Abstract
There is compelling evidence that the eosinophils bring negative biological outcomes in several diseases, including eosinophilic asthma and hypereosinophilic syndromes. Eosinophils produce and store a broad range of toxic proteins and other mediators that enhance the inflammatory response and lead to tissue damage. For instance, in asthma, there is a close relationship between increased lung eosinophilia, asthma exacerbation, and loss of lung function. The use of an anti-IL-5 therapy in severe eosinophilic asthmatic patients is efficient to reduce exacerbations. However, anti-IL-5-treated patients still display a relatively high amount of functional lung tissue eosinophils, indicating that supplemental therapies are required to damper the eosinophil functions. Our recent published works, suggest that compared to IL-5, IL-3 can more strongly and differentially affect eosinophil functions. In this review, we will summarize our and other investigations that have compared the effects of the three β-chain receptor cytokines (IL-5, GM-CSF and IL-3) on eosinophil biology. We will focus on how IL-3 differentially activates eosinophils compared to IL-5 or GM-CSF.
Keywords: IL-3, IL-5, GM-CSF, beta-chain cytokine receptors, eosinophils
INTRODUCTION
Mature eosinophils are non-dividing leukocytes that are recruited to tissues in response to parasites, allergens, and solid tumors, among other causes. Tissue eosinophils are implicated in harmful outcomes in a variety of diseases, including allergy, asthma and hypereosinophilic syndromes. Eosinophils perform their damaging functions through degranulation and the release of intracellularly stored toxic granule proteins and a variety of cytokines.1 IL-3, IL-5, and GM-CSF are critical cytokines involved in eosinophil development and biology. These three cytokines trigger intracellular signals via a common β-chain (βc) receptor, and yet can differentially affect eosinophil functions. Multiple, potential reasons may explain these differential properties among IL-3, IL-5, and GM-CSF. Obvious explanations are the regulation and trafficking of their specific α-chain receptors on the eosinophil surface, and their specific downstream intracellular signaling in a βc-chain-independent manner.2 We and others have shown that IL-3 is more potent than IL-5 or GM-CSF to increase eosinophil proteins, including CD48, CD13, semaphorin-7A and CD32.3–6 In addition, we have shown that when combined with TNF-α, IL-3 was more effective than IL-5 or GM-CSF to increase the expression level of a group of genes, including MMP-9, activin-A and the thymic stromal lymphopoietin receptor (TSLPR).7–9 Therefore, among the βc cytokines, IL-3 possesses unique characteristics to activate eosinophils.
Because IL-5 receptor alpha (IL5Rα) expression is typically limited to eosinophils and basophils, IL-5 is an ideal therapeutic target to reduce eosinophilia. Anti-IL-5 biologics (mepolizumab/Nucala® and reslizumab/Cinqair®) have been recently approved to treat severe eosinophilic asthma.10, 11 The use of anti-IL-5 therapies reduces asthma exacerbations by ~50% and allows reduction of high dose corticosteroid maintenance therapy in severe asthmatic patients with persistent eosinophilia. Unfortunately, these therapeutics do not completely block all exacerbations and are not effective in all patients with eosinophilic asthma.12, 13 This may be due, in part, to the limited effect of IL-5-neutralizing antibodies on airway eosinophils.14 Thus, it is important to study the mechanisms of action of other eosinophil-activating cytokines, such as IL-3. In this article, we will review how the three βc-chain cytokines differentially impact eosinophil biology and how their specific α-chain receptors are regulated on the cell surface. We will discuss critical biological functions for the eosinophil, including cell differentiation, survival, adhesion, degranulation, and migration. Finally, we will review what is known regarding the mechanisms involved in prolonged signaling and specific protein translation in IL-3-activated eosinophils. For the most part, we have limited our discussion to human eosinophils, as there are considerable differences between human and mouse eosinophils.15 that are beyond the scope of this review. A number of the studies reviewed are from the late 1980’s to early 1990’s because these earlier works concomitantly studied all three cytokines, thus allowing a better comparison of their differential functions.
I. SOURCE OF IL-3 AND ITS RELEVANCE IN EOSINOPHILIC ASTHMA
In addition to its effects on myeloid progenitors and macrophages, IL-3 activates eosinophils and basophils,16 which are key players in allergic inflammation. The main cellular sources of IL-3 are T cells and mast cells.17 In fact, all three βc family cytokines are produced by ex vivo-activated blood18 and airway19 T cells obtained from allergic asthmatics. Mast cells also secrete IL-3 and GM-CSF after allergen-mediated IgE stimulation.20 Interestingly, eosinophils and neutrophils can release preformed or newly synthetized IL-3 between 1 and 24 hours after activation with phorbol myristate (PMA).21
Along with IL-5 and GM-CSF, IL-3 was detected by immunohistochemistry in nasal polyps; however, levels of IL-3 were comparatively lower in tissue homogenates.22 In a report by Koller et al., an IL-3 sputum level of >30 ng/ml was measured in patients with cystic fibrosis and bronchial asthma, while IL-3 was undetectable in subjects with pneumonia.23 In addition, the amounts of sputum IL-3 strongly correlated with levels of eosinophil granule proteins, and with decreased lung function.23 In asthma, polymorphisms in the IL-3 gene have been associated with disease development.22, 24 IL-3 is elevated in serum from poorly controlled asthmatic patients;25 and, in a population of symptomatic asthmatics, IL-3 positive cells in the bronchoalveolar lavage (BAL) were associated with asthma severity.26 Of note, in addition to subjects with airway allergy or asthma, subjects with eosinophilic esophagitis also have elevated levels of IL-3 in their blood plasma.27 Together with IL-4, IL-5 and GM-CSF, IL-3 transcript elevation was observed in skin biopsies during an allergen-induced late-phase cutaneous reaction.28 Additionally, a local airway allergen challenge in mild asthma leads to increased levels of IL-3 in the BAL fluid.5, 29 Finally, and importantly, while airway eosinophils display reduced surface α-chain for the IL-5 receptor, they show increased surface α-chain for the IL-3 receptor compared to blood eosinophils.30, 31
II. COMMONALITIES AND DIFFERENCES AMONG IL-3, IL-5, AND GM-CSF EFFECTS ON EOSINOPHILS
A. Differentiation
Human eosinophils originate from CD34+ pluripotent hematopoietic stem cells (HSC) located in the red bone marrow. Downstream from the HSC, common myeloid progenitors (CMP) will differentiate into either granulocyte/macrophage progenitors (GMP), megakaryocyte/erythrocyte progenitors (MEP) or CD34+IL-5Rα+ eosinophil lineage-committed progenitors (EoP).32 These CD34+IL-5Rα+ EoP are the exclusive origin of mature human eosinophils and their numbers are increased in the bone marrow, circulation and tissues of patients with eosinophilic diseases.33 At the transcriptional level, the differentiation of CMP into CD34+IL-5Rα+ EoP requires downregulation of the GATA-1-binding transcription factor FOG1, allowing increased GATA1 function.34 PU.1 (SPI1)35 is then required for the final maturation into eosinophils. In addition, X-box binding protein 1 (XBP1) is a critical requirement for survival and terminal differentiation of murine EoP.36
EoP express other key receptors required for its development including IL-3Rα and GM-CSFRα, and the granule proteins eosinophil peroxidase (EPX) and major basic protein 1 (MBP).37 Therefore, IL-3, IL-5, and GM-CSF can all participate in EoP proliferation and differentiation until the mature non-proliferative stage is reached. IL-5 quickly matures eosinophils from the bone marrow, suggesting that IL-5 activates differentiation from existing eosinophil progenitor cells, rather than from stem cells. It is generally thought that IL-3 and GM-CSF are crucial in the early stage of eosinophil differentiation from CD34+ cells while IL-5 is required for their final maturation.38, 39 Yet, IL-5 deficient mice40–42 and anti-IL-5-treated humans14 retain a limited number of eosinophils. This suggests that the maturation of eosinophils does not require IL-5 and that eosinophilopoiesis can occur in an IL-5-independent manner. Pre-incubation of mouse bone marrow cells with IL-3 (not IL-5, GM-CSF, C-CSF, or CSF-1) significantly increases the numbers of eosinophils produced upon subsequence activation by IL-5.43 The combination of IL-3 plus IL-1 during the pre-incubation stage further enhances the number of IL-5-responsive eosinophil progenitors.43 IL-5 activation of human bone marrow induces colonies of highly pure and mature eosinophils, while IL-3 or GM-CSF induces 5 times more colonies that are comprised of less than 20% mature eosinophils and colonies that are contaminated with 5% neutrophils and basophils.44 In another study, human bone marrow cultured with IL-5 in liquid medium displayed 38% and 72% of differentiated eosinophils after 14 days and 28 days, respectively.45 At 21 days, contaminants found were surviving cells already present at the origin of the culture, including macrophages (10%) and lymphocytes/monocytes/neutrophil myelocytes (11%). In this same study, the addition of IL-3 to progenitors in semi-solid medium led to more eosinophil colonies compared to IL-5 or GM-CSF; although, in long-term (3 weeks) liquid medium cultures, IL-5 differentiated more eosinophils than IL-3, and much more than GM-CSF.45 This suggests that IL-5 activates less precursor types present in the bone marrow compared to IL-3, but it is more potent for the stimulation of EoP proliferation. Unfortunately, beyond recording eosinophil differentiation, these studies did not analyze the biology and functions of eosinophils differentiated in media lacking IL-5. For instance, it is legitimate to ask whether eosinophils matured without exogenous IL-5 are biologically similar to eosinophils matured with IL-5.
B. Survival
The number of eosinophils in the airways is associated with asthma severity.46 Delayed apoptosis and necrosis are required for eosinophil accumulation in tissues and are thus important factors in eosinophil pathogenesis. All three of the βc family cytokines (IL-3, IL-5, and GM-CSF) attenuate eosinophil death, particularly apoptosis.47 In a model of mouse bone marrow-derived eosinophils, IL-3 and GM-CSF showed little activity to generate eosinophils compared to IL-5.48 However, IL-5 and GM-CSF were equivalent in their ability to maintain the viability of differentiated eosinophils, while IL-3 was somewhat less effective.48 It was also reported that GM-CSF was more potent than IL-3 and IL-5 to prolong ex vivo eosinophil survival for 4 days.49 In another study, increasing concentrations of the three cytokines were used on blood eosinophils for 48 hours with no significant differences among the cytokines on cell survival, although IL-3 seemed a little inferior to IL-5 or GM-CSF for concentrations >100 pg/ml.50 In agreement with this study, we have recently published data showing human blood eosinophil survival in the presence of either of the three βc family cytokines.31 The cytokines were used at 2 ng/ml (~130 pM) and survival was determined from 2 to 16 days. IL-5, IL-3 and GM-CSF had a very similar effect on eosinophil survival during the first 6 days in culture. Longer-term cultures (12 days) demonstrated that GM-CSF is significantly superior to IL-5 or IL-3 in promoting survival.31 However, according to cytokine consumption analyses in this same study, IL-5 consumption by eosinophils is minimal over time. In contrast, as much as 2 ng of IL-3 was consumed by eosinophils in 48 hours, whereas only ~200 pg/ml of the initial 2 ng/ml of GM-CSF was used.31 In fact, while ~2 ng/ml of IL-5 were still present after 3 days of culture, IL-3 was already totally consumed. Therefore, the rapid consumption of IL-3 should be taken into account when comparing the effect of the three βc family cytokines on eosinophil survival, and studies examining eosinophil survival should compare the ability of the cytokines to prolong eosinophil survival at a constant concentration over time. In accordance with our observations, Ohnishi et al. had shown that any of the three βc family cytokines used at a very low dose (20 pg/ml) increased blood eosinophil survival from ~5% to ~80% after 4 days in culture.51 In a study by Tai et al., blood eosinophils from patients with hypereosinophilic syndrome were incubated for 10 days with IL-5, GM-CSF or IL-3.52 The authors concluded that all three cytokines significantly prolong eosinophil survival with IL-5>GM-CSF>IL-3. However, IL-5 was used at 0.5 ng/ml, whereas GM-CSF and IL-3 were used at a very high concentration (~150 ng/ml). It is our experience that high concentrations of cytokines (≥10ng/ml), can trigger eosinophil cytolysis. Therefore, under these conditions, it is uncertain whether IL-5 was more potent than IL-3 and GM-CSF to maintain eosinophil survival, or whether eosinophil lysis was induced by the high concentration of IL-3 and GM-CSF. In conclusion, it is safe to say that as long as they remain present in culture at a moderate level (≤2 ng/ml), all three βc family cytokines have a very similar effect on eosinophil survival.
C. Adhesion/Chemotactism/Migration
Adhesion is an important event in eosinophil biology. By means of eosinophil interaction with endothelial cells, adhesion directs the early step for eosinophil recruitment into the tissue, preceding extravasation.53 It is generally accepted that in vivo, expression of the α4β1 integrin on eosinophils interacting with the adhesion molecule VCAM-1 present on endothelial cells is critical for eosinophil recruitment into tissues.53 Ip et al. demonstrated that adhesion of eosinophils to epithelial cells was increased by IL-3 and IL-5, but not by GM-CSF.54 It may be important to note that, in this study, IL-3 and IL-5 were used at 20 ng/ml while GM-CSF was used at an even higher concentration (50 ng/ml). In another model, the effect of βc family cytokines on human blood eosinophil adhesion to serum-treated zymosan (STZ) coated with complement fragment iC3b was measured.55 All three cytokines strongly and rapidly (within 5 minutes) increased eosinophil adhesion to STZ via enhanced surface presence of the complement receptor type 3 (CR3).55 Reimert et al. showed that eosinophil adhesion to human serum albumin-coated wells was induced to a similar extent by all three cytokines.56 Of note, adhesion was CD18 (integrin β2)-dependent. Fattah et al. reported that all three cytokines increased eosinophil binding to IgG, ICAM-1 or VCAM-1, with IL-3 being about 10-fold less potent than either IL-5 or GM-CSF.57 This last study seems to be in disagreement with the work by Ip et al., although Ip used 20 ng/ml of cytokines, and at this high concentration, Fattah also observed no difference among the three cytokines to stimulate eosinophil adhesion on ICAM-1 and VCAM-1.
Following eosinophil differentiation in the bone marrow, mature eosinophils must enter the blood stream and migrate to the assaulted tissue. After extravasation through the vascular endothelium, the recruitment of the eosinophils to the inflamed site is directed by chemotactism toward a chemical gradient. Complement C5a, platelet activating factor (PAF), the eicosanoids (leukotriene B4 (LTB4) and prostaglandin D2), and the ligands for CC-chemokine receptor 3 (CCR3) (RANTES, MCP-4, and eotaxin 1–3) are the major chemoattractants for eosinophils.58 Warringa et al. compared chemotactism of eosinophils for 2.5 hours toward PAF, LTB4, C5a and IL-8 after a short pre-activation (30 min) with increasing concentrations of either GM-CSF or IL-3.59 Both GM-CSF and IL-3 increased eosinophil chemotactism toward LTB4 and IL-8, although at different cytokine concentrations. While the optimal concentration of GM-CSF for both LTB4- and IL-8-mediated chemotactism was as low as 15 pg/ml, 10 to 100 fold more IL-3 was required. Interestingly, only IL-3, not GM-CSF, strongly increased eosinophil chemotactism toward PAF, and the effective concentration of IL-3 was as low as 15 pg/ml.59 Of note, GM-CSF and IL-3 themselves were chemotactic for eosinophils during the 2.5 hour assay, although 10-fold more IL-3 (1.5 ng/ml) was required to observed a significant effect. This was confirmed by another paper that showed chemoattractant activity for the three βc family cytokines, with IL-5>GM-CSF>IL-3, on blood eosinophils from subjects having eosinophilia and asthma.60
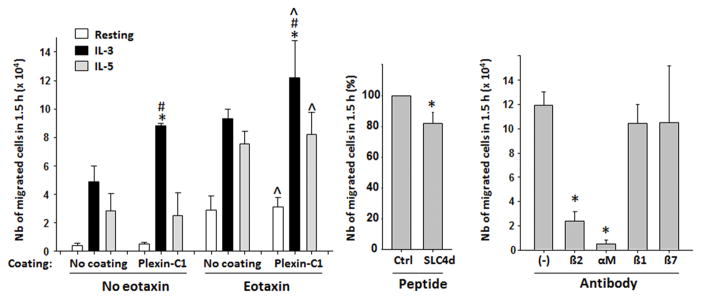
Cell migration is closely related to chemoattraction since chemoattraction leads to cell movement. However, different types of chemoattractants and environmental conditions can induce different types of cell migration. Cells in a liquid phase moving directionally toward an increasing gradient of a chemoattractant, such as PAF or LTB4, define “chemotaxis” while “chemokinesis” is defined by random and non-directional movement. Otherwise, “haptotaxis” is used to describe movement of cells along an insoluble extracellular matrix protein (ECM). We have previously reported that IL-3-activated eosinophils adhere to plexin-C1.5 Plexin-C1 is expressed by stromal cells and is known to only bind to semaphorin-7A.61 We wanted to know whether eosinophils could migrate on plexin-C1, and whether compared to IL-5, IL-3-activated eosinophil migration on plexin-C1 was enhanced. We had previously shown that compared to IL-5 or GM-CSF, IL-3 activation for 20 hours further enhanced semaphorin-7A levels on the eosinophil surface.5 Therefore, we anticipate that compared to IL-5, eosinophil pre-activation with IL-3 for 20 hours would induce greater migration on plexin-C1. As shown on Figure 1A, we found that eosinophil migration on plexin-C1 was increased by IL-3 activation compared to both non-activated cells and IL-5-activated cells. Eosinophil migration was further enhanced by the presence of the chemoattractant, eotaxin, which tended to limit the differential effect of IL-3 compared to IL-5. Migration on plexin-C1 without the requirement of a chemotactic compound (no eotaxin) indicates that eosinophils use a haptotaxic process on plexin-C1, similarly to their migration on an ECM protein such as periostin.62 Although, the only known ligand for plexin-C1 is sempahorin-7A,63 IL-3-activated eosinophil migration on plexin-C1 was significantly, yet only slightly, inhibited by a peptide inhibitor analog to the semaphorin-7A binding site for plexin-C1 (Figure 1B). In contrast, neutralizing antibodies to the αMβ2 integrin almost totally blocked eosinophil migration on plexin-C1 (Figure 1C). It is unknown whether the peptide inhibitor was not efficient in blocking plexin-C1/semphorin-7A interaction, or whether plexin-C1 possesses another ligand besides semaphorin-7A.
Figure 1. IL-3 increases eosinophil migration on plexin-C1.
Peripheral blood eosinophils were activated with IL-3 (2ng/ml), IL-5 (4ng/ml) or were left without cytokine (resting) for 20 h. Cells (3×105) were placed into Transwell inserts (polycarbonate membrane, 3μm pores, 6.5 mm, Corning, Lowee, MA) coated with plexin-C1 (R&D Systems, 10μg/ml, overnight at 4°C) or into uncoated inserts (no coating). Then the inserts were saturated with fetal bovine serum (FBS) for 1 hour at 37°C. Cell culture medium with or without eotaxin (10 nM) was added into the bottom chamber. One hundred fifty μl of 2×106 eosinophils were added to each insert, and the migrated cells in the bottom chamber were counted at 90 min. A/Graph represent an average ±SEM of 3 (no coating, no eotaxin) or 5 experiments. * indicates that IL-3-activated cells migrated more than IL-5-activated cells. # indicates that IL-3-activated eosinophils migrated more on plexin-C1 than on an uncoated membrane. ^ indicates that migration on plexin-C1was increased by eotaxin versus no eotaxin. B/Cells were activated with IL-3 for 20 hours and were added on plexin-C1-coated inserts pre-incubated with either SLC4d (CRGDQGGESSLSVSKWNTF; ProteGenix, France) or the control (Ctrl) peptide (KLGFTYVTIRVTYQIRVAG) (100 nM). The number of migrated cells in 90 min in the presence of SLC4d is a % of the migrated cells with control peptide. Graph is an average ±SEM of 3 experiments. * indicates difference is statistically significant. C/Cells were activated with IL-3 for 20 h, were incubated with the indicated neutralizing antibodies (10μg/ml) for 15 min and were seeded on plexin-C1-coated inserts. Migrated cells in the bottom chamber after 90 min were counted and the graph is an average ±SEM of 3 experiments. * indicates statistical differences from no antibody.
Moser et al. have reported that eosinophils migrate almost equally across a non-activated and IL-1-activated endothelial cell line monolayer after a 24 hour activation of eosinophils, with only 150 pg/ml of IL-5, IL-3 or GM-CSF.64 Yet, IL-3 activation tends to be less potent than IL-5 or GM-CSF. However, as described above, 150 pg/ml of IL-3 is rapidly consumed by eosinophils.31 It would be interesting to compare the effect of cytokine concentration on eosinophil migration. In another study, higher concentrations of the βc family cytokines (5 ng/ml) were added on blood eosinophils during transmigration through a layer of epithelial cells.65 In this condition, all three cytokines equally enhanced eosinophil migration compared to non-activated eosinophils, and increased migration was blocked by an antibody to the β2 integrin.65 Likewise, it has been reported that an addition of either of the three βc family cytokines at the beginning of an eosinophil migration assay through Matrigel® (a gelatinous mixture of ECM proteins such as laminin and collagen), significantly increased eosinophil migration; although, at a lower concentration, IL-3 seemed less efficient than IL-5 or GM-CSF.66
In summary, altogether these studies indicate that all three βc family cytokines increase eosinophil adhesion, chemotactism and migration. Although, at lower doses (~100 pg/ml), IL-3 was less efficient than IL-5 or GM-CSF. The decreased potency of IL-3 was probably partially due to the high consumption of IL-3 by eosinophils and as described later, the requirement of a longer activation time for IL-3 compared to IL-5 or GM-CSF. Yet, the three cytokines display differential effects on adhesion, chemoattraction, and migration depending on the substrate (IgG, VCAM-1, ICAM-1, iC3b or plexin-C1), and the chemoattractant (LTB4, IL-8 or PAF).
D. Degranulation
Degranulation remains the main event by which eosinophils can affect tissue integrity and inflammation. The release of stored mediators including toxic granule proteins, cytokines, chemokines, and growth factors from eosinophil granules may happen when 1) intact granules are discharged due to cell lysis, 2) granule contents are secreted through the cytoplasmic membrane (exocytosis), or 3) specific granule proteins are released by a process known as piecemeal degranulation (reviewed67). The release, or not, of toxic or pro-inflammatory intracellular materials in vivo is closely linked to the mechanism associated with eosinophil death (either apoptosis or necrosis or necroptosis). Apoptotic eosinophils are phagocytosed by macrophages and epithelial cells that digest eosinophil contents, while necrotic and necroptotic68 eosinophils lose membrane integrity and consequently release granules and mediators leading to tissue damage and inflammation. Importantly, eosinophil adhesion via interaction of its integrins (particularly αMβ2) with appropriate ligands is a required step for eosinophil degranulation.69, 70 In Reimert’s study mentioned above, GM-CSF-induced adhesion of eosinophil on albumin- or IgG-coated tissue culture plates preceded degranulation and the release of granule proteins by about 1 hour.56 One of the first in vitro models to study eosinophil degranulation was the binding of eosinophils on aggregated immunoglobulins (IgG or IgA).71 Notably, degranulation on immunoglobulins typically leads to eosinophil lysis and does not involve piecemeal release of cytokines.72 Therefore, the studies using IgG or IgA are exclusively measuring release of toxic proteins, such as EDN. Eosinophils activated with either IL-5, GM-CSF, or IL-3 for 1 hour and then cultured in the presence of secretory IgA for 4 hours release a very similar amount of the granule protein, EDN.73 In another study, eosinophils activated for 4 hours with GM-CSF, IL-5 or IL-3 (10 ng/ml) in human serum albumin-coated tissue culture plates released 12, 8, and 6% of their total EDN content, respectively.74 We have, however, recently shown that blood eosinophils activated for 20 hours with IL-3 and seeded on aggregated human IgG-coated on tissue culture plates, highly degranulate (25 % of total cellular EDN); whereas, activation with IL-5 results in release of only 10% of total cellular EDN.6 As described by us and others, eosinophil degranulation on aggregated IgG is αMβ2 integrin and CD32 (low affinity receptor for IgG; FCγRII)-dependent.6, 75, 76 The more potent ability for IL-3, compared to IL-5 or GM-CSF, to prime eosinophils to degranulate on IgG is probably due to increased production and a prolonged activation state of both the αMβ2 and CD32.6 The notion of a delayed and prolonged effect of IL-3 compared to IL-5 or GM-CSF, and the mechanisms involved in production and prolonged activation of αMβ2 and CD32 in IL-3-activated eosinophils, will be further developed below in the section on “Differential protein translation of semaphorin-7A and CD32 by IL-3”. Therefore, for longer periods of activation, IL-3 seems to be superior to IL-5 or GM-CSF to trigger eosinophil degranulation on immunoglobulins.
E. Gene and protein expression
Compared to IL-5 and GM-CSF, IL-3 is more potent in activating eosinophil’s release of certain proteins. We have shown that, compared to IL-5 or GM-CSF, the combination of IL-3 with TNF-α leads to a high mRNA expression level of several genes, including MMP-9 and activin-A.7, 8 MMP-9 and activin-A mRNA expression levels were dependent on the mRNA stabilization downstream of mitogen-activated protein (MAP) kinase signaling. More than IL-5, but similarly to GM-CSF, IL-3 potently induced newly synthetized CR3 when activation was prolonged for 24 hours.77 Calcium ionophore-induced leukotriene C4 (LTC4) production by blood eosinophils obtained from subjects with moderate-to-severe asthma is potentiated by all three βc family cytokines after 30 min, with IL-5>GM-CSF>IL-3.78 However, while the effect of GM-CSF and IL-5 declined after 1 hour, the effect of IL-3 was delayed but gradually augmented LTC4 release from 1 to 6 hours.78 Interestingly, the ionophore-induced release of LTC4 after incubation with IL-3 for 6 h was observed in eosinophils obtained from subjects with severe asthma, but not eosinophils from normal or mild asthmatic subjects.78
IL-3 has a strong effect on eosinophil surface proteins. Surprisingly, eosinophil activation for 24 hours with IL-3, but not IL-5 or GM-CSF, results in down-regulation of cell surface CCR3, via activation of the phosphatidylinositol-3 kinase (PI3K) pathway and receptor internalization.79 On a longer term (24 h), IL-3 reduced CCR3 mRNA expression levels. In contrast to the down-regulation of CCR3, a short activation (30 min) of blood eosinophils with ≥ 10 ng/ml of cytokines indicates that IL-3 was less effective than IL-5 or GM-CSF to induce eosinophil expression of CD11b (αM)50 However, we found that after 20 hours of activation, IL-3 augments both αM and β2 on the eosinophil surface.6 Similarly, Hartnel et al.,80 showed that compared to IL-5 or GM-CSF, long-term activation (24 h) led to a stronger effect of IL-3 on expression of the activation marker CD69 for cytokine concentrations ranging from 0.5 ng/ml to 10 ng/ml.50 CD13 (ANPEP) is another surface protein strongly increased by a 24 hour activation of eosinophils with IL-3 (1 ng/ml) compared to IL-5 or GM-CSF (both at 1 ng/ml), while CD13 mRNA was not changed.4 Furthermore, at a concentration >2ng/ml, IL-3 was the only of the three βc family cytokines to increase the production of surface CD48 on eosinophils at 18 h.3 Kinetic studies indicate that IL-3-induced CD48 production is significant only after 10 hours,3 suggesting that IL-3-induced IL-3 receptor augmentation may be required. Therefore, unlike IL-5 and GM-CSF, long-term activation with IL-3 enhances the production of a group of proteins that can dramatically impact the function of eosinophils.
In summary, compared to IL-5 and GM-CSF, long-term activation with IL-3 is better suited to induce expression and production of a subset of genes. Potential mechanisms include the regulation and the dynamics of the surface α-chain receptors and differential intracellular signaling, which we discuss in Sections III and IV.
III. THE COMMON BETA-CHAIN CYTOKINE RECEPTORS
IL-3, IL-5, and GM-CSF receptors are heterodimeric with a cytokine-specific α-chain and a common β-chain that initiates intracellular signaling. The cytokines bind to their respective α-chain with low affinity (nanomolar); however, subsequent recruitment of the β-chain results in a conformational change to a high affinity (picomolar) binding complex. βc is unique in that it is displayed as a preformed interlocked homodimer.81 At the point of insertion into the cell membrane the C-terminal tails are separated by over 120 Å, preventing interaction of βc subunits.81 Thus, a dynamic change in the receptor is required for signaling. The crystal structure of the human GM-CSF/GM-CSFRα/βc ternary complex revealed a hexamer structure composed of 2 binary complexes of GM-CSF/GM-CSFRα bound together via a single βc homodimer. Two hexamer complexes are packed head-to-head to form a dodecamer complex, which is required for signaling.82 The dodecamer formation provides a conformational structure that moves the central intracellular βc domains into close proximity (~10 Å versus ~120 Å in the hexamer formation) to enable JAK2 transphosphorylation and initiation of the JAK2/STAT5 signaling pathway.82, 83
The receptors for IL-3, GM-CSF and IL-5 can be controlled by their respective ligand and by other members of the βc family cytokines. IL-3Rα mRNA and protein levels are up-regulated by all three cytokines with GM-CSF>IL-5 or IL-3.31, 84–87 Increased eosinophil IL-3Rα occurs at the transcription level87 and is dependent on activation of the PI3K pathway.86 Of note, whereas IL- 5 or GM-CSF induces a rapid (within 3 h) and transient increase in IL-3Rα mRNA accumulation, the effect of IL-3 is delayed such that accumulation of mRNA does not peak until 24 h.85 This delayed effect of IL-3 is reminiscent of what we have observed for IL-3-mediated up-regulation of genes for activin,7 MMP-98, and IL-1β (unpublished observation). In contrast to their positive effect on IL-3Rα, the three βc cytokines induce a strong and prolonged down-regulation of IL-5Rα on human peripheral blood eosinophils, whereas surface GM-CSFRα remains generally constant.31, 84–87 Down-regulation of IL-5Rα occurs very rapidly (within 30 min) and is cytokine dose-dependent. Corresponding with the nearly undetectable level of cell surface IL-5Rα, eosinophils are no longer able to bind IL-587 or release EDN84 or superoxide86 upon subsequent IL-5 exposure. They do however, remain functionally intact in response to IL-3 or GM-CSF. We demonstrated that attenuation of membrane IL-5Rα (mIL-5Rα) corresponds to eosinophil release of a soluble form of the receptor (sIL-5Rα).84 Inhibition of matrix metalloproteinases (MMPs) reversed both the down-modulation of mIL-5Rα and the increase in sIL-5Rα,84 indicating that proteolytic release of mIL-5Rα may be a mechanism for accumulation of sIL-5Rα. Due to high affinity binding to IL-5, sIL-5Rα is a potent mIL-5Rα antagonist.88 In addition to surface cleavage, sIL-5Rα can also be generated by alternative splicing of the mIL-5Rα gene.88 Transcripts for sIL-5Rα are predominant in early eosinophil progenitors. IL-5 (but not IL-3 or GMCSF) induces a switch to expression of mIL-5Rα.89 Conversely, in mature human eosinophils, IL-5, IL-3, or GM-CSF down-regulate both receptor isoforms.86, 87
Importantly, much of the described ex vivo data have been recapitulated in vivo. We have demonstrated that eosinophils recruited to the airway following an allergen challenge of atopic subjects, have elevated surface levels of GM-CSFRα and IL-3Rα, and significantly less mIL-5Rα than their circulating counterparts.30, 31 The observed decrease in mIL-5Rα on allergen-induced BAL eosinophils has been confirmed by Julius, et al.90 We have also established the presence of sIL-5Rα in BAL fluid of allergic subjects.30 Concentrations were increased after an airway allergen challenge and were associated with increased levels of IL-5. We did not observe up-regulation of sIL-5Rα gene expression on BAL eosinophils compared to circulating eosinophils, suggesting that cell surface shedding of mIL-5Rα in response to IL-5 may contribute to the increase in sIL-5Rα. Gevaert et al. showed that sIL-5 is present in nasal polyp homogenates and that eosinophils from nasal polyps have less cell surface mIL-5Rα than blood eosinophils.91 Another study demonstrated sIL-5Rα in serum of patients with highly elevated circulating eosinophils.92 Concentrations of sIL-5Rα were associated with increased numbers of blood eosinophils and concentrations of serum IL-5.92
Taken together, these in vitro and in vivo studies indicate that IL-5Rα expression and function are regulated by multiple mechanisms including alternative IL-5Rα gene splicing and cell surface shedding of IL-5Rα. Conversely, IL-3Rα expression is increased in activated-eosinophils explaining the differential consequences of IL-3 and IL-5 stimulations.
IV. DIFFERENTIAL PROTEIN TRANSLATION OF SEMAPHORIN-7A AND CD32 BY IL-3
A. The ERK/RSK/RPS6 signaling
The βc family cytokines trigger an intracellular kinase cascade leading to the phosphorylation of a number of intracellular proteins. IL-3, IL-5, and GM-CSF activate (PI3K), the MAPK (ERK and p38) and the signal transducer and activator of transcriptions (STAT) pathways. Just downstream of the ligand-induced β-chain receptor activation, members of the proto-oncogene tyrosine kinase SRC (c-SRC), such as LYN (LCK/YES novel tyrosine kinase), and SYK, can tyrosine phosphorylate a number of intracellular proteins in eosinophils, including the β-chain receptor itself.93 Van der Bruggen et al.94 have analyzed the effect of the three βc family cytokines on general tyrosine phosphorylation in eosinophils. The optimal cytokine concentrations to induce tyrosine phosphorylation after 30 min were 150 pg/ml, 1.5 ng/ml and 15 ng/ml for GM-CSF, IL-5 and IL-3, respectively. At these optimal concentrations, high protein tyrosine phosphorylation was observed by activation with any of the cytokines between 5 and 60 min, but phosphorylation was dampened at the next 120 min time point.94 The weakest effect from IL-3 compared to the other two cytokines on early intracellular signaling was expected for the reason that fresh blood eosinophils possess less IL-3Rα than IL-5Rα and GM-CSFRα.85 Interestingly though, after 1 hour of activation, IL-3 and IL-5 have a very comparable strength to prime eosinophils in order to respond to a second activation with a ligand to a G protein-coupled receptor.95 Unlike for a short-term activation, long-term cytokine activation of eosinophils substantially changes the potential of the three βc family cytokines on eosinophil signaling. We have shown that IL-3 activation leads to a prolonged (> 48 h) phosphorylation of the ribosomal protein S6 (RPS6), downstream of p90S6K (RSK) and ERK phosphorylations, while IL-5 and GM-CSF could not maintain this intracellular signaling for more than a few hours.5 Intriguingly, prolonged signaling by IL-3 compared to IL-5 or GM-CSF has also been reported in basophils.96 Yet, we do not know whether the strong and rapid stimulation of IL-5 and GM-CSF quickly turns on a receptor containing an immunoreceptor tyrosine-based inhibitory motif (ITIM) that would damper the RSK/RPS6 signaling. It has been shown, for instance, that activation of the ITIM-containing receptor, CD300a suppresses some of the IL-5 and GM-CSF effects, including ERK phosphorylation, on eosinophils.97 Another explanation for the prolonged effect of IL-3 on eosinophils, is the enhanced presence of its specific receptor (IL-3Rα) at the cell surface between 4 and 24 hours after the beginning of activation.31, 85 Conversely, cell surface IL-5Rα and GM-CFRα levels are reduced or maintained, respectively, during eosinophil activation.31
B. IL-3 induces translation of a subset of mRNAs in eosinophils
For some genes, including MMP-9, TSLPR and activin-A, a differential effect of IL-3 compared to IL-5 or GM-CSF is not seen unless eosinophils are stimulated with the cytokines plus TNF-α.7–9 In TNF-α plus IL-3-activated eosinophils, the MMP-9 and activin-A mRNA expression levels were highly increased, and mRNA increased levels were controlled at least partially at the post-transcriptional level through increased mRNA stabilization. Conversely, the first protein that we found differentially increased by IL-3 used alone (no TNF-α) was semaphorin-7A, which was not altered at the mRNA expression level, but was enriched in the polyribosome cell compartment indicating increased semaphorin-7A mRNA translatability.5 Downstream of ERK, IL-3-induced increased semaphorin-7A translation was p90S6K-dependent. P90S6K is a well-known substrate for ERK, and is a RPS6-phosphorylating kinase.98 Although it was not evaluated in our study, semaphorin-7A increased translation was also likely RPS6-phosphorylation-dependent. This is because it has been suggested previously, in proliferating cells, that phosphorylation of RPS6 enhances protein synthesis from a subset of mRNAs by stimulating their binding to the 40S ribosomal subunit.99 It is thought that this mechanism involving RPS6 phosphorylation allows rapid translation of pre-existing transcripts. Semaphorin-7A mRNA is abundant in resting eosinophils; however, semaphorin-7A increased translation does not happen during the first 4 hours of activation with IL-3, raising the possibility that in addition to RPS6 phosphorylation, there is another factor requiring long-term cytokine stimulation. Importantly, while IL-3 induced the translation of semaphorin-7A by >10-fold, it increased global translation by less than 1.5-fold compared to GM-CSF.5 This demonstrates that IL-3 strongly targets a subset of mRNAs in eosinophils. Another identified IL-3-mRNA target is the low affinity IgG receptor, CD32.6 In humans, three different genes code for CD32: CD32A (FCGRIIA), CD32B (FCGRIIB) and CD32C (FCGRIIC). Though, FCGRIIA protein amount was not changed by IL-3, we were able to show the up-regulation of FCGRIIB/C protein in eosinophils, exclusively after long-term (20 h) activation with IL-3 (not with IL-5 or GM-CSF).6 In addition, as for semaphorin-7A, CD32B/C was also regulated at the translational level by the RSK pathway. The direct implication of RPS6-phosphorylation on semaphorin-7A and CD32B/C protein translation remains to be demonstrated. Importantly, all of the differential characteristics of in vitro IL-3-activated eosinophils were found in vivo in eosinophils recovered from the airways of allergen challenged patients, including increased surface expression of semaphorin-7A and CD32B/C, and activation of the p90S6K/RPS6 pathway.5, 6, 31 These in vivo data do not prove, yet they do suggest that IL-3 could be involved in these mechanisms in vivo as well.
CONCLUSION
Via their βc-chain receptor subunit, IL-3, IL-5 and GM-CSF have many common functions on eosinophils. The differential effect of IL-3, among the three βc cytokines, seems to be partially directed by the dynamic of their specific α-chain receptor on the cell surface. Freshly prepared blood eosinophils have low levels of surface IL-3Rα, which increases overtime when eosinophils are activated. Due to low level of IL-3Rα on fresh mature eosinophils, functions needing only low doses of cytokine, such as survival, are very similarly affected by either of the three cytokines. However, for functions requiring higher doses of cytokine and higher IL-3/IL-3Rα signaling, IL-3 displays typically weaker effects on short-term incubation. Conversely when eosinophil activation is prolonged (>10 hours), surface IL-3Rα is increased and IL-3 displays more potent effects on most of the eosinophil functions, compared to IL-5 or GM-CSF, which specific receptor decreases or remains at a constant level on the cell surface overtime. Among the critical enhanced functions due to prolonged IL-3-induced eosinophil activation and its consequent prolonged intracellular signaling (MAPK/p90S6K/RPS6), is the translatability of specific transcripts including semaphorin-7A and CD32B/C. Consequently for instance, IL-3-driven CD32 and integrin surface augmentation and activation lead eosinophils to strongly degranulate on IgG. To date, it remains important to identify the exhaustive list of proteins specifically augmented by IL-3 to further acknowledge the dramatic impact of IL-3 on the eosinophil biology. Because IL-3Rα is active on other critical cells besides the eosinophils, it remains important to identify specific mechanisms implicated in IL-3-induced eosinophil functions in order to propose new therapeutic targets to treat eosinophilia.
A summary of the differential functions of IL-3 compared to IL-5 and GM-CSF is shown in Table. 1.
Table 1.
Differential IL-3 functions on eosinophils, compared to IL-5 or GM-CSF
| Functions | Take-home messages |
|---|---|
| Differentiation | IL-3 and GM-CSF are crucial in the early stages, while IL-5 is important for final maturation during eosinophil differentiation. However, maturation can happen without IL-5. Human bone marrow activated with IL-5 forms colonies of highly pure and mature eosinophils, while IL-3 or GM-CSF forms more colonies, but with less mature eosinophils. |
| Survival | All three β-chain cytokines have a very similar effect on eosinophil survival, which requires only a low dose of cytokines. On long–term eosinophil cultures, the high consumption of IL-3 (1 ng of IL-3 per million eosinophils per day) must be taken into account when comparing cytokines. |
| Adhesion | IL-3, IL-5, and GM-CSF have relatively similar effects on eosinophil adhesion. Although, after a short-term activation a low dose of IL-3 may be less potent than IL-5 or GM-CSF to promote adhesion via the integrins. |
| Chemotactism/Migration | Activation with either of the cytokines may have differential effects on eosinophil chemoattraction depending on the chemoattractant. Long-term pre-activation with the cytokines indicates that IL-3 is more potent than IL-5 to induce eosinophil migration on plexin-C1 via semaphorin-7A and the αMβ2 integrin. At ng doses of cytokine for 24 h, IL-3, IL-5 and GM-CSF have similar efficacy to induce eosinophil transmigration through a layer of epithelial cells via the β2 integrin. |
| Degranulation | After a short-term (4 h) activation, eosinophil degranulation is slightly higher for GM-CSF and IL-5, compared to IL-3. However, a longer-term (20 h) activation places IL-3 ahead of the three cytokines via higher production and activation state of αMβ2 integrin and CD32. |
| Protein production | After a long-term activation (~24 h) activation, more potently than IL-5 or GM-CSF, IL-3 induces production of CD11b (αM), CD13, CD32B/C and CD48, CD69, CR3 and ICAM-1. |
| Signaling and Protein Translation | Whereas IL-5 and GM-CSF activate the ERK/p90S6K/RPS6 signaling for a few hours (1 to 6 h), IL-3 prolongs this signaling for >48 h. Subsequently, a subset of transcripts, including CD32B/C and semaphorin-7A are translated into proteins. |
| β-chain Receptor surface expression | Fresh mature blood eosinophils display a lower level of IL-3Rα compared to IL-5Rα and GM-CSFRα on their surface. However, eosinophil activation with either of the three β-chain cytokines leads to a strong increase of the IL-3Rα, maintenance of the GM-CSFRα, and reduction of the IL-5Rα. |
| Summary: the impact of each cytokine on eosinophil biology depends on the function that is analyzed. Although globally, it is safe to say that fresh blood and mature eosinophils possess a low level of IL-3Rα and require a higher dose of IL-3 compared to IL-5 and GM-CSF. Conversely, after >4 h of activation, unlike IL-5Rα and GM-CSFRα, surface IL-3Rα is upregulated, and IL-3 becomes more effective than IL-5 or GM-CSF to enhance some eosinophil functions, such as migration and degranulation. |
Acknowledgments
Funding Source: This work was supported by Program Project grant P01 HL088594 and Clinical and Translational Research Center grant UL1 RR025011 from the National Institutes of Health.
The projects described were supported by the National Institutes of Health Program Project Grant P01 HL088594 and the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Also, we thank Andrea Noll for helping with the revisions of this article.
Abbreviations
- BAL
bronchoalveolar lavage
- βc
β chain
- CCR3
CC-chemokine receptor 3
- EDN
eosinophil-derived neurotoxin
- EOS
eosinophils
- EoP
eosinophil lineage-committed progenitors
- FCGRII
receptor for Fc fragment of IgG, low affinity II
- IL5Rα
IL-5 receptor-α
- HA-IgG
heat aggregated IgG
- LTB4
leukotriene B4
- p90S6K
90-KDa ribosomal S6 kinase
- PAF
platelet activating factor
- SBP-Ag
segmental bronchoprovocation with an allergen
References
- 1.Valent P, Gleich GJ, Reiter A, Roufosse F, Weller PF, Hellmann A, Metzgeroth G, Leiferman KM, Arock M, Sotlar K, Butterfield JH, Cerny-Reiterer S, Mayerhofer M, Vandenberghe P, Haferlach T, Bochner BS, Gotlib J, Horny HP, Simon HU, Klion AD. Pathogenesis and classification of eosinophil disorders: a review of recent developments in the field. Expert Rev Hematol. 2012;5(2):157–176. doi: 10.1586/ehm.11.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geijsen N, Uings IJ, Pals C, Armstrong J, McKinnon M, Raaijmakers JA, Lammers JW, Koenderman L, Coffer PJ. Cytokine-specific transcriptional regulation through an IL-5Ralpha interacting protein. Science. 2001;293(5532):1136–1138. doi: 10.1126/science.1059157. [DOI] [PubMed] [Google Scholar]
- 3.Munitz A, Bachelet I, Eliashar R, Khodoun M, Finkelman FD, Rothenberg ME, Levi-Schaffer F. CD48 is an allergen and IL-3-induced activation molecule on eosinophils. J Immunol. 2006;177(1):77–83. doi: 10.4049/jimmunol.177.1.77. [DOI] [PubMed] [Google Scholar]
- 4.Braun RK, Foerster M, Workalemahu G, Haefner D, Kroegel C, Walker C. Differential regulation of aminopeptidase N (CD13) by transendothelial migration and cytokines on human eosinophils. Exp Lung Res. 2003;29(2):59–77. doi: 10.1080/01902140303766. [DOI] [PubMed] [Google Scholar]
- 5.Esnault S, Kelly EA, Johansson MW, Liu LY, Han S-H, Akhtar M, Sandbo N, Mosher DF, Denlinger LC, Mathur SK, Malter JS, Jarjour NN. Semaphorin 7A is expressed on airway eosinophils and upregulated by IL-5 family cytokines. Clin Immunol. 2014;150(1):90–100. doi: 10.1016/j.clim.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esnault S, Johansson MW, Kelly EA, Koenderman L, Mosher DF, Jarjour NN. IL-3 up-regulates and activates human eosinophil CD32 and alphaMbeta2 integrin causing degranulation. Clin Exp Allergy. 2017;47(4):488–498. doi: 10.1111/cea.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly EA, Esnault S, Johnson SH, Liu LY, Malter JS, Burnham ME, Jarjour NN. Human eosinophil activin A synthesis and mRNA stabilization are induced by the combination of IL-3 plus TNF. Immunol Cell Biol. 2016;94(7):701–708. doi: 10.1038/icb.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly EA, Liu LY, Esnault S, Quinchia Johnson BH, Jarjour NN. Potent synergistic effect of IL-3 and TNF on matrix metalloproteinase 9 generation by human eosinophils. Cytokine. 2012;58(2):199–206. doi: 10.1016/j.cyto.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook EB, Stahl JL, Schwantes EA, Fox KE, Mathur SK. IL-3 and TNFalpha increase thymic stromal lymphopoietin receptor (TSLPR) expression on eosinophils and enhance TSLP-stimulated degranulation. Clin Mol Allergy. 2012;10(1):8. doi: 10.1186/1476-7961-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keating GM. Mepolizumab: First Global Approval. Drugs. 2015;75(18):2163–2169. doi: 10.1007/s40265-015-0513-8. [DOI] [PubMed] [Google Scholar]
- 11.Markham A. Reslizumab: First Global Approval. Drugs. 2016;76(8):907–911. doi: 10.1007/s40265-016-0583-2. [DOI] [PubMed] [Google Scholar]
- 12.Cabon Y, Molinari N, Marin G, Vachier I, Gamez AS, Chanez P, Bourdin A. Comparison of anti-interleukin-5 therapies in patients with severe asthma: global and indirect meta-analyses of randomized placebo-controlled trials. Clin Exp Allergy. 2017;47(1):129–138. doi: 10.1111/cea.12853. [DOI] [PubMed] [Google Scholar]
- 13.Legrand F, Klion AD. Biologic therapies targeting eosinophils: current status and future prospects. J Allergy Clin Immunol Pract. 2015;3(2):167–174. doi: 10.1016/j.jaip.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167(2):199–204. doi: 10.1164/rccm.200208-789OC. [DOI] [PubMed] [Google Scholar]
- 15.Lee JJ, Jacobsen EA, Ochkur SI, McGarry MP, Condjella RM, Doyle AD, Luo H, Zellner KR, Protheroe CA, Willetts L, Lesuer WE, Colbert DC, Helmers RA, Lacy P, Moqbel R, Lee NA. Human versus mouse eosinophils: “that which we call an eosinophil, by any other name would stain as red”. J Allergy Clin Immunol. 2012;130(3):572–584. doi: 10.1016/j.jaci.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, Klunker S, Meyer N, O’Mahony L, Palomares O, Rhyner C, Ouaked N, Schaffartzik A, Van De Veen W, Zeller S, Zimmermann M, Akdis CA. Interleukins, from 1 to 37, and interferon-gamma: receptors, functions, and roles in diseases. J Allergy Clin Immunol. 2011;127(3):701–721. e701–770. doi: 10.1016/j.jaci.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 17.Stoeckle C, Simon HU. CD8(+) T cells producing IL-3 and IL-5 in non-IgE-mediated eosinophilic diseases. Allergy. 2013;68(12):1622–1625. doi: 10.1111/all.12311. [DOI] [PubMed] [Google Scholar]
- 18.Lantero S, Sacco O, Scala C, Rossi GA. Stimulation of blood mononuclear cells of atopic children with the relevant allergen induces the release of eosinophil chemotaxins such as IL-3, IL-5, and GM-CSF. J Asthma. 1997;34(2):141–152. doi: 10.3109/02770909709075659. [DOI] [PubMed] [Google Scholar]
- 19.Till S, Li B, Durham S, Humbert M, Assoufi B, Huston D, Dickason R, Jeannin P, Kay AB, Corrigan C. Secretion of the eosinophil-active cytokines interleukin-5, granulocyte/macrophage colony-stimulating factor and interleukin-3 by bronchoalveolar lavage CD4+ and CD8+ T cell lines in atopic asthmatics, and atopic and non-atopic controls. Eur J Immunol. 1995;25(10):2727–2731. doi: 10.1002/eji.1830251002. [DOI] [PubMed] [Google Scholar]
- 20.Wodnar-Filipowicz A, Heusser CH, Moroni C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature. 1989;339(6220):150–152. doi: 10.1038/339150a0. [DOI] [PubMed] [Google Scholar]
- 21.Kita H, Ohnishi T, Okubo Y, Weiler D, Abrams JS, Gleich GJ. Granulocyte/macrophage colony-stimulating factor and interleukin 3 release from human peripheral blood eosinophils and neutrophils. J Exp Med. 1991;174(3):745–748. doi: 10.1084/jem.174.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen JS, Eisma R, Leonard G, Kreutzer D. Interleukin-3 interleukin-5, and granulocyte-macrophage colony-stimulating factor expression in nasal polyps. Am J Otolaryngol. 1997;18(4):239–246. doi: 10.1016/s0196-0709(97)90003-x. [DOI] [PubMed] [Google Scholar]
- 23.Koller DY, Wojnarowski C, Herkner KR, Weinlander G, Raderer M, Eichler I, Frischer T. High levels of eosinophil cationic protein in wheezing infants predict the development of asthma. J Allergy Clin Immunol. 1997;99(6 Pt 1):752–756. doi: 10.1016/s0091-6749(97)80007-3. [DOI] [PubMed] [Google Scholar]
- 24.Miyake Y, Tanaka K, Arakawa M. IL3 rs40401 polymorphism and interaction with smoking in risk of asthma in Japanese women: the Kyushu Okinawa Maternal and Child Health study. Scand J Immunol. 2014;79(6):410–414. doi: 10.1111/sji.12171. [DOI] [PubMed] [Google Scholar]
- 25.Patil SP, Wisnivesky JP, Busse PJ, Halm EA, Li XM. Detection of immunological biomarkers correlated with asthma control and quality of life measurements in sera from chronic asthmatic patients. Ann Allergy Asthma Immunol. 2011;106(3):205–213. doi: 10.1016/j.anai.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson DS, Ying S, Bentley AM, Meng Q, North J, Durham SR, Kay AB, Hamid Q. Relationships among numbers of bronchoalveolar lavage cells expressing messenger-ribonucleic-acid for cytokines, asthma symptoms, and airway methacholine responsiveness in atopic asthma. J Allergy Clin Immunol. 1993;92(3):397–403. doi: 10.1016/0091-6749(93)90118-Y. [DOI] [PubMed] [Google Scholar]
- 27.Johnsson M, Bove M, Bergquist H, Olsson M, Fornwall S, Hassel K, Wold AE, Wenneras C. Distinctive blood eosinophilic phenotypes and cytokine patterns in eosinophilic esophagitis, inflammatory bowel disease and airway allergy. J Innate Immun. 2011;3(6):594–604. doi: 10.1159/000331326. [DOI] [PubMed] [Google Scholar]
- 28.Kay AB, Ying S, Varney V, Gaga M, Durham SR, Moqbel R, Wardlaw AJ, Hamid Q. Messenger RNA expression of the cytokine gene cluster, interleukin 3 (IL-3), IL-4, IL-5, and granulocyte/macrophage colony-stimulating factor, in allergen-induced late-phase cutaneous reactions in atopic subjects. J Exp Med. 1991;173(3):775–778. doi: 10.1084/jem.173.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson MW, Kelly EA, Busse WW, Jarjour NN, Mosher DF. Up-regulation and activation of eosinophil integrins in blood and airway after segmental lung antigen challenge. J Immunol. 2008;180(11):7622–7635. doi: 10.4049/jimmunol.180.11.7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, Jarjour NN, Busse WW, Kelly EAB. Decreased expression of membrane IL-5R alpha on human eosinophils: I. Loss of membrane IL-5 alpha on eosinophils and increased soluble IL-5R alpha in the airway after antigen challenge. J Immunol. 2002;169(11):6452–6458. doi: 10.4049/jimmunol.169.11.6452. [DOI] [PubMed] [Google Scholar]
- 31.Esnault S, Kelly EA, Shen ZJ, Johansson MW, Malter JS, Jarjour NN. IL-3 maintains activation of the p90S6K/RPS6 pathway and increases translation in human eosinophils. J Immunol. 2015;195(6):2529–2539. doi: 10.4049/jimmunol.1500871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mori Y, Iwasaki H, Kohno K, Yoshimoto G, Kikushige Y, Okeda A, Uike N, Niiro H, Takenaka K, Nagafuji K, Miyamoto T, Harada M, Takatsu K, Akashi K. Identification of the human eosinophil lineage-committed progenitor: revision of phenotypic definition of the human common myeloid progenitor. J Exp Med. 2009;206(1):183–193. doi: 10.1084/jem.20081756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gauvreau GM, Denburg JA. Human mast cell and basophil/eosinophil progenitors. Methods Mol Biol. 2015;1220:59–68. doi: 10.1007/978-1-4939-1568-2_4. [DOI] [PubMed] [Google Scholar]
- 34.Yamaguchi Y, Nishio H, Kishi K, Ackerman SJ, Suda T. C/EBPbeta and GATA-1 synergistically regulate activity of the eosinophil granule major basic protein promoter: implication for C/EBPbeta activity in eosinophil gene expression. Blood. 1999;94(4):1429–1439. [PubMed] [Google Scholar]
- 35.Ackerman SJ, Du J, Xin F, Dekoter R, McKercher S, Maki R, Singh H, Yamaguchi Y. Eosinophilopoiesis. Resp Med. 2000;94(11):1135–1138. doi: 10.1053/rmed.2000.0913. [DOI] [Google Scholar]
- 36.Bettigole SE, Lis R, Adoro S, Lee AH, Spencer LA, Weller PF, Glimcher LH. The transcription factor XBP1 is selectively required for eosinophil differentiation. Nat Immunol. 2015;16(8):829–837. doi: 10.1038/ni.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doyle AD, Jacobsen EA, Ochkur SI, McGarry MP, Shim KG, Nguyen DT, Protheroe C, Colbert D, Kloeber J, Neely J, Shim KP, Dyer KD, Rosenberg HF, Lee JJ, Lee NA. Expression of the secondary granule proteins major basic protein 1 (MBP-1) and eosinophil peroxidase (EPX) is required for eosinophilopoiesis in mice. Blood. 2013;122(5):781–790. doi: 10.1182/blood-2013-01-473405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson DS, Damia R, Zeibecoglou K, Molet S, North J, Yamada T, Kay AB, Hamid Q. CD34(+)/interleukin-5Ralpha messenger RNA+ cells in the bronchial mucosa in asthma: potential airway eosinophil progenitors. Am J Respir Cell Mol Biol. 1999;20(1):9–13. doi: 10.1165/ajrcmb.20.1.3449. [DOI] [PubMed] [Google Scholar]
- 39.Sehmi R, Baatjes AJ, Denburg JA. Hemopoietic progenitor cells and hemopoietic factors: potential targets for treatment of allergic inflammatory diseases. Curr Drug Targets Inflamm Allergy. 2003;2(4):271–278. doi: 10.2174/1568010033484007. [DOI] [PubMed] [Google Scholar]
- 40.Kopf M, Brombacher F, Hodgkin PD, Ramsay AJ, Milbourne EA, Dai WJ, Ovington KS, Behm CA, Kohler G, Young IG, Matthaei KI. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4(1):15–24. doi: 10.1016/s1074-7613(00)80294-0. [DOI] [PubMed] [Google Scholar]
- 41.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183(1):195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corry D, Folkesson H, Warnock M, Erle D, Matthay M, Wiener-Kronish J, Locksley R. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996;183:109–117. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Warren DJ, Moore MA. Synergism among interleukin 1, interleukin 3, and interleukin 5 in the production of eosinophils from primitive hemopoietic stem cells. J Immunol. 1988;140(1):94–99. [PubMed] [Google Scholar]
- 44.Sonoda Y, Arai N, Ogawa M. Humoral regulation of eosinophilopoiesis in vitro: analysis of the targets of interleukin-3, granulocyte/macrophage colony-stimulating factor (GM-CSF), and interleukin-5. Leukemia. 1989;3(1):14–18. [PubMed] [Google Scholar]
- 45.Clutterbuck EJ, Hirst EM, Sanderson CJ. Human interleukin-5 (IL-5) regulates the production of eosinophils in human bone marrow cultures: Comparison and interaction with IL-1, IL-3, IL-6, and GM-CSF. Blood. 1989;73:1504–1512. [PubMed] [Google Scholar]
- 46.Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323(15):1033–1039. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 47.Shen ZJ, Malter JS. Determinants of eosinophil survival and apoptotic cell death. Apoptosis. 2015;20(2):224–234. doi: 10.1007/s10495-014-1072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwartz C, Willebrand R, Huber S, Rupec RA, Wu D, Locksley R, Voehringer D. Eosinophil-specific deletion of IkappaBalpha in mice reveals a critical role of NF-kappaB-induced Bcl-xL for inhibition of apoptosis. Blood. 2015;125(25):3896–3904. doi: 10.1182/blood-2014-10-607788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker C, Virchow JC, Jr, Iff T, Bruijnzeel PL, Blaser K. T cells and asthma. I. Lymphocyte subpopulations and activation in allergic and nonallergic asthma. Int Arch Allergy Appl Immunol. 1991;94(1–4):241–243. [PubMed] [Google Scholar]
- 50.Yoshimura-Uchiyama C, Yamaguchi M, Nagase H, Fujisawa T, Ra C, Matsushima K, Iwata T, Igarashi T, Yamamoto K, Hirai K. Comparative effects of basophil-directed growth factors. Biochem Biophys Res Commun. 2003;302(2):201–206. doi: 10.1016/s0006-291x(03)00153-0. [DOI] [PubMed] [Google Scholar]
- 51.Ohnishi T, Kita H, Mayeno AN, Okada S, Sur S, Broide DH, Gleich GJ. Lidocaine in bronchoalveolar lavage fluid (BALF) is an inhibitor of eosinophil-active cytokines. Clin Exp Immunol. 1996;104(2):325–331. doi: 10.1046/j.1365-2249.1996.32737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tai PC, Sun L, Spry CJ. Effects of IL-5, granulocyte/macrophage colony-stimulating factor (GM-CSF) and IL-3 on the survival of human blood eosinophils in vitro. Clin Exp Immunol. 1991;85(2):312–316. doi: 10.1111/j.1365-2249.1991.tb05725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johansson MW. Activation states of blood eosinophils in asthma. Clin Exp Allergy. 2014;44(4):482–498. doi: 10.1111/cea.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ip WK, Wong CK, Wang CB, Tian YP, Lam CW. Interleukin-3, -5, and granulocyte macrophage colony-stimulating factor induce adhesion and chemotaxis of human eosinophils via p38 mitogen-activated protein kinase and nuclear factor kappaB. Immunopharmacol Immunotoxicol. 2005;27(3):371–393. doi: 10.1080/08923970500240925. [DOI] [PubMed] [Google Scholar]
- 55.Blom M, Tool AT, Kok PT, Koenderman L, Roos D, Verhoeven AJ. Granulocyte-macrophage colony-stimulating factor, interleukin-3 (IL-3), and IL-5 greatly enhance the interaction of human eosinophils with opsonized particles by changing the affinity of complement receptor type 3. Blood. 1994;83(10):2978–2984. [PubMed] [Google Scholar]
- 56.Reimert CM, Skov PS, Poulsen LK. A microtiter assay for activation of eosinophils. Simultaneous monitoring of eosinophil adhesion and degranulation. Allergy. 1998;53(2):129–138. doi: 10.1111/j.1398-9995.1998.tb03860.x. [DOI] [PubMed] [Google Scholar]
- 57.Fattah D, Page KR, Bezbaruah S, Priest RC, Horgan CM, Solari R. A rapid activation assay for human eosinophils based on adhesion to immobilized ICAM-1, VCAM-1 and IgG. Cytokine. 1996;8(3):248–259. doi: 10.1006/cyto.1996.0034. [DOI] [PubMed] [Google Scholar]
- 58.Luna-Gomes T, Bozza PT, Bandeira-Melo C. Eosinophil recruitment and activation: the role of lipid mediators. Front Pharmacol. 2013;4:27. doi: 10.3389/fphar.2013.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warringa RA, Koenderman L, Kok PT, Kreukniet J, Bruijnzeel PL. Modulation and induction of eosinophil chemotaxis by granulocyte-macrophage colony-stimulating factor and interleukin-3. Blood. 1991;77(12):2694–2700. [PubMed] [Google Scholar]
- 60.Sehmi R, Wardlaw AJ, Cromwell O, Kurihara K, Waltmann P, Kay AB. Interleukin-5 selectively enhances the chemotactic response of eosinophils obtained from normal but not eosinophilic subjects. Blood. 1992;79:2952–2959. [PubMed] [Google Scholar]
- 61.Spencer AY, Lallier TE. Mechanical tension alters semaphorin expression in the periodontium. J Periodontol. 2009;80(10):1665–1673. doi: 10.1902/jop.2009.090212. [DOI] [PubMed] [Google Scholar]
- 62.Johansson MW, Annis DS, Mosher DF. alpha(M)beta(2) integrin-mediated adhesion and motility of IL-5-stimulated eosinophils on periostin. Am J Respir Cell Mol Biol. 2013;48(4):503–510. doi: 10.1165/rcmb.2012-0150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, Tessier-Lavigne M, Comoglio PM. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99(1):71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- 64.Moser R, Fehr J, Olgiati L, Bruijnzeel PL. Migration of primed human eosinophils across cytokine-activated endothelial cell monolayers. Blood. 1992;79(11):2937–2945. [PubMed] [Google Scholar]
- 65.Ebisawa M, Liu MC, Yamada T, Kato M, Lichtenstein LM, Bochner BS, Schleimer RP. Eosinophil transendothelial migration induced by cytokines. II. Potentiation of eosinophil transendothelial migration by eosinophil-active cytokines. J Immunol. 1994;152(9):4590–4596. [PubMed] [Google Scholar]
- 66.Okada M, Matsuto T, Miida T, Inano K. Differences in the effects of cytokines on the expression of adhesion molecules in endothelial cells. Ann Med Interne (Paris) 1997;148(2):125–129. [PubMed] [Google Scholar]
- 67.Spencer LA, Bonjour K, Melo RC, Weller PF. Eosinophil secretion of granule-derived cytokines. Front Immunol. 2014;5:496. doi: 10.3389/fimmu.2014.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Radonjic-Hoesli S, Wang X, de Graauw E, Stoeckle C, Styp-Rekowska B, Hlushchuk R, Simon D, Spaeth PJ, Yousefi S, Simon HU. Adhesion-induced eosinophil cytolysis requires the RIPK3-MLKL signaling pathway which is counter-regulated by autophagy. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2017.01.044. [DOI] [PubMed] [Google Scholar]
- 69.Egesten A, Gullberg U, Olsson I, Richter J. Phorbol ester-induced degranulation in adherent human eosinophil granulocytes is dependent on CD11/CD18 leukocyte integrins. J Leukocyte Biol. 1993;53(3):287–293. doi: 10.1002/jlb.53.3.287. [DOI] [PubMed] [Google Scholar]
- 70.Horie S, Kita H. CD11b/CD18 (Mac-1) is required for degranulation of human eosinophils induced by human recombinant granulocyte-macrophage colony-stimulating factor and platelet-activating factor. J Immunol. 1994;152(11):5457–5467. [PubMed] [Google Scholar]
- 71.Abu-Ghazaleh RI, Fujisawa T, Mestecky J, Kyle RA, Gleich GJ. IgA-induced eosinophil degranulation. J Immunol. 1989;142(7):2393–2400. [PubMed] [Google Scholar]
- 72.Spencer LA, Szela CT, Perez SA, Kirchhoffer CL, Neves JS, Radke AL, Weller PF. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85(1):117–123. doi: 10.1189/jlb.0108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fujisawa T, Abu-Ghazaleh R, Kita H, Sanderson CJ, Gleich GJ. Regulatory effect of cytokines on eosinophil degranulation. J Immunol. 1990;144(2):642–646. [PubMed] [Google Scholar]
- 74.Horie S, Gleich GJ, Kita H. Cytokines directly induce degranulation and superoxide production from human eosinophils. J Allergy Clin Immunol. 1996;98(2):371–381. doi: 10.1016/s0091-6749(96)70161-6. [DOI] [PubMed] [Google Scholar]
- 75.Kaneko M, Horie S, Kato M, Gleich GJ, Kita H. A crucial role for beta 2 integrin in the activation of eosinophils stimulated by IgG. J Immunol. 1995;155(5):2631–2641. [PubMed] [Google Scholar]
- 76.Kaneko M, Swanson MC, Gleich GJ, Kita H. Allergen-specific IgG1 and IgG3 through Fc gamma RII induce eosinophil degranulation. J Clin Invest. 1995;95(6):2813–2821. doi: 10.1172/JCI117986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hartnell A, Kay AB, Wardlaw AJ. Interleukin-3-induced up-regulation of CR3 expression on human eosinophils is inhibited by dexamethasone. Immunology. 1992;77(4):488–493. [PMC free article] [PubMed] [Google Scholar]
- 78.Laviolette M, Ferland C, Comtois JF, Champagne K, Bosse M, Boulet LP. Blood eosinophil leukotriene C4 production in asthma of different severities. Eur Respir J. 1995;8(9):1465–1472. [PubMed] [Google Scholar]
- 79.Dulkys Y, Kluthe C, Buschermohle T, Barg I, Knoss S, Kapp A, Proudfoot AE, Elsner J. IL-3 induces down-regulation of CCR3 protein and mRNA in human eosinophils. J Immunol. 2001;167(6):3443–3453. doi: 10.4049/jimmunol.167.6.3443. [DOI] [PubMed] [Google Scholar]
- 80.Hartnell A, Robinson DS, Kay AB, Wardlaw AJ. CD69 is expressed by human eosinophils activated in-vivo in asthma and in-vitro by cytokines. Immunology. 1993;80(2):281–286. [PMC free article] [PubMed] [Google Scholar]
- 81.Carr PD, Gustin SE, Church AP, Murphy JM, Ford SC, Mann DA, Woltring DM, Walker I, Ollis DL, Young IG. Structure of the complete extracellular domain of the common beta subunit of the human GM-CSF, IL-3, and IL-5 receptors reveals a novel dimer configuration. Cell. 2001;104(2):291–300. doi: 10.1016/s0092-8674(01)00213-6. [DOI] [PubMed] [Google Scholar]
- 82.Hansen G, Hercus TR, McClure BJ, Stomski FC, Dottore M, Powell J, Ramshaw H, Woodcock JM, Xu Y, Guthridge M, McKinstry WJ, Lopez AF, Parker MW. The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell. 2008;134(3):496–507. doi: 10.1016/j.cell.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 83.Broughton SE, Hercus TR, Nero TL, Dottore M, McClure BJ, Dhagat U, Taing H, Gorman MA, King-Scott J, Lopez AF, Parker MW. Conformational changes in the GM-CSF receptor suggest a molecular mechanism for affinity conversion and receptor signaling. Structure. 2016;24(8):1271–1281. doi: 10.1016/j.str.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 84.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, Jarjour NN, Busse WW, Kelly EAB. Decreased expression of membrane IL-5R alpha on human eosinophils: II. IL-5 down-modulates its receptor via a proteinase-mediated process. J Immunol. 2002;169(11):6459–6466. doi: 10.4049/jimmunol.169.11.6459. [DOI] [PubMed] [Google Scholar]
- 85.Yoshimura-Uchiyama C, Yamaguchi M, Nagase H, Matsushima K, Igarashi T, Iwata T, Yamamoto K, Hirai K. Changing expression of IL-3 and IL-5 receptors in cultured human eosinophils. Biochem Biophys Res Commun. 2003;309(1):26–31. doi: 10.1016/s0006-291x(03)01526-2. [DOI] [PubMed] [Google Scholar]
- 86.Gregory B, Kirchem A, Phipps S, Gevaert P, Pridgeon C, Rankin SM, Robinson DS. Differential regulation of human eosinophil IL-3, IL-5, and GM-CSF receptor alpha-chain expression by cytokines: IL-3, IL-5, and GM-CSF down-regulate IL-5 receptor alpha expression with loss of IL-5 responsiveness, but up-regulate IL-3 receptor alpha expression. J Immunol. 2003;170(11):5359–5366. doi: 10.4049/jimmunol.170.11.5359. [DOI] [PubMed] [Google Scholar]
- 87.Wang P, Wu P, Cheewatrakoolpong B, Myers JG, Egan RW, Billah MM. Selective inhibition of IL-5 receptor alpha-chain gene transcription by IL-5, IL-3, and granulocyte-macrophage colony-stimulating factor in human blood eosinophils. J Immunol. 1998;160(9):4427–4432. [PubMed] [Google Scholar]
- 88.Tavernier J, Devos R, Cornelis S, Tuypens T, Vanderheyden J, Fiers W, Plaetinck G. A human high affinity interleukin-5 receptor (IL5R) is composed of an IL5-specific alpha chain and a beta chain shared with the receptor for GM-CSF. Cell. 1991;66(6):1175–1184. doi: 10.1016/0092-8674(91)90040-6. [DOI] [PubMed] [Google Scholar]
- 89.Tavernier J, Van der Heyden J, Verhee A, Brusselle G, Van Ostade X, Vandekerckhove J, North J, Rankin SM, Kay AB, Robinson DS. Interleukin 5 regulates the isoform expression of its own receptor alpha-subunit. Blood. 2000;95(5):1600–1607. [PubMed] [Google Scholar]
- 90.Julius P, Hochheim D, Boser K, Schmidt S, Myrtek D, Bachert C, Luttmann W, Virchow JC. Interleukin-5 receptors on human lung eosinophils after segmental allergen challenge. Clin Exp Allergy. 2004;34(7):1064–1070. doi: 10.1111/j.1365-2222.2004.01986.x. [DOI] [PubMed] [Google Scholar]
- 91.Gevaert P, Hellman C, Lundblad L, Lundahl J, Holtappels G, van Cauwenberge P, Tavernier J, Bachert C. Differential expression of the interleukin 5 receptor alpha isoforms in blood and tissue eosinophils of nasal polyp patients. Allergy. 2009;64(5):725–732. doi: 10.1111/j.1398-9995.2008.01885.x. [DOI] [PubMed] [Google Scholar]
- 92.Wilson TM, Maric I, Shukla J, Brown M, Santos C, Simakova O, Khoury P, Fay MP, Kozhich A, Kolbeck R, Metcalfe DD, Klion AD. IL-5 receptor alpha levels in patients with marked eosinophilia or mastocytosis. J Allergy Clin Immunol. 2011;128(5):1086–1092. e1081–1083. doi: 10.1016/j.jaci.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yousefi S, Hoessli DC, Blaser K, Mills GB, Simon HU. Requirement of Lyn and Syk tyrosine kinases for the prevention of apoptosis by cytokines in human eosinophils. J Exp Med. 1996;183(4):1407–1414. doi: 10.1084/jem.183.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van der Bruggen T, Kanters D, Tool AT, Raaijmakers JA, Lammers JW, Verhoeven AJ, Koenderman L. Cytokine-induced protein tyrosine phosphorylation is essential for cytokine priming of human eosinophils. J Allergy Clin Immunol. 1998;101(1 Pt 1):103–109. doi: 10.1016/S0091-6749(98)70200-3. [DOI] [PubMed] [Google Scholar]
- 95.Zhu Y, Bertics PJ. Chemoattractant-induced signaling via the Ras-ERK and PI3K-Akt networks, along with leukotriene C4 release, is dependent on the tyrosine kinase Lyn in IL-5- and IL-3-primed human blood eosinophils. J Immunol. 2011;186(1):516–526. doi: 10.4049/jimmunol.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kampfer SS, Odermatt A, Dahinden CA, Fux M. Late IL-3-induced phenotypic and functional alterations in human basophils require continuous IL-3 receptor signaling. J Leukoc Biol. 2017;101(1):227–238. doi: 10.1189/jlb.2A0715-292RR. [DOI] [PubMed] [Google Scholar]
- 97.Munitz A, Bachelet I, Eliashar R, Moretta A, Moretta L, Levi-Schaffer F. The inhibitory receptor IRp60 (CD300a) suppresses the effects of IL-5, GM-CSF, and eotaxin on human peripheral blood eosinophils. Blood. 2006;107(5):1996–2003. doi: 10.1182/blood-2005-07-2926. [DOI] [PubMed] [Google Scholar]
- 98.Erikson E, Maller JL. Substrate specificity of ribosomal protein S6 kinase II from Xenopus eggs. Second Messengers Phosphoproteins. 1988;12(2–3):135–143. [PubMed] [Google Scholar]
- 99.Thomas G. An encore for ribosome biogenesis in the control of cell proliferation. Nat Cell Biol. 2000;2(5):E71–72. doi: 10.1038/35010581. [DOI] [PubMed] [Google Scholar]