SUMMARY
The genes encoding ribosomal protein S15 (rpsO) and polynucleotide phosphorylase (pnp) occupy adjacent positions and are oriented in the same direction on the Escherichia coli chromosomes. The nucleotide sequence of the region controlling the expression of these two genes has been determined. Two in-phase gene fusions between pnp and lacZ were constructed. The fusions define the translational reading frame of the pnp gene and indicate that the expression of pnp is independent of the upstream rpsO gene. Transcript mapping with nuclease S1 demonstrated that the two genes are transcribed from separate promoters and that the rpsO-pnp intergenic space contains a strong transcriptional terminator. The transcriptional start points have been localized.
Keywords: Recombinant DNA, promoter, transcriptional start point and terminator, S1 mapping, operon, cloning vector, Tn5 transposon mutagenesis
INTRODUCTION
In E. coli the genes encoding elements of the transcription-translation apparatus are often cotranscribed and coregulated (for a review, see Lindahl and Zengel, 1982; Nomura et al., 1984). The gene encoding pnp is located immediately adjacent to, and downstream from, the gene encoding rpsO (Portier, 1982; Portier and Regnier, 1984). Upstream from the S15 gene in the same cluster is an operon containing genes encoding a minor initiator methionine tRNA, a p21 protein of unknown function, the NusA transcription termination-antitermination factor, the translation initiation factor IF2 and a pl5 protein of unknown function (Ishii et al., 1984; Nakamura and Mizusawa, 1985). The reason for this clustering and the interrelationships between these tightly linked genes is not understood.
The physiological role of PNPase within the bacterial cell is most likely related to instability and degradation of mRNA and/or stable RNA sequences. Mutations defective in PNPase and RNase II activity have been separately isolated and characterized (Reiner, 1969; Kaplan and Apirion, 1974). Attempts to recombine these two mutations within a single genotype have proven unsuccessful indicating that the two proteins most likely carry out separately and in parallel some essential step in RNA metabolism (Donovan and Kushner, 1983).
To gain some insight into the breakdown of RNA and the role of PNPase in this process, we have cloned the region of the chromosome surrounding the pnp gene. This was accomplished by inserting the transposon Tn5 into the bacterial chromosomes near the pnp locus and cloning large chromosome fragments selecting for the Km-resistance determinant (Crofton and Dennis, 1983). Our results using Tn5 insertion mutagenesis of plasmids carrying the pnp gene indicated that the pnp gene was confined to a 2-kb region within a 4.8-kb HindIII-EcoRI fragment and was oriented counterclockwise on the chromosome. Limited DNA sequence analysis indicated that the rpsO gene was located immediately upstream from pnp and oriented in the same direction (Portier, 1982). Here we have sequenced the 5′-flanking region controlling the expression of pnp and rpsO genes and mapped the transcripts from these genes.
MATERIALS AND METHODS
(a) Bacterial strains
Bacteria were cultured in either NY medium or M9 minimal salts medium supplemented with glucose (0.2%), thiamine (0.5 μg/ml) and either required aa (50 μg/ml) or Casamino acids (0.4%) (Miller, 1972). The host strains for recombinant plasmids were MC1000, araD139 Δ(araBCOIC-leu) Δlac-IOPZY; JM83, ara Δ(lac-pro) rpsL thi (ϕ80d-lacZΔM15); and JM103, Δ(lac pro) thi rpsL endA sbcB15 hsc-154 supE[F′traD36 proAB+ ladq lacZΔM15]. Physiological experiments were carried out using plasmid-containing derivatives of MC1000. Antibiotics were used at the following concentrations in both liquid and solid media: 100 μg Ap/ml; 10 μg Tc/ml; 20 μg Km/ml. For experimentation, bacteria were grown in liquid culture at 37 °C with continuous shaking and growth was monitored at A460. Experimentation was carried out at an A460 between 0.20 and 0.5. The activity of β-galactosidase per A460 unit of bacterial mass was assayed as described by Miller (1972). For transcript mapping experiments RNA was prepared as described by Dennis and Nomura (1975).
(b) DNA manipulations
Recombinant DNA techniques were according to Maniatis et al. (1982) and Messing (1983). Fragments of DNA were 3′ end-labeled with PolIk and [α-32P]dNTP or 5′ end-labeled with polynucleotide kinase and [γ-32P]ATP.
Mapping of the 3′ and 5′ ends of in vivo mRNA transcripts was carried out according to the procedure described by Favalaso et al. (1980). Fragments of DNA protected from SI nuclease digestion by RNA were analyzed for length on 8%, PA DNA sequencing gels. Molecular length standards were HpaII fragments of pBR322 end-labeled with PolIk and [α-32P]dCTP. The sizes of these fragments are listed in the legend to Fig. 5.
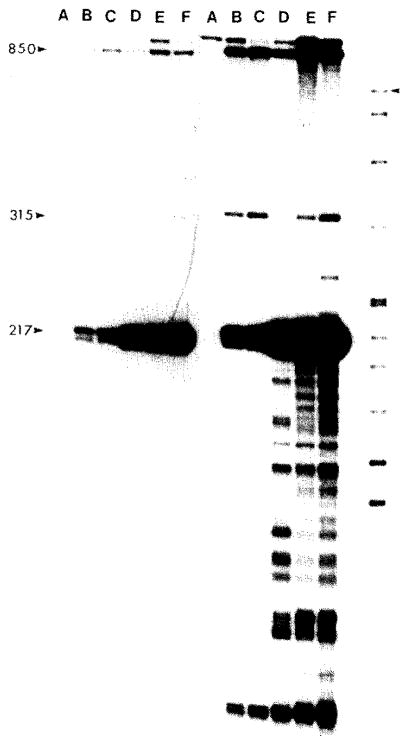
Fig. 5.
Nuclease S1 mapping of the 5′ ends of the S15 gene transcript. The 900-bp BumHi-PstI fragment isolated from plasmid pUB2 and containing about 650 bp of 5′-flanking sequence in front of the S15 gene was 5′ end-labeled with polynucleotide kinase and [γ-32P]ATP. The Pstl end of the fragment is at nt 263 within the coding region of the S15 gene and the Bam HI is from within the vector DNA sequences and is therefore never protected. The labeled fragment was hybridized to 5 μg RNA. digested with S1 nuclease, denatured and electrophoresed on an 8°, PA sequencing gel. The RNAs used for each sample are: (A) E. coli ribosomal RNA; (B)MC1000 RNA; (C)MC1000[pMBI] RNA;(D)MC1000[pSH122] RNA; (E) MC1000[pHEI] RNA; (F) MC1000[pMS31] RNA; last lane, MT standards. The sizes in nt of major and minor S1 protected fragments are indicated. The M T standards were generated by 3′ end-labeling HpaII cut pBR322 with PoIIk and [α-32P]dCTP. The standard lengths in nt from top to bottom are 622, 527, 404, 309, 242, 238, 217, 201, 190, 180, 160, 147, 122, 110, 90, 76, 67, 34, 26, 15 and 9. The position of the largest fragment is marked by an arrowhead. The left portion of the figure is a short and the right portion a long exposure of the autoradiogram.
RESULTS AND DISCUSSION
(a) Location of the pnp gene
The plasmid pHE1 carries the gene encoding ribosomal protein S15 and PNPase on a 4.8-kb HindIII-EcoRI fragment (Fig. 1). Tn5 insertion mutagenesis of pHE1 was used to localize and orient the position of the pnp gene (Crofton and Dennis, 1983). Insertions 37 and 67 define the outer limits of the sequence required to produce the fully active 84-kDal PNPase. Insertion 33 produces a 70-kDal polypeptide which retains activity and insertion 35 produces a 34-kDal polypeptide devoid of activity. This orients the pnp gene and positions the 5′ end about 150 bp to the left of the Hpal(4) restriction site.
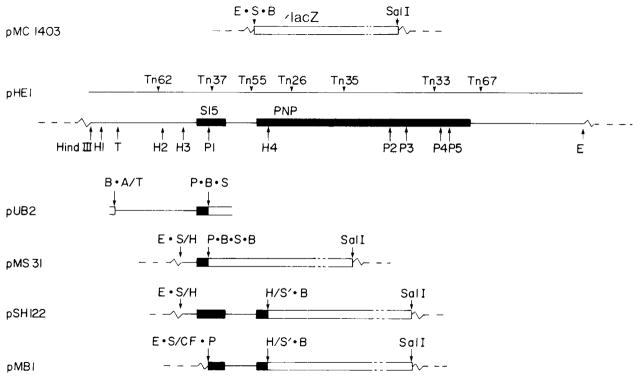
Fig. 1.
Structures of plasmids pMC1403, pHE1 and their derivatives. The plasmid pMC1403 contains a 6.8-kb EcoRI-SalI fragment carrying a 5′-deleted lacZ gene (‘lacZ) inserted between the EcoRI and the SaiI sites of pBR322. The plasmid pHE1 carries a 4.8-kb HindIII-EcoRI fragment from near 68 min on the E. coli chromosome that encodes ribosomal protein S15 (rpsO) and PNPase (pnp) (see solid bars). Open bars represent lacZ coding sequences. The positions of various Tn5 insertions used to position and orient the pnp gene are indicated. Restriction site abbreviations are: E, EcoRI; S, SmaI; B, BamHI; H, HpaI; T, TaqI; P, PstI and A, AccI; the centered dots indicate polylinker sequences; the slash symbols indicate ligation of specified sites. The dashed lines indicate pBR322 vector sequences. Constructions of the plasmids pUB2, pMS31, pSH122 and pMB1 were as follows. Plasmid pMS31 is a rpsO-lacZ fusion and was constructed by inserting the 262-bp HpaI[3]-PstI[1] fragment from pHE1 into the SmaI site of pMC1403. A short connector PstI-SmaI fragment obtained from pUC9 was used to fuse the PstI site on the fragment to the SmaI site in front of lacZ. This short connector fragment results in an out-of-frame fusion between the PstI site of rpsO and the SmaI site of lacZ (Fig. 3). Plasmid pSH122 is a pnp-lacZ fusion and was constructed by inserting the 843-bp HpaI[3–4] fragment into the SmaI site of pMC1403. One of these, pSH122, was shown by DNA sequence analysis to be an in-frame pnp-lacZ fusion (Fig. 3). This plasmid thus contains an active copy of the intact rpsO gene and an in-frame pnp-lacZ gene fusion. The plasmid pMB1 was constructed by inserting the 588-bp PstI[l]-BamHI fragment from pSH 122 between the SmaI and the BamHI site of pMC1403. The junction between the vector SmaI site and the fragment PstI site contains a short connector fragment from pUCl 3. The connector fragment contains a blunt end HindIII site (filled in with PolIk) at one end ligated to the vector SmaI site and a PstI site at the other end ligated to the PstI of the fragment. This plasmid contains only the distal half of rpsO and the in-frame pnp-lacZ fusion. A fourth plasmid, pUB2, is a derivative of pUC8 and was used as a source of DNA for some of the S1 nuclease transcript mapping experiments. The 900-bp TaqI-PstI fragment from pHE1 was inserted between the AccI and PstI sites in pUC8.
Deletion mapping has indicated that the 843-bp HpaI[3–4] fragment contains the sequences required for expression of the rpsO and pnp genes (Portier, 1982). The Pst1[1] restriction site within this fragment was shown by DNA sequencing to be within the coding sequence of rpsO. The Tn5 insertion 37, which is located immediately adjacent to the Pst1[1] site within the rpsO gene, does not obstruct the expression of the pnp gene, suggesting that the two linked genes may be under separate regulation (Crofton and Dennis, 1983).
(b) Nucleotide sequence analysis
Small restriction fragments from within the 843-bp HpaI[3–4] fragment were subcloned into M13 vectors and sequenced by the dideoxy chain termination method (Fig. 2). Our sequence is in general agreement with that recently published by Portier and Regnier (1984); differences are noted in the legend to Fig. 2. The coding sequence of the rpsO gene was identified by comparison with the S15 aa sequence (Morinaga et al., 1976). With a single exception there is exact correspondence between the nucleotide sequence of the gene and the published aa sequence of the protein. The nucleotide sequence contains an extra CAC histidine codon at nt 283 which is not present in the published aa sequence.
Fig. 2.
Nucleotide sequence of the 5′ controlling regions of the rpsO and pup genes. The nucleotide sequence of the HpaI[3–4] fragment from pHEI was determined (Sanger, 1976; Maxam and Gilbert, 1980). The coding sequence of rpsO extends from nt 148 to nt 417. The coding sequence of pup has not been unambiguously established but a likely candidate is the ORF with a TTG translation start at nt 663. The PstI at nt 262 and the HpaI site at nt 843 were used to construct rpsO and pnp fusions with lacZ. Some of the restriction sites used for S1 nuclease mapping of the rpsO and pnp gene transcripts are HpaII (212), PstI (262) and HpaII (721). The major transcription start and stop points for the in vivo S15 mRNA occur at about nt 46 and 458 (arrowhead and dot), respectively. The major start point for the in vivo PNPase mRNA occurs at about nt 583 (arrowhead). Sequences related to the consensus −10 and −35 sequences for RNA polymerase promoter function immediately upstream from the major start points are indicated. This entire sequence is identical to that of Portier and Regnier (1984) except that their sequence contains a single insertion of a C residue between nt 617 and 618.
An ORF beginning at nt 663 and continuing through the HpaI[4] site at nt 843 probably represents the coding sequence of PNPase (Portier and Regnier, 1984). The position of this ORF is entirely consistent with the position predicted by the Tn5 insertion analysis (Crofton and Dennis, 1983; see section a above) and the phase of the reading frame was established unambiguously by sequencing fusions to lacZ (see section c below). The sequence between nt418 and 663 presumably contains the promoter for pnp gene since the Tn5 insertion 37 near the PstI site within the rpsO gene fails to inactivate the gene encoding PNPase.
(c) Fusions of pnp with lacZ
The plasmid pMC1403 contains a 6.2-kb fragment inserted between the EcoRI and SalI sites of pBR322 (Fig. 1). At the EcoRI end the fragment carries an EcoRI, SmaI, BamHI polylinker fused to codon 8 of the lacZ gene (Casadaban et al, 1980). Expression of lacZ in this plasmid requires (i) insertion of a fragment of DNA carrying appropriately oriented transcription initiation and translation initiation signals into the polylinker site and (ii) maintenance of an in-phase translation reading frame across the fusion junction. Two in-phase pnp-lacZ gene fusions have been constructed, PSH122 and pMB1 (see Fig. 1), and their structures are illustrated in Fig. 1. The nucleotide sequence and the corresponding aa sequences across the fusion junctions are illustrated in Fig. 3.
Fig. 3.
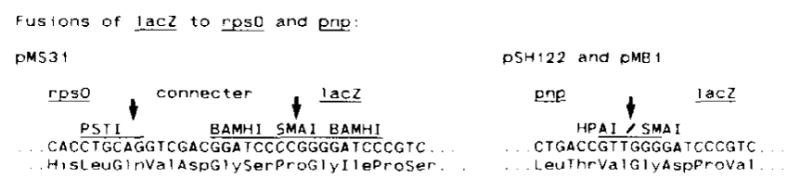
Nucleotide sequences of the rpsO and pnp fusion junctions with lacZ. The construction of plasmid pMS31, pSH122 and pMB1 is described in Fig. 1. The pMS31 fusion is out-of-phase whereas pSH122 and pMB1 are in-phase.
Both pnp-lacZ fusion plasmids gave high levels of β-galactosidase activity (Table I). The plasmid pSH122 contains about 660 bp of 5′-flanking sequence in front of the pnp gene (including the rpsO gene) whereas plasmid pMB1 contains about 400 bp of 5′-flanking sequence (see Fig. 1). This result suggests that a major transcription promoter for the pnp gene is located distal to the PstI site in rpsO. The two-fold higher level of activity in strains carrying pSH 122 compared to pMB1 could be due (i) to copy number effects of the plasmid, (ii) to some cotranscription or (iii) to transcriptional enhancement from the pnp promoter as a result of an active upstream rpsO gene. The location of a separate pnp promoter at a position downstream from the rpsO gene was also supported by the observation that insertion of a Km-resistance cassette (Vieira and Messing, 1982) into the PstI site at bp 262 within the rpsO gene in either orientation on plasmid pSH122 failed to abolish the LacZ + phenotype (not shown).
TABLE 1.
β-Galactosidase activity in strains carrying the pnp-lacZ and rpsO-lacZ. fusion plasmids
Bacterial strains were grown exponentially in M9 medium supplemented with glucose, Casamino acids and tryptophan. Samples of culture were removed and assayed according to the procedure of Miller (1972). Plasmids are shown in Fig. 1.
Specific enzyme activity is nanomoles of o-nitrophenyl-β-D-galactopyranoside hydrolyzed/min//A460 of bacterial culture at an assay temperature of 28 °C.
The strain carrying plasmid pMS31 exhibited a very low level of β-galactosidase activity and gave very light blue colonies on XGal indicator plates. The fusion junction on this plasmid was sequenced and, as expected, found to be out-of-phase (Fig. 3).
(d) Mapping of the rpsO and pnp transcripts
Nuclease S1 mapping of the in vivo transcripts from the rpsO and pnp genes and the lacZ fusion genes was carried out to identify promoters, terminators and processing signals in the DNA sequence. The results of these experiments are summarized in Fig. 4. Briefly, the major promoter for rpsO has a transcription start point at about nt 46, about 100 nt on the 5′ side of the S15 ATG initiation codon. Most of these transcripts are terminated at about nt 458, 40 nt downstream from the S15 TAA termination codon. The major promoter for pnp has a start point at about nt 583 and the coding sequence for pnp is believed to commence at nt 663. There is some evidence suggesting the existence of minor promoters and a small fraction of read-through transcripts in the vicinity of these two genes.
Fig. 4.
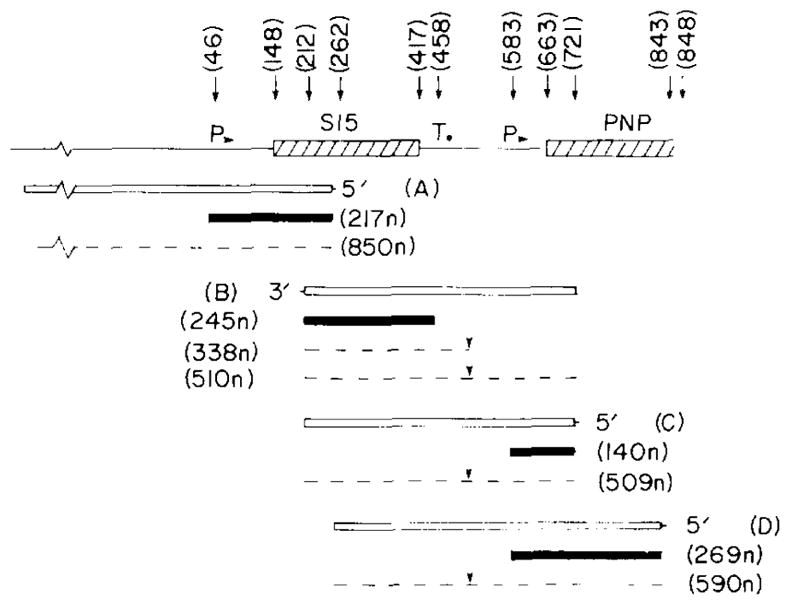
The transcription patterns of the rpsO and pnp genes. The results of the S1s nuclease transcript mapping experiments illustrated in Figs. 5,6 and 7 are summarized. At the top the DNA structure is depicted and relevant nt positions are identified. Restriction sites are: HpaII, 212 and 721; PstI, 262; BamHI, 848 (in lacZ). The major promoters (P) and terminators (T) are indicated. The end-labeled DNA probes (open boxes) are: A, 5′ end-labeled 900-bp BamHI-PstI fragment derived from plasmid pUB2; B, 3′ end-labeled 509-bp HpaII fragment from plasmid pHE1; C, 5′ end-labeled 509-bp HpaII fragment, and D, 5′ end-labeled 590-bp PstI-BamHI fragment from plasmid pSH122. Shaded boxes: major protected fragments (sizes in nt); dashed lines: minor protected fragments. Downward arrowheads over the read-through transcripts between rpsO and pnp: possible RNA-processing site.
The protection of the 5′ end label of a 900-nt restriction fragment terminating within the rpsO gene was used to identify the start point of the rpsO transcripts (Fig. 5). The major protected fragment was 217 nt in length and corresponds to the transcription start point at about nt 47. Minor start point corresponding to protected fragments of 315 and 850 (and possibly 100) nt were also observed. When the bacterial strain contained a high-copy-number plasmid carrying the 5′-flanking 150 nt in front of the rpsO gene, the abundance of the 217-nt protected fragment was substantially increased (lanes D, E and F) compared to the nonplasmid control (lane B) and the promoter-lacking plasmid control (lane C). One of the plasmid strains (lane F, pMS31) carries the out-of-phase rpsO-lacZ fusion at the PstI site within S15 and produces large amounts of the 217-nt transcript; translational frame shifting on this out-of-phase fusion mRNA apparently results in a small amount of β-galactosidase activity in this strain (Table I).
The 3′ end of the rpsO gene transcript was localized by hybridization of RNA to a 3′end-labeled DNA fragment spanning the distal portion of the S15 gene and about 300 nt of 3′-flanking sequence (Fig. 6). The major protected fragment 245 nt in length corresponds to an RNA 3′ terminus in the vicinity of nt 458 on the DNA sequence. This presumably represents a transcription termination point. A second minor fragment of 338 nt was also evident particularly in strains carrying rpsO plasmids (lanes D and F). This corresponds to a 3′ terminus near the – 35 sequence of the pnp promoter (see below); the sequence in this region exhibits dyad symmetry and may represent an RNA processing site for minor read-through transcripts. There may also be some protection of the entire 510-nt probe DNA fragment presumably by unprocessed read’ through transcripts.
Fig. 6.

Nuclease S1 mapping of the 3′ end of the S15 gene transcripts. A 509-bp HpaII fragment (nt 212–721) isolated from plasmid pHE1 and containing the distal portion and the 3′-flanking region of the S15 gene was 3′ end-labeled with PolIk and [α-12P]dCTP. The fragment was hybridized to 5 or 10 μg of RNA, digested with S1 nuclease, denatured and electrophoresed on an 8°, PA sequencing gel. The samples are: (A) E. coli ribosomal RNA; (B and C) 5 and 10 μg of MC1000 RNA; (D) MC1000[pHE1] RNA: (E) MC1000[pMS31] RNA; (F) MC1000[pSH122] RNA; (G) MC1000[pMB1] RNA; (P) untreated end-labeled fragment. The sizes in nt of the major protected fragments are indicated. The MT standards are as described in the legend to Fig. 5 and the 622-nt fragment is marked by an arrowhead.
The 5′ end of transcripts of the pnp gene were localized by hybridization to 5′ end-labeled DNA fragments spanning the rpsO-pnp intergenic space (Fig. 7). The shorter 509-nt long fragment (C in Fig. 4) ends in codon 21 (nt 723) of the pnp gene, whereas the longer fragment derived from pSH122 (D in Fig. 4) extends through the 181 nt of the pnp coding sequence and ends at the BamHI site within the connector sequence of the fusion gene. In the latter case, only RNA derived from the plasmid fusion genes will protect label at the BamHI site from S1 nuclease digestion. The shorter probe yields a major protected fragment of 140 nt and the amount of this fragment is enhanced by the presence of plasmids carrying the intact rpsO-pnp intergenic space (lanes C and D, middle panel). Using the longer probe with the end label at the BamHI connector site, a protected fragment of 269-nt was observed but only with RNA prepared from strains carrying the pnp-lacZ fusion plasmids (lanes C and D, right panel). Both the 140-nt and the 269-nt protected fragments correspond to a 5′ mRNA start site near nt 583 on the DNA sequence. In addition, some protection of the full-length fragment and a 180-nt fragment was observed (see, for example, Fig. 7, center panel, lane C′); this may be indicative of a limited read-through of transcripts from rpsO into pnp and partial processing of the read-through transcript.
Fig. 7.
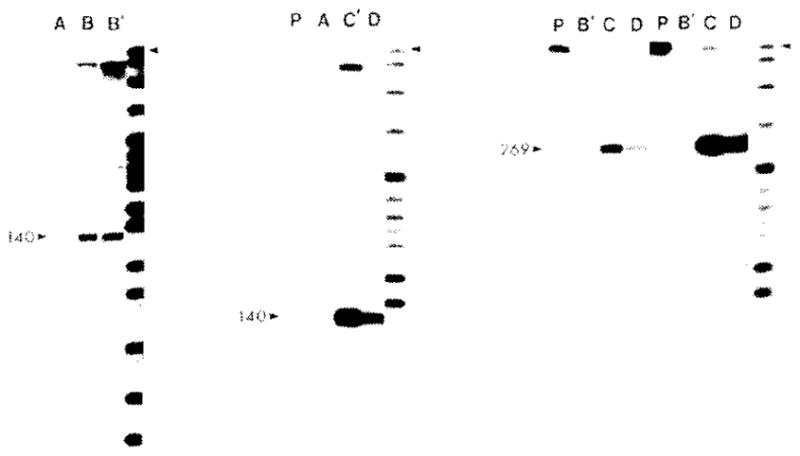
Nuclease S1 mapping of the 5′ ends of pnp gene transcripts. Two fragments, a 509-bp HpaII fragment (nt 212–721) isolated from plasmid pHE1 and a 590-bp PstI-BamHI fragment (nt 262–848) from plasmid pSH122, were 5′ end-labeled with polynucleotide kinase and [γ-32P]ATP. The HpaII fragment extends from within the coding region of S15 to codon 21 in the pnp gene. The PstI-BamHI fragment extends from within the coding region of the S15 gene through the coding region of the pnp gene and ends at the BamHI site just within the lacZ coding sequence of the fusion gene. The labeled fragments were hybridized to RNA, digested with S1 nuclease, denatured and electrophoresed on an 8°, PA sequencing gel. The samples are: (A) 10 μg E.coli ribosomal RNA; (B and B′) 10 or 20 μg of MC1000 RNA; (C and C′) 5 or 10 μg of MC1000[pSH122] RNA; (D) 5 μg of MC1000[pMB1]. The right portion of the figure shows two exposures of the same autoradiogram. The 509-bp probe was used in the right and center panels and the 590-bp probe was used in the left panel.
(e) Conclusions
The beginning of the coding sequence of the pnp gene has tentatively been assigned to the unusual TTG methionine codon located at position 663 in the nucleotide sequence. This assignment is supported (i) by Tn5 mutagenesis, (ii) by deletion mapping, (iii) by limited N-terminal aa sequence analysis of the PNPase protein and (iv) by the reading frame of in-phase fusions between pnp and lacZ on plasmids pSH122 and pMB1 (Portier and Regnier, 1984; Crofton and Dennis, 1983). The rpsO gene was located in front of the pnp gene.
The mapping of the 5′ and 3′ ends of the in vivo RNA transcripts indicates that there are two major promoters and one major terminator in the 5′-flanking region of the pnp gene. The upstream promoter with a start point at about nt 46 services the rpsO gene; the downstream promoter with a start point at about nt 583 services the pnp gene. The −10 and − 35 sequences preceding these transcription start points (Fig. 2) are closely related to the E. coli promoter consensus sequences (Rosenberg and Court, 1979). Transcription termination occurs at about nt 458 in the rpsO-pnp intergenic space. The sequence preceding this site exhibits dyad symmetry (nt 439–455) and the following sequence in which termination occurs contains six consecutive T residues (nt 456–461).
At this point it is still not possible to explain the clustering of macromolecular synthesis genes within the limited and defined regions of the bacterial chromosome. Such clustering may be conducive to efficient transcription. The genes encoding an initiator methionine tRNA, the termination-antitermination protein NusA and translation initiation factor IF2 are located immediately upstream of the rpsO and pnp genes (Ishii et al., 1984; Nakamura and Mizusawa, 1985). Our results indicate that there is little or no transcription read-through from the upstream genes and that the rpsO gene and the pnp gene are themselves driven by separate promoters and separated by an efficient termination signal.
Acknowledgments
This work was supported by the Medical Research Council of Canada (MA6340). We thank Deirdre de Jong-Wong for technical assistance.
Abbreviations
- aa
amino acid(s)
- Ap
ampicillin
- bp
base pairs
- kb
1000bp
- Km
kanamycin
- ORF
open reading frame
- nt
nucleotide(s)
- PA
polyacrylamide
- PNPase
polynucleotide phosphorylase
- PolIk
Klenow fragment of DNA polymerase I of E. coli
- Tc
tetracycline
- XGal
5-bromo-4-chloro-3-indolyl-β-D-galactoside
- [ ]
designates plasmid-carrier state
Footnotes
NOTE ADDED IN PROOF
P. Regnier and C. Portier (personal communication) have suggested that the start point of the pnp promoter is at nt 506 and that processing of this transcript by RNaseIII generates a new 5′ end at nt 583. The 5′ end at nt 583 is the one that we have identified as the pnp promoter.
References
- Casadaban MJ, Chon J, Cohen SN. In vitro gene fusions that join an enzymatically active β-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translation initiation signals. J Bacteriol. 1980;143:971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton S, Dennis PP. Cloning and orientation of the gene encoding polynucleotide phosphorylase in Escherichia coli. J Bacteriol. 1983;154:58–64. doi: 10.1128/jb.154.1.58-64.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis PP, Nomura M. Regulation of the expression of ribosomal protein genes in Escherichia coli. J Mol Biol. 1975;97:61–76. doi: 10.1016/s0022-2836(75)80022-2. [DOI] [PubMed] [Google Scholar]
- Donovan WP, Kushner SR. Amplification of ribo-nuclease II (rnb) activity in Escherichia coli K12. Nucl Acids Res. 1983;11:265–275. doi: 10.1093/nar/11.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloso J, Treisman R, Kamen R. Transcription maps of Polyoma virus specific RNA: analysis by 2-D nuclease S1 gel mapping. Methods Enzymol. 1980;65:718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Ishii S, Kuroki K, Iwamoto F. gene in the leader region of the nusA operon in Escherichia coli. Proc Natl Acad Sci USA. 1984;81:409–413. doi: 10.1073/pnas.81.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R, Apirion D. The involvement of ribonuclease I, ribonuclease II and polynucleotide phosphorylase in the degradation of stable RNA during carbon starvation in E. coli. J Biol Chem. 1974;249:149–151. [PubMed] [Google Scholar]
- Lindahl L, Zengel J. Expression of ribosomal genes in bacteria. Adv Genet. 1982;21:53–12. doi: 10.1016/s0065-2660(08)60297-7. [DOI] [PubMed] [Google Scholar]
- Maniatis T, Fritsch E, Sambrook J. A Laboratory Manual. Cold Spring Harbor Laboratory; Cold Spring Harbor. NY: 1982. Molecular Cloning. [Google Scholar]
- Maxam AM, Gilbert W. Sequencing end-labeled DNA with base specific chemical cleavages. Methods Enzymol. 1980;65:499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 cloning vectors. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miller J. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 1972. [Google Scholar]
- Morinaga T, Funatsu G, Funatsu M, Wittmann HG. Primary structure of the 16S rRNA binding protein S15 from Escherichia coli ribosomes. FEBS Lett. 1976;64:307–309. doi: 10.1016/0014-5793(76)80316-x. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Mizusawa S. In vivo evidence that the nusA and infB genes of E. coli are part of the same multi-gene operon which encodes at least four proteins. EMBO J. 1985;4:527–532. doi: 10.1002/j.1460-2075.1985.tb03660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M, Course R, Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- Portier C. Physical location and direction of transcription of the structural gene of Escherichia coli ribosomal protein SI5. Gene. 1982;18:261–266. doi: 10.1016/0378-1119(82)90164-0. [DOI] [PubMed] [Google Scholar]
- Portier C, Regnier P. Expression of the rpsO and pnp genes: Structural analysis of a DNA fragment carrying their control regions. Nucl Acids Res. 1984;12:6091–6102. doi: 10.1093/nar/12.15.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner AM. Isolation and mapping of polynucleotide phosphorylase mutants of E. coli. J Bacteriol. 1969;97:1431–1436. doi: 10.1128/jb.97.3.1431-1436.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M, Court D. Regulating sequences involved in promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulsen AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicira J, Messing J. The pUC plasmids, an M13 mp7-derived system for insertion mutagenesis and sequencing with universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]