Abstract
Background
The relative effect of hemoglobin A1c, blood pressure, and low‐density lipoprotein‐cholesterol (LDL‐C) (“ABC” factors) on the prevention of cardiovascular diseases (CVD) among patients with type 2 diabetes mellitus is poorly understood. This study aimed to evaluate the association of key clinical parameters on CVD risk using a multifactorial optimal control approach in Chinese primary care patients with type 2 diabetes mellitus.
Methods and Results
A population‐based retrospective cohort study was conducted on 144 271 Chinese type 2 diabetes mellitus primary care patients, aged 18 to 79 and without prior clinical diagnosis of CVD in 2008–2011. Cox regressions were conducted to examine the association between the combinations of ABC targets (hemoglobin A1c <7%, blood pressure <130/90 mm Hg, and LDL‐C <2.6 mmol/L) and risks of CVD (overall), coronary heart disease, stroke, and heart failure. Achieving more ABC targets incrementally reduced the incidence of total CVD and individual disease including coronary heart disease, stroke, and heart failure, irrespective of other patient characteristics. Compared with suboptimal control in all ABC levels, achieving any 1, 2, and all 3 ABC targets reduced the relative risk of CVD by 13% to 42%, 31% to 52%, and 55%, respectively. Among those achieving only 1 ABC target, LDL‐C reduction was associated with the greatest CVD risk reduction (42%), followed by blood pressure reduction (18%), and hemoglobin A1c reduction (13%).
Conclusions
To achieve the greatest risk reduction for the incidence of CVD, the ultimate goal of treatment should be to achieve target control of hemoglobin A1c, blood pressure, and LDL‐C. If it is not possible to achieve all 3 targets, efforts should be prioritized on treating the LDL‐C to minimize CVD risk.
Keywords: blood pressure, cardiovascular disease, diabetes mellitus, hemoglobin A1c, lipids
Subject Categories: Cardiovascular Disease; Epidemiology; Risk Factors; Primary Prevention; Diabetes, Type 2
Clinical Perspective
What Is New?
In Chinese primary care type 2 diabetes mellitus patients, attainment of all 3 ABC targets (hemoglobin A1c <7%, blood pressure <130/90 mm Hg, and low‐density lipoprotein‐C <2.6 mmol/L) was achieved in only one tenth of patients.
Achieving a greater number of ABC targets, irrespective of the patient's characteristics, incrementally reduced the risk of total cardiovascular disease (CVD) and all its subtypes including coronary heart disease, stroke, and heart failure.
The relative importance of ABC control for the primary prevention of CVD was found to be target low‐density lipoprotein‐C, followed by target blood pressure, followed by target hemoglobin A1c.
Patients benefitted the most from attaining all 3 ABC targets compared with those with suboptimal levels in all 3 ABC in the duration of diabetes mellitus <1 year group.
What Are the Clinical Implications?
To achieve the greatest risk reduction for the incidence of CVD, the ultimate goal of treatment should be to achieve target control of hemoglobin A1c, blood pressure, and low‐density lipoprotein‐C.
If it is not possible to achieve all 3 targets, efforts should be prioritized on treating the low‐density lipoprotein‐C to minimize CVD risk.
Our findings highlight the importance of optimal control of multifactorial risk factors at an earlier disease stage, and the need to promote and educate clinicians and patients of the importance of target control of the modifiable risk factors for the primary prevention of CVD.
Introduction
Diabetes mellitus (DM) remains a growing global health challenge. There are currently 415 million patients with DM worldwide, and this figure is predicted to increase to 642 million by 2040.1 Cardiovascular diseases (CVD) are the dominant cause of mortality in diabetic populations, contributing to 70% of deaths.2 The International Diabetes Federation and the American Diabetes Association strongly recommend maintaining optimal hemoglobin A1c (HbA1c), blood pressure (BP), and low‐density lipoprotein‐cholesterol (LDL‐C) (“ABC”) levels for the primary prevention of CVD.3, 4 Unfortunately, achieving target levels of all ABC risk factors remains a significant challenge as has been reported in Canada, the United States, the United Kingdom, and Europe.5, 6, 7, 8 The benefits of CVD risk reduction through better control of all 3 factors is beyond doubt, but little is known about the relative effects of controlling individual or various combinations of these risk factors in diabetic populations.
To date, there have only been a few studies that have explored the association between multifactorial risk factors and CVD.9, 10, 11, 12 These studies have typically examined the number of factors controlled as none, any 1, any 2, or all 3 risk factors, but the relative importance of these 3 risk factors remains unclear. In clinical practice, physicians need to understand the relative importance of treating individual factors to help them prioritize the treatment goals in patients who may not be able to tolerate treatment of all 3 conditions synchronously such as those who may be at increased risk of adverse effects, those with excessive polypharmacy, or to help minimize treatment burden.13, 14 Evidence on the relative importance of treating each factor or combinations of factors can help clinicians make the most informed decisions and can also be used for shared decision making in diabetic patients with multimorbidity. Previous studies included both type 1 diabetes mellitus (DM) and type 2 DM (T2DM) subjects, have included patients recruited from hospital‐based settings, have had inadequate sample sizes to observe sufficient end‐point events, or have been performed under very structured but artificial settings such as randomized clinical trials.9, 10, 11, 12 Given the potential difference in pathophysiology of CVD in type 1 DM and T2DM,15 and difference in disease severity in hospital‐based patients and primary care patients, findings from these previous studies may not be applicable to T2DM patients managed in a real‐world primary care outpatient setting. This is important given recent trends to shift delivery of diabetic care away from hospitals to primary care settings in most developed countries.16 Also, most previous studies have only considered baseline ABC measurements, ignoring the changes that may occur during the follow‐up period, and have not shown the results of subgroup analyses describing the association of multifactorial risk factors control on CVD in different higher‐risk subgroups such as smokers or those who are obese, which is an area where more knowledge is needed.
This study aimed to address some of the gaps in the existing literature by examining the association and relative importance of achieving target control for individual and combinations of ABC risk factors on CVD risk reduction among Chinese primary care patients with T2DM and to perform subgroup analyses to examine which patient characteristics are associated with CVD risk.
Methods
Study Design
This was a retrospective cohort study. All subjects were Chinese, aged from 18 to 79 years old, were clinically diagnosed with T2DM as identified by the International Classification of Primary Care‐2 (ICPC‐2) code of “T90,” without any CVD event before baseline, and were receiving health services for DM in 1 of the 74 general outpatient clinics of the Hong Kong Hospital Authority (HA). The HA is the government organization coordinating all public‐sector hospitals and primary care clinics in Hong Kong. Clinical records between August 1, 2008 and December 31, 2011 on all subjects meeting the inclusion criteria were retrieved through the HA's administrative database. For each patient, the date of the first time having the ABC value recorded was defined as their baseline and they were followed up until the date of occurrence of an outcome event, death, or last follow‐up as of the censoring date (November 30, 2015), whichever occurred first.
Consent of participants was not necessary as all data were anonymous and were extracted through the computerized administrative system of the HA. Ethics approval was received from all the regional Institutional Review Boards of the Hong Kong HA. The reported investigations have been carried out in accordance with the principles of the Declaration of Helsinki as revised in 2008.
CVD Identification
Outcomes of interest included the following 4 events:
CVD event including coronary heart disease (CHD), stroke, or heart failure;
CHD included ischemic heart disease, myocardial infarction, coronary death, and sudden death as identified using ICPC‐2 K74 to K76 or International Classification of Diseases, Ninth Edition, Clinical Modification 410.x to 414.x, 798.x;
Stroke including fatal and nonfatal was identified using ICPC‐2 K89 to K91 or International Classification of Diseases, Ninth Edition, Clinical Modification of 430.x to 438.x; and
Heart failure was identified using ICPC‐2 K77 or International Classification of Diseases, Ninth Edition, Clinical Modification 428.x.
Achieved ABC and Baseline Covariates
The achieved ABC value was defined as the average of annual ABC measurements from baseline to the date of last follow‐up. For instance, if the follow‐up period was 4 years, then the achieved mean value was calculated by averaging the baseline, 1‐, 2‐, 3‐, and 4‐year ABC values. Achieved values are commonly used to evaluate the association between clinical parameters and the incidence of morbidity.17, 18, 19 According to local guidelines, the recommended target values for HbA1c, BP, and LDL‐C were <7%, <130/80 mm Hg, and <2.6 mmol/L, respectively.20 All study subjects were stratified into 1 of the following 8 groups according to whether achieved ABC values achieved target or not (Group 1: none achieved the targets; Group 2: only HbA1c achieved the targets; Group 3: only BP achieved the targets; Group 4: only LDL‐C achieved the targets; Group 5: only HbA1c and BP achieved the targets; Group 6: only HbA1c and LDL‐C achieved the targets; Group 7: only BP and LDL‐C achieved the targets; Group 8: all achieved the targets). For example, if a patient had achieved HbA1c of 8%, BP of 125/75 mm Hg, and LDL‐C of 2.5 mmol/L, then this patient was stratified into Group 7.
The baseline covariates comprised the following:
Patient's sociodemographics: sex, age, and smoking status;
Laboratory results: lipid profile (LDL‐C and total cholesterol to high‐density lipoprotein‐cholesterol ratio), triglyceride, urine albumin‐to‐creatinine ratio, and estimated glomerular filtration rate.
Clinical characteristics: body mass index (BMI), Charlson's Index, treated hypertension defined as ICPC‐2 code of “K86” or “K87,” self‐reported duration of DM, and family history of DM.
Treatment modalities: use of insulin, metformin, sulfonylurea, other oral antidiabetic drugs, angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker, β‐blocker, calcium channel blocker, diuretic, other antihypertensive drugs, and statins.
All laboratory assays were performed in accredited laboratories by the College of American Pathologists, the Hong Kong Accreditation Service, or the National Association of Testing Authorities, Australia.
Data Analysis
Multiple imputation was adopted to deal with missing data for all baseline covariates other than ABC.21 This approach can effectively reduce unnecessary biases,21, 22 raise the power of the analysis, and produce more reliable and applicable models.23, 24, 25 In this study, each missing value was imputed 5 times by the chained equation method. The same analysis was performed for each of the 5 imputed data sets, and the 5 sets of results were combined using Rubin's rules.26
Descriptive statistics for the baseline covariates of each group were displayed after multiple imputation. Univariable linear (for continuous variables) or logistic (for binary variables) regressions were used to test whether there was any significant difference in baseline covariates across the groups. The incidence rate was estimated by an exact 95% CI based on a Poisson distribution for each group.27 The differences of the incidence of CVD across the 8 groups were examined using multivariable Cox proportional hazards regression models with the adjustment of all baseline covariates as shown above. The proportional hazards assumption was checked by examining plots of the scaled Schoenfeld residuals against time for the covariates. All models in the study satisfied the proportional hazards assumptions and showed no presence of multicollinearity. To avoid the association of specific standard target for ABC levels and potential bias because of severe disease at baseline, 3 sensitivity analyses: (1) changing HbA1c and BP targets to 7.5% and 140/90 mm Hg, respectively; (2) changing HbA1c and BP targets to 8% and 140/90 mm Hg, respectively; and (3) excluding subjects with follow‐up period less than 1 year were also conducted. Subgroup analyses were performed subsequently by stratifying sex, age groups (<65 years; ≥65 years), duration of DM (<1 year; ≥1 year), smoking status (smokers; nonsmokers), and BMI groups (<23 kg/m2; 23–24.9 kg/m2; BMI ≥25 kg/m2).
All significance tests were 2‐tailed and those with P<0.05 were considered statistically significant. Statistical analyses were performed in STATA Version 13.0.
Results
In total, there were 162 589 subjects who were Chinese, aged between 18 and 79 years old, clinically diagnosed with T2DM, with valid ABC measurement, and received their DM care in primary care clinics of HA between August 1, 2008 and December 31, 2011. After excluding 18 318 subjects with prior history of CVD history or without follow‐up after baseline, 144 271 T2DM patients were included in the main analysis. Data completion rates for each baseline covariate were >95%.
Table 1 shows the baseline characteristics for the overall cohort and for each of the groups after multiple imputation. In general, there were slightly more females (53%) than males (47%), mean age was 61.6 years (SD: 10.0 years), and the mean duration of DM was 6.2 years (SD: 6.3 years). In the study cohort, 40%, 31%, and 9% achieved 1, 2, and all 3 ABC targets, respectively; 47%, 37%, and 45% met the target levels for HbA1c, BP, and LDL‐C.
Table 1.
Baseline Characteristics of Subjects
| Factor | Total (N=144 271) | Group 1 (N=29 507) | Group 2 (N=22 382) | Group 3 (N=13 656) | Group 4 (N=21 270) | Group 5 (N=13 999) | Group 6 (N=18 337) | Group 7 (N=11 659) | Group 8 (N=13 461) | P Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Sociodemographic, % (n) | ||||||||||
| Male | 47.4% (68 360) | 46.0% (13 559) | 45.2% (10 122) | 45.2% (6175) | 50.2% (10 677) | 43.3% (6063) | 51.7% (9474) | 48.8% (5694) | 49.0% (6596) | <0.001a |
| Age (mean±SD), y | 61.63±10.02 (144 271) | 60.91±10.05 (29 507) | 63.62±9.72 (22 382) | 58.59±9.86 (13 656) | 61.63±10.00 (21 270) | 61.05±9.84 (13 999) | 64.26±9.56 (18 337) | 59.42±9.94 (11 659) | 61.86±9.89 (13 461) | <0.001a |
| Smoker | 11.7% (16 884) | 12.4% (3654) | 9.2% (2055) | 16.0% (2182) | 12.0% (2546) | 10.5% (1467) | 9.4% (1730) | 15.5% (1805) | 10.7% (1446) | <0.001a |
| Laboratory results, mean±SD | ||||||||||
| TC/HDL‐C ratio | 4.32±1.25 (144 271) | 4.80±1.23 (29 507) | 4.60±1.22 (22 382) | 4.69±1.29 (13 656) | 4.01±1.15 (21 270) | 4.51±1.18 (13 999) | 3.84±1.13 (18 337) | 3.91±1.10 (11 659) | 3.77±1.07 (13 461) | <0.001a |
| Triglyceride, mmol/L | 1.62±0.91 (144 271) | 1.72±0.91 (29 507) | 1.58±0.81 (22 382) | 1.61±0.84 (13 656) | 1.71±1.05 (21 270) | 1.51±0.78 (13 999) | 1.56±0.92 (18 337) | 1.60±1.02 (11 659) | 1.49±0.89 (13 461) | <0.001a |
| BMI, kg/m2 | 25.60±3.92 (144 271) | 26.13±4.03 (29 507) | 25.84±3.89 (22 382) | 24.91±3.69 (13 656) | 26.11±4.12 (21 270) | 24.93±3.69 (13 999) | 25.80±3.93 (18 337) | 24.91±3.79 (11 659) | 24.93±3.77 (13 461) | <0.001a |
| Urine ACR, mg/mmol | 7.14±55.71 (144 271) | 9.46±44.27 (29 507) | 7.75±38.34 (22 382) | 5.59±51.78 (13 656) | 8.09±37.27 (21 270) | 5.18±28.64 (13 999) | 7.26±40.46 (18 337) | 5.14±28.42 (11 659) | 4.74±31.79 (13 461) | <0.001a |
| Follow‐up measurements, mean±SD | ||||||||||
| Number of HbA1c | 4.98±1.26 (144 271) | 4.88±1.41 (29 507) | 4.77±1.37 (22 382) | 5.05±1.27 (13 656) | 5.15±1.16 (21 270) | 4.91±1.25 (13 999) | 4.97±1.17 (18 337) | 5.24±1.06 (11 659) | 5.05±1.09 (13 461) | <0.001a |
| Mean HbA1c, % | 7.21±0.99 (144 271) | 7.95±0.97 (29 507) | 6.48±0.38 (22 382) | 7.86±0.93 (13 656) | 7.83±0.80 (21 270) | 6.46±0.39 (13 999) | 6.47±0.40 (18 337) | 7.74±0.79 (11 659) | 6.44±0.41 (13 461) | <0.001a |
| Mean HbA1c, mmol/mol | 55.24±10.87 (144 271) | 63.43±10.64 (29 507) | 47.31±4.19 (22 382) | 62.37±10.20 (13 656) | 62.03±8.77 (21 270) | 47.08±4.26 (13 999) | 47.20±4.33 (18 337) | 61.11±8.64 (11 659) | 46.90±4.49 (13 461) | <0.001a |
| Number of BP | 5.32±1.23 (144 271) | 5.13±1.42 (29 507) | 5.26±1.32 (22 382) | 5.32±1.26 (13 656) | 5.35±1.19 (21 270) | 5.41±1.16 (13 999) | 5.41±1.11 (18 337) | 5.46±1.03 (11 659) | 5.48±1.01 (13 461) | <0.001a |
| Mean SBP, mm Hg | 132.52±11.41 (144 271) | 139.70±9.39 (29 507) | 138.65±8.45 (22 382) | 121.55±6.52 (13 656) | 138.46±8.45 (21 270) | 121.62±6.48 (13 999) | 137.71±7.83 (18 337) | 121.82±6.39 (11 659) | 121.87±6.29 (13 461) | <0.001a |
| Mean DBP, mm Hg | 74.14±7.57 (144 271) | 77.34±7.65 (29 507) | 75.59±7.71 (22 382) | 70.90±5.56 (13 656) | 76.65±7.39 (21 270) | 70.06±5.88 (13 999) | 75.10±7.52 (18 337) | 70.78±5.68 (11 659) | 69.86±5.87 (13 461) | <0.001a |
| Number of LDL‐C | 4.74±1.29 (144 271) | 4.57±1.43 (29 507) | 4.65±1.38 (22 382) | 4.77±1.31 (13 656) | 4.79±1.21 (21 270) | 4.81±1.26 (13 999) | 4.79±1.20 (18 337) | 4.89±1.12 (11 659) | 4.90±1.13 (13 461) | <0.001a |
| Mean LDL‐C, mmol/L | 2.71±0.59 (144 271) | 3.15±0.48 (29 507) | 3.11±0.44 (22 382) | 3.10±0.44 (13 656) | 2.23±0.30 (21 270) | 3.06±0.41 (13 999) | 2.22±0.31 (18 337) | 2.23±0.30 (11 659) | 2.22±0.31 (13 461) | <0.001a |
| Clinical characteristics, % (n) | ||||||||||
| Duration of T2DM, y | 6.22±6.33 (144 271) | 6.97±6.77 (29 507) | 4.95±5.62 (22 382) | 6.56±6.29 (13 656) | 7.82±6.89 (21 270) | 4.78±5.51 (13 999) | 5.45±6.00 (18 337) | 7.38±6.62 (11 659) | 5.32±5.67 (13 461) | <0.001a |
| Treated hypertension | 67.3% (97 104) | 70.3% (20 739) | 79.3% (17 746) | 43.3% (5914) | 73.1% (15 555) | 58.8% (8226) | 80.4% (14 735) | 49.3% (5744) | 62.7% (8445) | <0.001a |
| Family history of DM | 43.6% (62 940) | 45.7% (13 481) | 37.7% (8447) | 49.9% (6814) | 46.3% (9851) | 42.8% (5992) | 38.4% (7045) | 49.6% (5780) | 41.1% (5530) | <0.001a |
| eGFR <60 mL/min per 1.73 m2 | 3.3% (4704) | 3.4% (1006) | 4.1% (907) | 2.2% (296) | 3.6% (764) | 2.6% (362) | 3.8% (698) | 2.3% (273) | 3.0% (397) | <0.001a |
| Charlson's Index | 1.09±0.37 (144 271) | 1.08±0.35 (29 507) | 1.09±0.38 (22 382) | 1.07±0.33 (13 656) | 1.08±0.36 (21 270) | 1.09±0.40 (13 999) | 1.10±0.41 (18 337) | 1.08±0.37 (11 659) | 1.10±0.42 (13 461) | <0.001a |
| Treatment modalities, % (n) | ||||||||||
| Use of insulin | 1.3% (1872) | 2.0% (587) | 0.5% (105) | 1.6% (221) | 2.3% (482) | 0.4% (56) | 0.7% (129) | 1.8% (207) | 0.6% (85) | <0.001a |
| Use of metformin | 71.1% (102 513) | 76.3% (22 521) | 57.1% (12 778) | 75.9% (10 366) | 83.3% (17 711) | 56.8% (7947) | 67.8% (12 440) | 82.0% (9563) | 68.2% (9187) | <0.001a |
| Use of sulfonylurea | 53.1% (76 548) | 64.3% (18 968) | 37.7% (8435) | 61.1% (8344) | 70.3% (14 945) | 33.5% (4683) | 43.4% (7967) | 67.1% (7828) | 40.0% (5378) | <0.001a |
| Use of other oral diabetic drugs | 1.6% (2357) | 2.4% (714) | 0.5% (123) | 2.0% (274) | 3.0% (639) | 0.5% (68) | 0.8% (152) | 2.4% (279) | 0.8% (108) | <0.001a |
| Use of ACEI/ARB | 31.8% (45 860) | 35.5% (10 476) | 33.5% (7487) | 21.5% (2931) | 39.7% (8445) | 22.7% (3175) | 36.5% (6701) | 26.0% (3030) | 26.9% (3615) | <0.001a |
| Use of β‐Blocker | 25.7% (37 056) | 25.0% (7385) | 31.2% (6982) | 16.3% (2226) | 27.8% (5906) | 22.4% (3138) | 32.1% (5889) | 18.5% (2159) | 25.0% (3371) | <0.001a |
| Use of CCB | 38.6% (55 645) | 37.6% (11 105) | 46.1% (10 325) | 23.8% (3256) | 39.7% (8438) | 36.9% (5167) | 48.1% (8820) | 27.2% (3172) | 39.8% (5362) | <0.001a |
| Use of diuretic | 9.8% (14 094) | 10.6% (3125) | 11.7% (2623) | 6.4% (871) | 10.8% (2304) | 8.4% (1172) | 11.3% (2073) | 7.3% (850) | 8.0% (1076) | <0.001a |
| Use of other antihypertensive drugs | 8.5% (12 259) | 7.7% (2258) | 10.7% (2389) | 4.7% (643) | 8.9% (1896) | 7.3% (1018) | 11.6% (2127) | 5.9% (688) | 9.2% (1240) | <0.001a |
| Use of statin | 9.7% (14 024) | 6.1% (1810) | 7.2% (1610) | 6.3% (861) | 12.2% (2591) | 7.2% (1012) | 14.4% (2632) | 12.7% (1477) | 15.1% (2031) | <0.001a |
ACEI indicates angiotensin‐converting enzyme inhibitor; ACR, albumin–creatinine ratio; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CCB, calcium channel blocker; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; Group 1, no targets achieved; Group 2, only HbA1c target achieved; Group 3, only BP target achieved; Group 4, only LDL‐C target achieved; Group 5, only HbA1c and BP targets achieved; Group 6, only HbA1c and LDL‐C targets achieved; Group 7, only BP and LDL‐C targets achieved; Group 8, all targets achieved; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein–cholesterol; LDL‐C, low‐density lipoprotein–cholesterol; SBP, systolic blood pressure; T2DM, type 2 diabetes mellitus; TC, total cholesterol.
Significant at 0.05 level by univariate linear or logistic regression, as appropriate.
The number, unadjusted incidence rate, and hazard ratio of various CVD events for each group are shown in Table 2. The incidence rates of CVD ranged between 10.9 and 25.7 per 1000 person‐years among all groups during a median follow‐up period of 63.5 to 68.5 months. In general, the more ABC targets achieved, the lower the CVD risk. Compared with Group 1 (no targets achieved), achieving only 1 of the ABC targets (Groups 2–4), any 2 of the ABC targets (Groups 5–7), and all 3 ABC targets (Group 8) were associated with 13% to 42%, 31% to 52%, and 55% reduction in the incidence of CVD, respectively. Among Groups 2 to 4, achieving LDL‐C target only (Group 4) was associated with the largest reduction in CVD risk of 42% compared with Group 1. The relative CVD risk reduction for achieving LDL‐C and BP targets (Group 7) decreased further by 52% compared with Group 1. Similar results were obtained for CHD, stroke, and heart failure. The sensitivity analyses were conducted after changing the target for ABC levels and the exclusion of subjects with a follow‐up period of <1 year. The results were almost identical to the main analysis and the pattern was even more obvious for less stringent treatment targets.
Table 2.
Number, Incidence Rate, and Hazard Ratio of CVD Events
| Group 1 (N=29 507) | Group 2 (N=22 382) | Group 3 (N=13 656) | Group 4 (N=21 270) | Group 5 (N=13 999) | Group 6 (N=18 337) | Group 7 (N=11 659) | Group 8 (N=13 461) | |
|---|---|---|---|---|---|---|---|---|
| CVD | ||||||||
| Cumulative cases with event | 4033 | 2823 | 1248 | 1870 | 1168 | 1643 | 680 | 794 |
| Cumulative incidence rate | 13.7% | 12.6% | 9.1% | 8.8% | 8.3% | 9.0% | 5.8% | 5.9% |
| Person‐years | 156 871 | 117 803 | 74 577 | 114 118 | 75 529 | 95 935 | 62 651 | 70 798 |
| Median follow‐up, months | 68.5 | 66.5 | 68.5 | 66.5 | 67.5 | 63.5 | 65.5 | 63.5 |
| Incidence rate (95% CI)a | 25.71 (24.93, 26.51) | 23.96 (23.10, 24.86) | 16.73 (15.83, 17.69) | 16.39 (15.66, 17.15) | 15.46 (14.60, 16.38) | 17.13 (16.32, 17.97) | 10.85 (10.07, 11.70) | 11.22 (10.46, 12.02) |
| Hazard ratiob (95% CI) | Reference group | 0.87c (0.83, 0.92) | 0.82c (0.77, 0.87) | 0.58c (0.55, 0.62) | 0.69c (0.65, 0.74) | 0.57c (0.54, 0.61) | 0.48c (0.44, 0.52) | 0.45c (0.42, 0.49) |
| CHD | ||||||||
| Cumulative cases with event | 2078 | 1377 | 690 | 851 | 602 | 749 | 361 | 362 |
| Cumulative incidence rate | 7.0% | 6.2% | 5.1% | 4.0% | 4.3% | 4.1% | 3.1% | 2.7% |
| Person‐years | 162 587 | 121 877 | 76 106 | 116 540 | 77 032 | 97 995 | 63 398 | 71 782 |
| Median follow‐up, months | 70.5 | 68.5 | 69.5 | 67.5 | 67.5 | 64.5 | 65.5 | 63.5 |
| Incidence rate (95% CI)a | 12.78 (12.24, 13.34) | 11.30 (10.72, 11.91) | 9.07 (8.41, 9.77) | 7.30 (6.83, 7.81) | 7.81 (7.21, 8.46) | 7.64 (7.12, 8.21) | 5.69 (5.14, 6.31) | 5.04 (4.55, 5.59) |
| Hazard ratiob (95% CI) | Reference group | 0.87c (0.81, 0.94) | 0.86c (0.78, 0.94) | 0.54c (0.50, 0.59) | 0.71c (0.65, 0.78) | 0.56c (0.51, 0.61) | 0.50c (0.45, 0.56) | 0.43c (0.38, 0.48) |
| Stroke | ||||||||
| Cumulative cases with event | 1734 | 1202 | 514 | 801 | 498 | 677 | 270 | 347 |
| Cumulative incidence rate | 5.9% | 5.4% | 3.8% | 3.8% | 3.6% | 3.7% | 2.3% | 2.6% |
| Person‐years | 163 018 | 121 998 | 76 490 | 116 537 | 77 183 | 98 082 | 63 582 | 71 734 |
| Median follow‐up, months | 70.5 | 68.5 | 70.5 | 67.5 | 68.5 | 64.5 | 65.5 | 63.5 |
| Incidence rate (95% CI)a | 10.64 (10.15, 11.15) | 9.85 (9.31, 10.43) | 6.72 (6.16, 7.33) | 6.87 (6.41, 7.37) | 6.45 (5.91, 7.04) | 6.90 (6.40, 7.44) | 4.25 (3.77, 4.78) | 4.84 (4.35, 5.37) |
| Hazard ratiob (95% CI) | Reference group | 0.87c (0.79, 0.97) | 0.80c (0.69, 0.92) | 0.64c (0.58, 0.72) | 0.59c (0.51, 0.69) | 0.58c (0.52, 0.66) | 0.48c (0.40, 0.58) | 0.41c (0.35, 0.49) |
| Heart failure | ||||||||
| Cumulative cases with event | 982 | 699 | 252 | 495 | 215 | 405 | 141 | 156 |
| Cumulative incidence rate | 3.3% | 3.1% | 1.8% | 2.3% | 1.5% | 2.2% | 1.2% | 1.2% |
| Person‐years | 165 903 | 124 023 | 77 344 | 117 414 | 77 990 | 98 846 | 63 938 | 72 244 |
| Median follow‐up, months | 70.5 | 69.5 | 70.5 | 67.5 | 68.5 | 64.5 | 65.5 | 64.5 |
| Incidence rate (95% CI)a | 5.92 (5.56, 6.30) | 5.64 (5.23, 6.07) | 3.26 (2.88, 3.69) | 4.22 (3.86, 4.60) | 2.76 (2.41, 3.15) | 4.10 (3.72, 4.52) | 2.21 (1.87, 2.60) | 2.16 (1.85, 2.53) |
| Hazard ratiob (95% CI) | Reference group | 0.84c (0.77, 0.90) | 0.79c (0.71, 0.87) | 0.59c (0.54, 0.64) | 0.67c (0.61, 0.74) | 0.54c (0.49, 0.59) | 0.44c (0.39, 0.51) | 0.45c (0.40, 0.51) |
CHD indicates coronary heart disease; CVD, cardiovascular disease; Group 1, no target achieved; Group 2, only hemoglobin A1c (HbA1c) target achieved; Group 3, only blood pressure (BP) target achieved; Group 4, only low‐density lipoprotein cholesterol (LDL‐C) target achieved; Group 5: only HbA1c and BP targets achieved; Group 6, only HbA1c and LDL‐C targets achieved; Group 7, only BP and LDL‐C targets achieved; Group 8, all targets achieved.
Incidence rate (cases/1000 person‐years) with 95% CI based on Poisson Distribution.
Hazard ratios were adjusted by age, sex, smoking status, total cholesterol/high‐density lipoprotein cholesterol ratio, triglyceride, body mass index, urine albumin–creatinine ratio, duration of type 2 diabetes mellitus, diagnosed hypertension, family history of diabetes mellitus, estimated glomerular filtration rate, Charlson's Index, use of insulin, metformin, sulfonylurea, other oral diabetic drugs, angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker, β‐blocker, calcium channel blocker, diuretic, other antihypertensive drugs, and statin, calcium channel blocker, diuretic, other antihypertensive drugs, and statin.
Significant difference (P<0.05) by multivariable Cox proportional hazards regression.
Similar analyses were conducted on the following 11 subgroups: (1) females, (2) males, (3) age <65 years, (4) age ≥65 years, (5) duration of DM <1 year, (6) duration of DM ≥1 year, (7) smokers, (8) nonsmokers, (9) BMI <23 kg/m2, (10) BMI between 23 and 24.9 kg/m2 and (11) BMI ≥25 kg/m2 and the corresponding adjusted hazard ratios for the incidence of each outcome event were plotted against the 7 groups (using Group 1 as the reference group) in Figure 1. A similar pattern was observed for incidence of total CVD, CHD, heart failure, and stroke and the deviation from the overall cohort was small for each of the 11 subgroups. Among all subgroups, the largest relative risk reduction in CVD was 69% when all 3 targets were achieved compared with none in the group with duration of DM <1 year.
Figure 1.
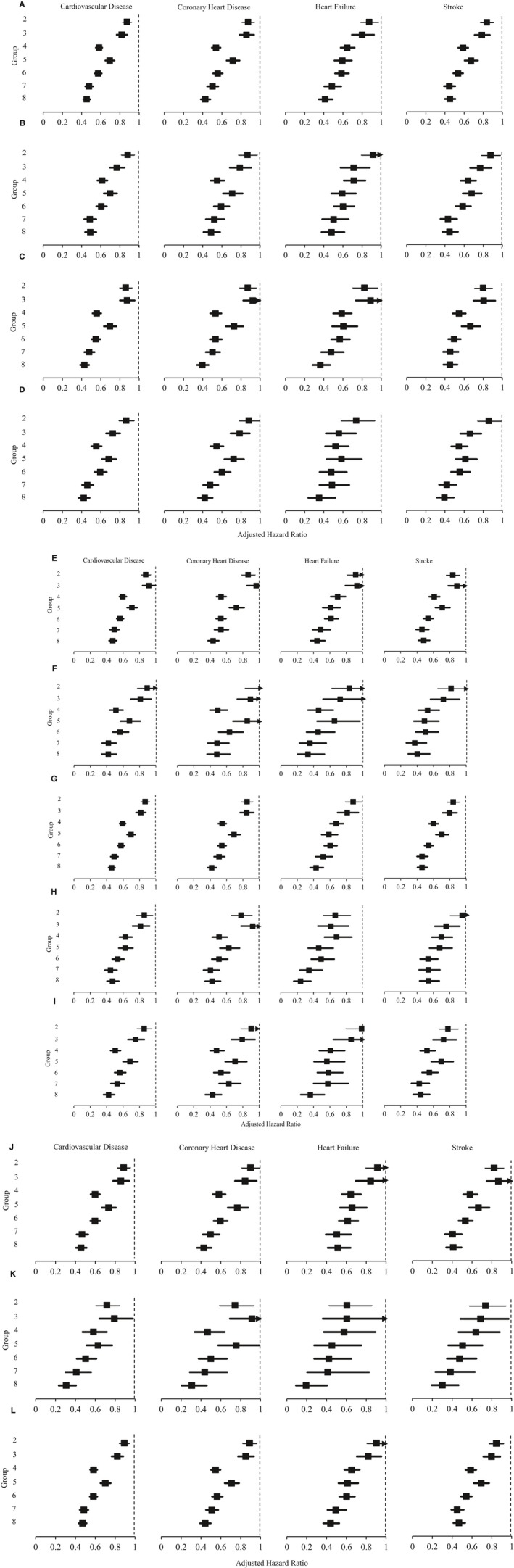
Adjusted hazard ratios for incidence of cardiovascular diseases among (A) all participants, (B) female, (C) male (D), age <65 years old, (E) age ≥65 years old, (F) duration of diabetes mellitus (DM) <1 year, (G) duration of DM ≥1 year, (H) smoker, (I) nonsmoker, (J) body mass index (BMI) <23 kg/m2, (K) BMI 23 to 25 kg/m2, and (L) BMI ≥25 kg/m2 by multivariable Cox proportional hazards regressions. Hazard ratios were adjusted by age, sex, smoking status, total cholesterol to high‐density lipoprotein cholesterol ratio, triglyceride, BMI, urine albumin‐to‐creatinine ratio, self‐reported duration of DM, diagnosed hypertension, family history of DM, estimated glomerular filtration rate, Charlson Index and the use of insulin, metformin, sulfonylurea, other oral diabetic drugs, angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker, β‐blocker, calcium channel blocker, diuretic, other antihypertensive drugs, and statin at baseline. Group 1 (no target achieved) was used as reference group; Group 2: only HbA1c (hemoglobin A1c) target achieved; Group 3: only blood pressure (BP) target achieved; Group 4: only low‐density lipoprotein‐cholesterol (LDL‐C) target achieved; Group 5: only HbA1c and BP targets achieved; Group 6: only HbA1c and LDL‐C targets achieved; Group 7: only BP and LDL‐C targets achieved; Group 8: all targets achieved.
Discussion
This is the first study to evaluate the relative association of target control of individual and combinations of ABC risk factors on the reduction of CVD among Chinese primary care patients with T2DM. In our study population, attainment of all 3 ABC targets was achieved in only one tenth of patients. Achieving a greater number of ABC targets, irrespective of the patient's characteristics, incrementally reduced the risk of total CVD and all its subtypes including CHD, stroke, and heart failure. Achieving target control of LDL‐C was associated with the most significant reduction of CVD risk, suggesting that the relative importance of attaining ABC targets for the primary prevention of CVD should be LDL‐C first, BP second, and HbA1c third. Among all subgroups, patients benefitted the most from attaining all 3 ABC targets compared with those with suboptimal levels in all 3 ABC in the group with duration of DM <1 year, suggesting that early optimization of the ABC risk factor control in earlier disease stage is important.
The key finding of this study was understanding that not all ABC risk factors are of equal importance in the reduction of CVD risk in diabetic patients. Among Chinese primary care patients with T2DM, the relative importance of ABC control for the primary prevention of CVD was found to be target lipid levels, followed by target BP, followed by target HbA1c. As earlier studies had only focused on the association of achieving target control for individual risk factors or number of risk factors, this study helps to fill a gap in the literature by providing information on the relative importance of achieving individual ABC targets or combinations of targets. A study comparing the effectiveness of lowering ABC levels by combining findings from epidemiological data and meta‐analyses found that the numbers needed to treat for the prevention of 1 CVD event over 5 years were 119 for 0.9% reduction in HbA1c, 44 for 1 mmol/L reduction in LDL‐C, and 34 for 10 mm Hg reduction in systolic blood pressure or 5 mm Hg reduction in diastolic blood pressure, and concluded that addressing lipid and BP control had much larger benefits on the prevention of CVD than glycemic control.28 In terms of the tolerability and safety of pharmacological treatments, glucose‐lowering therapies, in particular intensive glycemic control, can increase the risk of severe hypoglycemia and severe adverse events resulting in hospitalization or significant disability.29 In contrast, lowering of blood pressure or lipid levels can be achieved with drugs that have relatively less dangerous side effects.30, 31, 32 A recent review highlighted that effective low‐cost statin regimens (generic atorvastatin 40 mg daily costs around USD 2.6 per month) can reduce at least 2 mmol/L in patients with LDL‐C of 4 mmol/L or above, and lowering by 2 mmol/L reduces CVD risks by 45%.30 Weighing the benefits and risks of ABC factors based on current evidence, it appears that targeting lipid control should be prioritized if all 3 cannot be achieved.
Our findings were consistent with the results of the Steno‐2 randomized clinical trial, which also concluded the crucial importance of multifactorial interventions for established risk factors in DM management.12 Another recent study conducted in the United States demonstrated that diabetic patients achieving 1, 2, and all 3 ABC targets incrementally reduced CVD risk by 36%, 52%, and 62%, respectively, with similar associations observed in CHD risk.9 A large population‐based study also pointed out that 34.1%, 38.4%, 36.2%, and 29.3% of new onset of CVD, CHD, stroke, and heart failure events could be prevented with adequate control of risk factors in American diabetic patients.10 Another simulation study projected that 38.3% of CHD events were prevented by controlling all risk factors by applying the United Kingdom Prospective Diabetes Study Risk Engine.11 We found that patients with newly diagnosed DM received the greatest benefit from attaining all 3 ABC targets compared with those with suboptimal levels in all 3 ABC targets. Several previous studies also found that treatments were more effective for diabetic patients in an earlier disease stage, highlighting the importance of early optimal risk factor control to delay or reduce complications.33, 34, 35
Similar to other countries, it was observed that only a small proportion of Chinese diabetic patients achieve all 3 ABC targets. The local prevalence of 9% for 3‐factor target control is slightly lower than rates reported in other countries, where 41% (27% for age <40), 14.3%, 12.0%, 6.5% in the United Kingdom, the United States, Canada, and 8 European countries, respectively, achieved 3‐factor control. Clinical inertia has been postulated to be the main reason underlying the low prevalence of multitarget control. Clinical inertia refers to a failure or reluctance to initiate or intensify therapies when clinically indicated.36, 37 The causes for clinical inertia can be complex and multifactorial involving both clinician and patient barriers.36, 37 At the clinician level, barriers include time constraints for consultation, concern about the potential risk of harm, and lack of information and training needed to achieve therapeutic goals. At the patient level, barriers include anxieties about side effects of treatments, difficulties with adhering to treatment regimens, costs of medications, and other factors that contribute to excessive treatment burden. Regardless of the reason, while risk factor control remains suboptimal there is an urgent need to understand how to best manage patients in a real‐world setting where 3‐factor control may be the goal but is often not feasible.
There were several strengths to this study. First, a comprehensive evaluation on the association between ABC targets and CVD with subgroup analyses was able to be performed because of the large population‐based sample. Second, repeated measurements of ABC increased the reliability of the findings. Third, all data were extracted from a computerized administrative database, which helps assure data accuracy.
There were also several limitations. First, this was a retrospective cohort study, which can only provide associations but not causation. However, an identical conclusion was obtained in the sensitivity analysis, which minimized reverse causation. A further randomized clinical trial would be required to reappraise our results. Second, the clinical diagnosis of CVD depended on the ICPC‐2 and International Classification of Diseases, Ninth Edition, Clinical Modification coding from the database, which might be subject to misclassification bias. While there were no studies to audit the accuracy and completeness of diagnostic coding in this database, previous studies demonstrated a nearly excellent data completeness for drug prescription (99.98%), and all clinicians are needed to provide accurate coding for each episode of care in routine clinical practice in the HA.38, 39 Third, the specific optimal targets for ABC levels in this study were selected based on the current local guidance for the management of DM in effect at the time of the subject included. Although some international guidelines changed the BP target from 130/80 to 140/90 mm Hg recently, there is currently no consensus among international guidelines on the optimal targets. Similar results in the sensitivity analyses after changing to less stringent treatment targets were also obtained. Fourth, lifestyle factors such as diet and exercise were not available in the current study, but most of key factors such as laboratory and drug data could reflect the lifestyle and disease severity. Last, the current cohort only comprised Chinese patients with local target protocols. An external validation should be warranted to validate our model by using a Chinese population in other regions.
In T2DM primary care patients, regardless of other patient characteristics, achieving more ABC targets incrementally reduced the risk of CVD. These findings support current recommendations that control of all 3 ABC factors to target should be the ultimate goal for treatment. However, if it is not possible to achieve target control of all 3 parameters, efforts could be prioritized to achieving target LDL‐C levels. Among all subgroups, patients with newly diagnosed DM received the most benefit from attaining target control of all 3 ABC factors. These findings highlight the importance of optimal control of multifactorial risk factors at an earlier disease stage, and the need to promote and educate clinicians and patients about the importance of target control of the modifiable risk factors for the primary prevention of CVD.
Sources of Funding
This study was funded by the Health Services Research Fund, Food and Health Bureau, HKSAR Commissioned Research on Enhanced Primary Care Study (Ref. no. EPC‐HKU‐2). No funding organization had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation of the manuscript.
Disclosures
None.
Acknowledgments
The authors wish to acknowledge the contributions of the Risk Assessment Management Program for Diabetes Mellitus (RAMP‐DM) program team at the Hospital Authority head office, and the Chiefs of Service and RAMP‐DM program coordinators in each cluster, and the Statistics and Workforce Planning Department at the Hong Kong Hospital Authority.
(J Am Heart Assoc. 2017;6:e006400 DOI: 10.1161/JAHA.117.006400.)28862945
References
- 1. International Diabetes Federation . IDF Diabetes Atlas. 7 ed Brussels, Belgium: International Diabetes Federation; 2015. [Google Scholar]
- 2. Gilmer TP, O'Connor PJ, Rush WA, Crain AL, Whitebird RR, Hanson AM, Solberg LI. Predictors of health care costs in adults with diabetes. Diabetes Care. 2005;28:59–64. [DOI] [PubMed] [Google Scholar]
- 3. International Diabetes Federation Guideline Development Group . Global guideline for type 2 diabetes. Diabetes Res Clin Pract. 2014;104:1. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association . Standards of medical care in diabetes—2015. Diabetes Care. 2015;38:S70–S76.25537712 [Google Scholar]
- 5. Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in US diabetes care, 1999–2010. N Engl J Med. 2013;368:1613–1624. [DOI] [PubMed] [Google Scholar]
- 6. Stone MA, Charpentier G, Doggen K, Kuss O, Lindblad U, Kellner C, Nolan J, Pazderska A, Rutten G, Trento M. Quality of care of people with type 2 diabetes in eight European countries. Diabetes Care. 2013;36:2628–2638. DC_121759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Healthcare Quality Improvement Partnership . National diabetes audit (2013–2014 & 2014–2015)—report 1 care processes and treatment targets. 2016.
- 8. Grenier J, Leiter LA, Langer A, Goldin L, Teoh H, Connelly KA, Cheng AY, Tan MK, Fitchett D, McGuire DK. Glycemic control and cardiovascular risk factor management in patients with diabetes with and without coronary artery disease: insights from the diabetes mellitus status in Canada (DM‐SCAN) survey. Eur Heart J Qual Care Clin Outcomes. 2016;2:qcw013. [DOI] [PubMed] [Google Scholar]
- 9. Wong ND, Zhao Y, Patel R, Patao C, Malik S, Bertoni AG, Correa A, Folsom AR, Kachroo S, Mukherjee J. Cardiovascular risk factor targets and cardiovascular disease event risk in diabetes: a pooling project of the Atherosclerosis Risk in Communities Study, Multi‐Ethnic Study of Atherosclerosis, and Jackson Heart Study. Diabetes Care. 2016;39:668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vazquez‐Benitez G, Desai JR, Xu S, Goodrich GK, Schroeder EB, Nichols GA, Segal J, Butler MG, Karter AJ, Steiner JF. Preventable major cardiovascular events associated with uncontrolled glucose, blood pressure, and lipids and active smoking in adults with diabetes with and without cardiovascular disease: a contemporary analysis. Diabetes Care. 2015;38:905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wong ND, Patao C, Malik S, Iloeje U. Preventable coronary heart disease events from control of cardiovascular risk factors in US adults with diabetes (projections from utilizing the UKPDS risk engine). Am J Cardiol. 2014;113:1356–1361. [DOI] [PubMed] [Google Scholar]
- 12. Gæde P, Lund‐Andersen H, Parving H‐H, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. [DOI] [PubMed] [Google Scholar]
- 13. May CR, Eton DT, Boehmer K, Gallacher K, Hunt K, MacDonald S, Mair FS, May CM, Montori VM, Richardson A, Rogers AE, Shippee N. Rethinking the patient: using burden of treatment theory to understand the changing dynamics of illness. BMC Health Serv Res. 2014;14:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mair FS, May CR. Editorial. Thinking about the burden of treatment. BMJ. 2014;349:g6680. [DOI] [PubMed] [Google Scholar]
- 15. De Ferranti SD, De Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, Magge SN, Marx N, McGuire DK, Orchard TJ. Type 1 diabetes mellitus and cardiovascular disease a scientific statement from the American Heart Association and American Diabetes Association. Circulation. 2014;130:1110–1130. [DOI] [PubMed] [Google Scholar]
- 16. Gelding SV. Improving cardiovascular risk in type 2 diabetes: time to get personal. United Kingdom: Oxford University Press; 2016. [DOI] [PubMed] [Google Scholar]
- 17. Zhao W, Katzmarzyk PT, Horswell R, Li W, Wang Y, Johnson J, Heymsfield SB, Cefalu WT, Ryan DH, Hu G. Blood pressure and heart failure risk among diabetic patients. Int J Cardiol. 2014;176:125–132. [DOI] [PubMed] [Google Scholar]
- 18. Wan EYF, Fung CSC, Wong CKH, Chin WY, Lam CLK. Association of hemoglobin A1c levels with cardiovascular disease and mortality in Chinese patients with diabetes. J Am Coll Cardiol. 2016;67:456–458. [DOI] [PubMed] [Google Scholar]
- 19. Sundström J, Sheikhi R, Östgren CJ, Svennblad B, Bodegård J, Nilsson PM, Johansson G. Blood pressure levels and risk of cardiovascular events and mortality in type‐2 diabetes: cohort study of 34 009 primary care patients. J Hypertens. 2013;31:1603–1610. [DOI] [PubMed] [Google Scholar]
- 20. Food and Health Bureau HKSAR . Hong Kong reference framework for diabetes care for adults in primary care settings. 2010.
- 21. Royston P. Multiple imputation of missing values. Stata J. 2004;4:227–241. [Google Scholar]
- 22. Clark TG, Altman DG. Developing a prognostic model in the presence of missing data: an ovarian cancer case study. J Clin Epidemiol. 2003;56:28–37. [DOI] [PubMed] [Google Scholar]
- 23. Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147. [PubMed] [Google Scholar]
- 24. Steyerberg EW, van Veen M. Imputation is beneficial for handling missing data in predictive models. J Clin Epidemiol. 2007;60:979. [DOI] [PubMed] [Google Scholar]
- 25. Moons KG, Donders RA, Stijnen T, Harrell FE. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol. 2006;59:1092–1101. [DOI] [PubMed] [Google Scholar]
- 26. Rubin DB. Multiple Imputation for Nonresponse in Surveys. United States: John Wiley & Sons; 2004. [Google Scholar]
- 27. Ulm K. Simple method to calculate the confidence interval of a standardized mortality ratio (SMR). Am J Epidemiol. 1990;131:373–375. [DOI] [PubMed] [Google Scholar]
- 28. Yudkin J, Richter B, Gale E. Intensified glucose lowering in type 2 diabetes: time for a reappraisal. Diabetologia. 2010;53:2079–2085. [DOI] [PubMed] [Google Scholar]
- 29. Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal T, Hemmingsen C, Wetterslev J. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. Cochrane Libr. 2011; DOI: 10.1136/bmj.e1771. Available at http://www.leicestershirediabetes.org.uk/uploads/123/documents/BMJ%20Cp%20of%20Metformin%20and%20insulin%20v%20insulin%20type2.pdf. Accessed August 9, 2017. [DOI] [PubMed] [Google Scholar]
- 30. Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Blumenthal R, Danesh J, Smith GD, DeMets D. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–2561. [DOI] [PubMed] [Google Scholar]
- 31. Association AD . 8. Cardiovascular disease and risk management. Diabetes Care. 2015;38:S49–S57. [DOI] [PubMed] [Google Scholar]
- 32. Law M, Wald N, Morris J, Jordan R. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003;326:1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chan J, So W, Ko G, Tong P, Yang X, Ma R, Kong A, Wong R, Le Coguiec F, Tamesis B. The Joint Asia Diabetes Evaluation (JADE) Program: a web‐based program to translate evidence to clinical practice in Type 2 diabetes. Diabet Med. 2009;26:693–699. [DOI] [PubMed] [Google Scholar]
- 34. Alberti KGM, Zimmet P, Shaw J. International Diabetes Federation: a consensus on Type 2 diabetes prevention. Diabet Med. 2007;24:451–463. [DOI] [PubMed] [Google Scholar]
- 35. Colagiuri S, Cull CA, Holman RR. Are lower fasting plasma glucose levels at diagnosis of type 2 diabetes associated with improved outcomes? UK prospective diabetes study 61. Diabetes Care. 2002;25:1410–1417. [DOI] [PubMed] [Google Scholar]
- 36. Khunti S, Davies MJ, Khunti K. Clinical inertia in the management of type 2 diabetes mellitus: a focused literature review. Br J Diabetes. 2015;15:65–69. [Google Scholar]
- 37. Lin J, Zhou S, Wei W, Pan C, Lingohr‐Smith M, Levin P. Does clinical inertia vary by personalized A1c goal? A study of predictors and prevalence of clinical inertia in a US managed‐care setting. Endocr Pract. 2015;22:151–161. [DOI] [PubMed] [Google Scholar]
- 38. Wong MC, Jiang JY, Tang J‐L, Lam A, Fung H, Mercer SW. Health services research in the public healthcare system in Hong Kong: an analysis of over 1 million antihypertensive prescriptions between 2004–2007 as an example of the potential and pitfalls of using routinely collected electronic patient data. BMC Health Serv Res. 2008;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fung V, Cheung N, Szeto K, Ngai L, Lau M, Kong J. Hospital authority clinical vocabulary table–the past, the present, and the future. 2004 International Federation of Health Records Organization Congress Proceedings 2004.


