thrombin inhibitor, may also have similar benefits,24, 25 but human experiments are not yet evaluating this hypothesis. In our study, we noted that both RIVA use and DABI use were associated with a reduction in HF risk compared with warfarin in men as well as women with AF, thereby adding validity to the thrombin hypothesis discussed above.
With regard to all‐cause mortality, in harmony with our results, prior investigations have consistently shown an association between reduction in all‐cause mortality with RIVA use6, 19, 23 as well as DABI use,9, 10 compared with warfarin use in patients with AF. The mechanistic basis remains unclear and multiple hypotheses exist. Thrombin cascade inhibition mentioned above is one such hypothesis, while improved stroke and MI prevention with RIVA or DABI may also explain reductions in mortality. Selection bias is also possible because healthier individuals are more likely to be prescribed DOACs and hence have a survival advantage over warfarin candidates. Furthermore, in concordance with our results, RIVA use is shown to be similar to DABI use in terms of risk for all‐cause mortality in observational studies.23, 27 However, none of these referenced studies reported sex‐specific outcomes.
Limitations
Although the strengths of our analysis include nationally representative large sample of patients, and the use of propensity score matching to control for confounders, several limitations must be noted. Bias because of unmeasured confounders is still a possibility since propensity matching will not control for unmeasured confounders. In addition, our data include patients >65 years of age only, and therefore our findings may not generalize to younger patients. Also, the Medicare claims used for this study lack granular prognostic details such as AF burden, AF type, left ventricular ejection fraction, and degree of coronary artery disease. Furthermore, patients hospitalized for AF may have had troponin elevation because of the mechanism of demand–supply mismatch and could have received a diagnosis of MI. This is a known limitation of ICD‐9‐based MI outcome determination because ICD‐9 codes cannot differentiate MI caused by demand–supply mismatch and MI caused by plaque rupture.28 Similarly, hospitalization for AF could have had a clinical presentation of HF and hence such hospitalizations could have received a primary diagnosis of HF.29 Making such a distinction, although important, is beyond the scope of our data. Prospective studies with validated MI and HF outcome determination are needed to improve specificity of these outcomes. Finally, we had a short follow‐up (median 14 months) and hence it is yet to be determined whether these associations will stand with long‐term follow‐up.
Conclusions
Sex differences are possible in the association between oral anticoagulant use and the risk of MI, HF, and all‐cause mortality in patients with AF. Our finding that RIVA use may reduce the risk of MI in men may guide clinician decision making regarding the choice of anticoagulant in men with AF. Although it is reassuring to note that the DOACs are associated with a reduced risk of HF and all‐cause mortality in both sexes, our results also confirm the enormity of cardiovascular disease burden in women and the need for newer treatment strategies with proven benefit specific to women.
Sources of Funding
This study is supported by funding from the Agency for Healthcare Research and Quality (AHRQ; R01 HS023104), and by the Health Services Research and Development Service (HSR&D) of the Department of Veterans Affairs. The views expressed here are those of the authors and do not represent the Department of Veterans Affairs.
Disclosures
None.
Supporting information
Table S1. Covariates Included in the Cox‐Regression Models for Each of the Outcomes
(J Am Heart Assoc. 2017;6:e006381 DOI: 10.1161/JAHA.117.006381.)28862952
This article was handled independently by N.A. Mark Estes III, MD, as a guest editor.
References
- 1. Pokorney SD, Piccini JP, Stevens SR, Patel MR, Pieper KS, Halperin JL, Breithardt G, Singer DE, Hankey GJ, Hacke W, Becker RC, Berkowitz SD, Nessel CC, Mahaffey KW, Fox KA, Califf RM; ROCKET AF Steering Committee & Investigators; ROCKET AF Steering Committee Investigators . Cause of death and predictors of all‐cause mortality in anticoagulated patients with nonvalvular atrial fibrillation: data from ROCKET AF. J Am Heart Assoc. 2016;5:e002197 DOI: 10.1161/JAHA.115.002197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stewart S, Hart CL, Hole DJ, McMurray JJ. A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. [DOI] [PubMed] [Google Scholar]
- 3. Friberg L, Hammar N, Pettersson H, Rosenqvist M. Increased mortality in paroxysmal atrial fibrillation: report from the Stockholm Cohort‐Study of Atrial Fibrillation (SCAF). Eur Heart J. 2007;28:2346–2353. [DOI] [PubMed] [Google Scholar]
- 4. Violi F, Soliman EZ, Pignatelli P, Pastori D. Atrial fibrillation and myocardial infarction: a systematic review and appraisal of pathophysiologic mechanisms. J Am Heart Assoc. 2016;5:e003347 DOI: 10.1161/JAHA.116.003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; ACC/AHA Task Force Members . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 6. Gómez‐Outes A, Lagunar‐Ruíz J, Terleira‐Fernández AI, Calvo‐Rojas G, Suárez‐Gea ML, Vargas‐Castrillón E. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2016;68:2508–2521. [DOI] [PubMed] [Google Scholar]
- 7. Amsterdam E, Wenger N, Brindis R, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ; ACC/AHA Task Force Members . 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guideline. Circulation. 2014;130:e344–e426. [DOI] [PubMed] [Google Scholar]
- 8. Chatterjee S, Sharma A, Uchino K, Biondi‐Zoccai G, Lichstein E, Mukherjee D. Rivaroxaban and risk of myocardial infarction: insights from a meta‐analysis and trial sequential analysis of randomized clinical trials. Coron Artery Dis. 2013;24:628–635. [DOI] [PubMed] [Google Scholar]
- 9. Lauffenburger JC, Farley JF, Gehi AK, Rhoney DH, Brookhart MA, Fang G. Effectiveness and safety of dabigatran and warfarin in real‐world US patients with non‐valvular atrial fibrillation: a retrospective cohort study. J Am Heart Assoc. 2015;4:e001798 DOI: 10.1161/JAHA.115.001798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Douxfils J, Buckinx F, Mullier F, Minet V, Rabenda V, Reginster JY, Hainaut P, Bruyère O, Dogné JM. Dabigatran etexilate and risk of myocardial infarction, other cardiovascular events, major bleeding, and all‐cause mortality: a systematic review and meta‐analysis of randomized controlled trials. J Am Heart Assoc. 2014;3:e000515 DOI: 10.1161/JAHA.113.000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palamaner Subash Shantha G, Bhave P, Girotra S, Hodgson‐Zingman D, Mazur A, Giudici M, Chrischilles E, Vaughan Sarrazin M. Sex specific comparative effectiveness of oral anticoagulants in elderly patients with newly diagnosed atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2017;10:4. pii: e003418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ellis ER, Culler SD, Simon AW, Reynolds MR. Trends in utilization and complications of catheter ablation for atrial fibrillation in Medicare beneficiaries. Heart Rhythm. 2009;6:1267–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 14. Chen JY, Zhang AD, Lu HY, Guo J, Want FF, Li ZC. CHADS2 versus CHA2DS2‐VASc score in assessing the stroke and thromboembolism risk stratification in patients with atrial fibrillation: a systematic review and meta‐analysis. J Geriatr Cardiol. 2013;10:258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pisters R, Lane DA, Niewlaat R, deVos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 16. Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rassen JA, Shelat AA, Franklin JM, Glynn RJ, Solomon DH, Schneeweiss S. Matching by propensity score in cohort studies with three treatment groups. Epidemiology. 2013;24:401–409. [DOI] [PubMed] [Google Scholar]
- 18. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 20. Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook‐Bruns N, Fox KA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FW, Gibson CM; ATLAS ACS 2–TIMI 51 Investigators . Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19. [DOI] [PubMed] [Google Scholar]
- 21. Larsen TB, Rasmussen LH, Skjoth F, Due KM, Callréus T, Rosenzweig M, Lip GY. Efficacy and safety of dabigatran etexilate and warfarin in “real‐world” patients with atrial fibrillation: a prospective nationwide cohort study. J Am Coll Cardiol. 2013;61:2264–2273. [DOI] [PubMed] [Google Scholar]
- 22. Harenberg J, Marx S, Diener HC, Lip GY, Marder VJ, Wehling M, Weiss C. Comparison of efficacy and safety of dabigatran, rivaroxaban and apixaban in patients with atrial fibrillation using network meta‐analysis. Int Angiol. 2012;31:330–339. [PubMed] [Google Scholar]
- 23. Chan YH, Kuo CT, Yeh YH, Chang SH, Wu LS, Lee HF, Tu HT, See LC. Thromboembolic, bleeding, and mortality risks of rivaroxaban and dabigatran in Asians with nonvalvular atrial fibrillation. J Am Coll Cardiol. 2016;68:1389–1401. [DOI] [PubMed] [Google Scholar]
- 24. Zannad F, Stough WG, Regnault V, Gheorghiade M, Deliargyris E, Gibson CM, Agewall S, Berkowitz SD, Burton P, Calvo G, Goldstein S, Verheugt FW, Koglin J, O'Connor CM. Is thrombosis a contributor to heart failure pathophysiology? Possible mechanisms, therapeutic opportunities, and clinical investigation challenges. Int J Cardiol. 2013;167:1772–1782. [DOI] [PubMed] [Google Scholar]
- 25. Borissoff JI, Spronk HM, Heeneman S, ten Cate H. Is thrombin a key player in the ‘coagulation‐atherogenesis’ maze? Cardiovasc Res. 2009;82:392–403. [DOI] [PubMed] [Google Scholar]
- 26. Zannad F, Greenberg B, Dleland JG, Gheorghiade M, van Veldhuisen DJ, Mehra MR, Anker SD, Byra WM, Fu M, Mills RM. Rationale and design of a randomized, double‐blind, event‐driven, multicentre study comparing the efficacy and safety of oral rivaroxaban with placebo for reducing the risk of death, myocardial infarction or stroke in subjects with heart failure and significant coronary artery disease following an exacerbation of heart failure: the COMMANDER HF trial. Eur J Heart Fail. 2015;17:735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Graham DJ, Reichman ME, Wernecke M, Hsueh YH, Izem R, Southworth MR, Wei Y, Liao J, Goulding MR, Mott K, Chillarige Y, MaCurdy TE, Worrall C, Kelman JA. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. 2016;176:1662–1671. [DOI] [PubMed] [Google Scholar]
- 28. Diaz‐Garzon J, Sandoval Y, Smith SW, Love S, Schulz K, Thordsen SE, Johnson BK, Driver B, Jacoby K, Carlson MD, Dodd KW, Moore J, Scott NL, Bruen CA, Hatch R, Apple FS. Discordance between ICD‐coded myocardial infarction and diagnosis according to the universal definition of myocardial infarction. Clin Chem. 2017;63:415–419. [DOI] [PubMed] [Google Scholar]
- 29. Kociol RD, Pang PS, Gheorghiade M, Fonarow GC, O'Connor CM, Felker GM. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J Am Coll Cardiol. 2010;56:1071–1078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Covariates Included in the Cox‐Regression Models for Each of the Outcomes


