Abstract
Background
Transcatheter pulmonary valve implantation is approved for the treatment of dysfunctional right ventricle to pulmonary artery conduits. However, the literature is limited because of a small patient population, and it does not reflect changing procedural practice patterns over the last decade.
Methods and Results
A comprehensive search of Medline and Scopus databases from inception through August 31, 2016 was conducted using predefined criteria. We included studies reporting transcatheter pulmonary valve implantation in at least 5 patients with a follow‐up duration of 6 months or more. In 19 eligible studies, 1044 patients underwent transcatheter pulmonary valve implantation with a pooled follow‐up of 2271 person‐years. Procedural success rate was 96.2% (95% confidence intervals [CI], 94.6–97.4) with a conduit rupture rate of 4.1% (95% CI, 2.5–6.8) and coronary complication rate of 1.3% (95% CI, 0.7–2.3). Incidence of reintervention was 4.4 per 100 person‐years overall (95% CI, 3.0–5.9) with a marked reduction in studies reporting ≥75% prestenting (2.9 per 100 person‐years [95% CI, 1.5–4.3] versus 6.5/100 person‐years [95% CI, 4.6–8.5]; P<0.01). Pooled endocarditis rate was 1.4 per 100 person‐years (95% CI, 0.9–2.0).
Conclusions
Our study provides favorable updated estimates of procedural and follow‐up outcomes after transcatheter pulmonary valve implantation. Widespread adoption of prestenting has improved longer‐term outcomes in these patients.
Keywords: endocarditis, Melody valve, reintervention, transcatheter pulmonary valve
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Meta Analysis, Congenital Heart Disease
Clinical Perspective
What Is New?
Pooled data for >1000 patients suggest improving longevity for transcatheter pulmonary valve implantation, especially with universal adoption of pre‐stenting and low rates of endocarditis.
What Are the Clinical Implications?
The study provides updated estimates for procedural risk as well as medium‐term outcomes which should facilitate decision making by physicians and patients/families alike.
Introduction
Transcatheter pulmonary valve implantation (TPVI) was first performed by Bonhoeffer et al1 in 2000. Bonhoeffer's work led to the development of the Melody Transcatheter Pulmonary Valve (Medtronic Inc, Minneapolis, MN). The Melody valve received US Food and Drug Administration approval under Humanitarian Device Exemption (HDE) in 2010 and Pre‐Market Approval in 2015 for the treatment of right ventricular outflow tract (RVOT) conduit dysfunction. Use of the Melody valve has also been reported in off‐label, nonconduit cases in both small series2, 3 and cohort studies including both conduit and native RVOT implantation. More recently, the ES‐THV (Edwards Lifesciences LLC, Irvine, CA) has been implanted off‐label in the pulmonary valve (PV) position, and the Sapien XT (Edwards Lifesciences LLC, Irvine, California) valve has been approved by the US Food and Drug Administration for use in dysfunctional right ventricle to pulmonary artery (PA) conduits.
In the last 16 years, multiple reports of outcomes of patients post‐TPVI with the Melody valve and ES‐THV have been published; however, the largest series is comprised of only 155 patients.4 The longest duration of follow‐up was a median period of 4.6 years.5 In comparison, an estimated 50 000 adults underwent transcatheter aortic valve implantation between 2002 and 2012.6 Randomized studies in various populations of adult degenerative aortic stenosis are feasible and have been performed comparing transcatheter aortic valve implantion to surgical aortic valve replacement.7, 8, 9, 10 Because of patient numbers and disease heterogeneity, randomized trials for TPVI are not feasible; therefore, to improve the body of knowledge of the short‐ and long‐term outcomes post‐TPVI, a systematic statistical review and meta‐analyses of published observational reports is performed.
Methods
The study was reviewed and approved by the University of Alabama at Birmingham Institutional Review Board (Birmingham, AL).
Search Strategy
We queried the Medline and SCOPUS databases from inception until August 31, 2016 with the following MeSH terms: “Melody valve,” “transcatheter pulmonary valve,” “percutaneous pulmonary valve,” and “transcutaneous pulmonary valve.”
Study Characteristics
All observational studies, including case series and abstracts, were considered. Of these, studies that reported outcomes post‐TPVI in at least 5 patients with a minimum of 6 months follow‐up were included. There were instances of multiple publications from the same research group with overlapping patient populations. Caution was exercised in this scenario to carefully select only the publication with largest sample size and longest follow‐up. Two authors (A.C., M.A.L.) independently reviewed studies with overlapping authors/institutions, and studies were included by mutual consensus. Figure 1 shows the relevant details of the study selection process.
Figure 1.
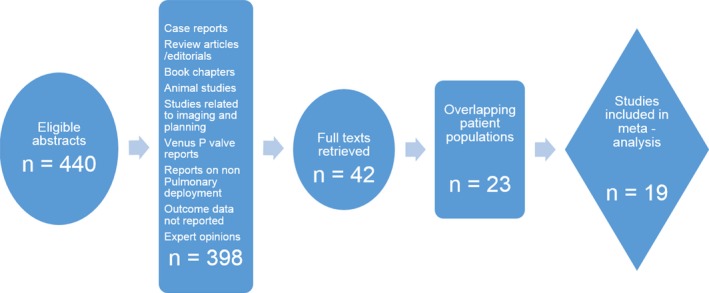
Flow diagram showing selection of studies for analysis.
Data Extraction
Two authors (A.C., J.S.W.) searched all titles and associated abstracts using the described search strategy. Full‐text articles for all potentially relevant studies were extracted and reviewed with focus on inclusion criteria and outcome reporting. From these articles, studies were selected for inclusion. The data from these studies were extracted into prestructured forms and reviewed for accuracy and validity by 2 authors (A.C., M.A.L.). Disagreements were resolved with mutual consensus between authors.
Outcome Measures
We analyzed the data primarily to evaluate procedural success of TPVI and to estimate rate of reintervention post‐TPVI. Definition of “success” was variable in the studies; however, it was most commonly defined as deployment of a transcatheter pulmonary valve (TPV) with acceptable gradient (<30 mm Hg peak to peak) and regurgitation (≤mild) without a need for surgical conversion before discharge. Reinterventions included catheter‐based intervention (balloon angioplasty/valvuloplasty or TPVI) on the TPV or surgical right ventricle to PA conduit replacement.
Secondary outcomes evaluated included procedural complications, survival postprocedure, and incidence of endocarditis in follow‐up. Endocarditis was defined as bloodstream infection classified in the respective study as possible or definite by the modified Duke criteria.11 Endocarditis events were further analyzed as TPV‐specific endocarditis (vegetation visualized on TPV or new dysfunction of TPV) and endocarditis leading to reintervention, explantation, or death. Additionally, a subgroup analysis was performed to ascertain relationship of prestenting with need for reintervention. To evaluate effect of prestenting, studies were divided into 2 groups—1 with ≥75% patients prestented before valve deployment and the other with <75% patients prestented. This cut off was chosen to reflect the change in practice in the United States pre‐ and post‐HDE approval of the Melody valve as reported by Armstrong et al.12
Statistical Analysis
Systematic and statistical analyses were carried out with Comprehensive Meta‐Analysis (version 3; Biostat, Englewood, NJ). Pooled event rates were computed using standard methods. Procedural outcomes are reported as percentages whereas follow‐up outcomes are reported as events per 100 person‐years (PY) of follow‐up. Heterogeneity was assessed with the I2 test (I2 >50 and Q‐statistic, P<0.05 implying substantial heterogeneity).13 Random‐effects modeling was utilized to account for studies’ heterogeneity and observational nature. Publication bias was assessed using the funnel plot method (Figure 2). All of the P values were 2‐tailed, with statistical significance specified at P<0.05 and confidence intervals (CI) computed at the 95% level. The analysis has been reported in accord with the PRISMA guidelines.14
Figure 2.
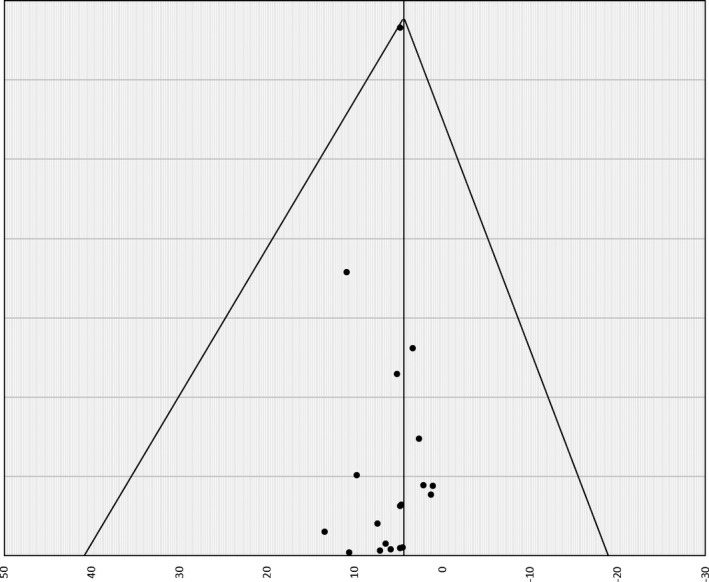
Funnel plot of rate of reinterventions using the SE method (95% CI).
Results
Nineteen observational studies2, 3, 4, 5, 12, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 met inclusion criteria and were included in the final analysis. Table 1 illustrates the baseline characteristics of the included studies. A total of 1044 patients underwent attempted TPVI. The pooled analysis yielded a follow‐up of 2271 PY. Thirteen studies solely used the Melody valve, 3 reported use of Edwards Sapien or Sapien XT THV, and 3 studies used both Melody and Edwards valve systems. Of the latter 3, Faza et al21 reported separate outcomes for Melody and Edwards cohorts. The Edwards cohort had overlapping patients with the study reported by Kenny et al.24 Hence, only the data for the Melody valve cohort were included for analysis from this article. Our data include TPVI attempted with the Melody valve system in 942 patients and Edwards THV system in 102 patients. Seven studies included at least 1 patient with a nonconduit RVOT whereas 2 studies reported solely on TPVI in patients with nonconduit RVOTs.
Table 1.
Studies Included and Demographic/Clinical Factors of Patients
| First Author, Year (Ref) | Patients Considered/Attempted | Age (y) | Median Follow‐up (y) | Valve Used | Primary Pathology | Native RVOT (Yes/No/Only) | Indication | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TOF/PA/PS | Ross | TGA/Truncus | Other | Stenosis | Regurg | Mixed | ||||||
| Wilson, 201527 | NA/25 | 34.0 | 3.5a | E | 17 | 6 | 2 | 0 | No | 8 | 7 | 10 |
| Cheatham, 201528 | 167/150 | 19.0 | 4.5 | M | 80 | 31 | 31 | 8 | No | 39 | 80 | 31 |
| Cools, 201519 | NA/111 | 19.3 | 2.4 | M | 71 | 22 | 18 | 0 | Yes | 49 | 36 | 26 |
| Jalal, 201523 | NA/10 | 16.3 | 0.9a | M | 7 | 0 | 2 | 1 | Yes | 3 | 2 | 5 |
| Biernacka, 201516 | 42/40 | 25.4 | 1.7a | M/E | 30 | 5 | 4 | 1 | Yes | 19 | 21 | 0 |
| Borik, 201517 | NA/51 | 20.2 | 4.2 | M | 35 | 5 | 10 | 1 | No | NA | NA | NA |
| Berman, 201415 | 25/24 | 8.0 | 1.3 | M | 18 | 2 | 5 | 0 | No | 5 | 7 | 13 |
| Armstrong, 201412 | 120/101 | 19.9 | 0.9 | M | 61 | 20 | 28 | 11 | No | 16 | 52 | 32 |
| Fraisse, 20145 | NA/64 | 21.4 | 4.6 | M | 35 | 8 | 15 | 6 | No | 14 | 7 | 43 |
| Meadows, 20143 | NA/31 | 24.0 | 1.3 | M | 28 | 0 | 1 | 2 | Only | 3 | 14 | 14 |
| Malekzadeh‐Milani, 20142 | NA/34 | 26.0 | 2.6 | M | 26 | 2 | 2 | 4 | Only | 18 | 16 | 0 |
| Faza, 201321 | NA/13 | 20.9 | 0.5 | M | 9 | 0 | 2 | 2 | No | 7 | 3 | 3 |
| Odemis, 201326 | NA/10 | 19.2 | 0.8 | M/E | NA | NA | NA | NA | Yes | NA | NA | NA |
| Butera, 201318 | 63/61 | 24.0 | 2.5 | M | 27 | 9 | 12 | 15 | No | 21 | 12 | 30 |
| Haas, 201322 | NA/22 | 21.7 | 0.5a | E | 13 | 2 | 4 | 3 | Yes | 2 | 11 | 9 |
| Eicken, 201120 | NA/102 | 21.5 | 1.0 | M | 61 | 8 | 23 | 10 | Yes | 36 | 18 | 48 |
| Kenny, 201124 | 36/34 | 30.3 | 0.5 | E | 16 | 11 | 1 | 8 | No | 15 | 19 | 2 |
| Martins, 201025 | 7/6 | 19.0 | 0.7 | M | 3 | 0 | 4 | 0 | No | 0 | 0 | 7 |
| Lurz, 20084 | 163/155 | 21.2 | 2.4 | M | 94 | 12 | 31 | 18 | Yes | 61 | 46 | 44 |
E indicates Edwards; M, melody; NA, not applicable; PA/PS, pulmonic atresia/stenosis; Ref, reference number; RVOT, right ventricular outflow tract; TGA, transposition of great vessels; TOF, tetralogy of fallot.
Mean follow‐up.
Patient Selection for TPVI
Eight studies4, 12, 15, 16, 18, 24, 25, 28 elaborated on the patient cardiac catheterization selection process before attempting TPVI. The pooled estimate of patients considered ineligible for TPVI was 7.7% (95% CI, 4.8–12.2%). In total, 52 of 623 patients were screened out as not suitable for TPVI. Reasons for exclusion included the following: coronary compression on balloon testing (n=18; 2.9%); conduit size not suitable for TPVI (n=18; 2.9%); conduit function better than anticipated on hemodynamic catheterization (n=8; 1.2%), branch PA stenosis (n=2; 0.3%) an exclusion criteria in early trials29; conduit stenosis adequately relieved by angioplasty alone (n=4; 0.6%); surgery needed for other congenital heart defects (n=1; 0.2%); and unspecified (n=1; 0.2%).
Procedural Outcomes
Success and procedural complications
Overall, 1019 of 1044 patients had a successful TPVI, yielding a pooled procedural success rate of 96.2% (95% CI, 94.6–97.4). Pooled procedural mortality was 1.5% (95% CI, 0.8–2.6%). There were a total of 5 procedural deaths. Two deaths were secondary to coronary compression or dissection whereas the other 3 patient deaths occurred when TPVI was utilized in a compassionate salvage procedure and mortality was likely not secondary to an adverse outcome of TPVI.
The adjusted pooled rate for total short‐term complications was 10.1% (95% CI, 7.3–13.8). The most frequent complication reported was conduit rupture: 4.1% (95% CI, 2.5–6.8). A total of 32 conduit ruptures were reported. The majority of ruptures were treated with covered stent and/or valve deployment. Only 5 required surgical repair. Other frequently observed complications were access‐site complications requiring blood transfusion or vascular surgery: 2.8% (95% CI, 1.7–4.6) and dislodgement of stent/valve system: 2.5% (95% CI, 1.3–4.8). Table 2 lists rates of all procedural complications. Notably, despite prescreening for coronary compression in most protocols, 5 patients had a coronary complication from either the bare metal stent used for prestenting or the valve system itself (pooled incidence rate, 1.3%; 95% CI, 0.7–2.3).
Table 2.
Pooled Estimates for Short‐Term Procedural Outcomes
| No. of Studies | Pooled Estimate (%) | I2 | P Value | |
|---|---|---|---|---|
| Procedural success | 19 | 96.2 (94.6–97.4) | 0.0 | 0.48 |
| Total complications | 17 | 10.1 (7.3–13.8) | 52.1 | 0.01 |
| Death | 19 | 1.5 (0.8–2.6) | 0.0 | 0.98 |
| Access complications | 16 | 2.8 (1.7–4.6) | 0.0 | 0.50 |
| Coronary compression/dissection | 17 | 1.3 (0.7–2.3) | 0.0 | 1.00 |
| Conduit rupture | 17 | 4.1 (2.5–6.8) | 43.6 | 0.03 |
| Pulmonary artery perforation/laceration | 17 | 2.1 (1.3–3.5) | 0.0 | 0.85 |
| Stent/valve dislodgement | 17 | 2.5 (1.3–4.8) | 38.5 | 0.05 |
| Surgical conversion | 18 | 3.4 (2.3–5.2) | 7.8 | 0.36 |
Surgical conversion for the management of complications occurred in 3.4% of cases (95% CI, 2.3–5.2). Reasons for surgical conversion were stent/valve dislodgement in 9 cases, conduit rupture in 5 cases, coronary compression in 4 cases, obstructed branch PA in 1 case, and stent compression causing acutely elevated gradient in another case.
Follow‐up Outcomes
Death
The adjusted pooled rate for death following discharge after a successful TPVI was 0.6 per 100 PY (95% CI, 0.3–0.9). There were a total of 17 deaths reported. Four of these were attributed to sepsis/endocarditis, 2 were secondary to progressive right ventricle dysfunction despite TPVI, 7 were considered to be unrelated to TPVI, whereas 4 were unexplained and may have been caused by arrhythmia.
Stent fractures
Reporting of stent fractures was not uniform across the studies included. Twelve studies reported on total and significant stent fractures, 2 reported total fractures only, whereas 2 others reported on significant stent fractures only (assumed to be either type 2 or 3 stent fractures per the Nordemeyer classification).29 Pooled incidence of all stent fractures was 4.4 per 100 PY (95% CI, 2.4–6.3) whereas type 2/3 stent fractures were noted in 1.3 per 100 PY (95% CI, 0.5–2.0).
Reintervention
Overall, 4.4 patients required a RVOT reintervention per 100 PY of follow‐up (95% CI, 3.0–5.9). Breaking these down, catheter‐based reinterventions accounted for 2.7 events per 100 PY (95% CI, 1.7–3.7) whereas adjusted rate for surgical reinterventions was 1.7 per 100 PY (95% CI, 1.2–2.2). There is significant heterogeneity in the studies in this regard, which is negated once we analyze studies after adoption of prestenting (Figure 3).2, 3, 4, 5, 12, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28 In total, 77.5% of reinterventions were done for restenosis, 15.2% for endocarditis, only 1.8% for valve insufficiency, and 5.2% for other indications.
Figure 3.
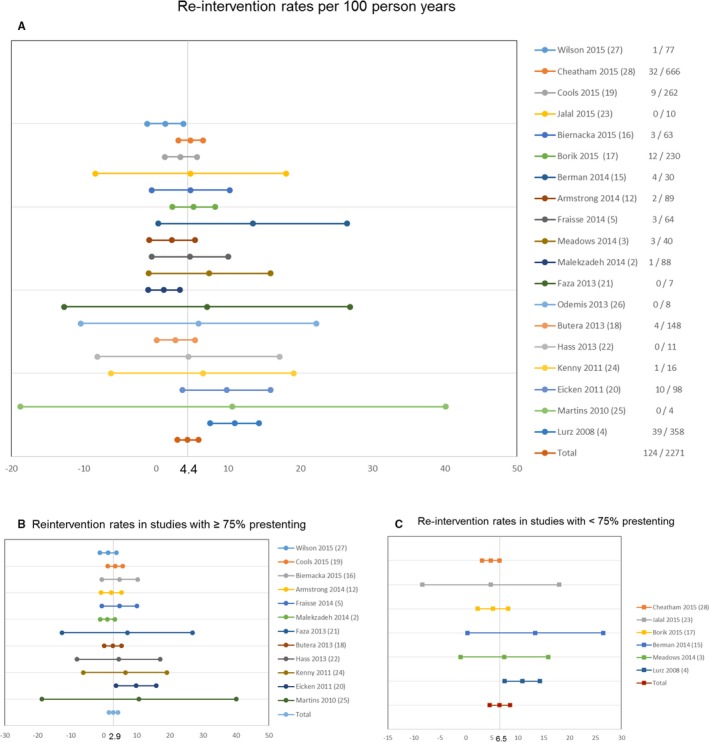
Forest plots depicting reintervention rates in the total pooled sample (A)—individual study event rates are noted in the right column (events/follow‐up in person‐years); reintervention rates contrasted among studies with <75% (B) and ≥75% presenting rates (C).
Effect of prestenting on stent fractures and reintervention
Studies with ≥75% prestenting had a much lower total rate of stent fractures (2.3 versus 7.2 per 100 PY; P<0.01). Reintervention was also less frequent in this group: 2.9 per 100 PY (95% CI, 1.5–4.3) as compared with 6.5 per 100 PY (95% CI, 4.6–8.5) for the studies with <75% prestenting (P<0.01). This difference persisted if we analyze catheter based or surgical reinterventions separately (Table 3).
Table 3.
Pooled Estimates for Outcomes in Follow‐up
| Parameter | No. of Studies | Events/100 PY | Lower CI | Upper CI | I2 | P Value |
|---|---|---|---|---|---|---|
| Death | 19 | 0.6 | 0.3 | 0.9 | 0.0 | 0.95 |
| Stent fracture | 14 | 4.4 | 2.4 | 6.3 | 79.7 | 0.00 |
| Type 2/3 stent fracture | 15 | 1.3 | 0.5 | 2.0 | 53.3 | <0.01 |
| Patients requiring reintervention | 19 | 4.4 | 3.0 | 5.9 | 51.4 | <0.01 |
| Catheter re‐interventions | 19 | 2.7 | 1.7 | 3.7 | 44.7 | 0.02 |
| Surgical reintervention | 19 | 1.7 | 1.2 | 2.2 | 0.0 | 0.56 |
| Endocarditis | 19 | 1.4 | 0.9 | 2.0 | 11.9 | 0.31 |
| TPV specific endocarditis | 19 | 0.6 | 0.3 | 0.9 | 0.0 | 0.70 |
| Endocarditis requiring explantation/death/reintervention | 19 | 0.6 | 0.3 | 0.9 | 0.0 | 0.88 |
| Effect of prestenting | ||||||
| Stent fracture | <0.01a | |||||
| ≥75% prestenting | 8 | 2.3 | 0.8 | 3.7 | 57.7 | 0.02 |
| <75% prestenting | 5 | 7.2 | 5.0 | 9.3 | 26.9 | 0.24 |
| Type 2/3 stent fracture | 0.01a | |||||
| ≥75% prestenting | 9 | 0.6 | 0 | 1.2 | 0.0 | 0.91 |
| ≤75% prestenting | 5 | 2.3 | 1.4 | 3.2 | 60.5 | 0.04 |
| Patients requiring reintervention | <0.01a | |||||
| ≥75% prestenting | 12 | 2.9 | 1.5 | 4.3 | 0 | 0.46 |
| <75% prestenting | 6 | 6.5 | 4.6 | 8.5 | 56.5 | 0.04 |
| Catheter reinterventions | <0.01a | |||||
| ≥75% prestenting | 12 | 1.5 | 0.7 | 2.3 | 1.5 | 0.43 |
| <75% prestenting | 6 | 4.4 | 3.3 | 5.5 | 0.0 | 0.56 |
| Surgical reinterventions | <0.01a | |||||
| ≥75% prestenting | 12 | 1.3 | 0.5 | 2.0 | 0.0 | 0.81 |
| <75% prestenting | 6 | 2.2 | 1.4 | 3.0 | 35.7 | 0.17 |
PY indicates person‐years; TPV, transcatheter pulmonary valve.
P value for subgroup analysis.
Endocarditis
Pooled incidence rate of endocarditis in these 19 studies was 1.4 per 100 PY (95% CI, 0.9–2.0). TPV‐specific endocarditis (vegetation involving TPV or new TPV dysfunction) and endocarditis requiring reintervention/TPV explantation or causing death occurred at a rate of 0.6 per 100 PY (95% CI, 0.3–0.9).
Discussion
Our meta‐analysis is the first attempt to systematically pool high‐quality observational studies to provide an estimate of procedural as well as follow‐up outcomes. We report pooled outcomes of more than 1000 patients with a follow‐up of 2271 PY. Because 20% of patients with congenital heart disease have RVOT or PV dysfunction,30 many patients undergo surgical RVOT conduit or PV placements during their lifetime and will need revisions with age. TPVI is an attractive option to minimize the need for repeated cardiac surgery for these patients. Although several studies have reported on the safety and feasibility of TPVI with excellent short‐term results4, 18, 28 and improvement in cardiopulmonary exercise capacity and quality of life,31, 32 direct comparisons between surgical valve replacement and TPVI have not been made. European Society of Cardiology guidelines33 do not distinguish between surgical and catheter interventions. American societies give TPVI a Class IIa recommendation.34 The pooled data from this meta‐analysis support TPVI as a highly successful intervention to treat RVOT dysfunction with an acceptable complication profile and low reintervention rate.
Procedural Success and Complications
The procedural success for TPVI is greater than 96%, with a complication rate of 10%. Our pooled estimate for procedural success is consistent with the high rates reported in the US IDE trial28 and postapproval study12 as well as the larger European studies.4, 19, 20 Failed TPVI procedures were commonly associated with a complication requiring surgical conversion, which occurred in over 3% of cases. We believe that procedural experience focusing on decreasing or treating these known complications will further increase TPVI success rates and decrease the frequency of surgical conversion.
Conduit rupture is a significant complication of TPVI. Berman et al15 reported a very high rate of 20.8% conduit rupture, although this study solely reported on a specific patient population weighing <30 kg with stenotic homograft conduits. In another series of 99 RVOT interventions, which included 76 TPVI procedures, homografts had a higher frequency of rupture.35 Interestingly, neither conduit size nor type/size of balloon used was associated with risk of rupture. This study classified conduit rupture severity as absent (grade 0), minimal rupture without extension (grade 1), moderate rupture with extension but contained and without hemodynamic instability (grade 2), and severe rupture with extension causing hemothorax or hemopericardium and with hemodynamic instability requiring blood transfusion (grade 3). Only 2 of 9 conduit ruptures reported were grade 3, and these were managed without surgery. Similarly, very few of the 32 conduit ruptures in our pooled analysis required surgical conversion, mostly managed with covered stents and/or valve implantation. The pooled rate of conduit rupture (4.1%) is comparable with Armstrong et al,12 who found an incidence of 5.9%.
Notable other procedural complications with important implications for the TPVI procedure are dislodgement of the stent/valve apparatus and PA perforation. Although these complications can sometimes be managed with catheter techniques, this meta‐analysis demonstrates that inability to retrieve or stabilize the dislodged/malpositioned valve or control PA bleeding results in surgical conversion. Six of the 9 valve dislodgements in our pooled sample were Edwards THV. Although determining whether this finding is significant is beyond the scope of this report, however, off‐label implantation of the Edwards THV into patched or native RVOT may be related to increased risk of valve dislodgement. Similarly, we have insufficient information to conclude if nonconduit Melody implants carry a higher risk of the same. Reports of Sapien use for TPVI are from early use of the device and growing familiarity and technical expertise may obviate this difference.
Eligibility for TPVI
Not all patients needing PV replacement are candidates for TPVI. Our pooled estimate of 7.7% of potential TPVI candidates found at the time of the procedure to be ineligible is comparable to both the US Melody IDE trial (10.2%)28 and the largest European study by Lurz et al (4.9%).4 The post–market approval study by Armstrong et al had a higher exclusion rate of 15.8%.12 It is important to point out that studies elaborating on the patient selection details predominantly did not include native RVOT candidates, hence this estimate should not be extrapolated to nonconduit implants. Because surgical conversion or death are predictable outcomes of coronary compression, this finding during balloon testing is a contraindication to TPVI. As expected, this is the most common reason for patient exclusion from TPVI. The occurrence of coronary compression on balloon testing (raw, 2.9%; pooled estimate, 3.7%) was similar to the 5% reported by Morray et al.36 They studied 404 patients considered for TPVI from 4 large‐volume centers included in US IDE trial and found that a large proportion of patients with coronary compression on balloon testing had anomalous coronary artery anatomy (15 of 21). The finding in this meta‐analysis supports the practice of careful preprocedural and procedural coronary artery imaging and balloon testing for coronary compression before prestenting or valve implantation.
Branch PA stenosis and unsuitable conduit size were other common reasons for exclusion. Two patients in our pooled sample were excluded because of distal PA stenosis, which would not have been an exclusion criterion in current clinical practice where concurrent intervention could be performed, if indicated. Unsuitable conduit size was another common reason for exclusion. Theoretically, the Melody valve mounted on a 24‐mm balloon or the Edwards THV could be implanted in some patients who were excluded secondary to large conduit or RVOT size, potentially decreasing the patient exclusion rate.
Reintervention
There is lack of data on the longevity of the Melody valve. Cheatham et al recently published a rate of 76±4% freedom from reintervention at 5 years28 in the US IDE trial cohort. They found higher rates of reintervention in patients with homografts, without prestenting, a higher post‐TPVI RVOT gradient (>25 mm Hg) and preimplantation moderate‐to‐severe tricuspid valve insufficiency. Our pooled rate of 4.8 per 100 PY also reflects a similar outcome. However, implantation practices have changed over the last decade and now place significant emphasis on prestenting with bare metal stents. Only 10% of cases were prestented in the first tertile of the US IDE trial.28 In comparison, the postapproval study by Armstrong et al observed prestenting in 76 of 101 patients.12 Similarly, Cools et al and Fraisse et al report prestenting rates of 96.4% and 96.9%, respectively.5, 19 Schievano et al, in their in vitro modeling study, showed that a stent‐in‐stent technique decreased mechanical stress on the TPV stent struts.37 There are increasing data to support that prestenting reduces stent fractures and hence reduces the need of reintervention. A recent meta‐analysis investigated the relationship between prestenting and stent fractures pooling data from 5 studies.38 Prestenting was associated with a 2‐fold reduction in all stent fractures and a 6‐fold reduction in type II/III stent fractures. However, the pooled sample only included 360 patients, of whom 207 were prestented. This study also showed a reduction in RVOT reintervention in prestented patients in a subgroup analysis including 191 patients only with 1 versus 6 reinterventions in prestented and non‐pre‐stented patients.
With a lack of clinical data regarding prestenting/stent fractures and reinterventions, we chose to analyze the pooled data in a different manner. Adopting Armstrong et al's postapproval study12 as a reflection of common clinical practice, we divided the studies into 2 groups—≥75% prestenting and <75% prestenting. We found a markedly lower rate of stent fractures (in total and type 2/3) as well as reintervention rates. The reduction in reintervention rates persisted for both catheter and surgical reinterventions. The rates of reintervention in the first group are more comparable to rates reported for surgical conduits and homografts.39 Thus, TPVI seems to be bridging the gap in longevity compared with surgical conduits as practice patterns have become more refined and standardized.
Endocarditis
Bloodstream infections have been recognized as a late complication post‐TPVI. Varying rates for endocarditis have been reported in different cohorts of patients. McElhinney et al reported an annualized rate of first episode of endocarditis and TPV‐related endocarditis of 2.4% per PY and 0.88% per PY, respectively.40 This was derived from 3 patient cohorts in North America and Europe and had a cumulative follow‐up of 687.1 PY. Malekzadeh‐Milani et al, in their study of 86 patients followed for a mean duration of 23.6±15 months, reported a rate of 5.8% (5 cases).41 Interestingly, all 5 of these cases could be classified as TPV‐related endocarditis. Buber et al reported a rate of 9.5% (14 cases) in their 147 patient study over a median follow‐up period of 19 months.42 Whereas vegetations were only noted on 4 valves, all 14 cases had a new elevation in RVOT gradient. The pooled rates for endocarditis and TPV‐specific endocarditis estimated in our study are considerably lower, with a cumulative follow‐up of over 2200 PY.
It is thought that TPVI in a smaller conduit is a risk for endocarditis given a higher residual gradient.40, 43 Also, a majority of cases reported have been associated with predisposing conditions, including noncompliance with antibiotic prophylaxis regimen with dental or other invasive procedures, and concomitant bacterial infection.4, 18, 40 Role of aspirin discontinuation has also been reported.41 In retrospective comparisons of Melody valve versus surgical RVOT conduits, Melody valves have been noted to have a higher rate of endocarditis.44, 45 The difference is pronounced when comparing Melody valves with homografts, but not for Contegra bovine valved conduit, suggesting possible tropism of micro‐organisms to the bovine material used in making the Melody and Contegra valves.45
Limitations
The primary study limitations are inherent to the meta‐analysis study design. Systematic pooling of different observational studies with differences in baseline characteristics, anatomic substrate, sample sizes, implantation techniques, valve type, and different follow‐up durations introduce imprecision in the results because of heterogeneity. Many studies included have small sample sizes and follow‐up durations and may lead to falsely low outcome rates, especially for endocarditis. There are variations in reporting how many patients were screened but considered ineligible for TPVI. Under‐reporting of outcomes has to be taken into account given that all of the data included are observational. We concede that there is variability in how data are presented in each study, criteria for implant, and adverse event reporting are also nonuniform. There may be under‐reporting of endocarditis, especially in the earlier studies. Moreover, there has been refinement of the procedure and improved outcomes as operators have become more experienced. It should be kept in mind that results from this analysis cannot be extrapolated to long‐term outcomes.
Because TPVI is now considered standard of care, randomized controlled trials proving the safety and efficacy of TPVI versus surgical right ventricle to PA conduit or PV implantation are not feasible. Furthermore, small sample size limitations in congenital heart disease makes pooling of multiple observational studies a logical approach to increase the evidence to support interventional therapies and outcomes. Thus, in spite of the inherent limitations, this meta‐analysis can clarify extensive and sometimes conflicting data by systematic organization and aggregation of available observational data. We hope that the pooled estimates can be used as a “worst‐case scenario,” especially given that it includes a significant number of patients from the early years of TPVI.
Conclusion
In our systematic review and meta‐analyses pooling studies reporting TPVI outcomes on conduit as well as nonconduit RVOTs with Melody and Edwards THV systems, TPVI was found to have an outstanding procedural success rate with an acceptable complication profile and low need for surgical conversion. We also demonstrate corroborative evidence of decrease in stent fractures and RVOT reinterventions with increase in adoption of prestenting with bare metal stents. Our analysis reports lower rates of infective endocarditis and TPV‐related endocarditis than previously reported; however, this finding should be evaluated with caution, given the chance of under‐reporting of events in observational data and the potentially catastrophic effects of TPV endocarditis. More experience needs to be gained with nonconduit RVOT TPVI procedures as well as with using the newer generations of Edwards Sapien XT and Sapien 3 systems to draw concrete conclusions about the same.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e006432 DOI: 10.1161/JAHA.117.006432.)28778940
References
- 1. Bonhoeffer P, Boudjemline Y, Saliba Z, Merckx J, Aggoun Y, Bonnet D, Acar P, Le Bidois J, Sidi D, Kachaner J. Percutaneous replacement of pulmonary valve in a right‐ventricle to pulmonary‐artery prosthetic conduit with valve dysfunction. Lancet. 2000;356:1403–1405. [DOI] [PubMed] [Google Scholar]
- 2. Malekzadeh‐Milani S, Ladouceur M, Cohen S, Iserin L, Boudjemline Y. Results of transcatheter pulmonary valvulation in native or patched right ventricular outflow tracts. Arch Cardiovasc Dis. 2014;107:592–598. [DOI] [PubMed] [Google Scholar]
- 3. Meadows JJ, Moore PM, Berman DP, Cheatham JP, Cheatham SL, Porras D, Gillespie MJ, Rome JJ, Zahn EM, McElhinney DB. Use and performance of the Melody Transcatheter Pulmonary Valve in native and postsurgical, nonconduit right ventricular outflow tracts. Circ Cardiovasc Interv. 2014;7:374–380. [DOI] [PubMed] [Google Scholar]
- 4. Lurz P, Coats L, Khambadkone S, Nordmeyer J, Boudjemline Y, Schievano S, Muthurangu V, Lee TY, Parenzan G, Derrick G, Cullen S, Walker F, Tsang V, Deanfield J, Taylor AM, Bonhoeffer P. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117:1964–1972. [DOI] [PubMed] [Google Scholar]
- 5. Fraisse A, Aldebert P, Malekzadeh‐Milani S, Thambo JB, Piéchaud JF, Aucoururier P, Chatelier G, Bonnet D, Iserin L, Bonello B, Assaidi A, Kammache I, Boudjemline Y. Melody (R) transcatheter pulmonary valve implantation: results from a French registry. Arch Cardiovasc Dis. 2014;107:607–614. [DOI] [PubMed] [Google Scholar]
- 6. Binder RK, Webb JG. TAVI: from home‐made prosthesis to global interventional phenomenon. Heart. 2012;98:30–36. [DOI] [PubMed] [Google Scholar]
- 7. Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J Jr, Kleiman NS, Chetcuti S, Heiser J, Merhi W, Zorn G, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte J, Maini B, Mumtaz M, Chenoweth S, Oh JK; U.S. CoreValve Clinical Investigators . Transcatheter aortic‐valve replacement with a self‐expanding prosthesis. N Engl J Med. 2014;370:1790–1798. [DOI] [PubMed] [Google Scholar]
- 8. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S; PARTNER Trial Investigators . Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 9. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG; PARTNER 2 Investigators . Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 10. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ; PARTNER Trial Investigators . Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med. 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 11. Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Bashore T, Corey GR. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638. [DOI] [PubMed] [Google Scholar]
- 12. Armstrong AK, Balzer DT, Cabalka AK, Gray RG, Javois AJ, Moore JW, Rome JJ, Turner DR, Zellers TM, Kreutzer J. One‐year follow‐up of the Melody transcatheter pulmonary valve multicenter post‐approval study. JACC Cardiovasc Interv. 2014;7:1254–1262. [DOI] [PubMed] [Google Scholar]
- 13. Higgins JPT, Green S. eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. Available at: www.handbook.cochrane.org. Accessed June 1, 2016. [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 15. Berman DP, McElhinney DB, Vincent JA, Hellenbrand WE, Zahn EM. Feasibility and short‐term outcomes of percutaneous transcatheter pulmonary valve replacement in small (<30 kg) children with dysfunctional right ventricular outflow tract conduits. Circ Cardiovasc Interv. 2014;7:142–148. [DOI] [PubMed] [Google Scholar]
- 16. Biernacka EK, Ruzyllo W, Demkow M, Kowalski M, Śpiewak M, Piotrowski W, Kuśmierczyk M, Banaś S, Różanski J, Hoffman P. Transcatheter pulmonary valve implantation in patients with right ventricular outflow tract dysfunction: early and mid‐term results. J Invasive Cardiol. 2015;27:E82–E89. [PubMed] [Google Scholar]
- 17. Borik S, Crean A, Horlick E, Osten M, Lee KJ, Chaturvedi R, Friedberg MK, McCrindle BW, Manlhiot C, Benson L. Percutaneous pulmonary valve implantation: 5 years of follow‐up: does age influence outcomes? Circ Cardiovasc Interv. 2015;8:e001745. [DOI] [PubMed] [Google Scholar]
- 18. Butera G, Milanesi O, Spadoni I, Piazza L, Donti A, Ricci C, Agnoletti G, Pangrazi A, Chessa M, Carminati M. Melody transcatheter pulmonary valve implantation. Results from the registry of the Italian Society of Pediatric Cardiology. Catheter Cardiovasc Interv. 2013;81:310–316. [DOI] [PubMed] [Google Scholar]
- 19. Cools B, Budts W, Heying R, Boshoff D, Eyskens B, Frerich S, Troost E, Gewillig M. Medium term follow‐up after percutaneous pulmonary valve replacement with the Melody® valve. IJC Heart Vasc. 2015;7:92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eicken A, Ewert P, Hager A, Peters B, Fratz S, Kuehne T, Busch R, Hess J, Berger F. Percutaneous pulmonary valve implantation: two‐centre experience with more than 100 patients. Eur Heart J. 2011;32:1260–1265. [DOI] [PubMed] [Google Scholar]
- 21. Faza N, Kenny D, Kavinsky C, Amin Z, Heitschmidt M, Hijazi ZM. Single‐center comparative outcomes of the Edwards SAPIEN and Medtronic Melody transcatheter heart valves in the pulmonary position. Catheter Cardiovasc Interv. 2013;82:E535–E541. [DOI] [PubMed] [Google Scholar]
- 22. Haas NA, Moysich A, Neudorf U, Mortezaeian H, Abdel‐Wahab M, Schneider H, De Wolf D, Petit J, Narayanswami S, Laser KT, Sandica E. Percutaneous implantation of the Edwards SAPIEN( ™ ) pulmonic valve: initial results in the first 22 patients. Clin Res Cardiol. 2013;102:119–128. [DOI] [PubMed] [Google Scholar]
- 23. Jalal Z, Malekzadeh‐Milani S, Hofbeck M, Al Najashi K, Thambo JB, Boudjemline Y. A new percutaneous pulmonary valve implantation technique for complex right ventricular outflow tracts: the “folded melody valve”. Catheter Cardiovasc Interv. 2015;85:604–610. [DOI] [PubMed] [Google Scholar]
- 24. Kenny D, Hijazi ZM, Kar S, Rhodes J, Mullen M, Makkar R, Shirali G, Fogel M, Fahey J, Heitschmidt MG, Cain C. Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: early phase 1 results from an international multicenter clinical trial. J Am Coll Cardiol. 2011;58:2248–2256. [DOI] [PubMed] [Google Scholar]
- 25. Martins JD, Ewert P, Sousa L, Freitas I, Trigo C, Jalles N, Matos P, Agapito A, Ferreira R, Pinto FF. Percutaneous pulmonary valve implantation: initial experience. Rev Port Cardiol. 2010;29:1839–1846. [PubMed] [Google Scholar]
- 26. Odemis E, Guzeltas A, Saygi M, Bakir I. Percutaneous pulmonary valve implantation; first experiences from Turkey. Anadolu Kardiyol Derg. 2013;13:409–410. [DOI] [PubMed] [Google Scholar]
- 27. Wilson WM, Benson LN, Osten MD, Shah A, Horlick EM. Transcatheter pulmonary valve replacement with the Edwards Sapien system: the Toronto experience. JACC Cardiovasc Interv. 2015;8:1819–1827. [DOI] [PubMed] [Google Scholar]
- 28. Cheatham JP, Hellenbrand WE, Zahn EM, Jones TK, Berman DP, Vincent JA, McElhinney DB. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015;131:1960–1970. [DOI] [PubMed] [Google Scholar]
- 29. Nordmeyer J, Khambadkone S, Coats L, Schievano S, Lurz P, Parenzan G, Taylor AM, Lock JE, Bonhoeffer P. Risk stratification, systematic classification, and anticipatory management strategies for stent fracture after percutaneous pulmonary valve implantation. Circulation. 2007;115:1392–1397. [DOI] [PubMed] [Google Scholar]
- 30. McElhinney DB, Hennesen JT. The Melody(R) valve and Ensemble(R) delivery system for transcatheter pulmonary valve replacement. Ann N Y Acad Sci. 2013;1291:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Batra AS, McElhinney DB, Wang W, Zakheim R, Garofano RP, Daniels C, Yung D, Cooper DM, Rhodes J. Cardiopulmonary exercise function among patients undergoing transcatheter pulmonary valve implantation in the US Melody valve investigational trial. Am Heart J. 2012;163:280–287. [DOI] [PubMed] [Google Scholar]
- 32. Muller J, Engelhardt A, Fratz S, Eicken A, Ewert P, Hager A. Improved exercise performance and quality of life after percutaneous pulmonary valve implantation. Int J Cardiol. 2014;173:388–392. [DOI] [PubMed] [Google Scholar]
- 33. Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, Gatzoulis MA, Gohlke‐Baerwolf C, Kaemmerer H, Kilner P, Meijboom F, Mulder BJ, Oechslin E, Oliver JM, Serraf A, Szatmari A, Thaulow E, Vouhe PR, Walma E; Task Force on the Management of Grown‐up Congenital Heart Disease of the European Society of Cardiology (ESC); Association for European Paediatric Cardiology (AEPC); ESC Committee for Practice Guidelines (CPG) . ESC guidelines for the management of grown‐up congenital heart disease (new version 2010). Eur Heart J. 2010;31:2915–2957. [DOI] [PubMed] [Google Scholar]
- 34. Feltes TF, Bacha E, Beekman RH III, Cheatham JP, Feinstein JA, Gomes AS, Hijazi ZM, Ing FF, de Moor M, Morrow WR, Mullins CE, Taubert KA, Zahn EM. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2607–2652. [DOI] [PubMed] [Google Scholar]
- 35. Boudjemline Y, Malekzadeh‐Milani S, Patel M, Thambo JB, Bonnet D, Iserin L, Fraisse A. Predictors and outcomes of right ventricular outflow tract conduit rupture during percutaneous pulmonary valve implantation: a multicentre study. EuroIntervention. 2016;11:1053–1062. [DOI] [PubMed] [Google Scholar]
- 36. Morray BH, McElhinney DB, Cheatham JP, Zahn EM, Berman DP, Sullivan PM, Lock JE, Jones TK. Risk of coronary artery compression among patients referred for transcatheter pulmonary valve implantation: a multicenter experience. Circ Cardiovasc Interv. 2013;6:535–542. [DOI] [PubMed] [Google Scholar]
- 37. Schievano S, Petrini L, Migliavacca F, Coats L, Nordmeyer J, Lurz P, Khambadkone S, Taylor AM, Dubini G, Bonhoeffer P. Finite element analysis of stent deployment: understanding stent fracture in percutaneous pulmonary valve implantation. J Interv Cardiol. 2007;20:546–554. [DOI] [PubMed] [Google Scholar]
- 38. Cardoso R, Ansari M, Garcia D, Sandhu S, Brinster D, Piazza N. Prestenting for prevention of melody valve stent fractures: a systematic review and meta‐analysis. Catheter Cardiovasc Interv. 2016;87:534–539. [DOI] [PubMed] [Google Scholar]
- 39. Batlivala SP, Emani S, Mayer JE, McElhinney DB. Pulmonary valve replacement function in adolescents: a comparison of bioprosthetic valves and homograft conduits. Ann Thorac Surg. 2012;93:2007–2016. [DOI] [PubMed] [Google Scholar]
- 40. McElhinney DB, Benson LN, Eicken A, Kreutzer J, Padera RF, Zahn EM. Infective endocarditis after transcatheter pulmonary valve replacement using the Melody valve: combined results of 3 prospective North American and European studies. Circ Cardiovasc Interv. 2013;6:292–300. [DOI] [PubMed] [Google Scholar]
- 41. Malekzadeh‐Milani S, Ladouceur M, Patel M, Boughenou FM, Iserin L, Bonnet D, Boudjemline Y. Incidence and predictors of Melody(R) valve endocarditis: a prospective study. Arch Cardiovasc Dis. 2015;108:97–106. [DOI] [PubMed] [Google Scholar]
- 42. Buber J, Bergersen L, Lock JE, Gauvreau K, Esch JJ, Landzberg MJ, Valente AM, Sandora TJ, Marshall AC. Bloodstream infections occurring in patients with percutaneously implanted bioprosthetic pulmonary valve: a single‐center experience. Circ Cardiovasc Interv. 2013;6:301–310. [DOI] [PubMed] [Google Scholar]
- 43. Patel M, Iserin L, Bonnet D, Boudjemline Y. Atypical malignant late infective endocarditis of Melody valve. J Thorac Cardiovasc Surg. 2012;143:e32–e35. [DOI] [PubMed] [Google Scholar]
- 44. O'Donnell C, Holloway R, Tilton E, Stirling J, Finucane K, Wilson N. Infective endocarditis following Melody valve implantation: comparison with a surgical cohort. Cardiol Young. 2017;27:294–301. [DOI] [PubMed] [Google Scholar]
- 45. Van Dijck I, Budts W, Cools B, Eyskens B, Boshoff DE, Heying R, Frerich S, Vanagt WY, Troost E, Gewillig M. Infective endocarditis of a transcatheter pulmonary valve in comparison with surgical implants. Heart. 2015;101:788–793. [DOI] [PubMed] [Google Scholar]


