Abstract
Insect serine proteases (SPs) and serine protease homologs (SPHs) participate in digestion, defense, development, and other physiological processes. In mosquitoes, some clip-domain SPs and SPHs (i.e. CLIPs) have been investigated for possible roles in antiparasitic responses. In a recent test aimed at improving quality of gene models in the Anopheles gambiae genome using RNA-seq data, we observed various discrepancies between gene models in AgamP4.5 and corresponding sequences selected from those modeled by Cufflinks, Trinity and Bridger. Here we report a comparative analysis of the 337 SP-related proteins in A. gambiae by examining their domain structures, sequence diversity, chromosomal locations, and expression patterns. One hundred and ten CLIPs contain 1 to 5 clip domains in addition to their protease domains (PDs) or non-catalytic, protease-like domains (PLDs). They are divided into five subgroups: CLIPAs (22). are clip1–5-PLD; CLIPBs (29), CLIPCs (12) and CLIPDs (14) are mainly clip-PD; most CLIPEs (33) have a domain structure of PD/PLD-PLD-clip-PLD0–1. While expression of the CLIP genes in group-1 is generally low and detected in various tissue- and stage-specific RNA-seq libraries, some putative GPs/GPHs (i.e. single domain gut SPs/SPHs) in group-2 are highly expressed in midgut, whole larva or whole adult libraries. In comparison, 46 SPs, 26 SPHs, and 37 multi-domain SPs/SPHs (i.e. PD/PLD-PLD≥1) in group-3 do not seem to be specifically expressed in digestive tract. There are 16 SPs and 2 SPH containing other types of putative regulatory domains (e.g. LDLa, CUB, Gd). Of the 337 SP and SPH genes, 159 were sorted into 46 groups (2–8 members/group) based on similar phylogenetic tree position, chromosomal location, and expression profile. This information and analysis, including improved gene models and protein sequences, constitute a solid foundation for functional analysis of the SP-related proteins in A. gambiae.
Keywords: phylogenetic analysis, gene duplication, chromosomal location, insect immunity, expression profiling, hemolymph protein, clip domain, serine protease cascade
Graphical Abstract
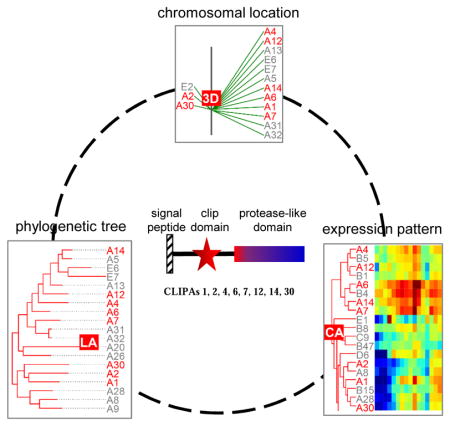
A group of eight CLIPAs with similar phylogenetic tree positions, chromosomal locations, and expression patterns
1. Introduction
Chymotrypsin-related serine proteases (SPs) form a large family of enzymes that hydrolyze peptide bonds at different rates and with various degrees of specificity (Rawlings and Barrett, 1993). For instance, trypsin cleaves at a high rate, specifically after most Lys and Arg residues, consistent with its role in protein digestion; pancreatic elastase cuts efficiently after any accessible small nonpolar residues (e.g. Ala) in many proteins, whereas human coagulation factor Xa cleaves only few protein substrates in plasma, after certain Arg residues and at a low rate (kcat). The S1 pocket of a protease interacts with the P1 residue of a protein substrate, governing its primary specificity (Schechter and Berger, 1967). Regulatory domains or regions in some nondigestive SPs provide additional specificity by localizing enzyme catalysis through specific interactions with activators, substrates, cofactors, and inhibitors (Kanost and Jiang, 2015; Krem and Di Cera, 2002). His, Asp and Ser residues in the active site of SPs are responsible for the acyl transfer mechanism of catalysis, with well-formed substrate binding clefts defining their specificities. SPs often contain a signal peptide guiding them to extracellular or granular locations, where they persist as inactive zymogens and then become activated by proteolytic cleavage at a particular peptide bond. In extracellular spaces, several SPs can constitute a cascade pathway in which one SP activates the zymogen of another in each step to trigger a rapid local response, such as blood coagulation or the complement system in mammals. In addition to active proteases, related serine protease homolog (SPH) genes encode SP-like sequences lacking one or more of the catalytic triad residues and, thus, proteolytic activity. Some cleaved SPHs are active as modulators of interacting SPs (Jiang et al., 2010; Park et al., 2010). While molecular mechanisms for such modulation are unclear, the SP-like fold and associated structural unit (e.g. clip domain) of SPHs are likely essential for the interactions that determine their biochemical functions.
SP-related proteins mediate insect immune responses (e.g. melanotic encapsulation, cytokine activation, and antimicrobial peptide induction) (Jiang et al., 2010). Like human clotting factors, insect SPs and SPHs form complex networks to stop bleeding and fight infection. In each insect species with a known genome, SP-related proteins form a large family with 60–400 members (Cao et al., 2015; Christophides et al., 2002; Ross et al., 2003; Waterhouse et al., 2007; Zhao et al., 2010; Zou et al., 2007; Zou et al., 2006). Their roles in defense and development have been explored in Drosophila melanogaster, Manduca sexta, Tenebrio molitor, and other insects (Kanost and Jiang, 2015; Park et al., 2010; Veillard et al., 2016). In mosquitoes, clip-domain SPs/SPHs have been named CLIPs (Waterhouse et al., 2007). As summarized by Cao et al. (2015), numbers of the clip-domain SP/SPH genes identified in genomes are 63 in Aedes aegypti, 55 in A. gambiae, 45 in D. melanogaster, 42 in M. sexta, and 49 in Tribolium castaneum.
Accurate gene models form a solid base for protein identification and elucidation of biochemical functions. Continuous efforts have been made to improve quality of the predicted genes after the initial genomes of D. melanogaster, A. gambiae, Apis mellifera, and other insects were published. The M. sexta genome project greatly benefited from next-generation sequencing, which provided RNA-seq data for the genome assembly, gene modeling and expression profiling (Kanost et al., 2016). We developed a method to select the best of the models from MAKER, Cufflink, Oases and Trinity programs (i.e. MCOT model) (Cao and Jiang, 2015). As this method has been automated and successfully applied in other insect genome projects (Cao and Jiang, 2017), we thought it would be interesting to test whether our method can further improve the latest release of A. gambiae gene models using the available RNA-seq data, with a focus on SP-like genes. Numerous discrepancies were identified between AgamP4.5 and corresponding AgMCOT models. To substantiate the observations and promote research on SP-related proteins in this species, we examined and improved the models in the official protein set (OPS), studied their domain organization and sequence diversity, classified them into the groups of CLIPs, GP(H)s and SP(H)s, and established an information system that contains systematic names, putative activation sites, predicted enzyme specificity, genomic locations, expression patterns, and phylogenetic relationships. Through further studies, we hope to establish a platform for comparing SP-related sequences from various insects and suggest functions for orthologs based on genetic and biochemical analyses in a few model species.
2. Materials and methods
2.1. Identification of A. gambiae SP-related proteins
OPS AgamP4.4 was downloaded from VectorBase (https://www.vectorbase.org/). Protein-coding genes were modeled using the MCOT pipeline (Cao and Jiang, 2015) by selecting the best for each gene from the OPS, TopHat-Cufflinks (Kim et al., 2013; Trapnell et al., 2012), Trinity (Haas et al., 2013) and Bridger (Chang et al., 2015) assemblies to constitute an AgMCOT protein set (unpublished data). Domains in the AgamP4.4 and AgMCOT sequences were identified by InterProScan5 v5.17 (Jones et al., 2014) in a local supercomputer. Proteins containing a chymotrypsin-like (i.e. S1 family), SP-related domain were extracted and pooled. After removal of redundant, alternatively spliced, and severely incomplete genes, the sequences were manually examined and improved according to characteristic features of the S1 SPs, such as signal peptide and conserved regions.
2.2. Properties of A. gambiae SP and SPH sequences
Sequences were separated into SPs or SPHs by examining the presence of a His-Asp-Ser catalytic triad as described before (Cao et al., 2015). Signal peptides were predicted using SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP/) (Petersen et al., 2011) and Signal-3L (http://www.csbio.sjtu.edu.cn/bioinf/Signal-3L/) (Shen and Chou, 2007). Some clip domains were identified by InterProScan5 and others by manual inspection of the sequences for a Cys doublet in the region close to the protease or protease-like domain (PD or PLD). SPs and SPHs with four additional Cys residues at particular locations (Cao et al., 2015; Jiang and Kanost, 2000) upstream of the doublet were designated CLIPs to indicate the presence of a clip domain (Kanost and Jiang, 2015). Residues 190, 216 and 226 (chymotrypsin numbering) (Perona and Craik, 1995) that form the primary substrate-binding pocket of PD were identified in the aligned sequences for predicting their substrate specificity (Cao et al., 2015).
2.3. Multiple sequence alignment and phylogenetic analysis
Multiple sequence alignments of the entire sequences in the CLIP, GP(H), and SP(H) groups were performed using MUSCLE (Edgar, 2004), one module of MEGA 7.0 (Kumar et al., 2016), under the default setting with maximum iterations changed to 1,000. The classification and naming were based on: 1) clip domain presence or absence, 2) position in a phylogenetic tree of non-CLIP SPs or SPHs, and 3) expression patterns. Neighbor-joining trees were constructed in a preliminary analysis of the SP-related sequences, and reliability of the trees was tested using a bootstrap method with 1,000 trials. Alignments of the three individual groups were converted to NEXUS format by MEGA, and phylogenetic analyses were conducted using MrBayes v3.2.6 (Ronquist et al., 2012) under the default model with the setting “nchains=12”. MCMC (Markov chain Monte Carlo) analyses were terminated after the standard deviations of two independent analyses were <0.01 for GP(H)s and CLIPs, and <0.02 for SP(H)s. FigTree 1.4.3 (http://tree.bio.ed.ac.uk/software/figtree/) was used to display the phylogenetic trees.
2.4. Chromosomal locations of the SP and SPH genes
For most of the SP-related genes, their genomic locations were available in the information lists of the AgamP4.4 or Cufflinks models. Retrieved position data were plotted using ArkMAP 2.0 (http://www.bioinformatics.roslin.ed.ac.uk/arkmap/) and improved using Adobe Illustrator.
2.5. Expression profiling of the SP-related genes
The 113 RNA-seq data sets of A. gambiae from previous research (Bonizzoni et al., 2012; Mead et al., 2012; Pinheiro-Silva et al., 2015; Rinker et al., 2013; Vannini et al., 2014) were downloaded from NCBI Sequence Read Archive (SRA) and converted to fastq format using SRA Toolkit. Reads were first trimmed with Trimmomatic (Bolger et al., 2014) to remove adaptors and low quality bases with the setting “SLIDINGWINDOW:4:30 LEADING:20 TRAILING:20 MINLEN:50”. Transcript sequences of the SP-like proteins in AgamP4.4 were replaced with the improved ones (Section 2.1). FPKM (fragments per kilobase of transcript per million mapped reads) values for genes in different libraries were calculated using Bowtie2 2.2.3 (Langmead and Salzberg, 2012) and RSEM 1.2.15 (Li and Dewey, 2011). FPKM values in libraries from biological replicates were averaged to represent gene expression in that type of samples. Hierarchically clustered gradient heatmaps of log2(FPKM+1) values were plotted using the clustermap function of Seaborn, a Python data visualization library, with the average linkage method and Euclidean matrix.
3. Results
3.1. Generation and classification of 337 reliable SP and SPH models in A. gambiae
In an effort to test if the MCOT approach (Cao and Jiang, 2015) is useful for improving the gene models in AgamP4.3 (https://www.vectorbase.org/), we upgraded the algorithm, automated data processing (Cao and Jiang, 2017), and generated an AgMCOT set by comparing, selecting, and naming the best models for individual genes from AgamP4.3, Cufflinks, Trinity and Bridger outputs (unpublished results). An InterPro domain scan of AgamP4.3 and AgMCOT sets resulted in a list of 732 SP-related sequences. After removing the redundant hits and sequences whose PD-like regions are shorter than 50% of a typical SP/SPH domain, we manually examined 366 candidates for flaws such as missing a signal peptide, incomplete PD/PLD domain, etc. After removal of isoforms of the same gene, the final list consists of 220 SPs and 117 SPHs (Table 1 and Table S1), one third of which have minor-to-major improvements as compared with the corresponding ones in AgamP4.5. Eighteen were absent in the AgamP4.5 release, and six of these eighteen (SP44, SP142, SPH12, SPH42, SPH113 and CLIPE32) were not detected in the genome assembly. Since the protein models we made are based on experimental data (i.e. RNA-seq reads), sequence comparison, model selection and manual curation, quality of the SP-related protein sequences is higher than Agam4.5, the newest official protein set (OPS) released on 2017-2-21.
Table 1.
Key structural features of the 337 SP-related proteins in A. gambiae
| name | T-C-E | activation cutting site | specificity | domain |
|---|---|---|---|---|
| CLIPA1 | LA3DCA | VVSH*SGEN | na | cH |
| CLIPA2 | LA3DCA | VVQR*TINE | na | 2cH |
| CLIPA3 | LA6bCC | LDVR*IVSN | na | cH |
| CLIPA4 | LA3DCA | EQNK*FNEI | na | cH |
| CLIPA5 | LA3DCK | KNGR*GVID | na | cH |
| CLIPA6 | LA3DCA | IGFR*ITGS | na | cH |
| CLIPA7 | LA3DCA | VGFR*ITGD | na | cH |
| CLIPA8 | LA3aCA | IMLR*FGEE | na | cH |
| CLIPA9 | LA3aCb | ? | na | cH |
| CLIPA10 | LA1qCE | KNPV*YVDG | na | cH |
| CLIPA12 | LA3DCA | VGFR*IGAG | na | cH |
| CLIPA13 | LA3DCf | IDVR*VGEE | na | cH |
| CLIPA14 | LA3DCA | IDIR*VGED | na | cH |
| CLIPA15 | LA2dCE | RKGR*VVGG | na | 5cH |
| CLIPA19 | LG2GCK | PRAL*STDL | na | cH |
| CLIPA20 | LA3aCf | ? | na | cH |
| CLIPA26 | LA2cCK | DEVH*LDFF | na | cH |
| CLIPA27 | LA5aCK | ISQA*VAGP | na | cH |
| CLIPA28 | LA3aCA | IGLR*AGLD | na | cH |
| CLIPA30 | LA3DCA | IEPR*LLND | na | cH |
| CLIPA31 | LA3DCG | ISLR*LNPE | na | cH |
| CLIPA32 | LA3DCJ | ISLR*LNPE | na | cH |
| CLIPB1 | LG2GCA | QMDR*IVGG | T(DGG) | cP |
| CLIPB2 | LG2GCK | VTDR*IIGG | T(DGG) | cP |
| CLIPB3a | LG2GCd | LADR*VIGG | T(DGD) | cP |
| CLIPB3b | LG2GCA | LADR*VIGG | T(DGG) | cP |
| CLIPB4 | LG2GCA | LTDR*VIGG | T(DGG) | cP |
| CLIPB5 | LG2mCA | TSDR*IFGG | T(DGG) | cP |
| CLIPB6 | LG2GCA | YTDR*IIGG | T(DGG) | cP |
| CLIPB7 | LG2dCA | LLMK*QKHS | na | cH |
| CLIPB8 | LG2FCA | YVAK*IRGG | T(DGG) | cP |
| CLIPB9 | LG2FCA | IGMR*IYGG | T(DGG) | cP |
| CLIPB10 | LG2FCA | LADR*IIGG | T(DGG) | cP |
| CLIPB11 | LF4GCA | SEDR*IAFG | T(DGG) | cP |
| CLIPB12 | LF4GCK | SNSR*ATWT | na | cH |
| CLIPB13 | LF1aCA | TVNR*IAHG | T(DGG) | cP |
| CLIPB14 | LG3aCK | FGVR*IIGG | T(DGG) | cP |
| CLIPB15 | LG4jCA | LADR*IYFG | T(DGG) | cP |
| CLIPB16 | LD4jCC | QCYR*GDFS | na | cH |
| CLIPB17 | LF2cCK | LLVK*IQDG | C(NGS) | 3cP |
| CLIPB18 | LF4GCK | SADR*MAYG | T(DGG) | cP |
| CLIPB19 | LG2GCK | TNTR*LIGS | T(EGG) | cP |
| CLIPB20 | LE3HCK | MDSG*SIGR | T(DGV) | cP |
| CLIPB36 | LG2GCA | TLRK*DTLT | na | cH |
| CLIPB41 | LD2mCf | VNTR*IIGG | T(DGG) | cP |
| CLIPB42 | LF4GCG | RVQL*IAYG | C(AGG) | cP |
| CLIPB43 | LF4GCJ | ? | na | cH |
| CLIPB44 | LF4GCC | TDDK*ISFG | T(DGG) | cP |
| CLIPB45 | LF3aCJ | IEEK*IANG | T(DGG) | cP |
| CLIPB46 | LG4jCK | ADEF*SFDS | T(DGG) | cP |
| CLIPB47 | LF2mCA | TVNK*IAFG | E(LGG) | 5cP |
| CLIPC1 | Lj4dCf | AVEL*IVDG | T(DGA) | cP |
| CLIPC2 | Lj2mCK | DQNL*IVGG | T(DGG) | cP |
| CLIPC3 | Lj2mCA | VVKL*IVGG | T(DGA) | cP |
| CLIPC4 | LH5BCA | IIDH*ISGR | T(DGG) | cP |
| CLIPC5 | LH5BCJ | LAYH*IIAG | T(DGS) | cP |
| CLIPC6 | LH5BCA | LTFH*IIDG | T(DGS) | cP |
| CLIPC7 | Lj2mCA | RQLK*GKGR | na | HcH |
| CLIPC9 | LH1aCA | KQFQ*IMHG | T(DGG) | cP |
| CLIPC10 | LH5BCf | LADH*IFNG | T(DGG) | cP |
| CLIPC12 | LE3HCK | PSWN*VWSD | T(DGG) | cP |
| CLIPC13 | LE3HCK | PLAL*VFSK | T(DGG) | cP |
| CLIPC14 | LE3HCK | PLAL*VYSE | T(DGG) | cP |
| CLIPD1 | LC2dCK | QLSK*IAGG | T(DGG) | cP |
| CLIPD2 | LC4aCf | DTER*IVGG | T(DGG) | cP |
| CLIPD3 | LC2cCE | SSGR*IVGG | T(DGG) | cP |
| CLIPD4 | LC2ECf | EHNR*VVGG | T(DGG) | cP |
| CLIPD6 | LC2ECA | QHNR*VVGG | T(DGG) | 2cP |
| CLIPD7 | LB4ECE | LQKR*IIGG | T(DGG) | cP |
| CLIPD8 | LB2dCE | PETR*IVGG | T(DGG) | cP |
| CLIPD9 | LB4ECE | RTNR*IVGG | T(DGG) | cP |
| CLIPD11 | Lj1aCC | DYYL*IYPI | T(DGA) | PHcH |
| CLIPD12 | LB4ECE | KSGR*VVGG | T(DGG) | cP |
| CLIPD13 | LB4ECE | AQRR*IVGG | T(DGG) | cP |
| CLIPD14 | Lj4dCf | ? | na | 2HcH |
| CLIPD20 | LC2ECG | THTR*VVGG | T(DGG) | cP |
| CLIPD22 | LB4ECE | PEPR*IVGG | T(DGG) | cP |
| CLIPE1 | LA4aCA | VISK*TPVV | na | 3cH |
| CLIPE2 | LA3DCH | VNDR*VSGT | na | 3cH |
| CLIPE4 | Lm3aCC | RLRK*GERV | na | 2HcH |
| CLIPE5 | Lm3aCC | IRQR*MSNG | T(DGG) | PHcH |
| CLIPE6 | LA3DCK | LGKR*FVPD | na | cH |
| CLIPE7 | LA3DCG | RNDH*GIGF | na | cH |
| CLIPE8 | Lr3aCb | ERRR*IESA | na | 2Hc |
| CLIPE9 | LN3GCG | IHAR*LQNK | T(DGG) | PHcH |
| CLIPE10 | Lm3aCC | GNTG*IVGP | T(DGG) | PHcH |
| CLIPE11 | LK4HCK | MLYR*FQQN | T(DGG) | PHcH |
| CLIPE12 | Lj2mCC | QEAK*QGKP | na | 2HcH |
| CLIPE13 | Lj2mCH | LGFF*IFGG | T(DGG) | PHc |
| CLIPE14 | LQ6aCG | ADER*IPPS | na | 2HcH |
| CLIPE15 | LK4dCA | LPLP*AFGR | T(DGG) | PHcH |
| CLIPE16 | LK4HCC | RYDF*SVNR | na | 2HcH |
| CLIPE17 | LK4HCC | RYDF*SVNR | na | 2HcH |
| CLIPE18 | Lj4jCK | SQCL*IFGG | T(DGG) | PHc |
| CLIPE19 | LP3aCG | YADI*SVGF | T(DGG) | PHcH |
| CLIPE20 | LQ6aCG | ADKL*IPPF | na | 2HcH |
| CLIPE21 | LP3DCE | PDQF*ISSG | T(DGG) | PHcH |
| CLIPE22 | LN3GCG | IHAR*LQNK | T(DGG) | PHcH |
| CLIPE23 | LN3GCG | INAR*LQNN | na | 2HcH |
| CLIPE24 | LN3GCH | IHTR*LQNN | T(DGG) | PHcH |
| CLIPE25 | LP6aCG | SSAT*AIGN | T(DGG) | PHcH |
| CLIPE26 | LQ3GCH | RLDA*SKFI | na | 2HcH |
| CLIPE27 | LQ3GCG | QRLK*DSKL | na | 2HcH |
| CLIPE28 | LQ3GCG | QRLK*DSKL | na | 2HcH |
| CLIPE29 | LK4HCK | MLYR*FQQN | na | 2HcH |
| CLIPE30 | Lr3aCE | VRQR*MENN | T(EAD) | PHc |
| CLIPE31 | Lr3aCG | ISKR*AGKS | na | 2Hc |
| CLIPE32 | LNnaCH | IHAR*LQSK | T(DGG) | PHcH |
| CLIPE33 | LN6aCH | IHTR*LQNN | T(DGG) | PHcH |
| CLIPE34 | LN3GCG | LQNK*QQIS | na | cH |
| GP1 | TD1aGD | DQSK*IVNG | C(GSG) | P |
| GP2 | TA3cGD | WFPR*IIGG | T(DGG) | P |
| GPH3 | TC1aGD | ? | na | H |
| GPH4 | TC4jGE | EVRS*IVGG | na | H |
| GP5 | TC1kGE | QGAR*IVGG | E(GID) | P |
| GP6 | TD4CGE | AGKR*IVGG | C(GSG) | P |
| GP7 | TD4CGE | AGKR*IVGG | T(DGG) | P |
| GP8 | TD6bGE | AGKR*IVGG | T(DGG) | P |
| GP9 | TD4CGE | VGHR*IVGG | T(DGG) | P |
| GP10 | TD4CGE | SGHR*IVGG | T(DGG) | P |
| GP11 | TD4dGE | RRAQ*IVGG | T(DAG) | P |
| GP12 | TA2AGE | SRPK*IVGG | C(SGG) | P |
| GP13 | TA2AGE | IRPP*IIEG | E(SGI) | P |
| GP14 | TD4jGE | KTYR*IVGG | C(GGD) | P |
| GP15 | TC1PGE | DGYR*VVGG | C(GGD) | P |
| GPH16 | TE1qGE | SENV*TANG | na | H |
| GP17 | TC1aGE | IWNR*IVGG | E(GID) | P |
| GPH18 | TC1cGE | VVGR*VADG | na | H |
| GP19 | TE1kGE | GGMR*VVNG | C(SGA) | P |
| GP20 | TA2AGE | TVNR*IIGG | C(SGN) | P |
| GP21 | TC1PGE | YVNR*VVGG | C(GGD) | P |
| GP22 | TC1PGE | YVNR*VVGG | C(GGD) | P |
| GP23 | TC1PGE | YVNR*VVGG | C(GGD) | P |
| GP24 | TD4CGE | NGHR*VVGG | T(DGG) | P |
| GP25 | TC1qGE | DSGR*IVGG | E(GAD) | P |
| GP26 | TD4CGE | VGQR*IVGG | T(DGG) | P |
| GP27 | TD1aGC | TTQR*IVGG | T(DRG) | P |
| GPH28 | TG1DGC | ENRL*ATYG | na | H |
| GPH29 | TG1DGC | TNRL*ATNG | na | H |
| GP30 | TB6aGC | HSGR*IVNG | T(DAS) | P |
| GP31 | TB6bGC | QSGR*IVNG | T(DSA) | P |
| GP32 | TB3aGC | QSGR*IVNG | T(DSA) | P |
| GP33 | TB3aGC | QSGR*IVNG | T(DTA) | P |
| GPH35 | TG1DGC | NQVR*IVSE | na | H |
| GPH36 | TE2HGC | ? | na | H |
| GPH37 | TG1DGC | ENRL*STYG | na | H |
| GPH38 | TG1DGC | PSPL*ATDG | na | H |
| GP39 | Tf2dGC | PNRR*IVNG | C(AGT) | P |
| GP40 | Tf1RGC | PNRR*IVNG | C(SGT) | P |
| GP41 | TH1GGC | RTNR*ITNG | E(SVS) | P |
| GP42 | TH1GGC | PSHR*ITNG | C(SGS) | P |
| GP43 | TH1GGC | PSHR*ITNG | C(SGS) | P |
| GP44 | TB2BGC | RADR*IVGG | T(DGG) | P |
| GP45 | TE1aGC | LLAK*VVNG | E(NVS) | P |
| GPH46 | Tj1FGB | PSAR*IVGG | na | H |
| GPH47 | TE1aGB | RNSR*IVNG | na | H |
| GPH48 | TA3cGB | VSPF*LVGG | na | H |
| GP49 | TH1FGB | PTHR*IVNG | C(SGS) | P |
| GPH50 | Tj1NGB | NNQR*VFGG | na | H |
| GP51 | Tj1RGB | DNAR*IVNG | C(GGS) | P |
| GP52 | TB2BGB | YNGR*IVGG | T(DGG) | P |
| GPH53 | TA1LGB | FLPF*IAGG | na | H |
| GPH54 | TA1LGB | AGPR*VTGG | na | H |
| GPH55 | TA1LGB | RSPR*LIGG | na | H |
| GP56 | TB1qGB | TSGR*IVGG | T(DGG) | P |
| GPH57 | TB2BGB | FQGR*IFGG | na | H |
| GP58 | TH1FGC | PSHR*VTNG | C(SGS) | P |
| GP59 | TC3FGB | WAGR*IVGG | C(GGD) | P |
| GP60 | TH1NGB | PSQR*IVNG | E(SVS) | P |
| GPH61 | TK1HGB | PDRR*INNG | na | H |
| GP62 | TC3FGB | WEGR*IVNG | C(GGD) | P |
| GP63 | TB2BGB | SLKK*IVGG | T(DGA) | P |
| GPH64 | TC3FGB | PNGR*IVGG | na | H |
| GP65 | TH1FGB | PTHR*ITNG | C(SGS) | P |
| GP66 | TH1NGB | PSAR*IVNG | E(SVS) | P |
| GPH67 | Tj1NGB | PRGR*VVGG | na | H |
| GPH68 | TM1NGB | KTPR*IRGG | na | H |
| GPH69 | TK1HGB | PSSR*ISNG | na | H |
| GP70 | TB2BGB | QNGR*IVGG | T(DGG) | P |
| GP71 | TH1FGB | PSHR*ITNG | C(SGS) | P |
| GP72 | TB2BGB | FSGR*IVGG | T(DGG) | P |
| GP73 | TH1FGB | PSHR*VVNG | C(SGS) | P |
| GP75 | TC2mGB | KGGR*IVGG | E(GVD) | P |
| GPH76 | TC3FGB | WKGR*IVGG | na | H |
| GPH77 | TK1HGB | RSSR*ISDG | na | H |
| GPH78 | TA1LGB | TLLR*DTIW | na | H |
| GP80 | TB6bGC | GLGR*IVNG | T(DGS) | P |
| GP81 | TH1NGB | PTRR*ITNG | E(SVS) | P |
| GP82 | TH1FGB | PSHR*IVNG | C(SGS) | P |
| GP83 | TB2BGC | VTGR*IFGG | T(DGG) | P |
| GPH84 | TA1LGB | TVVR*NVGF | na | H |
| GP85 | Tf1NGC | SLSK*VAGG | C(NGG) | P |
| GP86 | TG1RGC | PSGR*ITNG | C(SGT) | P |
| GPH87 | TC2mGB | FSPR*IAGG | na | H |
| GP88 | TB2BGC | ATGR*IVGG | E(SLA) | P |
| GPH89 | TK1HGC | RSQR*ILNG | na | H |
| GPH90 | TK1HGC | LNAR*ISGG | na | H |
| GPH91 | TK1HGB | PDRR*INNG | na | H |
| GP92 | TA4FGB | PSGR*VVGG | C(SGS) | P |
| GPH93 | TA4FGB | PQQR*LIGG | na | H |
| GPH94 | TK1HGB | RTGR*INNG | na | H |
| GPH95 | TK1HGB | RSAR*IADG | na | H |
| GP96 | TD3cGD | NMAR*VVGG | T(DGS) | P |
| GP97 | TE4jGD | RSSR*IVNG | E(GVS) | P |
| GP98 | TB2BGC | PSPF*IFGG | T(EGG) | P |
| GPH99 | TA4FGA | PERR*IFGG | na | H |
| GP100 | TB2BGC | KSAR*IVGG | T(DGS) | P |
| GPH101 | TG1DGC | GNKL*ATDG | na | H |
| GPH102 | TG1DGC | PYTY*SATY | na | H |
| GP103 | TD4CGE | SNHR*IVGG | T(DGG) | P |
| SP1 | PC2cSq | QQQR*IVGG | T(DGG) | P |
| SP2 | PD1BSG | PMAL*IIGG | E(GAD) | P |
| SP3 | PD1BSG | AANY*IVDG | E(GVD) | P |
| SP4 | PD1BSG | PLAP*IIGG | E(GSD) | P |
| SPH5 | PG1aSB | ? | na | H |
| SPH6 | PG2GSA | APER*LITS | na | H |
| SP7 | PG2HSA | VVDL*IVGG | T(DGA) | P |
| SPH8 | Pk1JSk | FDCG*VRGQ | na | H |
| SPH10 | PA4jSN | QSGR*ILNG | na | H |
| SP11 | Ph6bSN | GSQR*IIGG | T(DGG) | P |
| SPH12 | PAnaSN | QSGR*IVNG | na | H |
| SP13 | PA6aSN | QSGR*IING | T(DTA) | P |
| SPH14 | PA3aSN | RSGR*ITNS | na | H |
| SPH15 | PA6bSN | QSGR*IFNG | na | H |
| SPH17 | PF2NSE | ASFR*VLGG | na | H |
| SP18 | PF2NSE | TSSR*IVGG | T(DGG) | P |
| SP19 | PG2HSA | VVQL*IVGG | T(DGA) | P |
| SP20 | PA3aSN | QSGR*IVNG | T(EVA) | P |
| SP21 | PF2NSc | DASR*IVGG | T(DGG) | P |
| SP22 | PF2NSf | QEIR*IVGG | T(DGG) | P |
| SPH23 | PC2qSf | RNPK*IMHG | na | H |
| SP24 | PF2NSf | NNSK*IVGG | T(DGG) | P |
| SP25 | PF2NSB | RLTR*IVGG | T(DGG) | P |
| SP26 | PF2NSf | INER*IVGG | T(DGG) | P |
| SPH27 | PG3BSR | VTGV*VSFG | na | H |
| SP28 | Pm2cSL | YNNL*ILGG | C(GAT) | P |
| SPH29 | PP4dSN | GRRK*VQTN | na | H |
| SP30 | Ph1GSN | PFQR*ITNG | E(SVS) | P |
| SP31 | Ph1GSN | STDR*ITNG | E(SVS) | P |
| SP33 | Ph1GSN | STDR*VVNG | C(SGS) | P |
| SPH34 | PF2NSm | HGQR*IVAG | na | H |
| SPH35 | PA3aSN | QSGR*IING | na | H |
| SPH36 | PA3aSN | QSGR*IING | na | H |
| SPH37 | PA6aSN | QSGR*LVNG | na | H |
| SPH38 | Ph1DSN | GNWL*DTYG | na | H |
| SP39 | PA6aSL | HSGR*IVNG | T(DGS) | P |
| SP40 | PA6aSL | HSGR*IVNG | T(DGS) | P |
| SPH42 | PEnaSL | AFNR*TLFN | na | H |
| SP44 | PDnaSA | PTDA*IVRG | T(DGG) | P |
| SP45 | PG3mSR | MDNR*IVGG | T(DGA) | P |
| SPH46 | PE2PSA | ? | na | H |
| SPH47 | PE2PSA | ? | na | H |
| SPH48 | PE2PSA | EDGR*IFEN | na | H |
| SP49 | PA3aSL | HSGR*IING | T(DVS) | P |
| SPH50 | PJ3aSE | RDKR*MAAG | na | H |
| SPH51 | PG5aSA | EQEP*ECGD | na | H |
| SPH52 | Pk2mSA | ? | na | H |
| SP53 | PG2HSB | VVQL*IVGG | T(DGA) | P |
| SP54 | PL1kSA | KQGL*IFGG | E(SSV) | P |
| SP55 | PG4HSN | FYSF*GSGG | T(DGG) | P |
| SP56 | PG2dSN | VVGA*IVGG | T(DGG) | P |
| SP57 | PA3aSN | SSYR*IVNG | T(DGG) | P |
| SP58 | PA3aSN | MSFR*IVNG | T(DAG) | P |
| SP61 | PF4dSk | NSLR*VIGG | T(DGG) | P |
| SP62 | PD1qSc | GNDR*VVGG | C(GGD) | P |
| SP63 | PA1mSk | PDRR*IVNG | C(GSG) | P |
| SP64 | PF2NSR | RTNR*IVGG | T(DGG) | P |
| SP66 | Ph4GSN | HNNE*TLGN | T(DGS) | P |
| SP67 | Ph4GSc | QNNE*TLGN | T(DGV) | P |
| SP68 | PG3BSH | ISER*IIAY | T(DGG) | P |
| SP70 | PP1cSH | SEYL*IQNG | C(STT) | P |
| SP71 | PD2dSJ | DDSK*IVGG | C(GGD) | P |
| SP72 | PA4jSN | NGER*IVGG | T(DGG) | P |
| SP73 | PD1kSN | LGER*IVGG | T(DGG) | P |
| SP74 | PB4BSJ | SGNL*IVGG | T(DGG) | P |
| SP75 | PB4BSJ | ATNM*IVGG | T(DGG) | P |
| SP76 | PB4BSJ | WNNM*IVGG | T(DGG) | P |
| SP77 | PB4BSJ | TANM*VVGG | T(DGG) | P |
| SPH78 | PB1JSJ | AKRL*QIGG | na | H |
| SPH79 | PB1JSJ | GLGQ*AQNG | na | H |
| SPH80 | PB1JSf | AKGQ*QIGG | na | H |
| SP81 | PD3mSJ | IKPD*VANG | E(TGI) | P |
| SP101 | PQ3cSq | TIPL*IRKG | C(STT) | P3H |
| SP105 | PL3aSB | REGL*VKGG | E(GSI) | PH |
| SPH106 | PE3JSE | ? | na | 2H |
| SPH107 | PE3JSE | ? | na | >3H |
| SP108 | PP3JSE | SVYL*IHNG | C(STT) | PH |
| SP109 | PP3JSJ | SVYL*IHNG | C(STT) | P2H |
| SP111 | PG3BSf | DLTV*AYGG | T(DGG) | PH |
| SP112 | Pm1qSA | PMGL*VTKG | E(NAA) | PH |
| SPH113 | PEnaSJ | KYSC*IVGQ | na | 3H |
| SP114 | PL3aSN | RQEL*VKGG | E(GSI) | PH |
| SP115 | PN3KSR | SVYL*IHNG | E(SIT) | P2H |
| SP116 | PN6bSR | TIHL*VQNG | E(SIT) | P3H |
| SP117 | PN3KSR | SVYL*IHNG | E(SIT) | P3H |
| SP118 | PN6bSq | SVYL*IHNG | E(SIT) | PH |
| SP119 | PP3aSq | SIYL*IHNG | C(STT) | P3H |
| SP120 | PG3BSA | SHIE*SYGG | T(DGG) | PH |
| SP122 | Pm2mSA | VNLL*ITNG | C(STT) | PH |
| SP123 | PG3BSR | GFYG*AFGG | T(DGG) | PH |
| SPH124 | PG3BSN | VGYH*SFNA | na | 2H |
| SP125 | PP5aSL | TTYL*IHNG | C(STT) | P3H |
| SP126 | PL3aSR | RKGL*VKGG | E(GSI) | PH |
| SP127 | PN3KSL | TIHL*VHNG | E(SIT) | P3H |
| SP128 | Pm2cSL | RVNL*ILGG | C(GAA) | PH |
| SP130 | PP4dSL | SVFL*IHNG | C(GTT) | P3H |
| SPH132 | PE3aSR | ERRK*INGM | na | 2H |
| SP133 | PA3aSL | GFLR*IVNG | T(DGS) | PH |
| SP134 | Pm2mSA | RKVK*TIYL | E(SMT) | PH |
| SP135 | Pk3aSA | ALPK*QPSE | C(NTA) | PH |
| SP136 | PG4HSq | FYSF*GSGG | T(DGE) | P2H |
| SP137 | Pm4dSR | YAKL*ILGG | C(TSA) | PH |
| SP138 | Pk4aSN | RPIR*VAGS | C(NTA) | PH |
| SP139 | PG3BSH | ISSY*AFGG | T(DGG) | PH |
| SP140 | PP3cSH | SQYL*IHNG | C(STT) | P3H |
| SP141 | PP1cSH | SEYL*IQNG | C(STT) | P3H |
| SP142 | PPnaSH | SVYL*IHQG | C(SST) | P3H |
| SP143 | PG4dSH | IVPA*VSGG | T(DGG) | PH |
| SP144 | PP3JSH | TQYY*IHNG | C(SST) | P3H |
| SP201 | PJ3ESD | RTRK*IVGG | T(DGS) | CUBP |
| SP202 | PJ3ESm | KLNR*IVNG | T(DGS) | CUBP |
| SP203 | PJ3ESD | KTPT*IVNG | T(DGS) | CUBP |
| SP204 | PJ3ESD | RTPT*IVNG | T(DGS) | CUBP |
| SP205 | PJ3ESD | RTAK*IVGG | T(DGS) | CUBP |
| SP206 | PJ3ESD | RTSK*IVNG | T(DGS) | CUBP |
| SP207 | PL2cSB | ANPL*VTHG | E(SSV) | GdP |
| SP208 | Pk2cSA | IRSR*IIGG | C(SSS) | 2GdP |
| SP209 | Pk2cSE | FSHY*SING | C(VGV) | GdP |
| SP210 | Pk2cSA | FNRL*SING | C(VGV) | 2GdP |
| SP212 | PF1sSq | ESVR*IVGG | T(DGG) | Fig1 |
| SP213 | PG1eSA | YGAR*VVHG | T(DGG) | Fig1 |
| SP214 | PA1mSJ | ATKR*IVGG | T(DGG) | Fig1 |
| SPH216 | Ph5aSf | PTSQ*NIGL | na | Fig1 |
| SP217 | Pk2cSA | AEAY*IIGG | C(SAV) | Fig1 |
| SP218 | PC1kSB | NMLR*IIGG | T(DGG) | Fig1 |
| SP219 | Pk1aSR | LRSR*ITDG | E(SSV) | Fig1 |
| SPH220 | Pk2cSA | LEQR*IAGG | na | Fig1 |
T-C-E: identification codes for the phylogenetic trees (T) (Fig. 2), chromosomal (C) locations (Fig. 3), and expression (E) profiles (Figs. 4–6), as indicated in the figure legends. Activation cleavage sites (*) are predicted based on the domain scan results for SPs, usually before the conserved IVGG motif. For most clip-domain SPHs, activation cleavage sites are predicted to be next to R/K between Cys-3 and Cys-4 in the clip domain, based on the existing biochemical data. Enzyme specificity of SPs is predicted based on Perona and Craik (1995). T, trypsin; C, chymotrypsin; E, elastase; na, not applicable. Letters in parentheses are residues that determines the primary specificity. In the domain column, “c” stands for clip, “P” for serine protease or PD, and “H” for serine protease homolog or PLD. For SP201 to SPH220, detailed domain structures are shown in Fig. 1.
As CLIPs are key components of insect immune SP-SPH networks (Kanost and Jiang, 2015), we have identified 110 SP-related proteins that contain 1 to 5 clip domains and named the 55 newly discovered CLIPs based on an initial phylogenetic analysis (data not shown). To avoid confusion, we did not change names of the ones reported before (Christophides et al., 2002; Waterhouse et al., 2007), even though six CLIPs (A19, C7, E1, E2, E6, E7) are assigned to different clades in our new analysis. According to a preliminary analysis of the expression patterns and phylogenetic relationships, we named 100 of the other 227 proteins gut proteases (GPs) or homologs (GPHs), based on containing a single PD/PLD with a typical size of 230 residues and higher expression level than CLIPs. The 127 SP(H)s were named by considering their domain structures: SP1–SP81 contain a single PD/PLD, SP101–SP144 contain 2 to 4 PDs/PLDs, and SP201–SPH220 contain a PD/PLD along with other non-clip regulatory domains (Table S1). Consequently, the SPs/SPHs are divided into three groups: 110 CLIPs, 100 GP(H)s and 127 SP(H)s. In the following, “SP-related proteins” or “SPs/SPHs” refer to all or part of the 337 regardless of their groups, whereas “SP(H)s” specify those in the third group.
3.2. General structural features of the 337 SP-related proteins
Consistent with their expected extracellular functions, 324 of the 337 sequences (except for SP133, 214; SPH10, 14, 35, 42, 106, 107, 113, 220; CLIPs B46, D22 and E22) are predicted to have a signal peptide for secretion (Table S1). The presence of catalytic residues His, Asp and Ser in the conserved motifs of TAAHC, DIAL and GDSGGP was used to predict if a protein is an active SP after activation. It is possible that some of the 220 SPs are catalytically inactive due to the lack of other essential structural features not considered. In contrast, none of the 117 SPHs are expected to be active proteases due to substitution of 1–3 of the catalytic residues, even though overall folding of the PDs and PLDs is likely similar due to sequence conservation.
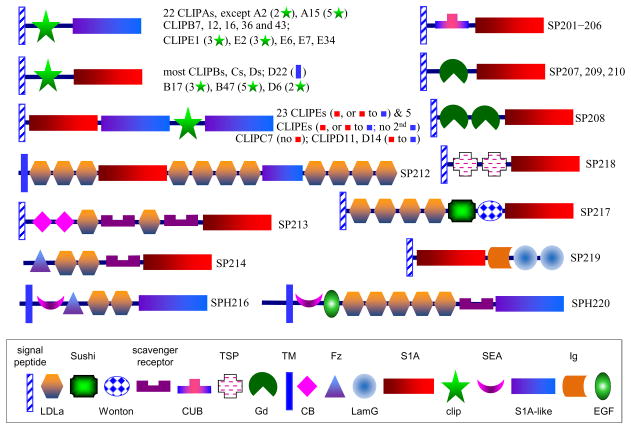
Members of the CLIP, GP(H) and SP(H) groups differ in domain structure. Most of the 100 GP(H)s and 72 SP(H)s (SP1 to SP81) consist of a signal peptide, a pro-region, and a PD/PLD. The CLIPs in subgroups A–D contain a signal peptide, 1 to 5 clip domains, and a PD/PLD (Fig. 1). CLIPD22 has a transmembrane region 20 residues away from its amino terminus. CLIPEs, as well as CLIPs C7, D11 and D14, have a structure of signal peptide-PD/PLD-PLD-clip-PLD0–1. Thirty-seven SP(H)s (SP101 to SP144) have two or more PD/PLD domains. In eighteen of the multi-domain SP(H)s (SP201 to SPH220), we identified thirteen types of other domains, namely LDLa for low-density lipoprotein receptor class A (21), CUB for C1r/s, Uegf & Bmp1 (6), Gd for Gastrulation defective (6), SR for scavenger receptor (3), CB for chitin binding (2); LamG for laminin G (2), Fz for frizzled (2), TSP for thrombospondin (2), Ig for immunoglobulin (1), SEA for sperm protein, enterokinase and agrin (1), EGF for epidermal growth factor (1), Sushi (1), and Wonton (1) (Fig. 1). Numbers in parentheses are total numbers of the domains identified in all these proteins. These structural modules probably function in interactions of the proteases with themselves or partners and form SP-SPH cascades to mediate physiological processes and to guide proper domain interactions needed to control catalytic activities and localize proteolytic reactions. This notion is consistent with the conserved domain structures of SP217-ModSP, SP212-Nudel, CLIPA15-Masquerade, and several other orthologous groups in a phylogenetically wide range of holometabolous insects, including beetles, moths, bees, mosquitos, and flies (Christophides et al., 2002; Ross et al., 2003; Waterhouse et al., 2007; Zou et al., 2007; Zou et al., 2006). Drosophila ModSP, Nudel, and Masquerade are 1:1 orthologs of the mosquito proteins.
Fig. 1.
Domain organization of 128 multi-domain SPs and SPHs in A. gambiae. Signal peptide and other structural elements (see symbols in inset) were predicted as described in Section 2.2. The schematic diagrams are not drawn to scale.
3.3. Phylogenetic relationships, genome locations and expression patterns of the 110 CLIPs
Clip domains constitute the largest group of regulatory structures in the SP-related proteins of A. gambiae. These disulfide-bridged units exist in insect and crustacean SPs/SPHs involved in defense, development and other processes (Kanost and Jiang, 2015). In total, 126 clip domains were identified in 63 SPs and 47 SPHs – seven CLIPs have 2, 3 or 5 clip domains (Fig. 1). Seventy-one CLIPs have one clip domain at the amino terminus; 23 CLIPEs, CLIPs C7, D11 and D14 have a CLIP domain between PLDs; other 5 CLIPEs have their clip domain at the carboxyl end. In this study, we have identified 55 CLIPs not previously annotated: 8 CLIPA (19, 20, 26–28, 30–32), 9 CLIPB (3b, 36, 41–47), 4 CLIPC (9, 12–14), 7 CLIPD (9, 11–14, 20, 22), and 27 CLIPE (8–34). Together with those reported before, 22 CLIPAs, 29 CLIPBs, 12 CLIPCs, 14 CLIPDs, and 33 CLIPEs exist in A. gambiae. A majority of the mature CLIPEs have a distinct domain organization of P(L)D-PLD-clip-PLD0–1. The PD, PLD, and clip domain may organize into higher structures to perform complex functions.
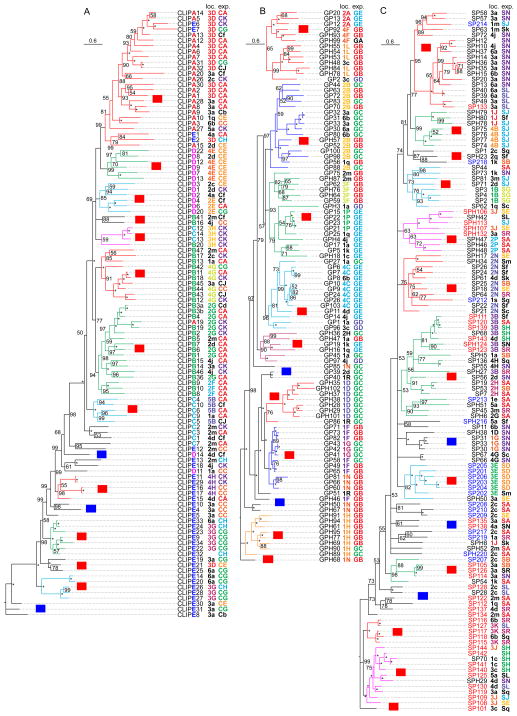
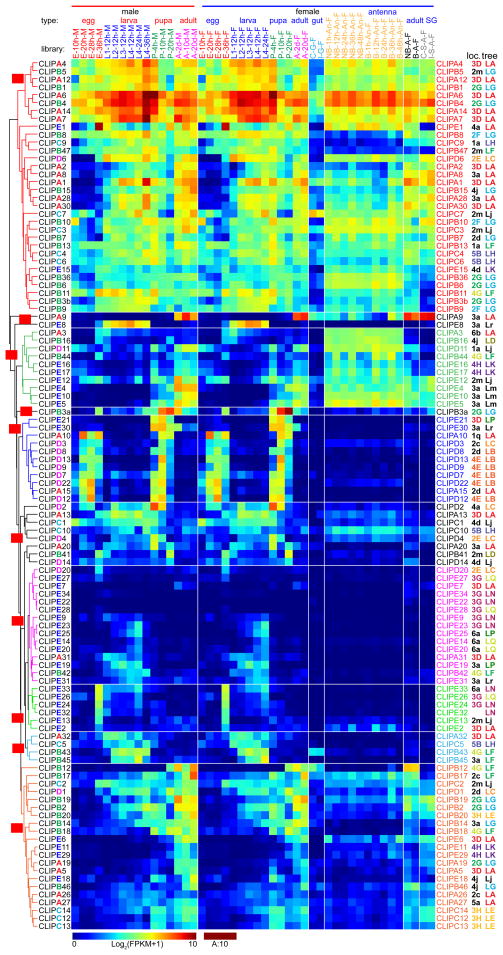
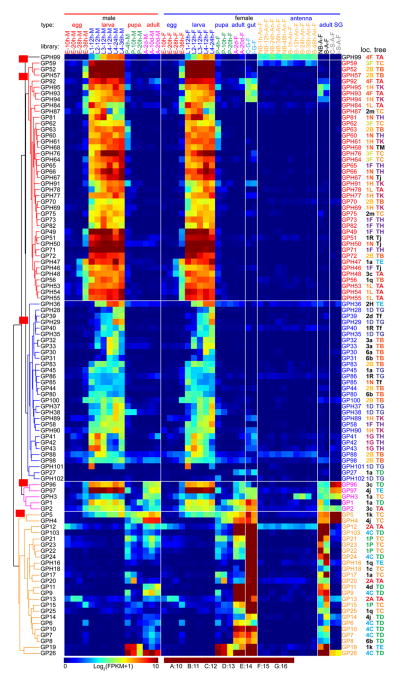
A phylogenetic tree based on alignment of complete sequences of the 110 CLIPs reveals evolutionary relationships among them (Fig. 2A). Separation of the five clades is obvious: all but one CLIPA and CLIPs E1, E2, E6, E7 form a monophyletic group with a probability (P) of 99; most CLIPDs (apart from D11, D14) form two groups (P: 99 and 85); CLIPBs and CLIPA19 forms three groups (P: 88, 100, 94); CLIPCs (except for C7) form three groups (P: 100); most CLIPEs and C7 form seven groups (P: 95–100). The grouping of relevant genes generally agrees with their locations in ten regions (i.e. 2E–G, 3D, 3G, 3H, 4E, 4G, 4H, 5B) of the chromosomes 2R (2nd half), 3 and X (Fig. 3). Apparently, rounds of gene duplication have given rise to the clusters of closely related CLIP genes. Since regulatory elements may be duplicated along with the coding regions in members of a gene cluster, we anticipated and then observed a considerable level of consistency in expression patterns (Fig. 4, Table S2) among genes with similar sequence and chromosomal location. For example, genes of CLIPA1, 2, 4, 6, 7, 12, 14, and 30 in tree group “LA” (Fig. 2A) reside in the same region of chromosome 3L (Fig. 3, location group “3D”) and have similar expression profiles (Fig. 4, expression group “CA”). We have observed 14 similar three-way agreements (phylogenetic tree, chromosomal location and expression pattern), each with 2 to 8 genes and involving a total of 46 CLIPs. Transcript levels of the CLIPAs, Bs and Cs in expression group “CA” are much higher than those of CLIPEs in “CG” and “CH”, especially in the adults. The profiles of CLIPs A9, A10, B3a, B12, B17, B19, C1, C14, D2, D12, E4, and E8 mRNA levels are distinct in the 45 cDNA libraries, making them attractive targets for functional studies.
Fig. 2.
Phylogenetic relationships of the 110 CLIPs (A), 100 GP(H)s (B) and 127 SP(H)s (C) in A. gambiae. Entire sequences of the proteins in each group were aligned and the phylogenetic tree was constructed using MrBayes as described in Section 2.3. Probability values for branches are indicated near the branching points, with “*” representing 100. Subtrees of similar sequences in various colors are assigned with a series of group IDs, which start with L for CLIPs, T for gut protease (homologs), or P for serine protease (homologs). Those on red background with the 2nd letter in capital represent reliable monophyletic groups. Other branches are assigned with IDs on blue background with the 2nd letter in lowercase. Chromosomal location (Fig. 2) and expression (Fig. 4) IDs are listed next to gene names, in various colors based on the IDs. In panel A, the letter A, B, C, D, or E in CLIP gene names are marked with different colors. In panel C, SP1xx are colored red and SP2xx blue. CLIPE32, SP44, SP142, SPH12, SPH42, and SPH113 gene are not identified in the genome assembly and, thus, without location IDs.
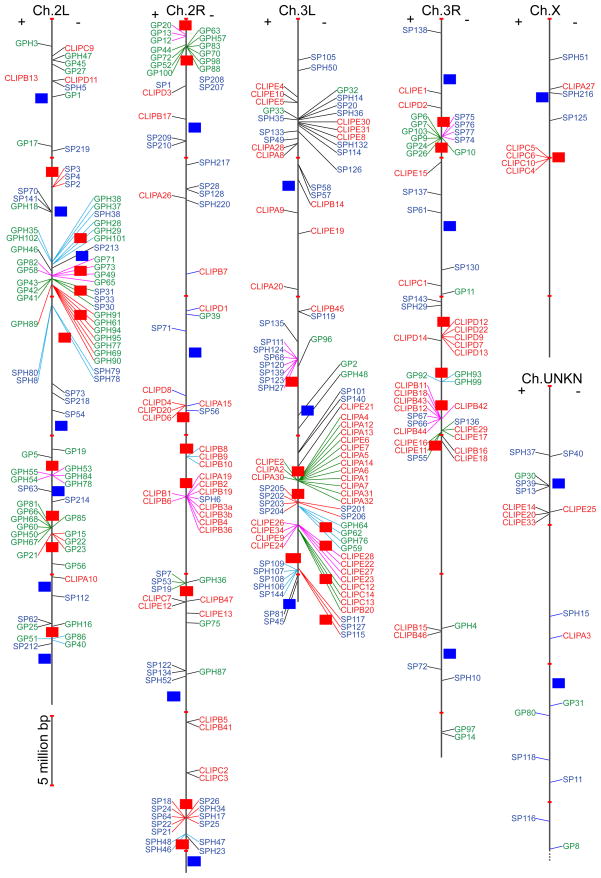
Fig. 3.
Chromosomal locations of the 331 genes coding for A. gambiae SP-related proteins. As indicated by the scale bar, positions of the genes are plotted in proportion on chromosomes, with “+” and “−” indicating positive and negative strands on the left and right, respectively. Note that CLIPE32, SP44, SP142, SPH12, SPH42, and SPH113 genes are not found in the genome assembly. Names of CLIPs (red), GP(H)s (green) and SP(H)s (blue) are linked to their locations by straight lines. Adjacent genes with high sequence similarities are grouped by lines in the same color and marked by a series of location IDs on red background for different chromosomal segments. Regions in between are labeled with IDs on blue background. In these IDs, the 1st letter (1–6) represent chromosome (Ch.) 2L, 2R, 3L, 3R, X and UNKN (unknown), respectively, and the 2nd letter in capital for gene clusters or in lowercase for other regions.
Fig. 4.
Transcript profiles of the 110 CLIPs in A. gambiae. The CLIP mRNA levels in 45 types of the tissue samples, as represented by log2(FPKM+1) values, are shown in the hierarchically clustered gradient heatmap from blue (0) to maroon (10). The values, rounded to the closest integers and, if equal to 10, converted to A, are used to label the color blocks. Subtrees of genes with similar expression patterns are in different colors and assigned with group IDs beginning with C (for CLIP) on red background. The 2nd letter in capital indicates a reliable monophyletic group. Group IDs with the 2nd letter in lowercase are assigned to other branches. The letter A, B, C, D, or E in CLIP gene names are marked on the left with different colors. See Fig. 4 legend for descriptions of library types, chromosomal location IDs, and phylogenetic tree IDs.
3.4. Evolution, location, and expression of the 100 putative GP(H)s in A. gambiae
By definition, GP(H)s are serine proteases and their homologs expressed in midgut tissues. While experimental evidence is needed for naming, only two libraries are available for midguts, both from the blood-fed female adults (Mead et al., 2012). As described in Section 3.1, we tentatively named them by integrating information from the preliminary analyses of gene expression, sequence similarity and chromosome locations. The profiles of GP(H) mRNA levels demonstrated four major expression groups (Fig. 5): “GB” for 18 GPs and 22 GPHs highly expressed in the larval stages; “GC” for 20 GPs and 10 GPHs mostly expressed at lower levels in larvae; “GD” for 4 GPs and GPH3 expressed in larvae and in adults at lower levels; “GE” for 20 GPs and 4 GPHs expressed at low levels in pupae and male adults and at high levels in female adults. GP19 and GP26 expression in pupae and adults are very high, particularly in the midgut of female adults. GP10, 13–15, 17, 24, 103, GPH16 and 18 transcripts are more abundant in female than male adults and peak in midgut after blood feeding. The expression of GP5, 6, 7, 13, and GPH4 in midgut was higher after feeding on normal blood than infectious blood containing Plasmodium falciparum (Mead et al., 2012). GP5 and GPH4 mRNA levels were high in salivary glands, and GPH99 in antennae.
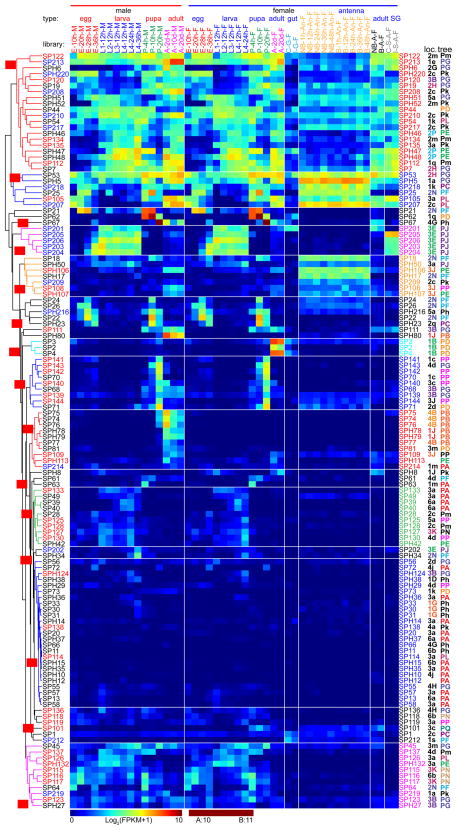
Fig. 5.
Transcript profiles of the 100 GP(H)s in A. gambiae. mRNA levels of the gut serine proteases or their homologs in 45 types of tissue samples, represented by log2(FPKM+1) values, are shown in the hierarchically clustered gradient heatmap from blue (0) to maroon (≥10). The values, rounded to the closest integers and, if ≥10, converted to A (10), B (11) … G (16), are used to label the color blocks. Subtrees of genes with similar expression patterns are in different colors and assigned with group IDs GA through GE (G for gut) on red background. In the library names, E, egg, L, larva, P, pupa, A, adult; h, hour, d, day; F, female, M, male; An, antenna, S, salivary gland; NB, no blood feeding; B, blood fed; C, control, and I, infected. Chromosomal location IDs (loc.) (Fig. 2) and phylogenetic tree IDs (tree) (Fig. 3) are labeled on the right next to the gene names in different colors based on their IDs.
Most GP(H) genes are located in 14 regions on the left (location IDs: 1D, 1F, 1G, 1H, 1J, 1L, 1N, 1P, 1R, 3F) and right (2A, 2B, 4C, and 4F) arms of chromosomes 2 and 3 (Fig. 3). Genes located in each of these regions are generally consistent with their positions (“TA”–“TE”, “TG”, “TH”, and “TK”) in the phylogenetic tree (Fig. 2B). It appears that extensive gene duplication has resulted in large clusters of GP(H) genes, whose transcription is regulated in a similar manner for each gene group. We have identified 16 such three-way agreement groups, each involving 2 to 7 members whose gene locations, tree positions, and expression patterns are the same. Among the 65 in these groups, the GPH28, 29, 35, 37, 38, 101 and 102 genes (tree ID: “TG”, Fig. 2B) reside in the “1D” region of chromosome 3L (Fig. 3), have similar expression profiles (expression ID: “GC”, Fig. 5). GP6, 7, 9, 10, 24, 26 and 103 genes have the tree ID “TD”, location ID “4C”, and expression ID “GE”; GPH61, 69, 77, 91, 94, and 95 are in “TK”, “1H” and “GB”.
3.5. Features of the 127 SP(H) genes in A. gambiae
Most SP(H) genes are found in ten regions of chromosomes 2 (location groups: 1G, 2H, 2N, 2P) and 3 (3B, 3E, 3J, 3K, 4B) (Fig. 3). In region “3E”, a recently evolved cluster of six genes encode CUB-domain SPs 201–206, five of which are identical in tree position (“PJ”) (Fig. 2C) and expression group (“SD”) (Fig. 6). The two gene doublets encoding Gd-domain SPs 207–210 are likely products of two rounds of gene duplication, even though they are 5.2 Mb apart (Fig. 3). Such evolutionary events are proposed based on structural similarity and phylogenetic relationships of these SPs (Fig. 2C). There are twelve gene dyads, three triads, one tetrad, one pentad, and one hexad, based on similar gene locations, tree positions, and expression groups. For instance, SPs 74–77 genes in tree group “PB” (Fig. 2C) and region “4B” of chromosome 3R (Fig. 3) are mainly expressed in adult males (Fig. 6, group “SJ”).
Fig. 6.
Transcript profiles of the 127 SP(H)s in A. gambiae. The serine protease (homolog) mRNA levels in 45 types of tissue samples, as represented by log2(FPKM+1) values, are shown in the hierarchically clustered gradient heatmap from blue (0) to maroon (≥10). The values, rounded to the closest integers and, if equal to 10 and 11, converted to A and B, are used to label the color blocks. Subtrees of genes with similar expression patterns are in different colors and assigned with group IDs beginning with S (for serine in SPs or SPHs) on red background. The 2nd letter in capital indicates a reliable monophyletic group. Group IDs with the 2nd letter in lowercase are assigned to other branches. SP1xx are colored red and SP2xx blue on the left. See Fig. 4 legend for descriptions of library types, chromosomal location IDs, and phylogenetic tree IDs.
Judged on the basis of their log2(FPKM+1) values, most of the SP(H) genes are expressed at low levels in the RNA samples of whole insects (Fig. 6). Transcript levels of group-SA and -SB genes are moderate-to-high in most libraries, whereas high mRNA abundances are detected in a few tissue types for genes in groups Sc, SD, Sf, SG, SH, and SJ. High expression of SP2, SP3 and SP4 in adult females (but not males) led us to consider their possible involvement in reproduction.
4. Discussion
4.1. Improving the AgamP4.3 gene models using AgMCOT
Correct modeling of protein-encoding genes based on genome and cDNA sequences is important for guiding functional studies of their protein products. Several programs have been developed to fulfil this goal, with varying degrees of success. We took an integrated approach that compares and selects the best from models predicted by different programs for a single and then all genes in M. sexta (Cao and Jiang, 2015). In this study, we employed the same method to improve AgamP4.3 and generated AgMCOT, which represents a collection of the selected protein models. We then focused on SP-related proteins by manually examining the corresponding ones and validating improvements in 117 of the 337 SP-like sequences in AgamP4.5. Since parallel study of the Drosophila SP-like genes resulted in fewer than 10 such corrections (data not shown), we think the room is still large for improving AgamP4.5 and even the genome assembly. It is possible that some of the SP-like genes not detected in the genome assembly but with good evidence from RNA-seq data are located near their close relatives in a chromosomal region that has not been assembled. Models for proteins other than SPs/SPHs in AgMCOT should be used to validate and improve the respective sequences in the latest OPS. The power of AgMCOT stems from genome-independent assemblies of RNA-seq data that are integrated with the MAKER/OPS or Cufflink models during selection and manual curation.
4.2. Functional importance of the A. gambiae CLIPs
Specific genetic traits of A. gambiae cause developmental arrest and melanotic encapsulation of Plasmodium cynomolgi ookinetes (Collins et al., 1986). Since phenoloxidases (POs) are key enzymes that catalyze melanization, the proteolytic activation of PO zymogens (i.e. proPOs) by an SP-SPH system has been studied by reverse genetic methods. Knocking down CLIPs B4 or B8 led to reduced melanization of Sephadex beads, and silencing CLIP B1, B9 or B10 had lesser effects (Paskewitz et al., 2006). RNAi silencing of CLIP A8/B4/B8/B14/B15/B17 and A2/A5/A7 decreased and increased melanization of Plasmodium berghei ookinetes and oocysts (Volz et al., 2006; Volz et al., 2005; Zhang et al., 2016), respectively. Recombinant CLIPB9Xa, activated by bovine clotting factor Xa, cleaved M. sexta proPOs and generated POs with a low specific activity (An et al., 2011). Melanization has a functional link to TEP1 activation via CLIPA2 (Yassine et al., 2014) and CLIPA30 (i.e. SPCLIP1) (Povelones et al., 2013). Formation of a complex of TEP1, LRIM1 and APL1C is required in defense against malaria parasites and bacteria in A. gambiae. Transcriptome analysis showed that CLIPC2 was preferentially induced in midgut of A. gambiae by P. fusarium infection (Blumberg et al., 2013). RNAi screening revealed CLIPA26’s role in transcriptional regulation and SPH51’s role in phagocytosis (Lombardo et al., 2013). Together, these studies have begun to elucidate CLIPs’ functions in the mosquito immune responses.
Interestingly, CLIPs A2, A5, A7 and A30, encoded by the same cluster of genes in region “3D” on chromosome 3L, all regulate melanization and/or TEP1 activation. Such functional relatedness also exists in the gene triplet of CLIPs B8, B9 and B10, as well as in the doublet of CLIPs B1 and B4. One explanation is that during neo- or sub-functionalization, duplicated genes may maintain certain levels of their ancestor’s original functions. On the other hand, if dosage increase of the gene copies is detrimental to the host, one or more of the copies may encounter functional loss. Since molecular functions are mostly unclear for proteins encoded by the 15 CLIP genes in “3D”, it would be exciting to find out what functions these copies of original genes have. With the tree, location, and expression IDs available, RNAi of entire gene clusters may produce mutant phenotypes that are masked by functional redundancy in single knockdown tests. Once a strong phenotype is found, scaling down the targets should reveal the culprit(s).
4.3. Functions and expression regulation of the putative GPs and GPHs in A. gambiae
GPs had been studied for years before the A. gambiae genome was published. These include GP5 (Sp24D), GP6 (Antryp6), GP7 (Antryp5), GP9 (Antryp3), GP10 (Antryp7), GP12 (ISP13), GP13 (AgChyL), GP22 (Anchym1), GP23 (Anchym2), GP24 (Antryp2), GP26 (Antryp1), GP97 (AgESP) and GP103 (Antryp4) (Dimopoulos et al., 1997; Han et al., 1997; Muller et al., 1995; Muller et al., 1993; Rodrigues et al., 2012; Shen et al., 2000; Vizioli et al., 2001). Expression patterns of GP5, GP13, GP22 and GP26, for example, vary dramatically, due to the regulatory elements in their genes (Giannoni et al., 2001; Shen and Jacobs-Lorena, 1998; Skavdis et al., 1996). While these studies support the naming, mRNA profiles and structure features (e.g. size, domain, similarity) of the 100 G(P)Hs provide additional evidence for the classification. Nonetheless, we must point out that experimental data are necessary to validate their identities as GPs and GPHs. For instance, GP5 mRNA level is much higher in thorax than gut and in adult males than females (Han et al., 1997) and GP97 is involved in the Plasmodium invasion of midgut and salivary glands (Rodrigues et al., 2012).
In the larvae, dietary proteins are likely processed by the 37 GPs in expression groups GB and GC (Fig. 5). The transcript levels are much higher for genes in group GB (18 GPs, 22 GPHs) than in group GC (20 GPs, 10 GPHs). We do not know their protein levels or catalytic activities to estimate their relative contributions to digestion but, if all things (e.g. translation, stability) are the same, why would the larvae make similar or more GPHs than GPs in the midgut (Fig. 5, Table S2)? In other words, what physiological roles do these non-catalytic proteins play in the midgut? Do they protect the host cells from damage caused by excessive GPs or toxic molecules taken up from the environment? Bacillus thuringiensis israelensis, a naturally occurring soil bacterium, is used as a biological control agent to kill mosquito larvae in water (Shaalan and Canyon, 2009). Its insecticidal crystal proteins require proper cleavage by GPs to form active toxins. Under- or over-processing of the protoxins by GPs may both impact their effectiveness and, therefore, call for further studies of the mixture of GPs and GPHs.
It is also possible that GPs and GPHs in expression group GC serve a function different from those in group GB. The proteins in group GC may be constitutively synthesized at low levels and released as samplers to produce a basal level of amino acids from ingested food. If the level exceeds a threshold when dietary proteins are present, GPs and GPHs in the GB group are then expressed at high level and released for the bulk digestion and protection of larval tissues, respectively. Such a scenario was reported in adult females of Aedes aegypti (Noriega and Wells, 1999).
GP1, GP2, GP96, GP97 and GPH3 in group D, GP5, GP88 and GP98 are expressed in larvae, pupae and adults, whereas the other 20 GPs and 3 GPHs in group GE are mainly expressed in pupae and adults (Fig. 5). It is clear that different gene sets are employed by the mosquito for digestion in larvae and adults. The GP(H) transcript levels in pupae are generally low except for GP19, GP20 and GP26. Roles of these putative GPs in tissue remodeling need exploration in the pupae, and so do the tissue specificity and sex dichotomy of GPs in the adults.
4.4. Functions and transcription of the A. gambiae SPs and SPHs
Even though some of the 126 SP(H)s have interesting domain structures (Fig. 1, Table S1), their functions are poorly explored, except for SP2, SP3, SP4, SPH51, and SP213 (Danielli et al., 2000; Gorman et al., 2000; Lombardo et al., 2013; Mancini et al., 2011). Consistent with their specific expression in the adult females (Section 3.5), SP2, SP3 and SP4 proteins are detected in the lower reproductive tissues to process transferred male proteins. SP213 (GRAAL or Sp22D) may mediate immune responses, as its constitutive expression in adult hemocytes, fat body and midgut epithelial cells is induced 1.5 fold after wounding or bacterial infection. SP212 and SP217 are orthologs of Drosophila Nudel and ModSP, which are involved in embryonic development and immune responses, respectively.
Of the 25, 44, 40 and 17 SPs/SPHs in expression groups SA–SB, Sc–SJ, Sk–SN and Sq–SR 13, 20, 9, and 13 have two or more domains (Fig. 6). The transcript levels in groups Sk–SN were the lowest, slightly higher and more evenly distributed in these libraries for groups Sq–SR, a lot higher in some tissue types for groups Sc–SJ, and the highest in most of the libraries for groups SA–SB. Specific expression of the genes in Sc–SJ (e.g. SP2, SP3 and SP4) in RNA-seq libraries is interesting, which may provide clues for their functional elucidation.
5. Conclusions
Serine proteases and their homologs constitute a large family of proteins in A. gambiae. We generated the AgMCOT gene set and made improvements in the SP and SPH sequences of AgamP4.5. Extensive RNA-Seq data not only enhanced the quality of AgMCOT models but also revealed the expression patterns of 220 SPs and 117 SPHs. We also identified close connections among phylogenetic relationships, chromosomal locations, and expression profiles for 159 genes in 46 groups. Structural features and other information of the SP-related proteins are provided to facilitate research on their physiological functions. We have identified thirteen types of cystine- stabilized domains in 127 SP(H)s, which may allow molecular recognition to occur among members of SP-SPH cascade pathways in the malaria mosquito.
Supplementary Material
Detailed information of the 337 SP-related proteins in A. gambiae
For each protein, its systemic name, alias, T-C-E (see Table 1 footnotes), AgamP4.5 and gene IDs, AgamP4.5 comment and improvement, chromosomal location, gene coordinates and orientation, exon number, amino acid sequence, predicted activation cleavage site, putative enzyme specificity of SP, length, domain structure, and clip sequence (if available) are listed. In AgamP4.5 comments, N, I and C stand for amino-terminal, internal and carboxyl-terminal regions, respectively.
Expression of the A. gambiae SP/SPH genes in different cDNA libraries
Transcript profiles of the 337 SP-related genes in the 113 RNA-seq data sets. FPKM values are shown in the heat map from cyan to white and then to red. Descriptions of the cDNA libraries are provided, including SRA run IDs, library names, detailed descriptions, and corresponding names used in Figs. 4–6.
Identify 337 SP/SPH genes, improve 117 of their models, and classify them into 110 CLIPs, 100 GPs/GPHs and 127 SP(H)s
Analyze the domain organization of CLIPs A–E and identify 13 other types of putative regulatory domains in 18 SP(H)s
Reveal relationships among phylogenetic tree positions, chromosomal locations and expression patterns of 159 SPs/SPHs
Acknowledgments
We thank Dr. Michael Kanost at Kansas State University for his insightful comments, which greatly helped the manuscript improvement. This work was supported by NIH grants AI112662 and GM58634. We would like to thank the mosquito scientists for producing the RNA-seq data, especially the researchers in Pirbright Institute who have deposited their data in NCBI SRA but not yet published their analyses. Computation for this project was done at OSU High Performance Computing Center, supported in part through the NSF grant OCI-1126330. This work was approved for publication by the Director of Oklahoma Agricultural Experimental Station and supported in part under project OKLO2450.
Abbreviations
- SP
serine protease
- SPH
(non-catalytic) serine protease homolog
- PD
SP catalytic domain
- PLD
protease-like domain in SPH
- LDLa
low-density lipoprotein receptor class A repeat
- SR
scavenger receptor
- TSP
thrombospondin
- CUB
C1r/C1s, Uegf, Bmp1
- MSP
modular serine protease
- CLIP
clip-domain SP or SPH
- GP and GPH
gut serine protease and gut serine protease homolog
- PO and proPO
phenoloxidase and its precursor
- PAP
proPO activating protease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An CJ, Budd A, Kanost MR, Michel K. Characterization of a regulatory unit that controls melanization and affects longevity of mosquitoes. Cellular and Molecular Life Sciences. 2011;68:1929–1939. doi: 10.1007/s00018-010-0543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg BJ, Trop S, Das S, Dimopoulos G. Bacteria- and IMD Pathway-Independent Immune Defenses against Plasmodium falciparum in Anopheles gambiae. Plos One. 2013:8. doi: 10.1371/journal.pone.0072130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics (Oxford, England) 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzoni M, Afrane Y, Dunn WA, Atieli FK, Zhou G, Zhong D, Li J, Githeko A, Yan G. Comparative transcriptome analyses of deltamethrin-resistant and -susceptible Anopheles gambiae mosquitoes from Kenya by RNA-Seq. PloS one. 2012:7. doi: 10.1371/journal.pone.0044607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, He Y, Hu Y, Zhang X, Wang Y, Zou Z, Chen Y, Blissard GW, Kanost MR, Jiang H. Sequence conservation, phylogenetic relationships, and expression profiles of nondigestive serine proteases and serine protease homologs in Manduca sexta. Insect biochemistry and molecular biology. 2015;62:51–63. doi: 10.1016/j.ibmb.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Jiang H. Integrated modeling of protein-coding genes in the Manduca sexta genome using RNA-Seq data from the biochemical model insect. Insect biochemistry and molecular biology. 2015;62:2–10. doi: 10.1016/j.ibmb.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Jiang H. Integrated modeling of structural genes using MCuNovo. Insect Genomics, Methods in Molecular Biology. 2017 doi: 10.1007/978-1-4939-8775-7_5. (submitted) [DOI] [PubMed] [Google Scholar]
- Chang Z, Li G, Liu J, Zhang Y, Ashby C, Liu D, Cramer CL, Huang X. Bridger: a new framework for de novo transcriptome assembly using RNA-seq data. Genome biology. 2015;16:30. doi: 10.1186/s13059-015-0596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, Blass C, Brey PT, Collins FH, Danielli A, Dimopoulos G, Hetru C, Hoa NT, Hoffmann JA, Kanzok SM, Letunic I, Levashina EA, Loukeris TG, Lycett G, Meister S, Michel K, Moita LF, Muller HM, Osta MA, Paskewitz SM, Reichhart JM, Rzhetsky A, Troxler L, Vernick KD, Vlachou D, Volz J, von Mering C, Xu JN, Zheng LB, Bork P, Kafatos FC. Immunity-related genes and gene families in Anopheles gambiae. Science. 2002;298:159–165. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- Collins FH, Sakai RK, Vernick KD, Paskewitz S, Seeley DC, Miller LH, Collins WE, Campbell CC, Gwadz RW. Genetic Selection of a Plasmodium-Refractory Strain of the Malaria Vector Anopheles-Gambiae. Science. 1986;234:607–610. doi: 10.1126/science.3532325. [DOI] [PubMed] [Google Scholar]
- Danielli A, Loukeris TG, Lagueux M, Muller HM, Richman A, Kafatos FC. A modular chitin-binding protease associated with hemocytes and hemolymph in the mosquito Anopheles gambiae. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:7136–7141. doi: 10.1073/pnas.97.13.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos G, Richman A, Muller HM, Kafatos FC. Molecular immune responses of the mosquito Anopheles gambiae to bacteria and malaria parasites. Proc Natl Acad Sci U S A. 1997;94:11508–11513. doi: 10.1073/pnas.94.21.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic acids research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoni F, Muller HM, Vizioli J, Catteruccia F, Kafatos FC, Crisanti A. Nuclear factors bind to a conserved DNA element that modulates transcription of Anopheles gambiae trypsin genes. Journal of Biological Chemistry. 2001;276:700–707. doi: 10.1074/jbc.M005540200. [DOI] [PubMed] [Google Scholar]
- Gorman MJ, Andreeva OV, Paskewitz SM. Sp22D: a multidomain serine protease with a putative role in insect immunity. Gene. 2000;251:9–17. doi: 10.1016/s0378-1119(00)00181-5. [DOI] [PubMed] [Google Scholar]
- Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, Couger MB, Eccles D, Li B, Lieber M, Macmanes MD, Ott M, Orvis J, Pochet N, Strozzi F, Weeks N, Westerman R, William T, Dewey CN, Henschel R, Leduc RD, Friedman N, Regev A. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nature protocols. 2013;8:1494–1512. doi: 10.1038/nprot.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YS, Salazar CE, Reese-Stardy SR, Cornel A, Gorman MJ, Collins FH, Paskewitz SM. Cloning and characterization of a serine protease from the human malaria vector, Anopheles gambiae. Insect Mol Biol. 1997;6:385–395. doi: 10.1046/j.1365-2583.1997.00193.x. [DOI] [PubMed] [Google Scholar]
- Jiang H, Kanost MR. The clip-domain family of serine proteinases in arthropods. Insect Biochem Mol Biol. 2000;30:95–105. doi: 10.1016/s0965-1748(99)00113-7. [DOI] [PubMed] [Google Scholar]
- Jiang H, Vilcinskas A, Kanost MR. Immunity in lepidopteran insects. Advances in experimental medicine and biology. 2010;708:181–204. doi: 10.1007/978-1-4419-8059-5_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P, Binns D, Chang HY, Fraser M, Li W, McAnulla C, McWilliam H, Maslen J, Mitchell A, Nuka G, Pesseat S, Quinn AF, Sangrador-Vegas A, Scheremetjew M, Yong SY, Lopez R, Hunter S. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30:1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanost MR, Arrese EL, Cao X, Chen YR, Chellapilla S, Goldsmith MR, Grosse-Wilde E, Heckel DG, Herndon N, Jiang H, Papanicolaou A, Qu J, Soulages JL, Vogel H, Walters J, Waterhouse RM, Ahn SJ, Almeida FC, An C, Aqrawi P, Bretschneider A, Bryant WB, Bucks S, Chao H, Chevignon G, Christen JM, Clarke DF, Dittmer NT, Ferguson LC, Garavelou S, Gordon KH, Gunaratna RT, Han Y, Hauser F, He Y, Heidel-Fischer H, Hirsh A, Hu Y, Jiang H, Kalra D, Klinner C, Konig C, Kovar C, Kroll AR, Kuwar SS, Lee SL, Lehman R, Li K, Li Z, Liang H, Lovelace S, Lu Z, Mansfield JH, McCulloch KJ, Mathew T, Morton B, Muzny DM, Neunemann D, Ongeri F, Pauchet Y, Pu LL, Pyrousis I, Rao XJ, Redding A, Roesel C, Sanchez-Gracia A, Schaack S, Shukla A, Tetreau G, Wang Y, Xiong GH, Traut W, Walsh TK, Worley KC, Wu D, Wu W, Wu YQ, Zhang X, Zou Z, Zucker H, Briscoe AD, Burmester T, Clem RJ, Feyereisen R, Grimmelikhuijzen CJ, Hamodrakas SJ, Hansson BS, Huguet E, Jermiin LS, Lan Q, Lehman HK, Lorenzen M, Merzendorfer H, Michalopoulos I, Morton DB, Muthukrishnan S, Oakeshott JG, Palmer W, Park Y, Passarelli AL, Rozas J, Schwartz LM, Smith W, Southgate A, Vilcinskas A, Vogt R, Wang P, Werren J, Yu XQ, Zhou JJ, Brown SJ, Scherer SE, Richards S, Blissard GW. Multifaceted biological insights from a draft genome sequence of the tobacco hornworm moth, Manduca sexta. Insect Biochem Mol Biol. 2016;76:118–147. doi: 10.1016/j.ibmb.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanost MR, Jiang HB. Clip-domain serine proteases as immune factors in insect hemolymph. Curr Opin Insect Sci. 2015;11:47–55. doi: 10.1016/j.cois.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome biology. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krem MM, Di Cera E. Evolution of enzyme cascades from embryonic development to blood coagulation. Trends Biochem Sci. 2002;27:67–74. doi: 10.1016/s0968-0004(01)02007-2. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo F, Ghani Y, Kafatos FC, Christophides GK. Comprehensive genetic dissection of the hemocyte immune response in the malaria mosquito Anopheles gambiae. PLoS pathogens. 2013:9. doi: 10.1371/journal.ppat.1003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini E, Tammaro F, Baldini F, Via A, Raimondo D, George P, Audisio P, Sharakhov IV, Tramontano A, Catteruccia F, della Torre A. Molecular evolution of a gene cluster of serine proteases expressed in the Anopheles gambiae female reproductive tract. Bmc Evol Biol. 2011:11. doi: 10.1186/1471-2148-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead EA, Li M, Tu Z, Zhu J. Translational regulation of Anopheles gambiae mRNAs in the midgut during Plasmodium falciparum infection. BMC Genomics. 2012;13:366. doi: 10.1186/1471-2164-13-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HM, Catteruccia F, Vizioli J, della Torre A, Crisanti A. Constitutive and blood meal-induced trypsin genes in Anopheles gambiae. Exp Parasitol. 1995;81:371–385. doi: 10.1006/expr.1995.1128. [DOI] [PubMed] [Google Scholar]
- Muller HM, Vizioli I, della Torre A, Crisanti A. Temporal and spatial expression of serine protease genes in Anopheles gambiae. Parassitologia. 1993;35(Suppl):73–76. [PubMed] [Google Scholar]
- Noriega FG, Wells MA. A molecular view of trypsin synthesis in the midgut of Aedes aegypti. Journal of Insect Physiology. 1999;45:613–620. doi: 10.1016/s0022-1910(99)00052-9. [DOI] [PubMed] [Google Scholar]
- Park JW, Kim CH, Rui J, Park KH, Ryu KH, Chai JH, Hwang HO, Kurokawa K, Ha NC, Soderhall I, Soderhall K, Lee BL. Beetle Immunity. Adv Exp Med Biol. 2010;708:163–180. doi: 10.1007/978-1-4419-8059-5_9. [DOI] [PubMed] [Google Scholar]
- Paskewitz SM, Andreev O, Shi L. Gene silencing of serine proteases affects melanization of Sephadex beads in Anopheles gambiae. Insect Biochemistry and Molecular Biology. 2006;36:701–711. doi: 10.1016/j.ibmb.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Perona JJ, Craik CS. Structural basis of substrate specificity in the serine proteases. Protein Sci. 1995;4:337–360. doi: 10.1002/pro.5560040301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nature methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Pinheiro-Silva R, Borges L, Coelho LP, Cabezas-Cruz A, Valdes JJ, do Rosario V, de la Fuente J, Domingos A. Gene expression changes in the salivary glands of Anopheles coluzzii elicited by Plasmodium berghei infection. Parasit Vectors. 2015;8:485. doi: 10.1186/s13071-015-1079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povelones M, Bhagavatula L, Yassine H, Tan LA, Upton LM, Osta MA, Christophides GK. The CLIP-Domain Serine Protease Homolog SPCLIP1 Regulates Complement Recruitment to Microbial Surfaces in the Malaria Mosquito Anopheles gambiae. Plos Pathogens. 2013:9. doi: 10.1371/journal.ppat.1003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings RD, Barrett AJ. Evolutionary families of peptidases. Biochem J. 1993;290:205–218. doi: 10.1042/bj2900205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinker DC, Pitts RJ, Zhou X, Suh E, Rokas A, Zwiebel LJ. Blood meal-induced changes to antennal transcriptome profiles reveal shifts in odor sensitivities in Anopheles gambiae. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:8260–8265. doi: 10.1073/pnas.1302562110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues J, Oliveira GA, Kotsyfakis M, Dixit R, Molina-Cruz A, Jochim R, Barillas-Mury C. An Epithelial Serine Protease, AgESP, Is Required for Plasmodium Invasion in the Mosquito Anopheles gambiae. Plos One. 2012:7. doi: 10.1371/journal.pone.0035210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J, Jiang H, Kanost MR, Wang Y. Serine proteases and their homologs in the Drosophila melanogaster genome: an initial analysis of sequence conservation and phylogenetic relationships. Gene. 2003;304:117–131. doi: 10.1016/s0378-1119(02)01187-3. [DOI] [PubMed] [Google Scholar]
- Schechter I, Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967;27:157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Shaalan EAS, Canyon DV. Aquatic insect predators and mosquito control. Trop Biomed. 2009;26:223–261. [PubMed] [Google Scholar]
- Shen HB, Chou KC. Signal-3L: A 3-layer approach for predicting signal peptides. Biochem Biophys Res Commun. 2007;363:297–303. doi: 10.1016/j.bbrc.2007.08.140. [DOI] [PubMed] [Google Scholar]
- Shen Z, Edwards MJ, Jacobs-Lorena M. A gut-specific serine protease from the malaria vector Anopheles gambiae is downregulated after blood ingestion. Insect Molecular Biology. 2000;9:223–229. doi: 10.1046/j.1365-2583.2000.00188.x. [DOI] [PubMed] [Google Scholar]
- Shen ZC, Jacobs-Lorena M. Nuclear factor recognition sites in the gut-specific enhancer region of an Anopheles gambiae trypsin gene. Insect Biochemistry and Molecular Biology. 1998;28:1007–1012. doi: 10.1016/s0965-1748(98)00089-7. [DOI] [PubMed] [Google Scholar]
- Skavdis G, SidenKiamos I, Muller HM, Crisanti A, Louis C. Conserved function of Anopheles gambiae midgut-specific promoters in the fruitfly. Embo J. 1996;15:344–350. [PMC free article] [PubMed] [Google Scholar]
- Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley D, Pimentel H, Salzberg S, Rinn J, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature protocols. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini L, Dunn WA, Reed TW, Willis JH. Changes in transcript abundance for cuticular proteins and other genes three hours after a blood meal in Anopheles gambiae. Insect Biochemistry and Molecular Biology. 2014;44:33–43. doi: 10.1016/j.ibmb.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillard F, Troxler L, Reichhart JMM. Drosophila melanogaster clip-domain serine proteases: Structure, function and regulation. Biochimie. 2016;122:255–269. doi: 10.1016/j.biochi.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Vizioli J, Catteruccia F, della Torre A, Reckmann I, Muller HM. Blood digestion in the malaria mosquito Anopheles gambiae - Molecular cloning and biochemical characterization of two inducible chymotrypsins. European Journal of Biochemistry. 2001;268:4027–4035. doi: 10.1046/j.1432-1327.2001.02315.x. [DOI] [PubMed] [Google Scholar]
- Volz J, Muller HM, Zdanowicz A, Kafatos FC, Osta MA. A genetic module regulates the melanization response of Anopheles to Plasmodium. Cell Microbiol. 2006;8:1392–1405. doi: 10.1111/j.1462-5822.2006.00718.x. [DOI] [PubMed] [Google Scholar]
- Volz J, Osta MA, Kafatos FC, Muller HM. The roles of two clip domain serine proteases in innate immune responses of the malaria vector Anopheles gambiae. Journal of Biological Chemistry. 2005;280:40161–40168. doi: 10.1074/jbc.M506191200. [DOI] [PubMed] [Google Scholar]
- Waterhouse RM, Kriventseva EV, Meister S, Xi Z, Alvarez KS, Bartholomay LC, Barillas-Mury C, Bian G, Blandin S, Christensen BM, Dong Y, Jiang H, Kanost MR, Koutsos AC, Levashina EA, Li J, Ligoxygakis P, Maccallum RM, Mayhew GF, Mendes A, Michel K, Osta MA, Paskewitz S, Shin SW, Vlachou D, Wang L, Wei W, Zheng L, Zou Z, Severson DW, Raikhel AS, Kafatos FC, Dimopoulos G, Zdobnov EM, Christophides GK. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science. 2007;316:1738–1743. doi: 10.1126/science.1139862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassine H, Kamareddine L, Chamat S, Christophides GK, Osta MA. A Serine Protease Homolog Negatively Regulates TEP1 Consumption in Systemic Infections of the Malaria Vector Anopheles gambiae. Journal of Innate Immunity. 2014;6:806–818. doi: 10.1159/000363296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, An CJ, Sprigg K, Michel K. CLIPB8 is part of the prophenoloxidase activation system in Anopheles gambiae mosquitoes. Insect Biochemistry and Molecular Biology. 2016;71:106–115. doi: 10.1016/j.ibmb.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Wang GHH, Dong ZMM, Duan J, Xu PZZ, Cheng TCC, Xiang ZHH, Xia QYY. Genome-wide identification and expression analysis of serine proteases and homologs in the silkworm Bombyx mori. BMC genomics. 2010;11:405. doi: 10.1186/1471-2164-11-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Evans JD, Lu Z, Zhao P, Williams M, Sumathipala N, Hetru C, Hultmark D, Jiang H. Comparative genomic analysis of the Tribolium immune system. Genome Biology. 2007:8. doi: 10.1186/gb-2007-8-8-r177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Lopez DL, Kanost MR, Evans JD, Jiang H. Comparative analysis of serine protease-related genes in the honey bee genome: possible involvement in embryonic development and innate immunity. Insect Mol Biol. 2006;15:603–614. doi: 10.1111/j.1365-2583.2006.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed information of the 337 SP-related proteins in A. gambiae
For each protein, its systemic name, alias, T-C-E (see Table 1 footnotes), AgamP4.5 and gene IDs, AgamP4.5 comment and improvement, chromosomal location, gene coordinates and orientation, exon number, amino acid sequence, predicted activation cleavage site, putative enzyme specificity of SP, length, domain structure, and clip sequence (if available) are listed. In AgamP4.5 comments, N, I and C stand for amino-terminal, internal and carboxyl-terminal regions, respectively.
Expression of the A. gambiae SP/SPH genes in different cDNA libraries
Transcript profiles of the 337 SP-related genes in the 113 RNA-seq data sets. FPKM values are shown in the heat map from cyan to white and then to red. Descriptions of the cDNA libraries are provided, including SRA run IDs, library names, detailed descriptions, and corresponding names used in Figs. 4–6.