Abstract
Background
The role of adiponectin in patients with acute coronary syndromes is incompletely defined. This study investigated adiponectin levels in patients with acute coronary syndromes and the association between adiponectin and 30-day infarct size and 1-year clinical outcomes.
Methods
Retrospective analysis of 120 participants with acute coronary syndromes enrolled in the Immediate Myocardial Metabolic Enhancement During Initial Assessment and Treatment in Emergency care Trial. Blood levels were tested three times within 24 h of onset of ischaemic symptoms. Infarct size was measured at 30 days. The 1-year clinical outcome was the composite of all-cause mortality or hospitalization for heart failure.
Results
Using linear mixed models, log adiponectin levels decreased by −0.005 μg/mL per hour (p = 0.035). After stratifying the analysis by gender, there was no decrease in log adiponectin in men; however, levels decreased by −0.01 μg/mL per hour in women (p = 0.02). Results of multivariable regression models showed no association between log adiponectin and infarct size (β = −1.1, p = 0.64). Log adiponectin levels did not predict 1-year outcomes using Cox-proportional hazard models.
Conclusion
There was a small decrease in plasma adiponectin shortly after symptoms of ischaemia, more noticeable in women. No relationship was found between adiponectin and infarct size or clinical outcomes. This adds to evidence showing no clear association between adiponectin and adverse outcomes in patients with acute coronary syndromes.
Keywords: Acute coronary syndromes, adiponectin, adipose tissue, infarct size, mortality
Introduction
Adipose tissue is an active endocrine organ that secretes bioactive molecules known as adipokines.1,2 These molecules play key roles in regulating energy, metabolic and inflammatory processes.2 One of these classes of molecules, adiponectin, has drawn special attention due to its potential anti-inflammatory, insulin-sensitizing and anti-atherogenic properties.3,4 Plasma adiponectin levels are lower in obese individuals and have a protective effect against the development of type 2 diabetes.5,6 Women have higher concentrations of adiponectin than do men.7 In addition, adiponectin levels are inversely correlated with other cardiovascular risk factors, such as hyperlipidaemia, hypertension and C-reactive protein (CRP) levels.8–10 However, in people with an underlying coronary artery disease (CAD), the role of adiponectin in acute and chronic progression of atherosclerotic vascular disease is less clear.10
Several observational studies have demonstrated an association between low adiponectin levels and stable CAD,11–14 while other studies have not been able to establish a link between serum levels of adiponectin and the development of cardiovascular disease.15–17 Furthermore, studies in patients with acute coronary syndromes (ACS) have shown that higher plasma adiponectin levels are associated with greater disease severity and a higher risk of adverse outcomes.18–20 Animal studies indicate that a lack of adiponectin increases the size of an infarct following myocardial ischaemia.21 However, no human studies have examined the association between infarct size and serum adiponectin levels in patients who developed an ACS.
In most studies that have examined the relationship between adiponectin and CAD, adiponectin was measured in patients with stable CAD22 or late after the onset of symptoms of myocardial ischaemia.23 In this study, using data from the Immediate Myocardial Metabolic Enhancement During Initial Assessment and Treatment in Emergency care (IMMEDIATE) Trial, we investigated changes in serum levels of adiponectin early in the course of ACS and the association between these serum levels and various patient characteristics. In addition, we examined the association between serum levels of adiponectin and 30-day infarct size, as well as 1-year clinical outcomes in patients with ACS.
Methods
Study sample
This study analysed data collected from participants enrolled in the IMMEDIATE Trial, the methodology of which has been published elsewhere.24 In brief, this was a randomized, placebo-controlled, double-blind, multi-centre clinical effectiveness trial of glucose–insulin–potassium (GIK) that enrolled patients with suspected ACS between 2006 and 2011. Paramedics, aided by electrocardiograph (ECG)-based decision support, randomized and enrolled patients aged ≥30 years with a high probability of ACS.24 Participants were given either GIK solution or an identical-appearing placebo. This study was based on the IMMEDIATE Trial biological mechanism cohort (‘biocohort’), which consisted of individuals who consented to participate in this biocohort, were confirmed as having ACS and were treated with GIK or placebo for at least 8 h. Enrolment in the biocohort began after the trial was started and only included 6 out of 13 study centres. We restricted this analysis to biocohort participants with at least one frozen blood sample available for measurement at any of the three examined time points, as described below.
Data collection
During the 12-h infusion of study drug in the IMMEDIATE Trial, blood levels were drawn at three time points: (1) initial measurement, as soon as feasible after hospital arrival; (2) 6 h after the start of study drug; and (3) 12 h after the start of study drug or upon discontinuation of the infusion. Using time from acute symptom onset to study drug administration, and time for blood measurements, we re-calculated time from symptom onset to the first, second and third measurements, using the exact time of the blood drawing rather than the designation as being the initial, 6- or 12-h samples. Participants returned for sestamibi perfusion and left ventricular (LV) function imaging studies at 30 days. Standardized interpretation of these studies was performed at the SPECT core laboratory at Tufts Medical Center. The core laboratory of Tufts Clinical and Translational Science Institute (CTSI) performed biomarker measurements.
Biomarkers measured included serum levels of glucose, insulin, free fatty acids and high-sensitivity CRP (hs-CRP). The measurement of adiponectin was not part of the IMMEDIATE Trial biocohort; however, using serum samples that were stored promptly at −20°C, in 2013 we measured adiponectin levels. After thawing the samples, serum levels of adiponectin were measured by Human Adiponectin Platinum enzyme-linked immunosorbent assay (ELISA) kit (eBioscience Inc., San Diego, CA). Not all participants had adiponectin measurements at all three time points, but if at least one measurement was available, that participant was included in our data analyses. Additional blood samples were collected at 30 days for the measurement of haemoglobin A1C (Hgb A1C) and brain-type natriuretic peptide (BNP). Other covariates collected included patients'; demographic characteristics, vital signs, medical history, and medications used at home, in the hospital and upon discharge.
For 1-year clinical outcomes, we used the composite outcome of all-cause mortality or hospitalization for heart failure (HF) as adjudicated in the IMMEDIATE Trial.24,25 For all rehospitalizations during the follow-up period, source documents were provided for review by a Clinical Events Committee, to determine whether the hospitalization was related to HF.25
Data analysis
Descriptive statistics were used to describe baseline characteristics of the study population. Adiponectin and hs-CRP values were logarithmically transformed to obtain normal distributions. Because participants in the IMMEDIATE Trial received GIK or placebo therapy, we first tested whether adiponectin levels differed between treatment arms before combining the data. Mixed models with random and fixed effects were used to analyse changes in serum levels of adiponectin over the three previously described time points. Time was treated as a continuous variable. Regression models were used to examine the relationship between clinical characteristics (as the predictors) and the first available measurement of adiponectin (as the outcome) for each participant. Since adiponectin levels tend to be higher in women,26 all analyses were further stratified according to gender.
Univariate and multivariable linear regression models were used to study the association between baseline or first available measurement of adiponectin (as the predictor) and 30-day infarct size (as the outcome). Adiponectin was examined continuously and in quartiles, with the first quartile as the referent. Variables in multivariable regression models included those determined to be clinically significant or statistically significant in univariate analysis at p = 0.1. Infarct sizes ranged from 0% to 59% of LV mass, where 0% represented those who did not develop an infarct and 59% was assigned to those who died (n = 4) before imaging, based on the fact that the largest infarct size measured in the study was 58%. In addition, Spearman rank correlations between adiponectin and infarct size were estimated. Sensitivity analyses were performed by removing participants who did not develop an infarct and those who died. Cox-proportional hazards models were used to estimate univariate hazard ratios (HRs) for the association of one unit increase in adiponectin with 1-year composite outcome of all-cause mortality or hospitalization for HF. We also ran the analysis using quartiles of adiponectin. We checked assumptions needed for proportional hazards analyses by plotting and testing Schoenfeld residuals as related to time. All analyses were done using R, version 3.0.2.
Results
Characteristics of study population
A total of 120 participants had at least one frozen blood sample available for adiponectin measurements, and 50 subjects had all three levels available. There were no significant differences in clinical characteristics, or in serum adiponectin levels, between the GIK and placebo groups, and the participants were combined into a single cohort for the purpose of these analyses. The mean age of this study cohort was 64 years, 70% were men, 97% were White and 23% had a history of diabetes mellitus (Table 1). Approximately 88% of participants had a confirmed diagnosis of acute myocardial infarction, and the remaining were diagnosed with unstable angina. The median time from the onset of ischaemic symptoms until the first, second and third blood measurements was 4, 7.5 and 13.5 h, respectively. Women were older than men and included higher percentages of people with previously diagnosed diabetes and HF (Table 1). Other clinical characteristics, time from symptom onset to adiponectin measurements and clinical outcomes were similar between men and women (Table 1).
Table 1.
Baseline characteristics of adiponectin cohort.
| Biocohort characteristics | All participants (N = 120) | Men (N = 84) | Women (N = 36) |
|---|---|---|---|
| Age, mean (SD), years | 64.1 (12.4) | 62.7 (11.7) | 70.0 (12.7) |
| Race, White, n (%) | 116 (96.7) | 83 (98.8) | 33 (91.7) |
| BMI, mean (SD) | 28.9 (7.4) | 28.9 (7.6) | 28.8 (6.8) |
| Chest pain on presentation, n (%) | 105 (87.5) | 74 (88.1) | 31 (86.1) |
| Medical history, n (%) | |||
| Diabetes | 27 (22.5) | 15 (17.9) | 12 (33.3) |
| Heart failure | 10 (8.3) | 5 (6.0) | 5 (13.9) |
| Hypertension | 76 (63.3) | 51 (60.7) | 25 (69.4) |
| Myocardial infarction | 37 (30.8) | 27 (32.1) | 10 (27.8) |
| ACI-TIPI score, mean (SD), % | 83.0 (13.1) | 84.0 (13.6) | 82.1 (11.8) |
| Hospital reperfusion treatment, n (%) | |||
| Percutaneous coronary intervention | 97 (80.8) | 69 (82.1) | 28 (77.8) |
| Coronary artery bypass graft | 1 (0.8) | 1 (1.2) | 0 (0) |
| Confirmed AMI diagnosis, n (%) | 106 (88.3) | 73 (86.9) | 33 (91.7) |
| Time from symptom onset to adiponectin measurement, median (IQR), h | |||
| First measurement | 4.0 (3.2–5.7) | 3.9 (3.3–5.2) | 4.5 (2.9–7.1) |
| Second measurement | 7.5 (7.0–8.5) | 7.5 (6.9–8.3) | 7.8 (7.1–8.9) |
| Third measurement | 13.5 (13.0–14.6) | 13.5 (12.9–14.2) | 13.9 (13.1–15.1) |
| Randomized to GIK, n (%) | 55 (45.8) | 40 (47.6) | 15 (41.7) |
| One-year outcomes, n (%) | |||
| All-cause mortality | 10 (8.3) | 4 (4.8) | 6 (16.7) |
| Hospitalization due to heart failure | 9 (7.5) | 3 (3.6) | 6 (16.7) |
SD: standard deviation; BMI: body mass index; ACI-TIPI: acute cardiac ischaemia time-insensitive predictive instrument; AMI: acute myocardial infarction; IQR: inter quartile range; GIK: glucose–insulin–potassium.
Changes in serum adiponectin levels
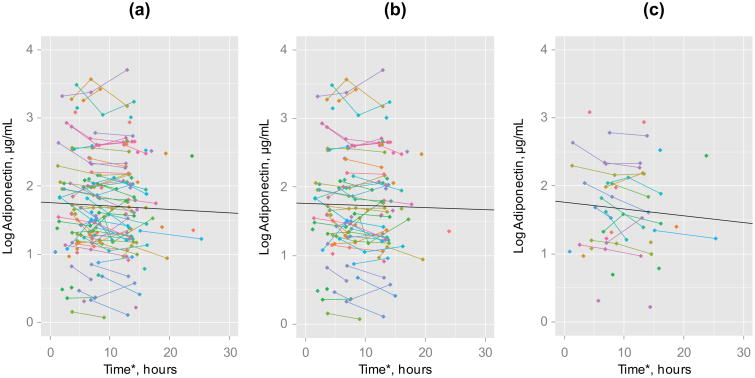
The mean and standard deviation (SD) values of the first, second and third adiponectin levels before log transformation were 7.62 (6.58), 6.79 (6.08) and 7.12 (5.99) μg/mL, respectively. Figure 1 shows a scatter plot illustrating the individual changes in log adiponectin levels over time for all participants and for men and women. This figure shows a wide range of starting values of log adiponectin and relatively small changes over time. Using linear mixed models, log adiponectin levels decreased by −0.005 μg/mL per hour (p = 0.035, Table 2). The estimated mean log adiponectin levels at 4, 8 and 14 h after symptom onset were 1.74, 1.72 and 1.69 μg/mL, respectively (Table 2). After stratifying the analysis by gender, men failed to have a significant decrease in log adiponectin levels over time; on the other hand, log adiponectin levels decreased by −0.01 μg/mL per hour in women (p = 0.02, Table 2).
Figure 1.
Individual changes in log adiponectin levels: (a) all participants, (b) men and (c) women. *Time from symptom onset to adiponectin measurement. Data points without lines represent those participants with only one adiponectin measure available. Regression line was obtained using linear mixed models.
Table 2.
Changes in log adiponectin levels.
| Mixed model | All participants (N = 120) | Men (N = 84) | Women (N = 36) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| β | SE | p | β | SE | p | β | SE | p | |
| Intercept | 1.76 | 0.08 | <0.001 | 1.76 | 0.09 | <0.001 | 1.76 | 0.14 | <0.001 |
| Timea (h) | −0.005 | 0.002 | 0.030 | −0.003 | 0.003 | 0.33 | −0.01 | 0.005 | 0.015 |
|
| |||||||||
| Log adiponectin measurement | Estimated meanb | Estimated meanb | Estimated meanb | ||||||
|
| |||||||||
| 4 ha | 1.74 | 1.75 | 1.72 | ||||||
| 8 h | 1.72 | 1.74 | 1.68 | ||||||
| 14 ha | 1.69 | 1.72 | 1.62 | ||||||
SE: standard error; β: regression coefficient.
Time from symptom onset to adiponectin measurement.
Estimated from linear mixed models.
Adiponectin and clinical characteristics
Log adiponectin levels decreased by increasing age (β coefficient = −0.01, p = 0.02), but no other patient characteristics were found to have significant association with plasma log adiponectin (Table 3). In addition, there was no association of baseline log hs-CRP, 30-day BNP, and Hgb A1C levels with the plasma log adiponectin (Table 3). Although not statistically significant, history of diabetes mellitus, history of heart failure, higher HbA1C levels, and higher hs-CRP levels tend to be associated with an increased in adiponectin levels in men, while these same factors showed a different relationship in women (Table 3).
Table 3.
Association between baseline clinical characteristics and log adiponectin levels.a
| All participants (N = 120) | Men (N = 84) | Women (N =36) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | β | p | β | p | β | p |
| Age (years) | −0.01 | 0.02 | −0.01 | 0.05 | −0.01 | 0.25 |
| Men | 0.07 | 0.63 | – | – | – | – |
| BMI (kg/m2) | 0.001 | 0.90 | <0.0001 | 0.93 | 0.002 | 0.92 |
| History of diabetes | −0.001 | 0.10 | 0.25 | 0.24 | −0.35 | 0.15 |
| History of MI | −0.06 | 0.66 | −0.03 | 0.85 | −0.04 | 0.83 |
| History of HF | −0.26 | 0.26 | 0.03 | 0.93 | 0.56 | 0.08 |
| Hgb A1C (%) | 0.02 | 0.69 | 0.06 | 0.41 | −0.09 | 0.46 |
| Log hs-CRP (mg/L) | 0.08 | 0.47 | 0.14 | 0.34 | −0.03 | 0.92 |
| BNP (pg/mL) | −0.0003 | 0.80 | −0.001 | 0.98 | −0.0001 | 0.58 |
β: regression coefficient; BMI: body mass index; BNP: brain-type natriuretic peptide; hs-CRP: high-sensitivity C-reactive protein; Hgb A1C: glycosylated haemoglobin; HF: heart failure; MI: myocardial infarction.
First available adiponectin measurement for each participant was used in this analysis.
Adiponectin and 30-day infarct size
There was no significant correlation between log-transformed serum levels of adiponectin and 30-day infarct size (r = −0.02, p = 0.86). Unadjusted and multivariable adjusted linear regression models showed no significant association between 30-day infarct size and log adiponectin levels (Table 4). In addition, stratifying the analysis by gender log adiponectin levels was not associated with infarct size for either gender (Table 4). Examination of the quartile analysis of adiponectin similarly showed no relationship between adiponectin and infarct size (Table 4). Excluding patients who died and were analysed using imputed values of large infarct size (59%) yielded similar results, for both continuous and quartiles of adiponectin.
Table 4.
Association between log adiponectin and 30-day infarct size.a
| All participants (N = 90) | Men (N = 64) | Women (N = 26) | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| β | p | β | p | β | p | |
| Unadjusted models | ||||||
| Age (years) | 0.19 | 0.21 | 0.09 | 0.62 | 0.46 | 0.14 |
| Men | −0.07 | 0.99 | – | – | – | – |
| BMI (kg/m2) | 0.11 | 0.62 | 0.18 | 0.43 | −0.15 | 0.78 |
| History of diabetes | −1.38 | 0.74 | −1.92 | 0.71 | −0.62 | 0.94 |
| History of MI | −4.52 | 0.27 | −3.79 | 0.40 | −7.64 | 0.45 |
| History of HF | −2.35 | 0.77 | −13.52 | 0.16 | 15.21 | 0.26 |
| Log hs-CRP (mg/L) | 2.56 | 0.45 | 2.72 | 0.47 | 2.73 | 0.75 |
| BNP (pg/mL) | 0.004 | 0.20 | 0.004 | 0.19 | 0.003 | 0.70 |
| GIK treatment | −9.60 | 0.006 | −10.32 | 0.01 | −7.81 | 0.29 |
| Log adiponectin, per 1 μg/mL | −2.39 | 0.32 | 0.08 | 0.98 | −8.92 | 0.07 |
| Adiponectin quartiles | ||||||
| Quartile 1 | Ref | Ref | Ref | Ref | Ref | Ref |
| Quartile 2 | −5.81 | 0.27 | 2.85 | 0.65 | 1.98 | 0.85 |
| Quartile 3 | −0.39 | 0.94 | 2.90 | 0.61 | −1.86 | 0.86 |
| Quartile 4 | −5.27 | 0.30 | 1.71 | 0.77 | −10.90 | 0.29 |
| Adjusted model | ||||||
| Age (years) | 0.19 | 0.21 | 0.07 | 0.69 | 0.47 | 0.16 |
| Men | 0.88 | 0.82 | – | – | – | – |
| GIK treatment | −9.62 | 0.006 | −10.24 | 0.01 | −8.91 | 0.25 |
| Log adiponectin, per 1 μg/mL | −1.10 | 0.64 | 0.52 | 0.85 | −5.30 | 0.32 |
| Adiponectin quartilesb | ||||||
| Quartile 1 | Ref | Ref | Ref | Ref | Ref | Ref |
| Quartile 2 | −4.65 | 0.37 | 0.49 | 0.94 | 7.58 | 0.47 |
| Quartile 3 | −0.06 | 0.99 | 1.01 | 0.86 | 8.48 | 0.45 |
| Quartile 4 | −2.81 | 0.57 | 1.34 | 0.81 | −0.85 | 0.94 |
β: regression coefficient; BMI: body mass index; BNP: brain-type natriuretic peptide; GIK: glucose–insulin–potassium; Hgb A1C: glycosylated haemoglobin; HF: heart failure; hs-CRP: high-sensitivity C-reactive protein; MI: myocardial infarction.
First available adiponectin measurement for each participant was used in this analysis.
Adjusted for the same variables as continuous adiponectin (age, male gender, and GIK treatment).
Adiponectin and 1-year composite outcome
There were 14 participants in the adiponectin cohort who either died or were hospitalized for HF during the 1-year follow-up period. Nine participants were hospitalized for HF, and 10 participants died during the 1-year follow-up period. In the unadjusted Cox-proportional models, log-transformed adiponectin levels and quartiles of adiponectin were not associated with the 1-year composite outcome (Table 5). Due to the low number of outcomes in this cohort, we did not run multivariable adjusted models, and we were unable to stratify the composite outcome by gender.
Table 5.
Association between log adiponectin and 1-year composite outcomes of all-cause mortality or hospitalization for heart failure (N = 120).a
| All-cause mortality (no. of outcomes = 10) | Hospitalization for heart failure (no. of outcomes = 9) | Composite of all-cause hospitalization mortality and for heart failure (no. of outcomes = 14) | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Unadjusted models | HR | p | HR | p | HR | p |
| Age | 1.11 | <0.001 | 1.08 | 0.011 | 1.09 | <0.001 |
| Sex, men | 0.28 | 0.046 | 0.19 | 0.021 | 0.30 | 0.025 |
| BMI | 0.81 | 0.043 | 0.76 | 0.016 | 0.82 | 0.021 |
| DM | 1.44 | 0.60 | 1.64 | 0.48 | 1.32 | 0.64 |
| History of MI | 5.68 | 0.012 | 2.94 | 0.10 | 3.12 | 0.035 |
| History of HF | 9.74 | <0.001 | 18.0 | <0.001 | 10.3 | <0.001 |
| Log hs-CRP (mg/L) | 3.25 | 0.024 | 3.91 | 0.034 | 3.20 | 0.014 |
| BNP (pg/mL) | 1.00 | 0.16 | 1.00 | 0.29 | 1.00 | 0.13 |
| GIK treatment | 0.29 | 0.12 | 0.14 | 0.07 | 0.31 | 0.07 |
| Log adiponectin, per 1 μg/mL | 0.54 | 0.20 | 0.43 | 0.10 | 0.51 | 0.09 |
| Adiponectin quartiles | ||||||
| Quartile 1 | Ref | Ref | Ref | Ref | Ref | Ref |
| Quartile 2 | 0.20 | 0.14 | 0.50 | 0.42 | 0.50 | 0.33 |
| Quartile 3 | 0.69 | 0.61 | 0.58 | 0.50 | 0.74 | 0.64 |
| Quartile 4 | 0.20 | 0.15 | 0.25 | 0.21 | 0.17 | 0.10 |
BMI: body mass index; BNP: brain-type natriuretic peptide; GIK: glucose–insulin–potassium; Hgb A1C: glycosylated haemoglobin; HF: heart failure; HR: hazard ratio; hs-CRP: high-sensitivity C-reactive protein; MI: myocardial infarction.
First available adiponectin measurement for each participant was used in this analysis.
Discussion
In this study of participants with ACS in the IMMEDIATE Trial, plasma adiponectin levels decreased slightly in the first few hours after the onset of ACS symptoms. This suggests that adiponectin may respond to the acute phase of CAD. The exact mechanism of the decrease in plasma adiponectin levels shortly following the onset of ACS is not known. One possible explanation is that rupture of the coronary plaque may lead to decrease in plasma adiponectin levels. Animals and human studies show that adiponectin is found in injured vessels rather than in intact vascular walls.27 Therefore, it is possible that adiponectin targets ruptured plaques resulting in its consumption in the circulating plasma.27,28 The findings of this study are in agreement with the previous literature. In one report, mean plasma adiponectin concentrations declined significantly 24 h (6.2 μg/mL) and 72 h (5.8 μg/mL) after myocardial infarction compared to the concentrations on admission (8.1 μg/mL).29 In addition, a recent study showed that plasma adiponectin levels fluctuated before and after percutaneous coronary intervention in subjects with ST-elevation myocardial infarction. Here, we observed a slight decrease in adiponectin values within the first 24 h of symptom onset.30 Although this reduction was minimal, the results of our study and others suggest that levels of systemic adiponectin can vary depending on the time of sampling after presentation.
When the analysis was stratified by gender, the small decrease observed in adiponectin levels was only noticeable in women. The mechanisms behind those gender differences are unclear. One possible explanation is variations in medical therapy at the very early stages of ACS between men and women. A study by Sullivan et al.,31 examining the duration of time for sequence of care in patients with suspected ACS, using data from the IMMEDIATE Trial, found significant delays in women compared to men. Therefore, the differences in access to early treatments may have affected adiponectin levels shortly after symptoms of ACS. Nevertheless, biological differences between genders could explain our findings. In the general population, women have higher plasma adiponectin levels than men,7,32 and several reports show that sex hormones affect adiponectin and may result in the differences seen between genders.33 Future research is required to explore the changes in adiponectin levels early after ACS between men and women, possibly focusing on the effect of early management of ACS on adiponectin.
When studying the association between plasma adiponectin levels on admission and patient's clinical and demographic characteristics, older individuals had lower adiponectin levels on admission. Other clinical characteristics that are known to be correlated with plasma adiponectin34 were not associated with this biomarker in our cohort. For example, in the general population, plasma adiponectin levels were highly correlated with Hgb A1C, markers of inflammation and body mass index (BMI).14 However, few observational studies in patients with established CAD did not find an association between plasma adiponectin and the presence of diabetes, and CRP levels.35,36 In addition, a study in subjects with CAD found no correlation between adiponectin and BMI in those with a BMI over 30 kg/m2.37 The average BMI in our cohort was around 29 kg/m2, and similarly, we did not observe an association between BMI and adiponectin. The findings in our study suggest the possibility that the role of systemic inflammation, body weight and glucose metabolism, as part of the relationship between adiponectin and atherosclerosis, may be decreased in the course of CAD.
Adiponectin-deficient mice have been found to have a larger infarct size following myocardial ischaemia and reperfusion compared with control mice.21 In addition, intracoronary injections of adiponectin in animals have shown to decrease infarct size.38 In contrast to animal models, we could not establish such a correlation between infarct size and plasma adiponectin levels. It is important to note that the lack of association between adiponectin and infarct size could be attributed to the heterogeneous cohort in this study. We included subjects with unstable angina, who did not develop an infarct, together with subjects who had a measurable infarct. This can bias the results towards the null. Nevertheless, when we ran a sensitivity analysis excluding subjects with infarct size of 0, the association remained non-significant. In addition, using only unadjusted hazard models, plasma adiponectin levels were not associated with the composite clinical outcomes or all-cause mortality or hospitalization for HF, during the 1-year follow-up period. The findings in our study support those from a number of observational studies that have found no association between plasma adiponectin levels and clinical outcomes, including future acute coronary events in patients with various degrees of CAD.15–17
This study has several limitations. Our sample size is small, especially for the stratified and sensitivity analyses, which might explain the non-significant p-values. Also, the lack of association between adiponectin and several clinical characteristics may be attributed to the number of subjects included. However, it represents the first study on serial measures of adiponectin assessed early in the course of ACS, and its relation with infarct size. In addition, the number of clinical outcomes was too small to perform adjusted analysis, limiting conclusions about the effect of adiponectin on all-cause mortality or hospitalization for HF. Not all participants in the biocohort had three blood measurements available for analysis. Nevertheless, we used a linear mixed model analysis, which has the ability to accommodate missing data points often encountered in longitudinal datasets.39 We used frozen samples for adiponectin measurements. However, adiponectin is considered to be a relatively stable adipokine with stable concentrations even after long periods of freezing; therefore, this would unlikely affect our results.40 Due to the nature of the design of this study, we do not have plasma adiponectin levels before the development of ischaemia and at the 30-day follow-up when infarct size was measured. Finally, in this study, we only measured total adiponectin levels; however, some evidence suggests that high-molecular weight adiponectin be more biologically active.41 Therefore, measuring specific adiponectin isoforms can provide additional prognostic information than that seen with total adiponectin.
The strength of this study is a unique data set that examines changes in plasma adiponectin levels in patients with ACS shortly after symptoms of ischaemia and the association of those levels with 30-day infarct size. We utilized data collected from a randomized controlled trial and had serial measurements of adiponectin within the first 24 h of ischaemic symptoms. There is an interest in the potential clinical use of adiponectin, and several pre-clinical and clinical trials are presently being conducted on drugs that can increase adiponectin levels, including intracoronary injections during ischaemia.38,42 However, the effect of plasma adiponectin levels in the setting of ACS remains unclear.
Conclusion
Plasma adiponectin levels tend to slightly decrease after symptoms of ischaemia in patients with ACS. This reduction, although weak, was more noticeable in women; but the clinical importance of this decrease in plasma adiponectin levels is unclear. However, this might indicate that adiponectin plasma levels may vary depending on the time of measurement. In this sample of patients with ACS, there was no relationship between plasma adiponectin levels and infarct size measured at 30 days, nor with composite clinical outcomes. This study adds to current evidence that has found no clear relationship between plasma adiponectin levels and adverse clinical outcomes in patients with ACS. Further studies, in larger samples of participants, will be required to explore gender differences in adiponectin levels shortly after ACS symptoms and the relationship between adiponectin and infarct size.aFirst available adiponectin measurement for each participant was used in this analysis.
Acknowledgments
Funding: The IMMEDIATE Trial was funded by the National Institutes of Health (NIH) cooperative agreement from National Heart, Lung and Blood Institute (U01HL077821, U01HL077826, U01HL077823). The IMMEDIATE Trial is registered at www.ClinicalTrials.gov (NCT00091507). The project described was supported by the National Center for Advancing Translational Sciences, NIH (Grant Numbers UL1 TR000073 and UL1 TR001064). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Hadeel Alkofide is supported by a scholarship from King Saud University, Riyadh, Saudi Arabia.
Footnotes
Declaration of conflicting interests: The authors declare that they have no conflicts of interest.
References
- 1.Bluher M. Clinical relevance of adipokines. Diabetes Metab J. 2012;36:317–327. doi: 10.4093/dmj.2012.36.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci. 2010;1212:E1–E19. doi: 10.1111/j.1749-6632.2010.05875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H, Cui J, Zhang C. Emerging role of adipokines as mediators in atherosclerosis. World J Cardiol. 2010;2:370–376. doi: 10.4330/wjc.v2.i11.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubota N, Terauchi Y, Yamauchi T, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 5.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29:2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 6.Aprahamian TR, Sam F. Adiponectin in cardiovascular inflammation and obesity. Int J Inflamm. 2011;2011:376909. doi: 10.4061/2011/376909. 8 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rathmann W, Haastert B, Herder C, et al. Differential association of adiponectin with cardiovascular risk markers in men and women? The KORA survey 2000. Int J Obes. 2007;31:770–776. doi: 10.1038/sj.ijo.0803471. [DOI] [PubMed] [Google Scholar]
- 8.Spranger J, Kroke A, Mohlig M, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–228. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- 9.Ouchi N, Kihara S, Funahashi T, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 10.Hopkins TA, Ouchi N, Shibata R, et al. Adiponectin actions in the cardiovascular system. Cardiovasc Res. 2007;74:11–18. doi: 10.1016/j.cardiores.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouchi N, Kihara S, Arita Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 12.Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura Y, Shimada K, Fukuda D, et al. Implications of plasma concentrations of adiponectin in patients with coronary artery disease. Heart. 2004;90:528–533. doi: 10.1136/hrt.2003.011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pischon T, Girman CJ, Hotamisligil GS, et al. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 15.Lindsay RS, Resnick HE, Zhu J, et al. Adiponectin and coronary heart disease: the Strong Heart Study. Arterioscler Thromb Vasc Biol. 2005;25:e15–e16. doi: 10.1161/01.ATV.0000153090.21990.8c. [DOI] [PubMed] [Google Scholar]
- 16.Lawlor DA, Davey Smith G, Ebrahim S, et al. Plasma adiponectin levels are associated with insulin resistance, but do not predict future risk of coronary heart disease in women. J Clin Endocrinol Metab. 2005;90:5677–5683. doi: 10.1210/jc.2005-0825. [DOI] [PubMed] [Google Scholar]
- 17.Sattar N, Wannamethee G, Sarwar N, et al. Adiponectin and coronary heart disease: a prospective study and meta-analysis. Circulation. 2006;114:623–629. doi: 10.1161/CIRCULATIONAHA.106.618918. [DOI] [PubMed] [Google Scholar]
- 18.Cavusoglu E, Ruwende C, Chopra V, et al. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur Heart J. 2006;27:2300–2309. doi: 10.1093/eurheartj/ehl153. [DOI] [PubMed] [Google Scholar]
- 19.Pilz S, Mangge H, Wellnitz B, et al. Adiponectin and mortality in patients undergoing coronary angiography. J Clin Endocrinol Metab. 2006;91:4277–4286. doi: 10.1210/jc.2006-0836. [DOI] [PubMed] [Google Scholar]
- 20.Lindberg S, Pedersen SH, Mogelvang R, et al. Usefulness of adiponectin as a predictor of all cause mortality in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Am J Cardiol. 2012;109:492–496. doi: 10.1016/j.amjcard.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 21.Tao L, Gao E, Jiao X, et al. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–1416. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 22.Wilson SR, Sabatine MS, Wiviott SD, et al. Assessment of adiponectin and the risk of recurrent cardiovascular events in patients presenting with an acute coronary syndrome: observations from the Pravastatin Or atorVastatin Evaluation and Infection Trial-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Am Heart J. 2011;161:1147–1155.e1. doi: 10.1016/j.ahj.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Schnabel R, Messow CM, Lubos E, et al. Association of adiponectin with adverse outcome in coronary artery disease patients: results from the AtheroGene study. Eur Heart J. 2008;29:649–657. doi: 10.1093/eurheartj/ehn009. [DOI] [PubMed] [Google Scholar]
- 24.Selker HP, Beshansky JR, Sheehan PR, et al. Out-of-hospital administration of intravenous glucose-insulin-potassium in patients with suspected acute coronary syndromes: the IMMEDIATE randomized controlled trial. JAMA. 2012;307:1925–1933. doi: 10.1001/jama.2012.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selker HP, Udelson JE, Massaro JM, et al. One-year outcomes of out-of-hospital administration of intravenous glucose, insulin, and potassium (GIK) in patients with suspected acute coronary syndromes (from the IMMEDIATE [Immediate Myocardial Metabolic Enhancement During Initial Assessment and Treatment in Emergency Care] Trial) Am J Cardiol. 2014;113:1599–1605. doi: 10.1016/j.amjcard.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onat A, Hergenc G, Dursunoglu D, et al. Relatively high levels of serum adiponectin in obese women, a potential indicator of anti-inflammatory dysfunction: relation to sex hormone-binding globulin. Int J Biol Sci. 2008;4:208–214. doi: 10.7150/ijbs.4.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okamoto Y, Arita Y, Nishida M, et al. An adipocyte-derived plasma protein, adiponectin, adheres to injured vascular walls. Horm Metab Res. 2000;32:47–50. doi: 10.1055/s-2007-978586. [DOI] [PubMed] [Google Scholar]
- 28.Ouchi N, Kihara S, Arita Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 29.Kojima S, Funahashi T, Sakamoto T, et al. The variation of plasma concentrations of a novel, adipocyte derived protein, adiponectin, in patients with acute myocardial infarction. Heart. 2003;89:667. doi: 10.1136/heart.89.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trifunovic D, Stankovic S, Marinkovic J, et al. Time-dependent changes of plasma adiponectin concentration in relation to coronary microcirculatory function in patients with acute myocardial infarction treated by primary percutaneous coronary intervention. J Cardiol. 2015;65:208–215. doi: 10.1016/j.jjcc.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Sullivan AL, Beshansky JR, Ruthazer R, et al. Factors associated with longer time to treatment for patients with suspected acute coronary syndromes: a cohort study. Circ Cardiovasc Qual Outcomes. 2014;7:86–94. doi: 10.1161/CIRCOUTCOMES.113.000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 33.Bottner A, Kratzsch J, Muller G, et al. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab. 2004;89:4053–4061. doi: 10.1210/jc.2004-0303. [DOI] [PubMed] [Google Scholar]
- 34.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 35.Von Eynatten M, Hamann A, Twardella D, et al. Relationship of adiponectin with markers of systemic inflammation, atherogenic dyslipidemia, and heart failure in patients with coronary heart disease. Clin Chem. 2006;52:853–859. doi: 10.1373/clinchem.2005.060509. [DOI] [PubMed] [Google Scholar]
- 36.Ang DS, Welsh P, Watt P, et al. Serial changes in adiponectin and BNP in ACS patients: paradoxical associations with each other and with prognosis. Clin Sci. 2009;117:41–48. doi: 10.1042/CS20080506. [DOI] [PubMed] [Google Scholar]
- 37.Knobler H, Benderly M, Boyko V, et al. Adiponectin and the development of diabetes in patients with coronary artery disease and impaired fasting glucose. Eur J Endocrinol. 2006;154:87–92. doi: 10.1530/eje.1.02054. [DOI] [PubMed] [Google Scholar]
- 38.Kondo K, Shibata R, Unno K, et al. Impact of a single intracoronary administration of adiponectin on myocardial ischemia/reperfusion injury in a pig model. Circ Cardiovasc Interv. 2010;3:166–173. doi: 10.1161/CIRCINTERVENTIONS.109.872044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards LJ. Modern statistical techniques for the analysis of longitudinal data in biomedical research. Pediatr Pulmonol. 2000;30:330–344. doi: 10.1002/1099-0496(200010)30:4<330::aid-ppul10>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 40.Shand B, Elder P, Scott R, et al. Biovariability of plasma adiponectin. Clin Chem Lab Med. 2006;44:1264–1268. doi: 10.1515/CCLM.2006.227. [DOI] [PubMed] [Google Scholar]
- 41.Pischon T, Hotamisligil GS, Rimm EB. Adiponectin: stability in plasma over 36 hours and within-person variation over 1 year. Clin Chem. 2003;49:650–652. doi: 10.1373/49.4.650. [DOI] [PubMed] [Google Scholar]
- 42.Phillips SA, Kung JT. Mechanisms of adiponectin regulation and use as a pharmacological target. Curr Opin Pharmacol. 2010;10:676–683. doi: 10.1016/j.coph.2010.08.002. [DOI] [PubMed] [Google Scholar]