Abstract
Fibroblast growth factor-21 (FGF21) levels are elevated in patients with primary mitochondrial disorders but have not been studied in patients with inborn errors of metabolism (IEM) known to have secondary mitochondrial dysfunction. We measured plasma FGF21 by ELISA in patients with and without IEM. FGF21 levels were higher in patients with IEM compared to without IEM (370 pg/dL vs. 0–65 pg/dL). Further study of FGF21 as a biomarker in IEM is warranted.
1. Background
Secondary mitochondrial dysfunction is observed as a consequence of dysfunction in pathways of intermediary metabolism. A well-known example occurs in acute metabolic decompensations in patients with organic acidurias (OA) and fat oxidation disorders (FAOD) which are often characterized by hyperlactatemia and the urinary excretion of mitochondrial and Kreb's cycle metabolites [1]. Speculation about the mechanism of secondary mitochondrial dysfunction in some inborn errors of metabolism (IEM) has revolved around the depletion of the Coenzyme-A pools subsequent to the overproduction of organic and dicarboxylic acids, a paucity of Kreb's cycle intermediates or direct effects on oxidative phosphorylation (OXPHOS) by elevated levels of cytotoxic intermediates [6]. Moreover, mitochondrial oxidative stress has been implicated as a pathological factor in a broader range of IEM including urea cycle disorders and phenylketonuria [4], [7]. There is little known about how mitochondrial energy production is regulated in IEM patients in the bioenergetically balanced or decompensated states despite the fact that it has long been implicated in the group's pathological manifestations.
Fibroblast growth factor 21 (FGF21) is an important hepatokine in both intermediary and mitochondrial energy metabolism. FGF21 has been shown to stimulate fatty acid oxidation and ketogenesis, reduce insulin secretion, increase insulin sensitivity and inhibit overall growth through PPAR-gamma and beta-Klotho signaling pathways on multiple tissue types, including the brain, adipose and muscle [5]. Additionally, FGF21 may modulate OXPHOS through AMPK and SIRT1 activation [2].
Irrespective of its actions on metabolism, FGF21 has recently been proposed as a clinical biomarker for primary mitochondrial disorders, in particular those that manifest as myopathy [3]. In one study by [8], FGF21 levels were more sensitive and specific than traditional biomarkers of mitochondrial dysfunction such as creatine kinase, lactate and pyruvate. Given that FGF21 levels are elevated in primary mitochondrial disorders and many IEM demonstrate secondary mitochondrial dysfunction we hypothesized FGF21 levels would be significantly higher in patients with IEM.
2. Methods
2.1. Study population
After approval from the Children's National Office for the Protection of Human Subjects, plasma samples were collected from patients with diagnosed IEM (n = 42) seen for health supervision (well) visits in the Metabolic Disorders Clinic at Children's National, Washington, DC. Control samples (n = 7) were from de-identified, waste plasma samples from patients without a known IEM and were collected and analyzed at the same time as the IEM patients' samples.
2.2. FGF21 levels
FGF21 levels were determined in plasma of patients and control samples using the Human FGF-21 ELISA kit from BioVendor (Cat# RD191108200R) following manufacturer's recommendations. The lower limit of detection for the assay was 30 pg/mL. Leucine (Leu) and phenylalanine (Phe) levels were measured for clinical care using a Biochrom 30 analyzer in a CLIA certified lab as previously described. Statistical analysis was done using GraphPad Prism (v7). Averages were calculated for groups (Controls, IEM, mitochondrial IEM and non-mitochondrial IEM, Leu and Phe levels). Correlation between Leu and FGF21 levels was tested by calculating the Pearson coefficient.
3. Results
The study population included 7 control patients without IEM, 4 with respiratory chain complex 1 deficiency, 6 with urea cycle disorders (1 orinithine transcarbamylase deficiency, 1 carbamoyl phosphate synthase-1 deficiency, 1 citrullinemia, 2 arginosuccinate lyase deficiency, 1 with proximal UCD that had not been molecularly characterized), 4 fatty acid oxidation disorder (2 very-long chain acyl-CoA dehydrogenase deficiency, 2 carnitine palmitoyltransferase-2 deficiency), 7 organic aciduria (4 propionic acidemia, 2 isovaleric aciduria, 1 undiagnosed OA), 6 maple syrup urine disease, 4 phenylketonuria, 7 homocystinuria (3 classic homocystinuria, 2 methionine synthase deficiency, 1 cobalamin-c disease).
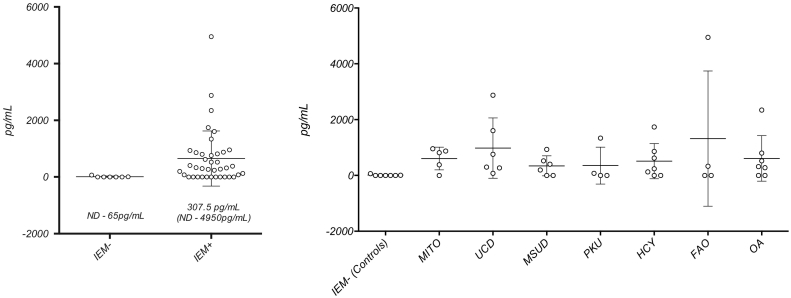
Fig. 1's first panel shows the difference in FGF21 levels between patients with IEM and those without IEM (370 pg/dL vs. 0–65 pg/dL). FGF21 levels were not significantly higher in patients with mitochondrial IEM (UCD, OA, FAO) compared to non-mitochondrial IEM (PKU, MSUD, Homocystinuria) (908 pg/dL vs 415 pg/dL). Fig. 1's second panel shows the distribution of levels by broad categories of IEM.
Fig. 1.
FGF21 levels in patient with and without IEM. IEM − = Control subjects without an IEM, IEM + = all subjects with a diagnosed IEM, MITO = known primary mitochondrial disorder, UCD = Urea Cycle Disorder, MSUD = Maple Syrup Urine Disease, PKU = Phenylketonuria, HCY = Homocystinuria (Classic), FAO = Fatty Acid Oxidation Disorder, OA = Organic aciduria.
Because Leu levels are widely accepted by clinicians as markers of biochemical control in MSUD patients, we looked for a correlation between Leu and FGF21 levels analyzed from the same plasma sample (N = 6; r2 = 0.0542, p = 0.1), observing a tendency towards a moderate positive correlation that was not statistically significant. We were unable to perform the same analysis for the PKU patients due to small sample size (N = 4), but did note that the PKU patient with the highest average Phe level (13.8 mg/dL vs 4.8, 7.7 and 9.8 mg/dL) over the year preceding the FGF21 measurement, had the highest FGF21 level of the PKU group (1340 pg/mL vs 75, 0 and 0 pg/mL).
4. Discussion
FGF21 levels were generally higher in patients with IEM compared to unaffected controls and the levels within the IEM group show a high degree of variability. In this sample's patients with MSUD FGF21 levels tended to correlate with plasma leucine although this was not a statistically significant observation. These data suggest that elevation of FGF21 is a common finding in patients with IEM and that perturbations might potentially correlate with disease severity in some disorders.
The multiple effects of FGF21 on metabolism as well as the multiple effects of metabolic states (catabolic vs anabolic) on FGF21 levels do not allow us to easily say why FGF21 levels should be high in IEM patients. Certainly FGF21 may be elevated for the same reasons they are postulated to be high in primary mitochondrial disorders, namely that inefficient energy metabolism leads to a cellular bioenergetic state that is biochemically similar to starvation [9]. Alternatively, for cases wherein the dietary management is based on macromolecular restriction (protein in PKU and MSUD, long-chain fat in FAOD) FGF21 levels could be affected. What speaks against this latter point in our study is that fact that all of the IEM groups had individuals with elevated FGF21 levels and not all of them are treated with (or adherent to) dietary restriction.
Further studies of FGF21 in IEM patients are warranted particularly if FGF21 continues to prove useful as a clinical discriminator of mitochondrial dysfunction. There are currently very few informative biomarkers for IEM disease severity, dietary adherence and clinical outcomes. Future studies of FGF21 in IEM should be larger, disorder-focused, longitudinal, account for clinical factors such as genotype and clinical disease severity as well as measure traditional biomarkers (amino acids, ammonia, etc.) and markers of oxidant stress.
Footnotes
Disclosures: None of the authors have any conflicts to disclose.
References
- 1.Blau N., editor. Laboratory Guide to the Methods in Biochemical Genetics. first ed. Vol. 1. Springer-Verlag; Berlin: 2008. [Google Scholar]
- 2.Chau M.D., Gao J., Yang Q., Wu Z., Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc. Natl. Acad. Sci. U. S. A. 2010;107(28):12553–12558. doi: 10.1073/pnas.1006962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis R.L., Liang C., Edema-Hildebrand F., Riley C., Needham M., Sue C.M. Fibroblast growth factor 21 is a sensitive biomarker of mitochondrial disease. Neurology. 2013;81(21):1819–1826. doi: 10.1212/01.wnl.0000436068.43384.ef. [DOI] [PubMed] [Google Scholar]
- 4.Felipo V., Butterworth R.F. Mitochondrial dysfunction in acute hyperammonemia. Neurochem. Int. 2002;40(6):487–491. doi: 10.1016/s0197-0186(01)00119-x. [DOI] [PubMed] [Google Scholar]
- 5.Goetz R. Metabolism: adiponectin—a mediator of specific metabolic actions of FGF21. Nat. Rev. Endocrinol. 2013;9(9):506–508. doi: 10.1038/nrendo.2013.146. [DOI] [PubMed] [Google Scholar]
- 6.Saudubray J.-M. 5th Edition ed. Springer; New York: 2013. Inborn Metabolic Diseases: Diagnosis and Treatment. [Google Scholar]
- 7.Sitta A., Barschak A.G., Deon M., de Mari J.F., Barden A.T., Vanzin C.S.…Vargas C.R. L-carnitine blood levels and oxidative stress in treated phenylketonuric patients. Cell. Mol. Neurobiol. 2009;29(2):211–218. doi: 10.1007/s10571-008-9313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suomalainen A., Elo J.M., Pietilainen K.H., Hakonen A.H., Sevastianova K., Korpela M.…Tyynismaa H. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet Neurol. 2011;10(9):806–818. doi: 10.1016/S1474-4422(11)70155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyynismaa H., Carroll C.J., Raimundo N., Ahola-Erkkila S., Wenz T., Ruhanen H.…Suomalainen A. Mitochondrial myopathy induces a starvation-like response. Hum. Mol. Genet. 2010;19(20):3948–3958. doi: 10.1093/hmg/ddq310. [DOI] [PubMed] [Google Scholar]