Abstract
Organophosphate (OP) nerve agents and pesticides trigger a common mechanism of neurotoxicity resulting from critical targeting and inhibition of acetylcholinesterases (AChE) in central and peripheral synapses in the cholinergic nervous system. Therapeutic countermeasures have thus focused on either administering an oxime post-exposure, that can rapidly reactivate OP-inhibited AChE, or by preventing OP poisoning through administering pre-exposure treatments that scavenge OPs before they inhibit their physiological AChE targets. While several pyridinium aldoxime antidotes are currently approved, their utility is impaired due to their inability to cross the blood-brain barrier (BBB) efficiently. The present study utilized a macaque (Ma) model to demonstrate the efficacy of a novel zwitterionic and centrally acting oxime RS194B to reactivate sarin- and paraoxon-inhibited macaque AChE and butyrylcholinesterase (BChE) in vitro and to further assess the capacity of RS194B to effect a reversal of clinical symptoms following sarin inhalation in vivo. In vitro, oxime reactivation of MaAChE and MaBChE was shown to be comparable to their human orthologs, while the macaque studies indicated that IM administration of 62.5mg/kg of RS194B and 0.28mg/kg atropine after continuous exposure to 49.6 ug/kg sarin vapor, rapidly reactivated the inhibited AChE and BChE in blood and reversed both early and advanced clinical symptoms of sarin-induced toxicity following pulmonary exposure within one hour. The rapid cessation of autonomic and central symptoms, including convulsions, observed in macaques bodes well for the use of RS194B as an intra- or post-exposure human treatment and validates the macaque model in generating efficacy and toxicology data required for approval under the FDA Animal rule.
Introduction
Exposure to organophosphate (OP) neurotoxins, such as nerve agents and pesticides, results in irreversible inhibition of acetylcholinesterase (AChE) in neuromuscular junctions, peripheral and central neuronal synapses and on circulating red blood cells (1–3). Following inhalation, OPs with high vapor pressures rapidly enter through the pulmonary system, diffuse into tissues, cross the blood-brain barrier resulting in overstimulation at cholinergic synapses in the central nervous system (CNS) and potential lethal toxicity. Traditionally, treatment for OP toxicity has involved post-exposure therapy using an oxime capable of reactivating the OP-inhibited AChE, in combination with the muscarinic antagonist (atropine) and an anti-convulsant (diazepam) to control seizures (1–4). Since the early studies of Irwin Wilson and colleagues, who demonstrated the site direction of nucleophilic oximes (5), most synthetic oxime reactivators have belonged to the quaternary cationic, pyridinium aldoxime family e.g. pralidoxime (2-PAM) or its analogues: HI-6, obidoxime, MMB4. However, efficacy of these oximes is limited by steric constraints of an impacted active center gorge limiting pyridinium aldoxime attack, poor oral bioavailability, rapid clearance, and their inability to rapidly cross the blood-brain-barrier (BBB)(6–8). Recent clinical trials using pesticide-poisoned individuals have shown uneven clinical benefits with these oximes (9–11).
In the last decade, as OP threats have spread widely due to terrorism and to increased agricultural use of pesticides, research has focused on the development of two types of countermeasures: (i) prophylactic stoichiometric and catalytic bio-scavengers (2,12–17), an advanced candidate being butyrylcholinesterase (BChE) that has been shown to prevent OP toxicity in several animal models, and (ii) novel centrally acting, bioavailable oxime reactivators that can restore inhibited AChE activity in the brain post-OP exposure (4,7,8). Because the pharmacokinetics following parenteral delivery of large doses of stoichiometric bioscavengers of high molecular weight, such as BChE, are suboptimal for immediate post-exposure and longer term protection (17), administration of aerosolized (aer) rHuBChE via the pulmonary route is also under consideration to provide a protective pulmonary shield against inhaled volatile agents (18–19). A more recent approach to extend the duration of protection involves combining protective pretreatment with a scavenger and post-treatment of symptoms and reactivation of inhibited AChE with oximes (19,20).
Because currently approved pyridinium aldoximes are quaternary and do not rapidly diffuse to the brain, several efforts have been directed to identifying broad spectrum, centrally acting oximes with enhanced ability to mitigate OP toxicity. Of many novel oximes synthesized and evaluated to date, only a few types have shown the potential to cross the BBB. These include pro-drug analogs of PAM (21), phenoxyalkyl pyridinium, detergent-like oximes (22), conjugation of various pyridinium aldoximes to a glucose C-6 Sox transporter (23), various nucleophiles that encompass association with the active center and peripheral site on AChE (24–26) and low molecular weight zwitterions, whose neutral species enables rapid blood-brain barrier passage (27,28).
To date, a leading candidate to emerge is a zwitterion of simplified structure, RS194B, which in mouse studies, exhibits highly favorable pharmacokinetics, extended oral bioavailability in both brain and plasma, low toxicity (LD50 >500mg/kg) and good antidotal action following OP exposure with protective indices up to 45 when administered both prior to and after exposure [28].
The life threatening nature of OP nerve agent exposure requires that any counter-measure treatment be extensively tested under the Animal Rule (21 CFR 601.90 for biological products) for future regulatory approval. Based on prior studies in mice, the present investigation employed a non-human primate model to assess the efficacy of RS194B. Initially, in vitro studies were used to compare RS194B-assisted reactivation of AChE and BChE from macaques and humans following exposure to the pesticide, paraoxon (POX) and an analogue of the nerve agent sarin; the latter forming the same RP and SP conjugates as do the sarin enantiomers.
In vivo studies showed that post-exposure treatment with RS194B by IM administration rapidly reversed both early and advanced classic clinical symptoms in macaques following a 18–28 minute head only exposure to sarin vapor. Together, these data indicate that post-sarin exposure, RS194B results in rapid reactivation of erythrocyte AChE and plasma BChE activity in circulation, consistent with survival in macaques, even for those exhibiting a panoply of symptoms leading to tremors and convulsions.
2. Methods and Materials
Animal studies were conducted in compliance with the Animal Welfare Act and other federal statutes and regulations stated in the Guide for the Care and Use of Laboratory Animals (NRC Publication, 1996). Procedures with macaques received prior approval by Institutional Animal Care and Use Committees at Battelle Laboratories and were performed at Battelle’s Biomedical Research Center, a fully accredited facility by the Association for Assessment and Accreditation of Laboratory Animal Care, International. This study was performed using good documentation practices consistent with, but not in strict accordance with, the U.S. Food and Drug Administration (FDA) Good Laboratory Practices (GLP) regulations 21 CFR 58.
2.1 Expression and purification of rMaBChE, Ma RBC-AChE, rHuBChE and rHuAChE
For the in vitro studies, tetrameric CHO-derived rMaBChE and rHuBChE were produced and purified using procainamide Sepharose chromatography as previously described [18,29]. RBC Ma-AChE was prepared from 7.5 ml of rhesus macaque blood, which was centrifuged at 1,000 RPM for 15 min. Collected RBCs were washed three times with PBS before being lysed in water to a final volume of 4 ml. Recombinant HuAChE was produced in HEK 293 cells (27, 28).
BChE and AChE activity was assayed using 1 mM butyrylthiocholine or acetylthiocholine (Sigma-Aldrich) and 0.5 mM 5,5-dithiobis 2-nitrobenzoic acid (DTNB) in 50 mM sodium phosphate buffer, pH 8.0, at 22°C. The formation of product was followed by monitoring the increase in absorbance of 5-thio-2- nitrobenzoic acid at 412 nm using a molar extinction coefficient of 13,600 M−1. Activity was reported as U/ml where 1 U represents 1 µmole of acetyl- or butyrylthiocholine per min. In the AChE activity assay, 20 µM ethopropazine was used as a BChE-specific inhibitor. The specific activity of MaBChE (900 U/mg) is slightly higher than HuBChE (700 U/mg). Background levels in macaque blood generally range from 2.5 to 6.5 U/ml AChE and 3.5 to 8.5 U/ml BChE.
2.2 RS194B oxime
RS194B was prepared as previously described (27,28) and was formulated for IM injection at concentrations of 50–75 mg/ml. RS194B was dispersed in sterile water and then titrated with ~18% HCl to a pH of 5.5–6.5, resulting in clarity of the solution. It was allowed to stand for at least 24 hr at 4C before injection at room temperature. The concentration of ionized species is slightly above isotonicity.
2.3. Assays for reactivation of AChE and BChE
AChE from human and macaque sources was inhibited by ethylparoxon (POX) (Sigma-Aldrich) or a non-volatile sarin analogue with a cyanocoumarin leaving group replacing the fluorine at concentrations ranging from 1–10 uM (typically ~ 1.5 to 10-fold above the stoichiometry of active sites) (27,28). After inhibition at 22°C proceeded to between 90–98% within 3–5 min, the reactants were immediately placed over two successive spin columns at 4C to remove excess OP. The spin column excluded fractions were then diluted another 10–100-fold; reactivation with RS194B was initiated and allowed to approach equilibrium reactivation. First-order approaches to equilibrium were analyzed in terms of an overall bimolecular rate constant and an equilibrium reactivation level. Control samples without prior OP inhibition, but with added reactivator, were measured in parallel. All experiments were performed at 37°C in 0.01% bovine serum albumin, pH 7.4, 0.1M sodium phosphate over the RS194B concentration range (0.1mM to 15mM). The overall reactivation constant, kr (M−1min−1) was calculated from k2/Kox, where Kox (M) is the Michaelis-Menten type constant reflecting an apparent dissociation constant for the oxime, and k2 (min−1) is the maximal rate constant.
For reactivation kinetic studies of OP-inhibited AChE, we used the racemic sarin analog with a cyanocoumarin leaving group affording safety with its non-volatility. It forms the same methylphosphonate conjugate with the cholinesterases as does sarin with its fluoride leaving group, although the ratio of enantiomers formed from the racemic mixtures may differ. In the case of AChE, its small acyl pocket only allows for a 110-fold preference for the SP enantiomer of the methylphosphonate (30). However, as considered for mutant cholinesterases, the ratio of the Sp and Rp conjugates formed for BChE inhibition may well differ.
2.4. Administration of sarin
A transparent sarin exposure system for head-only inhalation exposure of macaques to sarin vapor was fabricated and tested at Battelle Biomedical Research Center, West Jefferson, OH, to allow visualization of symptoms with progressive exposure. Prior to challenges, macaques were acclimated to sitting in a restraint chair that would be used during prophylaxis and challenge. Once, secured in the exposure chair within the hood and connected to the inhalation exposure system, respiratory monitoring was initiated. One day before dosing, the head hair of each NHP was removed with clippers to minimize GB vapor adsorption/desorption issues. Animals were lightly anesthetized using Telazol at a 1 to 6 mg/kg IM dose prior to pretreatment with aer-rHuBChE which was administered using a nebulizer 24–48 hours before challenge.
Only the data from the 8 surviving BChE-pretreated and sarin-exposed macaques are described in this report; 3 of which received no oxime (Fig.2) while 5 received oxime (Fig.3). A control group of 5 BChE pretreated macaques, that received no oxime and did not survive (#43681, 43781, 44011, 43342 and 43303) are mentioned in the legend to Fig. 3 and a second control group of 5 sarin-exposed but untreated macaques (#42230, 41387, 42042, 44512) are mentioned in the Discussion with no data shown.
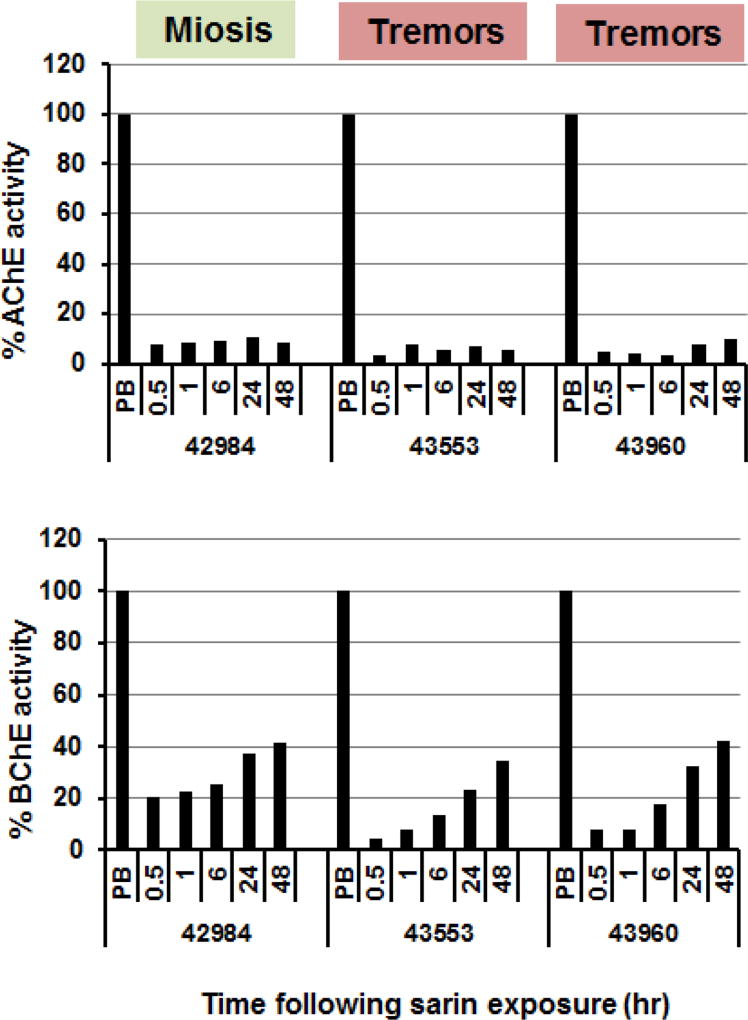
Figure 2.
Reactivation of blood AChE (upper panel) and BChE (lower panel) in three surviving macaques (#42984, 43553 and 43960) following inhalation exposure to sarin. Values are related to pre-exposure levels in the corresponding macaque. The respective macaques received sarin exposure for 10.8, 17.7 and 17.3 min and exhibited the symptoms including tremors following miosis in two of the three animals. Colors from green to red signify the severity of clinical symptoms. Animals were pretreated with aerrHuBChE using a nebulizer and received no oxime.
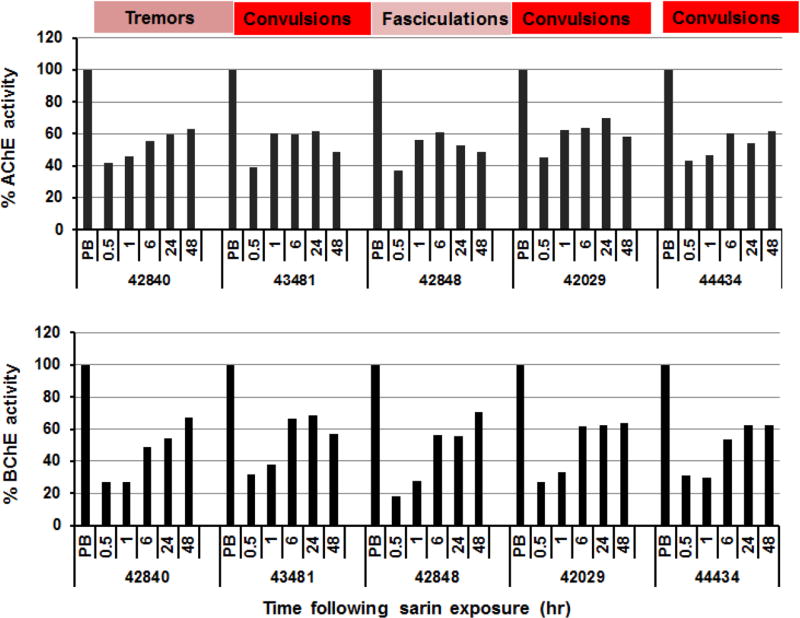
Figure 3.
Post-exposure reactivation by IM RS194B of RBC-AChE and plasma BChE in five macaques (#42840, 43481, 42848, 42029, 44434) exposed to inhaled sarin (49.6 µg/kg). Values are shown as percent of pre-exposure levels in the individual macaque prior to sarin exposure. The time intervals between cessation of sarin administration and injection of RS194B in the surviving animals was 2.75, 0.9, 1.5, 4.2 and 4.5 min respectively. All animals were administered oxime (62.5 mg/kg) IM with atropine (0.28 mg/kg) IM and all survived. Colors from pale pink to red signify the severity of clinical symptoms. The clinical signs indicated were observed at 27.6min (#42840), 33.1min (#43481), 20.3mins (#42840), 19.3 mins (#42029) and 20.5 mins (#44434) following initiation of sarin administration. By contrast, five control sarin-exposed macaques, which did not receive the RS194B, exhibited the typical clinical signs of sarin poisoning including tremors and convulsions and died with AChE levels at 3–4% of baseline in those animals where an initial sample measurement was possible. Animals were pretreated with aer-rHuBChE.
Baseline respiratory parameters were collected for a minimum of 5 minutes prior to challenge. A vapor jet generated a controlled sarin vapor ranging in concentrations between 0.5 and 2.0 mg/m3 A MINICAMS gas chromatograph (GC), or equivalent, was used to monitor sarin concentrations continuously. Buildup and washout, stability, and reproducibility of sarin vapor within the head-only exposure chamber were measured. An in-line respiratory monitoring system is used to measure inhaled volumes by the macaques during challenge. A 10-min washout period followed each animal’s head-only exposure. The helmet was then removed and the head decontaminated with Reactive Skin Decontamination Lotion (RSDL, Emergent BioSolutions, Gaithersburg, MD). After a 5-min reaction time, the RSDL was rinsed off using a water mist sprayer and cotton swabs. One 1-ml blood samples were collected from the saphenous vein 1 hr before sarin exposure and at 30 min, 60 min, 6 hr, 24 hr, and 48 hr post-exposure.
2.5. In vivo treatment and challenge of macaques
In these studies, macaques were pretreated 24–48hr prior to sarin exposure with doses of liquid aerosolized rHuBChE (aer-rHuBChE) (18,19) deposited in the lungs (8 mg/kg) based on many reports indicating the LD50 of inhaled sarin to be 12–15 µg/kg and an LCt50 of ~70 mg.min/m3 [31–34]. However, this pretreatment dose, given 24–48 hr prior to exposure, proved to be well below protective levels, because the accumulated dose of sarin administered to the macaques by inhalation was 49.6 µg/kg.
Macaques were then observed for clinical signs of sarin toxicity that included a progressive sequence of miosis, muscle fasciculations, labored breathing, salivation, tremors and convulsions during and following sarin exposure which lasted 11–28 mins to achieve the 49.6 ug/kg dose. At time intervals, ranging from 0.9 to 4.5 min following cessation of sarin administration, RS194B at 62.5 mg/kg and atropine at 0.28 mg/kg IM were administered to prevent further decline in the macaques’ condition and onset of a full cholinergic crisis and fatality. Animals were examined for cessation of symptoms typical of excessive cholinergic stimulation over the periods typically lasting 0.5 – 1 hr and for survival for 48 hr after challenge. Blood was drawn prior to and at 0.5, 1, 6, 24 and 48 hr following sarin exposure in the absence of oxime (3 animals) and in the presence of RS194B oxime given with atropine (5 animals) and assessed for AChE and BChE levels. Whole blood samples (20 µl) from macaques were first diluted 10-fold in water and tested for plasma BChE and RBC-AChE activity.
3. Results
3.1. Concentration dependent reactivation of sarin- and paraoxon- inhibited RBC-Ma-AChE and rMaBChE by RS194B in vitro
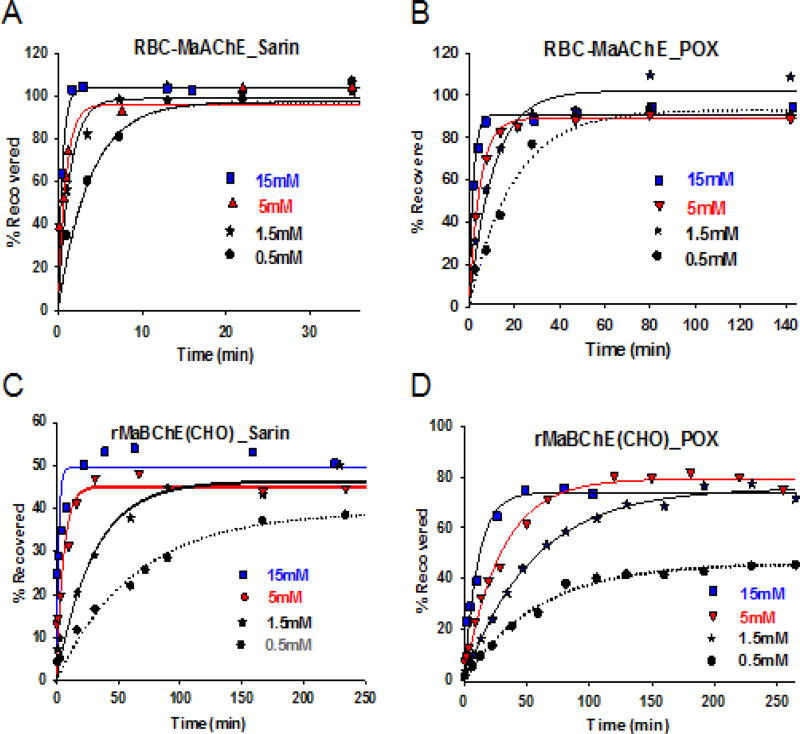
To assess whether the reactivation of macaque AChE was comparable to that previously shown for rHuAChE (28), RBC-Ma-AChE (Fig. 1A and 1B) and rMaBChE (Fig. 1C and 1D) were inhibited by the non-volatile sarin analog or POX and rates of recovery by RS194B assessed as a function of concentration and time.
Fig. 1.
RS194B concentration dependence for reactivation at 37°C of macaque RBC-AChE and recombinant CHO-derived (rMaBChE) inhibited by a sarin analogue and paraoxon (POX) in vitro. (A) reactivation of isopropyl methylphosphonyl AChE from macaque blood MaRBC-AChE (B) reactivation of diethylphosphoryl Ma RBC AChE, (C) reactivation of isopropyl methylphosphonyl rMaBChE and (D) reactivation of diethylphosphoryl rMaBChE. Oxime was added at 0.5mM (grey), 1.5 mM (black), 5mM (red) and 15mM (blue).
Having demonstrated the capacity for RS194B to reactivate Ma-RBC-AChE and rMaBChE in vitro efficiently and rapidly, the overall reactivation constants (kr), apparent dissociation constants (KOX) and rate constants (k2) of RBC-MaAChE and CHO-rMaBChE, inhibited by sarin or POX, were assessed and compared with current measurements of Kox and k2 for the human enzymes. The results, summarized in Table 1, indicate that: (i) RS194B reactivation rates of Ma-RBC-AChE-sarin conjugates and Hu-rAChE-sarin conjugates are comparable, (ii) reactivation of Ma-RBC-AChE-paraoxon and Hu-AChE-paraoxon conjugates are also comparable, (iii) reactivation of BChE conjugates from each species is slower than that for the respective AChE conjugates, (iv) reactivation rates for sarin conjugates are more rapid than for POX conjugates.
Table I.
Concentration Dependence of Reactivation Rates for Recombinant MaBChE, RBC MaAChE, Recombinant HuBChE and HuAChE Following Inhibition by a Sarin Analogue or Paraoxon.
| RS194B | 15mM | 5mM | 1.5mM | 0.5mM | ||||
|---|---|---|---|---|---|---|---|---|
| Sarin | kobs | A | kobs | A | kobs | A | kobs | A |
| rMaBCHE (CHO) | 0.55 | 49 | 0.12 | 39 | 0.02 | 34 | 0.015 | 26 |
| RBC-MaAChE | 2.4 | 104 | 0.85 | 83 | 0.52 | 86 | 0.39 | 72 |
| rHuBChE (CHO) | 1.8 | 93 | 0.35 | 76 | 0.18 | 61 | 0.05 | 57 |
| rHuAChE (HEK) | 2.5 | 53 | 1.82 | 77 | 0.83 | 56 | 0.44 | 51 |
| RS194B | 15mM | 5mM | 1.5mM | 0.5mM | ||||
|---|---|---|---|---|---|---|---|---|
| POX | kobs | A | kobs | A | kobs | A | kobs | A |
| rMaBCHE (CHO) | 0.069 | 86 | 0.024 | 91 | 0.023 | 73 | 0.020 | 37 |
| RBC-MaAChE | 0.52 | 91 | 0.15 | 91 | 0.067 | 105 | 0.038 | 91 |
| rHuBChE (CHO) | 0.21 | 86 | 0.09 | 93 | 0.034 | 84 | 0.032 | 58 |
| rHuAChE (HEK) | 0.27 | 41 | 0.16 | 47 | 0.046 | 41 | 0.021 | 35 |
kobs as a first order rate constant is in min−1. A is the percent reactivation compared with an uninhibited control carried through the same spin column and dilution sequence. Letters in parentheses denote the Chinese hamster ovary and human embryonic kidney cell expression systems. RBC isolated and washed erythrocytes
There is some variance in the extent of reactivation. This likely arises from four factors: (a) dilution factors from the double spin columns to remove free organophosphate, (b) formation of residual phosphoryl or phosphonyl oxime that could prove inhibitory (35), (c) residual retention of excess organophosphate or fractional aging, (d) the rapid recovery rates at high RS194B concentrations limiting precise time withdrawal of kinetic samples. For AChE, the rate of inactivation with the SP enantiomer of the sarin analog (30) is much more rapid than the RP enantiomer, so only a single enantiomeric conjugate should be formed.
3.2. Reactivation of AChE and BChE activity in macaque blood samples following inhalation exposure to sarin in the absence of oxime
While both human and macaque AChE and BChE inhibition by sarin was rapidly reversed in the presence of RS194B in vitro, minimal spontaneous reactivation in the absence of oxime was observed in vitro during the intervals monitored (Fig. 1). In vivo-reactivation patterns were first examined in blood samples taken at various time intervals from three macaques which survived sarin-exposure in the absence of oxime. Interestingly, while no reactivation of RBC-AChE was observed after exposure, a slow, but significant and progressive increase in plasma BChE activity did occur in vivo; reaching 30–40% by 24–48 hr in surviving macaques (Fig. 2). The slightly faster reactivation observed in macaque #42984 in the present study may correlate with a lower dose of inhaled sarin.
3.3 Rapid RS194B oxime reactivation of blood AChE and BChE activities in macaques following sarin inhalation
In vivo macaque studies were performed to determine if post-exposure treatment with oxime RS194B could impact survival. Thus, animals exhibiting late signs of toxicity following sarin inhalation (49.6µg/kg), were administered 62.5 mg/kg RS194B and atropine (0.28 mg/kg) IM at 0.78 – 4.32 min following termination of the sarin challenge which ranged from 11–28 minutes. Each macaque was monitored for reactivation of RBC-AChE and plasma BChE, reversal of cholinergic symptoms and survival past 48 hr. The predominant symptoms observed at the time of oxime/atropine administration, ranging from fasciculations to convulsions, are shown in Fig. 3. Macaques #42840, 43481, 42848, 42029, 44434 all exhibited rapid, sustained recovery of blood AChE and BChE activities and ultimately survived despite exhibiting advanced clinical signs prior to the RS194B-atropine treatment. For example, following sarin exposure, RBC-AChE activity increased from ~4% of baseline in the absence of oxime to ~40% by 30 min and 48–63% by 48 hr following a single administration of RS194B. On the other hand, plasma BChE activity, which spontaneously increased from baseline levels of ~6% up to 34–42%, underwent a further RS194B-mediated increase reaching 57–71% reactivation by 48 hr. The recovery of cholinesterase levels by RS194B in the blood, along with the rapid cessation of central symptoms (tremors, convulsions), suggest a roughly parallel time course for RS194B-mediated reactivation of AChE in the brain.
3. Discussion
These studies have utilized a non-human primate model to demonstrate post-exposure efficacy of the zwitterionic RS194B oxime to protect macaques from lethal toxicity following inhalation exposure to sarin vapor; the nerve agent employed and documented in terrorists attacks in Tokyo and on a far larger scale in Syria (36). As a consequence of the volatility and high vapor pressure of sarin compared to other OP nerve agents (37), inhalation into the lungs represents the predominant route of exposure from which the soluble and volatile compound rapidly distributes into the brain and other body tissues in exposed individuals. Similar to the volatile anesthetics, partial pressure may be a determinant of blood-brain barrier transfer for sarin and accumulation within the brain. Thus, rapid distribution into the brain by a post-exposure antidote is a prerequisite for survival following pulmonary sarin exposure. A non-human primate is the best suited model, since it most closely replicates human pulmonary ventilation-perfusion system and encompasses a weight range of a small child. It is also important to recognize similarities in respiratory physiology and anatomy within the primates, where access to the terminal bronchi and alveoli is not impeded by an elaborate olfactory system found in rodents or dogs. While OP toxicity results in inhibition of AChE in the brain and blood, it should be noted that in non-human primates, unlike rodents, comparable inhibition of soluble BChE in plasma also occurs following OP exposure (18).
Initial in vitro reactivation data demonstrated that RBC-MaAChE activity is restored by RS194B nearly as efficiently as in rHuAChE when inhibited by either sarin or POX, although reactivation of the POX conjugate was 5–8-fold slower than the Sp conjugate of sarin as previously shown for OP-AChE conjugates in rodents (27,28). Thus, it appears that the two ethoxy groups of the tetrahedral diethylphosphoryl conjugates are able to retard the oximes from accessing the impacted POX-conjugated phosphorus. In terms of recovery, reactivation of POX-inhibited MaBChE and MaAChE reached 80%, a likely consequence of the BChE acyl pocket being able to accommodate the symmetric POX equivalently. By contrast, in the case of sarin, only one of the two occupying enantiomers, Sp, is capable of rapid reactivation, which could be one factor why BChE reactivation of sarin inhibition approached only 50% in the macaques. Previous in vitro studies which compared inhibition and reactivation of AChE from several animal species also indicated that the inhibitory potency of macaque AChE with POX was similar to that of human AChE with only moderate differences in the reactivation constants between the two enzymes [38].
Five macaques treated with RS194B within minutes of termination of a sarin challenge exhibited dramatic reversal of classic clinical symptoms within one hour in the four macaques with the most severe signs (e.g tremors and convulsions) and thought to have little chance of surviving. Macaque #42848 exhibited less severe signs of toxicity (e.g. fasciculations, miosis and salivation) and may have survived in the absence of the oxime antidote. This compares with five control sarin-exposed macaques which did not receive the RS194B and died between 38.5 to 369 mins following sarin initiation (data not shown).
Interestingly, in the presence of oxime, reactivation of both blood AChE and plasma BChE activities reached similar levels (~60–70%) by 48 hr in macaques, unlike previous rodent studies, where AChE was more selectively reactivated. This rapid recovery of cholinesterase activity by RS194B and rapid cessation of central effects (convulsions) suggest efficient passage of oxime through the blood-brain barrier in macaques, as shown previously in mice (28). Several studies conducted over many years established that respiratory and cardiovascular depression associated with OP exposure is a consequence of AChE inhibition in both central and peripheral nervous systems (1,39). The protective efficacy observed here is also consistent with pharmacokinetic studies in mice (28) that showed rapid penetration of RS194B into the brain within 5–10 minutes to attain near steady-state concentrations within the first 40 min of administration. However, in contrast to mice, which showed a rapid ~10-fold decline over 2 hrs of RS194B in plasma, ChE levels in macaque blood continued to increase or remained high and unchanged for up to 48 hrs post-sarin challenge. This is in agreement with initial RS194B pharmacokinetic studies in macaques (not shown) and suggests that residual oxime remains or is replenished in the macaque circulation for longer time intervals (Fig.3). It is also consistent with the zwitterionic properties of RS194B and the formation of a neutral species that exhibit more sustained residual tissue and plasma levels. The capacity to cross the blood-brain barrier, to be absorbed orally and to remain in the plasma after a single injection offers RS194B a significant advantage over 2-PAM and several pyridinium aldoxime analogs (HI-6, obidoxime and MMB4) that are unable to enter intracellular sites in tissues or the CNS and thus are rapidly cleared (40,41).
In this context, IM injection of oxime MMB4, which does not cross the blood-brain barrier, efficiently reactivated cyclosarin-inhibited AChE in blood samples from macaques administered IM with 1LD50 dose of cyclosarin 5 mins before MMB4 treatment. However in this study, it is not clear to what extent the MMB4 protected the animals, since macaques received a higher dose of atropine (1mg/kg) 15 mins prior to cyclosarin exposure; suppressing the incidence and severity of the clinical signs of cyclosarin toxicity (42). In the case of OP pesticides, humans administered a 2g loading dose plus a steady infusion of 2-PAM following poisoning with two diethylphosphoryl-containing pesticides, chlorpyrifos and quinalphos, variably reactivated BChE, but was not sustained past 10–12 hr (43). In addition, in the treatment of methamidophos poisoning in man, multiple dosing of a pyridinium aldoxime over a sustained period was required to reverse toxicity (11).
In contrast to sarin analog-inhibited Ma-RBC-AChE and Ma-rBChE in vitro, where inhibition was sustained but reversed by RS194B, enhancement of sarin-inhibited BChE activity in macaques in vivo appears more complex; involving a slow oxime-independent and a rapid oxime-dependent reactivation. Slow recovery of BChE activity has also been previously observed in macaques exposed to POX in vivo (44) and in vitro (45).
Our findings in macaques following pulmonary sarin exposure suggest different outcomes from prior studies of systemic sarin treatment in guinea pigs (46). Despite the attractiveness of low carboxylesterase activity in this animal model, initial in vitro studies with guinea pig liver microsomes showed rapid metabolism of RS194B, which is in contrast to the absence of metabolic decomposition of RS194B in mouse, rat, monkey and human microsomes (B. Capacio, USARMICD, unpub. data). In addition, guinea pig AChE shows more resistance (47,48) to inhibition by certain organophosphates. Such interspecies variability requires an assessment of antidote efficacy in multiple species and, for volatile OP’s, administration by the very means that replicate vapor exposure in the environment.
The macaques in this study received sub-stoichiometric doses of rHuBChE as a prophylactic treatment, thus additional studies are underway to ascertain the extent to which prior inhalation of BChE contributed to the efficacy of RS 194B. Efficient distribution of IM RS194B via the circulation to the pulmonary system and into epithelial cells and bronchial secretions may effect a localized BChE reactivation, resulting in enhanced/extended protection from aer-BChE pretreated macaques. In this context, it is important to note that, while 4/5 control BChE-untreated sarin-exposed macaques experienced convulsions and tremors (three of which died), none of four macaques pretreated with rHuBChE using a nebulizer (3 of which died) exhibited convulsions at the same dose (>5 m/m3)(data not shown). This suggests that even at sub-optimal doses, sufficient aer-BChE was present to minimize the amount of agent reaching the brain resulting in some clinical protection. In this context, Saxena et al (49), showed that suboptimal doses of plasma BChE delayed cardiac and neural toxicity signs, but not survivability in sarin-exposed Gottingen minipigs. Such findings raise the attractive prospect of developing a parenteral, post-exposure IM loading dose/oral maintenance dose schemes, in combination with pulmonary administration of a bio-scavenger as a complementary and synergistic means to counteract terrorism and minimize its consequences.
Conclusion
Identifying efficacious antidotes capable of reactivating nerve agent or pesticide-inhibited brain AChE in the central and peripheral nervous systems has proven elusive despite many years of intense effort. In addition, the lack of efficacious protective antidotes has been exacerbated by variations in clinical efficacy of the few available oximes approved for human use. In this context, the choice of animal models for regulatory approval is critical. The efficacy of RS194B to reactivate both sarin- and POX-inhibited MaAChE and MaBChE conjugates in vitro and to effect a reversal of severe clinical symptoms with subsequent survival of macaques exposed to sarin in vivo bodes well for its development as a protective treatment in humans, as well as the utility of the NHP model for regulatory approval.
Supplementary Material
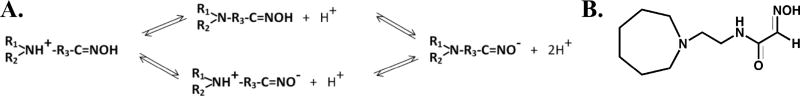
Scheme 1.
(A) Ionization equilibria of the hydroxyiminoacetamidoalkyl cyclic amines showing the transition from cations to anions to proceed through zwitterionic and neutral species. The oximate-oxime and amine protonation in the azepine ring have nearly identical pKa’s ~8.8. The neutral species facilitates crossing the blood-brain barrier and oral bioavailability, while the cation will be attracted to the active center gorge and in solution the anion is the nucleophilic, general base catalyst. (B) The lead structure, RS 194B of over 100 congeners synthesized, is shown on the right.
Acknowledgments
The authors thank Dr. Mark Perry and Dr. Thomas Snider for protocol discussions and performing the aerosol-rHuBChE administrations and the sarin vapor exposure of macaques. Thanks are extended to Dr. Ben Capacio, USAMRICD, for performing the metabolic oxime studies and to Dr. Lori Urban for critical reading of the manuscript.
Funding: This work was supported by the Defense Threat Reduction Agency grant # HDTRA1-15-1-0026 to YJR and National Institutes of Health NS UO1-058046 to PT.
Abbreviations
- aer-BChE
aerosol butyrylcholinesterase
- AChE
acetylcholinesterase
- Ma
macaque
- OP
organophosphate
- POX
paraoxon
- IM
intramuscular
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement
The authors have no competing interests.
References
- 1.Taylor P. Anticholinesterases. In: Brunton LL, editor. Goodman and Gilman’s Pharmacological Basis of Therapeutics. 12. Chapter 10. 2009. pp. 239–254. 13th Edition, July 2017. [Google Scholar]
- 2.Somani SM, Romano JA., Jr . Chemical Warfare Agents: Toxicity at Low Levels. CRC Press; Boca Raton: 2001. p. 447. [Google Scholar]
- 3.Gupta RC. Toxicology of Organophosphate & Carbamate Compounds. Academic Press; New York: 2006. p. 763. [Google Scholar]
- 4.Shih T-M, Koplovitz I, Kan RK, McDonough JH. In search of an effective in vivo reactivator for organophosphorus nerve agent-inhibited acetylcholinesterase in the central nervous system. Advanced Studies in Biology. 2012;4:451–478. [Google Scholar]
- 5.Wilson IB, Ginsburg S. Reactivation of acetylcholinesterase inhibited by alkylphosphates. Arch. Biochem. Biophys. 1955;54:569–71. doi: 10.1016/0003-9861(55)90075-8. [DOI] [PubMed] [Google Scholar]
- 6.Kovalevsky A, Blumenthal DK, Cheng X, Taylor P, Radic Z. Limitations in current acetylcholinesterase structure-based design of oxime antidotes for organophosphate poisoning. Ann. N.Y. Acad. Sci. 2016 doi: 10.111/nyas13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorecki L, Korabecny J, Musilek K, Malinak D, Nepovimova E, Dolezal R, Jun D, Soukup O, Kuci K. SAR study to find optimal cholinesterase reactivator against organophosphorus nerve agents and pesticides. Arch. Toxicol. 2016;90:2831–2859. doi: 10.1007/s00204-016-1827-3. [DOI] [PubMed] [Google Scholar]
- 8.Worek F, Wille T, Koller M, Thiermann H. Toxicology of organophosphorus compounds in view of an increasing terrorist threat. Arch. Toxicol. 2016;90:2131–2145. doi: 10.1007/s00204-016-1772-1. [DOI] [PubMed] [Google Scholar]
- 9.Buckley NA, Eddleston M, Li Y, Bevan M, Robertson J. Oximes for acute organophosphate pesticide poisoning. Cochrane Database Syst. Rev. 2011;2:CD005085. doi: 10.1002/14651858.CD005085.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Eddleston M, Eyer P, Worek F, Juszczak E, Alder N, Mohamed F, Senarathna L, Hittarage A, Azher S, Jeganathan K, Jayamanne S, von Meyer L, Dawson AH, Sheriff MH, Buckley NA. Pralidoxime in acute organophosphorus insecticide poisoning - a randomised controlled trial. PLoS Med. 2009;6(6):1–12. doi: 10.1371/journal.pmed.1000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinritz D, Eyer F, Worek F, Thiermann H, John H. Repetitive obidoxime treatment induced increase of red blood cell acetylcholinesterase activity even in a late phase of a severe methamidophos poisoning: A case report. Toxicol. Lett. 2016;244:121–123. doi: 10.1016/j.toxlet.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Lenz DE, Yeung D, Smith JR, Sweeney RE, Lumley LA, Cerasoli DM. Stoichiometric and catalytic scavengers as protection against nerve agent toxicity: a mini review. Toxicology. 2007;233(1–3):31–39. doi: 10.1016/j.tox.2006.11.066. [DOI] [PubMed] [Google Scholar]
- 13.Doctor BP BP, Saxena A A. Bioscavengers for the protection of humans against organophosphate toxicity. Chem. Biol. Interact. 2005;157–158:167–71. doi: 10.1016/j.cbi.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Wille T, Worek F, Thiermann H. Catalytic bioscavengers in nerve agent poisoning: A promising approach? Toxicology Letters. 2016;244:143–148. doi: 10.1016/j.toxlet.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Mata DG, Rezk PE, Sabnekar P, Cerasoli DM, Nageswararao C. Investigation of evolved paraoxonase 1 variants for prevention of organophosphorus pesticide compound intoxication. J. Pharmacol. Exp. Ther. 2014;349:549–558. doi: 10.1124/jpet.114.213645. [DOI] [PubMed] [Google Scholar]
- 16.Goldsmith M, Eckstein S, Ashani Y, Greisen P, Jr, Leader H, Sussman JL, Aggarwal N, Ovchinnikov S, Tawfik DS, Baker D, et al. Catalytic efficiencies of directly evolved phosphotriesterase variants with structurally different organophosphorus compounds in vitro. Arch. Toxicol. 2016;90:2711–2724. doi: 10.1007/s00204-015-1626-2. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg YJ, Jiang X, Mao L, Hernandez-Abanto SM, Lee K. Development of a prophylactic butyrylcholinesterase bioscavenger to protect against insecticide toxicity using a homologous macaque model. In: Soloneski S, Larramendy M, editors. Insecticides Basic and Others Applications. First. Tech. Press; 2012. pp. 79–100.7. [Google Scholar]
- 18.Rosenberg YJ, Laube B, Mao L, Jiang X, Hernandez-Abanto SM, Lee KD, Adams R. Pulmonary delivery of an aerosolized recombinant butyrylcholinesterase pretreatment protects against aerosolized paraoxon insecticide. Chem. Biol. Interact. 2012;203:167–171. doi: 10.1016/j.cbi.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg YJ, Fink JB. Creation of a protective pulmonary bioshield against inhaled organophosphates using an aerosolized bioscavenger. Ann. N. Y. Acad. Sci. 2016;1374(1):151–8. doi: 10.1111/nyas.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caranto GR, Waibel KH, Asher JM, Larrison RW, Brecht KM, Schutz MB, Raveh L, Ashani Y, Wolfe AD, Maxwell DM, et al. Amplification of the effectiveness of acetylcholinesterase for detoxification of organophosphorus compounds by bis-quaternary oximes. Biochem. Pharmacol. 1994;47(2):347–57. doi: 10.1016/0006-2952(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 21.DeMar JC, Clarkson ED, Ratcliffe RH, Campbell AJ, Thangavelu SG, Herdman CA, Leader H, Schulz SM, Marek E, Medynets MA, Ku TC, Evans SA, Khan FA, Owens RA, Nambiar MP, Gordon RK. Pro-PAM therapy for central and peripheral cholinesterases. Chem. Biol. Interact. 2010;187:191–198. doi: 10.1016/j.cbi.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chambers JE, Chambers HW, Funck KE, Meek EC, Pringle RB, Ross MK. Efficacy of novel phenoxyalkyl pyridinium oximes as brain-penetrating reactivators of cholinesterase inhibited by surrogates of sarin and VX. Chem. Biol. Interact. 2016;259(Pt B):154–159. doi: 10.1016/j.cbi.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Garcia GA, Campbell AJ, Olson J, Moorad-Doctor D, Morthole VI. Novel oximes as blood-brain barrier penetrating cholinesterase reactivators. Chem. Biol. Interact. 2010;187(1–3):199–206. doi: 10.1016/j.cbi.2010.02.033. [DOI] [PubMed] [Google Scholar]
- 24.Mercey G, Verdelet T, Renou J, Kliachyna M, Baati R, Nachon F, Jean L, Renard PY. Reactivators of acetylcholinesterase inhibited by organophosphorus nerve agents. Acc. Chem. Res. 2012;45:756–66. doi: 10.1021/ar2002864. [DOI] [PubMed] [Google Scholar]
- 25.Cadieux CL, Wang H, Zhang Y, Koenig JA, Shih T-M, McDonough J, Koh J, Cerasoli D. Probing the activity of a non-oxime reactivator for acetylcholinesterase inhibited by organophosphate nerve agents. Chem. Biol. Interact. 2016;259B:133–141. doi: 10.1016/j.cbi.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McHardy SF, Bohmann JA, Corbett MR, Campos B, Tidwell MW, Thompson PM, Mencha TA, Reeves TE, Cantrell WR, Jr, Bauta WE, et al. Design, synthesis and characterization of novel, non-quaternary reactivators of GF-inhibited human acetylcholinesterase. Bioorg. & Medicinal Chem. Lett. 2014;24:1711–1714. doi: 10.1016/j.bmcl.2014.02.049. [DOI] [PubMed] [Google Scholar]
- 27.Sit RK, Radić Z, Gerardi V, Zhang L, Garcia E, Katalinić M, Amitai G, Kovarik Z, Fokin VV, Sharpless KB, Taylor P. New structural scaffolds for centrally acting oxime reactivators of phosphylated cholinesterases. J. Biol. Chem. 2011;286(22):19422–19430. doi: 10.1074/jbc.M111.230656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radić Z, Sit RK, Kovarik Z, Berend S, Garcia E, Zhang L, Amitai G, Green C, Radić B, Fokin VV, Sharpless KB, Taylor P. Refinement of structural leads for centrally acting oxime reactivators of phosphylated cholinesterases. J Biol. Chem. 2012;287(15):11798–809. doi: 10.1074/jbc.M111.333732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg YJ, Saxena A, Sun W, Jiang X, Chilukuri N, Luo C, Doctor BP, Lee KD. Demonstration of in vivo stability and lack of immunogenicity of a polyethyleneglycol-conjugated recombinant CHO-derived butyrylcholinesterase bioscavenger using a homologous macaque model. Chem. Biol. Interact. 2010;187(1–3):279–86. doi: 10.1016/j.cbi.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 30.Kovarik Z, Radic Z, Berman HA, Simeon-Rudolph V, Reiner E, Taylor P. Acetylcholinesterase active centre and gorge conformations analysed by combinatorial mutations and enantiomeric phosphonates. Biochem. J. 2003;373 doi: 10.1042/BJ20021862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anzueto A, deLemos R, Seidenfeld J, Moore G, Hamil H, Johnson D, Jenkinson SG. Acute inhalation toxicity of soman and sarin in baboons. Fundam. Appl. Toxicol. 1990;14(4):676–687. doi: 10.1016/0272-0590(90)90293-s. [DOI] [PubMed] [Google Scholar]
- 32.Bide RW, Armour SJ, Yee E. GB toxicity reassessed using newer techniques for estimation of human toxicity from animal inhalation toxicity data: new method for estimating acute human toxicity (GB) J. Appl. Toxicol. 2005;25(5):393–409. doi: 10.1002/jat.1074. [DOI] [PubMed] [Google Scholar]
- 33.Cresthull P, Koon WS, McGrath FP, Oberst FW. Inhalation effects (incapacitation and mortality) for monkeys exposed to GA, GB, and GF vapors. U.S. Army Chemical Center, MD. 1957 Report No. 2179. [Google Scholar]
- 34.Callaway S, Crichton D. Chemical Defence Experimental Establishment. Porton Down, U.K: 1954. Porton Technical Paper No. 424: The production of casualties in monkeys with GB vapour. [Google Scholar]
- 35.Luo C, Saxena A, Smith M, Garcia G, Radic Z, Taylor P, Doctor BP. Phosphoryl oxime inhibition of acetylcholinesterase is inhibited by edrophonium. Biochemistry. 38:9937–9947. doi: 10.1021/bi9905720. [DOI] [PubMed] [Google Scholar]
- 36.Dolgin E. Syrian gas attack reinforces the need for better anti-sarin drugs. Nature Med. 19:1194–1195. doi: 10.1038/nm1013-1194. [DOI] [PubMed] [Google Scholar]
- 37.Jang YJ, Kim K, Tsay OG, Atwood DA, Churchill DG. Update of: Destruction and detection of chemical warfare agents. Chem. Revs. 2015;PR1-PR, 7(6):195–205. doi: 10.1021/acs.chemrev.5b00402. [DOI] [PubMed] [Google Scholar]
- 38.Worek F, Aurbek N, Wille T, Eyer P, Thiermann H. Kinetic analysis of interactions of paraoxon and oximes with human, Rhesus monkey, swine, rabbit, rat and guinea pig acetylcholinesterase. Toxicol. Lett. 2011;200(1–2):19–23. doi: 10.1016/j.toxlet.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Edery H, Geyer MA, Taylor P, Berman HA. Target sites for acetylcholinesterase on the ventral surface of the medulla oblongata: Hypotension elicited by organophosphorus agents. J. Auton. Pharmacol. 1986;6:195–205. doi: 10.1111/j.1474-8673.1986.tb00645.x. [DOI] [PubMed] [Google Scholar]
- 40.Hong SP, Gibbs ST, Kobs DJ, Osheroff MR, Johnson JD, Burback BL. Pharmacokinetics of MMB4 DMS in rats, rabbits, and dogs following a single iv administration. Int. J. Toxicol. 2103;32(2):30S–37S. doi: 10.1177/1091581813488954. [DOI] [PubMed] [Google Scholar]
- 41.Sidell FR, Groff WA. Intramuscular and intravenous administration of small doses of 2-pyridinium aldoxime methochloride to man. J. Pharm. Sci. 1971;60:1224–1228. doi: 10.1002/jps.2600600823. [DOI] [PubMed] [Google Scholar]
- 42.Harvilchuck JA, Hong SP, Richey JS, Osheroff MR, Johnson JD. In vivo acetylcholinesterase reactivation in male guinea pigs and rhesus macaques following cyclosarin exposure and treatment with 1,1'-methylenebis{4-[(hydroxyimino)methyl] pyridinium} dimethanesulfonate. Int. J. Toxicol. 2013;32(4 Suppl):99S–107S. doi: 10.1177/1091581813498778. [DOI] [PubMed] [Google Scholar]
- 43.Konickx LA, Worek F, Jayamanne S, Thiermann H, Buckely HA, Eddleston M. Reactivation of plasma butyrylcholinesterase by pralidoxime chloride in patients poisoned by WHO class II toxicity organophosphorus insecticides. Toxicol. Sci. 2013;136:274–283. doi: 10.1093/toxsci/kft217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenberg YJ, Gearhart J, Mao L, Jiang X, Hernandez-Abanto S. Protection against paraoxon toxicity by an intravenous pretreatment with polyethylene-glycol-conjugated recombinant butyrylcholinesterase in macaques. Chem. Biol. Interact. 2014;210:20–25. doi: 10.1016/j.cbi.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Worek F, Diepold C, Eyer P. Dimethylphosphoryl-inhibited human cholinesterases: inhibition, reactivation, and aging kinetics. Arch. Toxicol. 1999;73:7–14. doi: 10.1007/s002040050580. [DOI] [PubMed] [Google Scholar]
- 46.Wilhelm CM, Snider TH, Babin MC, Jett DA, Platoff GE, Jr, Yeung DT. A comprehensive evaluation of the efficacy of leading oxime therapies in guinea pigs exposed to organophosphorus chemical warfare agents or pesticides. Tox. Appl. Pharmacol. 2014;281:254–265. doi: 10.1016/j.taap.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruark CD, Chapleau RR, Mahle DA, Gearhart JM. Organophosphorus inhibition and characterization of recombinant guinea pig acetylcholinesterase. Protein Pept. Lett. 2015;22:862–8. doi: 10.2174/0929866522666150728114754. [DOI] [PubMed] [Google Scholar]
- 48.Cadieux CL, Broomfield CA, Kirkpatrick MG, Kazanski ME, Lenz DE, Cerasoli DM. Comparison of human and guinea pig acetylcholinesterase sequences and rates of oxime assisted reactivation. Chem. Biol. Interact. 2010;187:229–233. doi: 10.1016/j.cbi.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 49.Saxena A, Sun W, Dabisch PA, Hulet SW, Hastings NB, Jakubowski EM, Mioduszewski RJ, Doctor BP. Pretreatment with human serum butyrylcholinesterase alone prevents cardiac abnormalities, seizures and death in Gottingen minipigs exposed to sarin vapor. Biochem. Pharmacol. 2011;82:1984–1993. doi: 10.1016/j.bcp.2011.09.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.