Abstract
Murine noroviruses (MNV) are highly prevalent in laboratory mice, can cause persistent infections, and have been shown to infect macrophages, dendritic cells, and B cells. To address the potential impact of MNV infection on research outcomes, numerous studies have been conducted with various mouse models of human disease and have generated mixed results ranging from no impact to significant disease. Many of these studies included histologic evaluations after MNV infection, and similarly these results have been variable as to whether MNV induces lesions despite the fact that localization of MNV by viral culture and molecular techniques have demonstrated systemic distribution regardless of mouse immune status. The aim of this review is to summarize the histologic findings that have been reported with MNV infection in several mouse models. The studies demonstrate that experimental infection of MNV in wild-type mice results in minimal to no histologic changes. In contrast, immunodeficient mice consistently have detectable MNV-induced lesions that are typically inflammatory and, in the most severe cases, accompanied by necrosis. In these, the liver is commonly affected with more variable lesions reported in the lung, gastrointestinal tract, mesenteric lymph nodes, brain, and spleen. In specific disease models including atherosclerosis, MNV infection had a variable impact that was dependent upon the mouse model, viral strain, timing of infection, or other experimental variables. It is important to recognize the reported MNV lesions to help discern the possible influence of MNV infection on data generated in mouse models.
Keywords: atherosclerosis, Caliciviridae, calicivirus, histopathology, laboratory animals, murine norovirus, pathology, laboratory mice, STAT1−/−, IFNαβγR−/−
Noroviruses are nonenveloped, single-stranded, positive-sense RNA viruses in the family Caliciviridae and are best known for their ability to cause acute gastroenteritis in humans.8 These viruses are considered species-specific and have been reported in animals including pigs, cows, sheep, cats, dogs and rats.8,30,39,44,48,52 A norovirus infecting laboratory mice was first described in 2003, and was designated murine norovirus 1 (MNV-1).22 This virus caused lethal infection in mice deficient in signal transducer and activator of transcription 1 (STAT1−/−) or the interferon αβ and γ receptors (IFNαβγR−/−), both of which are important in the immune response against viral pathogens.41 Evaluation by histopathology after infection of these mice with MNV-1 revealed pneumonia, hepatitis, encephalitis, meningitis, and vasculitis of the cerebral vessels. Interestingly however, many strains of mice – including wild-type and other immunodeficient strains such as recombination activating gene-deficient (RAG−/−) mice which lack T and B cells – did not succumb to lethal disease after infection with MNV-1. While this initial isolate of MNV caused only transient infection in mice, discovery and characterization of additional MNV strains revealed that the virus can cause persistent infections in laboratory mice and is shed in the feces for prolonged periods.15
Prevalence
MNV was the first norovirus to be grown in cell culture.50 The ability to grow the virus in vitro allowed for the development of diagnostic assays including immunohistochemistry to detect viral antigens, serologic assays for detection of anti-MNV antibodies, and molecular assays to detect viral RNA.9,15,16,22,49 Initial large scale prevalence data on MNV infection revealed that 22.1% of 12,639 serum samples from laboratory mice in the United States and Canada were positive for antibodies to MNV.16 Another large scale study likewise confirmed the high prevalence of MNV infection with a rate of 32.4% for laboratory mice in North America and Europe, and other studies have reported rates as high as 64%.26,27,32,40 These data indicate that MNV infection in laboratory mice is widespread and worldwide and therefore has the potential to interfere with research results.
Effects on Research
As a result of this novel discovery of a norovirus in laboratory mice, the variable clinical disease seen depending on the type of mouse infected, and the high prevalence of infection in research mice, further study is warranted to reveal and characterize the potential impact of this virus on research using MNV infected mice. Importantly, MNV has a tropism to infect macrophages and dendritic cells, and more recently has been reported to infect B cells.18,50 Infection of immune cells therefore raises the possibility that MNV may be a confounding factor in mouse models of inflammatory disease. Surprisingly, results have been variable in that some mouse models are altered by MNV infection, while others do not show any changes. For example, MNV has been shown to cause Paneth cell abnormalities in a mouse model of Crohn’s disease and to exacerbate inflammatory bowel disease (IBD) in a Helicobacter bilis-driven mouse model of IBD.4,29 Additionally, MNV has been reported to disrupt the epithelial barrier and increase inflammation in the stomach and colon of Il10-deficient mice.3 Effects outside of the gastrointestinal system have also been reported with MNV infection resulting in increased atherosclerosis in low-density lipoprotein receptor-deficient mice.36 MNV infection also altered another commonly used mouse model of atherosclerosis, the apolipoprotein E (ApoE)-deficient mice, but that effect was variable.12 Interestingly however, a number of studies have also reported a lack of effect of MNV infection on research mouse models. MNV infection did not alter inflammatory bowel disease in Il10-deficient mice or the development of colon cancer in Smad3-deficient mice when inflammation was driven by H. bilis.13,28 MNV infection did not disrupt the intestinal microbiota in Swiss Webster or C57BL/6 mice.34 Infection of mice with MNV also had little or no impact on studies of mice infected with murine cytomegalovirus, Friend retrovirus, vaccinia virus or influenza A virus, and did not alter a mouse model of diet-induced obesity and insulin resistance.1,5,10,35 Collectively, these studies highlight the variable effect that MNV has on mouse models of disease suggesting that research using MNV-infected mice should be carefully scrutinized for potential confounding effects.
Histologic Lesions Caused by MNV Infection in Mice
As described above, MNV infection may alter some mouse models of disease but may have little or no impact on other models. This variable response may be due to a number of factors including the particular disease model being studied or differences in pathogenicity of the infecting MNV strain. A large number of MNV strains have been described and are likely the result of genetic recombination and/or the lack of a proofreading activity in the RNA-dependent RNA polymerase of RNA viruses.2,6,14,32 Therefore, determining whether MNV infection is a confounding factor in a particular research study using infected mice should be evaluated on a case by case basis.
A comprehensive summary has been compiled of whether histologic changes have been reported in particular tissues as a result of MNV infection in laboratory mice (Tables 1–5). Additional information including histologic changes and detailed methods including infectious challenge, the mouse genetic background, sex, health status, and housing type (if reported), and any other infections or treatments are provided in the Supplemental Materials (Supplemental Tables 1–5). The intent of these summary tables are to assist the pathologist, veterinarian, and scientist with identifying the currently available and published information on the histologic findings in MNV-infected mice. The intent is not a comprehensive review of MNV itself, and so the reader is referred to a number of excellent reviews for more information on MNV and noroviruses in general.20,21,23,51
Table 1.
Histologic Changes in Mice Naturally Infected with MNV.a
| Reference | Mouse Strain | MNV Strain | Liver | Lung | MLN | Peritoneum | Small Intestine | Large Intestine | Brain | Spleen | Stomach |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 | NOD.CB17-Prkdcscid/J | MNV-5, MNV-6 | − | n/r | − | n/r | − | − | n/r | − | n/r |
| 24 | NOD/ShiLtJ | MNV-5, MNV-6 | − | n/r | − | n/r | − | − | n/r | − | n/r |
| 38 | Tac:SW | endemic | + | n/r | − | n/r | − | n/r | n/r | − | n/r |
| 45 | 129S6/SvEv-Stat1tm1Rds | MNV-07 | + | + | + | n/r | − | − | n/r | + | n/r |
| 45 | 129S6/SvEvTac | MNV-07 | + | − | − | n/r | + | − | n/r | + | n/r |
| 45 | het 129S6/SvEv-Stat1tm1Rds | MNV-07 | + | − | − | n/r | − | − | n/r | + | n/r |
| 49 | Rag1−/−/IFNγR−/− | endemic | + | + | + | + | − | n/r | n/r | n/r | n/r |
| 49 | OT1Rag1−/−/IFNγR−/− | endemic | + | + | n/r | + | − | n/r | n/r | n/r | n/r |
| 49 | OT2Rag1−/−/IFNγR−/− | endemic | + | + | n/r | n/r | − | n/r | n/r | n/r | n/r |
| 49 | Rag1−/−/Stat1−/− | endemic | + | + | + | + | − | n/r | n/r | n/r | n/r |
| 49 | Rag2−/− | endemic | − | − | − | − | − | n/r | n/r | n/r | n/r |
+ = histologic changes reported, − = no histologic changes, n/r = not reported, MLN= mesenteric lymph node
The histologic changes seen in MNV-infected mice from endemic colonies with natural exposure (i.e., via other infected animals) are summarized in Table 1. For these studies, the dose and route of inoculation are not controlled and often the MNV strain or isolate is unknown or uncharacterized. Generally, MNV-associated liver lesions are significant only in immunodeficient STAT1−/− and IFNγR−/− mice (often in combination with RAG deficiency). These lesions are characterized as mild to severe multifocal to diffuse inflammatory lesions with variable necrosis or fibrosis.45,49 In contrast, only minimal to mild hepatic lesions have been reported in immunocompetent outbred Swiss Webster (SW) mice and wild-type 129 mice. In SW mice, there were small foci of inflammatory cells including neutrophils, lymphocytes, or macrophages in 80% of livers, and were not consistently associated with MNV IHC positive staining.38 In 129 wild-type mice, 15% of livers had hepatic veins with adhesions of mononuclear leukocytes (diagnosed as mild vasculitis by the authors) with occasional mild inflammatory foci.45 Pneumonia has been reported in naturally infected STAT1−/− and IFNγR−/−mice, but not wild-type mice, indicating the importance of the interferon response in controlling inflammation against viral infections such as MNV.45,49 Interestingly, although noroviruses are gastrointestinal pathogens, there was only one report that described histologic changes in the intestines of 129 mice naturally infected with MNV and these changes were limited to enlargement of Peyer’s patches with increased germinal centers.45 This suggests that MNV is well adapted to its immunocompetent host and causes no or only subtle disease in the intestines after natural infection.
Controlled experimental MNV infections in wild-type mice allow for the evaluation of histologic lesions attributable to MNV without other confounding variables such as immunodeficiency, unknown inoculating dose, viral strain, or timing of infection (Table 2). The majority of studies conducted in wild-type mice infected with MNV show no overt clinical signs of disease or histologic lesions. However, histologic changes were recently reported in wild-type 129 mice including mild to moderate lesions in the liver (defined as 1–2 or 5 to 10 foci of inflammatory cells per 10 fields at 10× objective, respectively) and reactive changes in the lymphoid tissues including enlargement of small intestinal Peyer’s patches with increased germinal centers, splenic red pulp hyperplasia and activation of the white pulp.45 Therefore, the strain or stock of the wild-type mouse, as well as the particular infecting MNV strain or isolate, are likely important determinants of whether histologic changes will be seen after infection.
Table 2.
Histologic Changes in Wild-Type Mice Experimentally Infected with MNV.a
| Reference | Mouse Strain | MNV Strain | Liver | Lung | MLN | Peritoneum | Small Intestine | Large Intestine | Brain | Spleen | Stomach |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | C3H/HeJBirZtm | MNV/Hannover1/2007/DEU | n/r | n/r | n/r | n/r | n/r | − | n/r | n/r | − |
| 3 | C57BL/6JZtm | MNV/Hannover1/2007/DEU | n/r | n/r | n/r | n/r | n/r | − | n/r | n/r | − |
| 19 | 129S6/SvEvTac | MNV-1.CW3 or MNV-3 | n/r | n/r | n/r | n/r | + | n/r | n/r | n/r | n/r |
| 22 | 129 | MNV-1 | − | − | n/r | n/r | n/r | n/r | n/r | − | n/r |
| 33 | 129S6/SvEvTac | MNV-1.CW3 | − | − | n/r | n/r | + | n/r | n/r | + | n/r |
| 45 | 129S6/SvEvTac | MNV-07 | + | n/r | n/r | n/r | n/r | n/r | n/r | − | n/r |
| 45 | 129S6/SvEvTac | MNV-1.CW3 | − | n/r | n/r | n/r | n/r | n/r | n/r | + | n/r |
+ = histologic changes reported, − = no histologic changes, n/r = not reported, MLN= mesenteric lymph node
In contrast to immunocompetent mice, experimental infection of mice with known immunodeficiencies results in significant disease (Table 3). Most of these reports involve mice that lack genes important in the type I and type II interferon response to viruses such as STAT1 and IFN receptors.41 In general, histologic changes such as inflammation, necrosis, and apoptosis were consistently reported in the liver (Figures 1, 2, 3, and 4,) and spleen of these immunodeficient mice following infection with MNV. Inflammation in the lungs and small intestines were also reported. Collectively, these reports indicate that experimental MNV infection in immunodeficient STAT1−/− and IFNαβγR−/−mice results in systemic lesions with significant hepatitis. This is in contrast to immunocompetent mice where experimental and natural infections lead to minimal lesions, such as small hepatic foci of inflammatory cells including neutrophils, lymphocytes or macrophages38 or mild centrilobular accumulations of mononuclear leukocytes45; neither group reported severe hepatitis in immunocompetent mice. Additionally, we report previously unpublished findings of MNV infection in mice with Toll-like receptor 4 (TLR4) deficiencies both with and without concurrent infection with the bacteria Klebsiella and Proteus. TLR4 is a component of the innate immune system recognizing lipopolysaccharide (LPS) from Gram-negative bacteria, but has also been reported to be involved in the recognition of viruses.17 In these TLR4-deficient mice, MNV infection alone or concurrently with Klebsiella and Proteus infection did not appreciably increase lesions in the mesenteric lymph nodes or colitis (C. Hsu, unpublished data).
Table 3.
Histologic Changes in Immunodeficient Mice Experimentally Infected with MNV.a
| Reference | Mouse Strain | MNV Strain | Liver | Lung | MLN | Peritoneum | Small Intestine | Large Intestine | Brain | Spleen | Stomach |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 22 | Stat1−/− | MNV-1 | + | + | n/r | n/r | n/r | n/r | n/r | + | n/r |
| 22 | Rag2−/−/Stat1−/− | MNV-1 | + | + | n/r | n/r | n/r | n/r | + | n/r | n/r |
| 22 | IFNαβγR−/− | MNV-1 | n/r | n/r | n/r | n/r | n/r | n/r | + | n/r | n/r |
| 31 | 129S6/SvEv-Stat1tm1Rds | MNV-1 (cDNA derived) | + | n/r | n/r | n/r | + | n/r | n/r | + | n/r |
| 31 | 129S6/SvEv-Stat1tm1Rds | MNV-1 VF1 (ORF4) deficient | + | n/r | n/r | n/r | − | n/r | n/r | + | n/r |
| 33 | 129S6/SvEv-Stat1tm1Rds | MNV-1.CW3 | − | + | n/r | n/r | + | n/r | n/r | + | n/r |
| 42 | AG129 (IFNαβγR−/−) | MNV-1.CW3 | n/r | n/r | n/r | n/r | + | n/r | n/r | n/r | n/r |
| 45 | 129S6/SvEv-Stat1tm1Rds | MNV-07 | + | n/r | n/r | n/r | n/r | n/r | n/r | + | n/r |
| 45 | 129S6/SvEv-Stat1tm1Rds | MNV-1.CW3 | + | n/r | n/r | n/r | n/r | n/r | n/r | + | n/r |
| 46 | C57BL/6-Stat1tm1Dlv | pCW3 (MNV-1) | + | n/r | n/r | n/r | n/r | n/r | n/r | + | n/r |
| 46 | C57BL/6-Stat1tm1Dlv | pCW3 PCR6 | + | n/r | n/r | n/r | n/r | n/r | n/r | + | n/r |
| 46 | C57BL/6-Stat1tm1Dlv | pCR6 | − | n/r | n/r | n/r | n/r | n/r | n/r | + | n/r |
| 46 | C57BL/6-Stat1tm1Dlv | pCR6 PCW3 | + | n/r | n/r | n/r | n/r | n/r | n/r | + | n/r |
| Unpublished | Tlr4−/− | MNV-4 | n/r | n/r | − | n/r | n/r | − | n/r | n/r | n/r |
| Unpublished | Tlr4−/− + Klebsiella and Proteus | MNV-4 | n/r | n/r | − | n/r | n/r | − | n/r | n/r | n/r |
+ = histologic changes reported, − = no histologic changes, n/r = not reported, MLN= mesenteric lymph node
Figure 1.
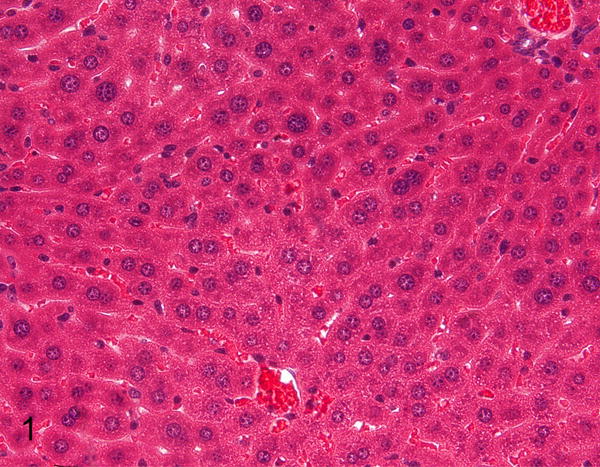
Normal liver, STAT1−/− mouse. Hematoxylin and eosin (HE).
Figure 2.
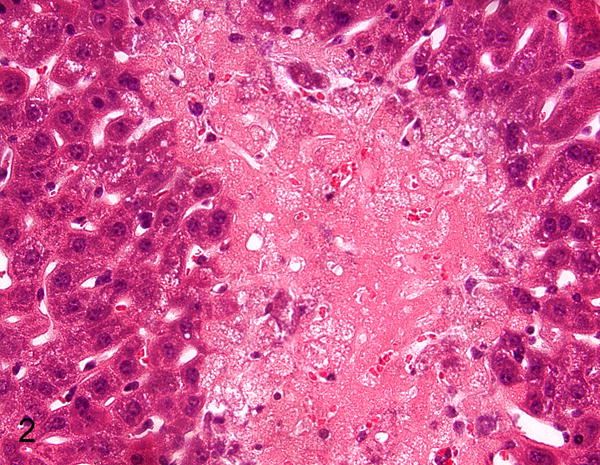
Hepatic necrosis, liver, STAT1−/− mouse infected with murine norovirus (MNV). HE. Figures 1 and 2 are reprinted from “STAT1-dependent innate immunity to a Norwalk-like virus,” Karst SM, et al. Science. 2003;299(5612):1575–8 with permission from AAAS.
Figure 3.
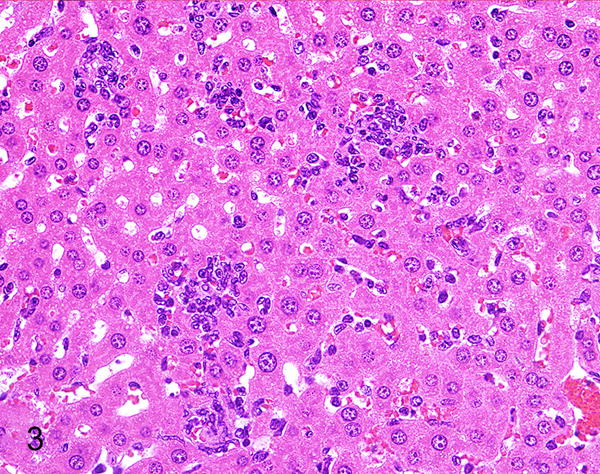
Hepatitis, liver, 2.5-month-old Rag1−/−/IFNγR−/− mouse. There are small granulomas and inflammatory cells in sinusoids. HE.
Figure 4.
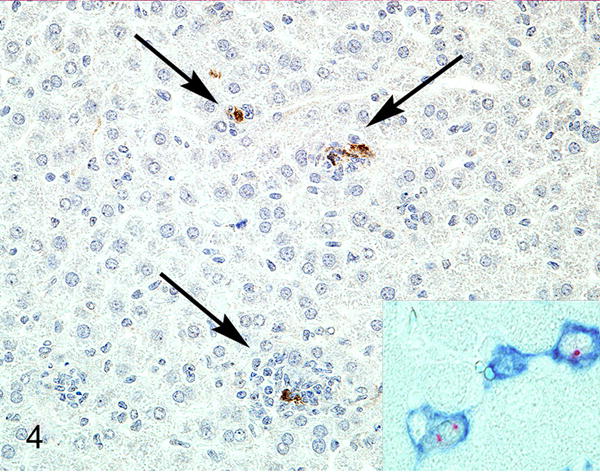
Liver, 2.5-month-old Rag1−/−/IFNγR−/− mouse. There is expression of MNV-1 ProPol in inflammatory cells (arrows). Inset: hepatitis, liver, Rag1−/−/STAT1−/− mouse. The sinusoidal lining cells have F4/80-positive cell membranes (blue) and MNV ProPol labeling (red) of the cytoplasm. Immunohistochemistry for MNV-1 ProPol. Figures 3 and 4 are reprinted from “Pathology of immunodeficient mice with naturally occurring murine norovirus infection,” Ward JM, et al. Toxicol Pathol. 2006;34(6): 708–15 with permission from SAGE Publications.
Since noroviruses are gastrointestinal pathogens, much of the research on the impact of MNV has been conducted on mouse models of gastrointestinal disease (Table 4) including dextran sodium sulfate (DSS)-induced colitis, mice with disrupted expression of the autophagy related 16-like 1 gene (Atg16L1), and mice deficient in multi-drug resistance gene 1a (FVB.129P2-Abcb1atm1Bor; Mdr1a−/−) (Figures 5 to 8) or in interleukin-10 (B6.129P2-Il10tm1Cgn/J;Il10−/−). DSS is administered orally to mice to cause direct damage to the intestinal epithelium as a model of inflammatory bowel disease (IBD), while mutations in the autophagy gene ATG16L1 have been associated with Crohn’s disease.4,7,43 Mdr1a−/− mice have an altered intestinal epithelial barrier, while Il10−/− mice have impaired regulatory T-cells resulting in IBD.7,43 MNV infections in different mouse models of gastrointestinal disease varied in their ability to cause intestinal histologic lesions, perhaps due to the particular MNV strain used for infection, timing of MNV infection, or whether another experimental infectious agent was present. For example, we recently reported that MNV-4 did not alter IBD in mice lacking the immunoregulatory cytokine IL-10 when intestinal disease was driven by infection with Helicobacter bilis, however others have reported histologic changes as a result of MNV infection in Il10−/− mice with a different strain of MNV and without H. bilis.3,13 Additionally, mice with hypomorphic expression of the Atg16L1 protein (Atg16L1HM) displayed inflammatory hallmarks of Crohn’s disease after DSS treatment when infected with MNV CR6, which persistently infects mice, but not with MNV-1.CW3, which is not persistent.4 This histologic change was also seen when Atg16L1HM mice were infected with MNV CR6 prior to DSS treatment, but not when MNV CR6 was given concurrently with DSS administration, suggesting that timing of MNV infection is important. Finally, with MNV infection in 129-Smad3tm1Par/J × 129S6/SvEvTac-Rag2tm1Fwa (Smad3−/−/Rag2−/−) double-knockout mice, B6.129S1-Nod2tm1Flv/J (Nod2−/−) mice with and without H. bilis infection, and Mdr1a−/− mice fed a purified or high-fat diet, we found that MNV-4 infection did not cause histologic changes in these mice (unpublished data). Overall, the impact of MNV infection in mouse models of gastrointestinal disease varies widely, from having little to no impact to causing significantly increased inflammation in the gastrointestinal tract. Conversely, it has even been reported that MNV infection provides protection against inflammation induced by Citrobacter rodentium infection and antibiotic treatment.25
Table 4.
Histologic Changes in Mice of Gastrointestinal Disease Models Experimentally Infected with MNV.a
| Reference | Mouse Strain | MNV Strain | Liver | Lung | MLN | Peritoneum | Small Intestine | Large Intestine | Brain | Spleen | Stomach |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | C3Bir.129P2-Il10tm1Cgn/JZtm | MNV/Hannover1/2007/DEU | n/r | n/r | n/r | n/r | n/r | + | n/r | n/r | + |
| 3 | B6.129P2-Il10tm1Cgn/JZtm | MNV/Hannover1/2007/DEU | n/r | n/r | n/r | n/r | n/r | + | n/r | n/r | + |
| 3 | Germ-free B6.129P2-Il10tm1Cgn/JZtm | MNV/Hannover1/2007/DEU | n/r | n/r | n/r | n/r | n/r | + | n/r | n/r | n/r |
| 3 | ASF B6.129P2-Il10tm1Cgn/JZtm | MNV/Hannover1/2007/DEU | n/r | n/r | n/r | n/r | n/r | + | n/r | n/r | + |
| 4 | Atg16L1HM | MNV CR6 | n/r | n/r | n/r | n/r | + | n/r | n/r | n/r | n/r |
| 4 | wild type | MNV CR6 | n/r | n/r | n/r | n/r | − | n/r | n/r | n/r | n/r |
| 4 | Atg16L1HM | MNV-1.CW3 | n/r | n/r | n/r | n/r | + | n/r | n/r | n/r | n/r |
| 4 | wild type | MNV-1.CW3 | n/r | n/r | n/r | n/r | + | n/r | n/r | n/r | n/r |
| 4 | Atg16L1HM + DSS | MNV CR6 prior to DSS | n/r | n/r | n/r | n/r | + | + | n/r | n/r | n/r |
| 4 | wild type + DSS | MNV CR6 prior to DSS | n/r | n/r | n/r | n/r | − | − | n/r | n/r | n/r |
| 4 | Atg16L1HM + DSS | MNV-1.CW3 prior to DSS | n/r | n/r | n/r | n/r | + | − | n/r | n/r | n/r |
| 4 | wild type + DSS | MNV-1.CW3 prior to DSS | n/r | n/r | n/r | n/r | − | − | n/r | n/r | n/r |
| 4 | Atg16L1HM + DSS | MNV CR6 concurrent to DSS | n/r | n/r | n/r | n/r | − | − | n/r | n/r | n/r |
| 4 | wild type + DSS | MNV CR6 concurrent to DSS | n/r | n/r | n/r | n/r | − | − | n/r | n/r | n/r |
| 4 | Atg16L1HM + DSS + TNFα or IFNγ blocking antibodies | MNV CR6 | n/r | n/r | n/r | n/r | + | + | n/r | n/r | n/r |
| 4 | Atg16L1HM + DSS + broad spectrum Abx | MNV CR6 | n/r | n/r | n/r | n/r | − | − | n/r | n/r | n/r |
| 11 | C57BL/6J + Salmonella typhimurium | MNV-1.CW3, MNV-4 | n/r | n/r | n/r | n/r | n/r | − | n/r | n/r | n/r |
| 13 | B6.129P2-Il10tm1Cgn/J + Helicobacter bilis | MNV-4 | n/r | n/r | − | n/r | n/r | − | n/r | n/r | n/r |
| 25 | Germ-free C57BL/6J | MNV.CR6, MNV.CW3, or MNV.SKI | n/r | n/r | n/r | n/r | + | − | n/r | n/r | n/r |
| 25 | C57BL/6J + Abx | MNV.CR6 | n/r | n/r | n/r | n/r | + | n/r | n/r | n/r | n/r |
| 25 | C57BL/6J + Abx + Citrobacter rodentium | MNV.CR6 | n/r | n/r | n/r | n/r | n/r | + | n/r | n/r | n/r |
| 29 | FVB.129P2-Abcb1atm1Bor (Mdr1a−/−) + Helicobacter bilis | MNV-4 | n/r | n/r | + | n/r | − | + | n/r | + | n/r |
| 28 | 129-Smad3tm1Par/J + Helicobacter bilis | MNV-4 | n/r | n/r | n/r | n/r | n/r | − | n/r | n/r | n/r |
| Unpublished | 129-Smad3tm1Par/J × 129S6/SvEvTac-Rag2tm1Fwa (Smad3−/−/Rag2−/−) | MNV-4 | − | − | − | n/r | − | − | n/r | n/r | n/r |
| Unpublished | B6.129S1-Nod2tm1Flv/J + Helicobacter bilis | MNV-4 | n/r | n/r | − | n/r | − | − | n/r | n/r | n/r |
| Unpublished | FVB.129P2-Abcb1atm1Bor (Mdr1a−/−) + AIN93M diet | MNV-4 | n/r | n/r | − | n/r | n/r | − | n/r | n/r | n/r |
| Unpublished | FVB.129P2-Abcb1atm1Bor (Mdr1a−/−) + high fat diet | MNV-4 | n/r | n/r | − | n/r | n/r | − | n/r | n/r | n/r |
+ = histologic changes reported, − = no histologic changes, n/r = not reported, MLN = mesenteric lymph node, ASF = altered Schaedler flora, Abx = antibiotics, DSS = dextran sulfate sodium
Figures 5 and 6.
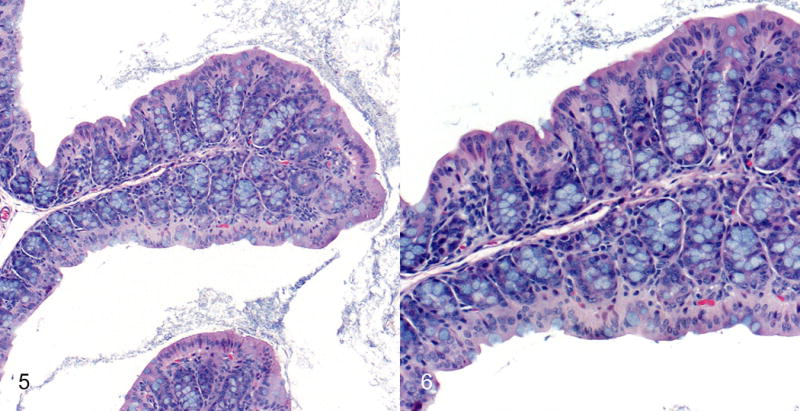
Normal proximal colonic mucosa; Mdr1a−/− mouse infected with H. bilis only (IBD score of 6). Note the regular short glands and abundant goblet cells. Hematoxylin and eosin (HE).
Figures 7 and 8.
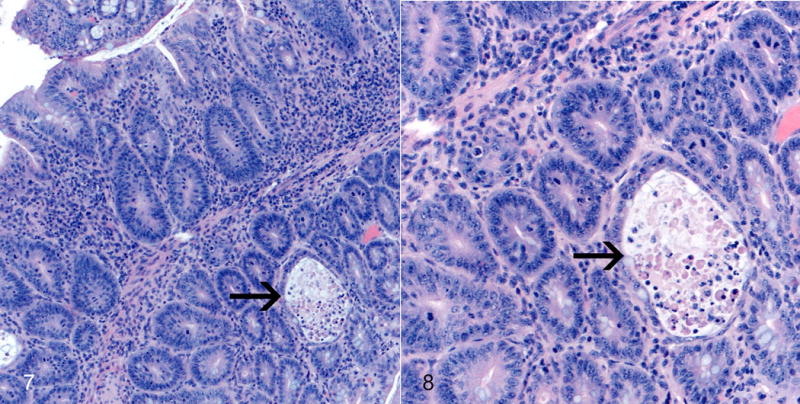
Colitis, proximal colon; Mdr1a−/− mouse coinfected with MNV4 and H. bilis. There is severe proliferative and lymphohistiocytic colitis with crypt abscess (arrow, Fig. 7). Note the thickened mucosa, abundant mitotic figures, loss of goblet cells, and crypt abscess (arrow, Fig. 8). HE. Figures 5–8 are reprinted from “Murine norovirus: an intercurrent variable in a mouse model of bacteria-induced inflammatory bowel disease,” Lencioni KC, et al. Comp Med. 2008;58(6):522–33 with permission from AALAS.
Despite the fact that noroviruses are gastrointestinal pathogens, MNV may infect research mice used to study a wide array of diseases beyond the gastrointestinal system. Since MNV is highly prevalent in many research institutions and has been reported to infect macrophages, dendritic cells, and B cells, MNV has the potential to alter research outcomes in diseases for which these cells are important including atherosclerosis, diabetes and obesity (Table 5). Our laboratory has evaluated the impact of MNV infection on atherosclerosis in B6.129P2-Apoetm1Unc/J (Apoe−/−) (Figures 9 and 10) and in low density lipoprotein receptor (Ldlr)-deficient mice (B6.129S7-Ldlrtm1her/J), and we also include previously unpublished data from our laboratory on the lack of impact that MNV infection had on the development of atherosclerosis in ApoE/TLR3 double knockout mice.36,37 Interestingly, although a major component of atherosclerotic plaques is lipid-laden macrophages, and MNV has as tropism for infecting macrophages, we found a variable influence of MNV infection on the development of atherosclerosis. This data underscores the importance of continuing to investigate the impact that MNV infection may have on various mouse models of disease.
Table 5.
Histologic Changes in Mice of Other Disease Models Experimentally Infected with MNV.a
| Reference | Mouse Strain | MNV Strain | Model | Diet | Liver | MLN | Aorta |
|---|---|---|---|---|---|---|---|
| 12 | B6.129P2-Apoetm1Unc/J | MNV-4 | Atherosclerosis | Normal | n/r | n/r | + |
| 12 | B6.129P2-Apoetm1Unc/J | MNV-4 | Atherosclerosis | High fat, high cholesterol | n/r | n/r | − |
| 35 | C57BL/6J | MNV-4 | Diet-Induced Obesity and Insulin Resistance | High fat | − | + | n/r |
| 36 | B6.129S7-Ldlrtm1her/J | MNV-4 | Atherosclerosis | High fat, high sucrose | n/r | n/r | − |
| 36 | B6.129S7-Ldlrtm1her/J | MNV-4 | Atherosclerosis | High fat, high cholesterol | n/r | n/r | + |
| 37 | B6.129S7-Ldlrtm1her/J | MNV-4 | Atherosclerosis | High fat, high cholesterol | n/r | n/r | − |
| Unpublished | Apoe−/−/Tlr3−/− | MNV-4 | Atherosclerosis | Normal | n/r | n/r | − |
+ = histologic changes reported, − = no histologic changes, n/r = not reported, MLN= mesenteric lymph node
Figure 9.
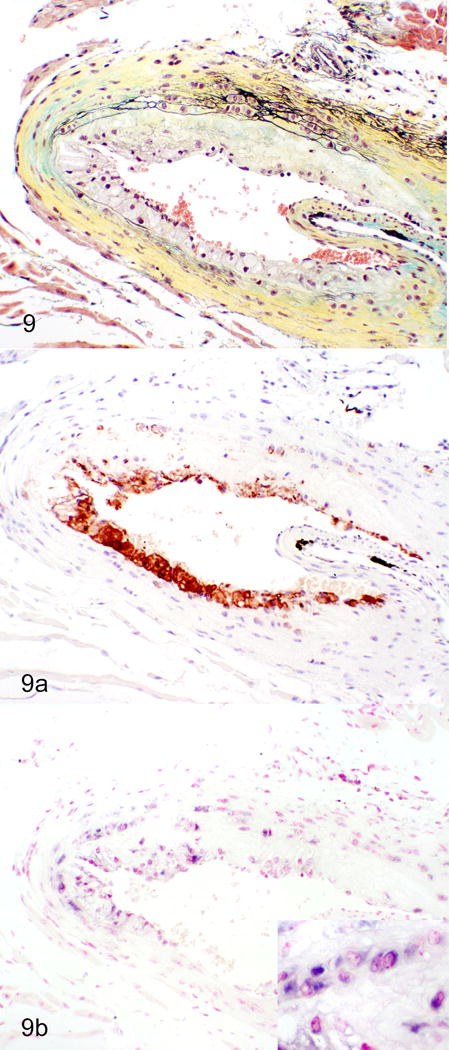
Atherosclerosis, aortic sinus; ApoE−/− mouse infected with MNV-4. Increased atherosclerosis in MNV-4 infected mice. Cells within atherosclerotic lesions are predominantly macrophages and positive for MNV-4 RNA (purple staining) by ISH (Inset: higher magnification). a) Movat’s pentachrome. b) Immunohistochemistry for the macrophage marker, Mac-2 (brown signal). c) In situ hybridization (ISH) for MNV-4 (nuclear fast red counterstain).
Figure 10.
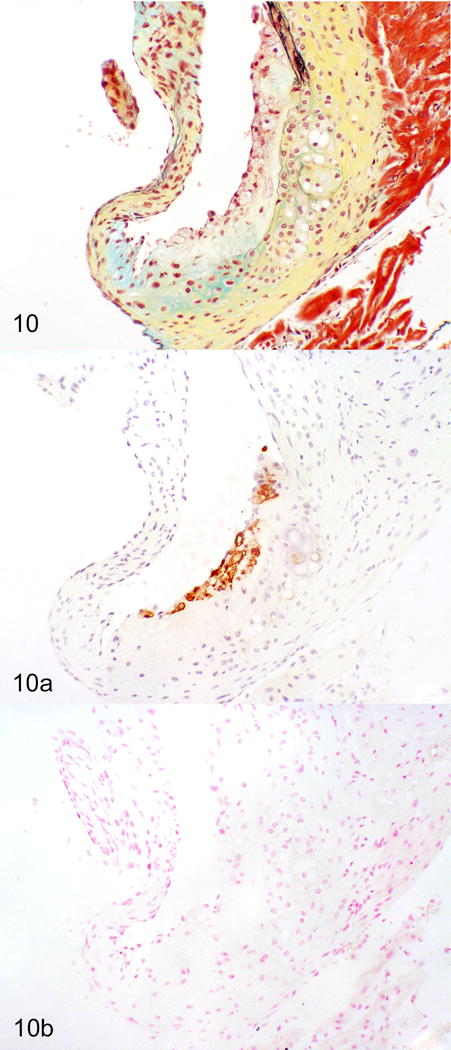
Atherosclerosis, aortic sinus, ApoE−/− mice. There is no signal for MNV-4 in this uninfected tissue. a) Movat’s pentachrome. b) Immunohistochemistry for the macrophage marker, Mac-2 (brown signal). c) In situ hybridization (ISH) for MNV-4 (nuclear fast red counterstain). Figures 9 and 10 are reprinted from “Murine norovirus infection variably alters atherosclerosis in mice lacking apolipoprotein E,” Hsu CC, et al. Comp Med, 2015;volume(issue):pages with permission from AALAS.
Many studies have employed immunohistochemistry, immunofluorescence, in situ hybridization, or in situ RT-PCR to localize virus in tissue sections derived from MNV-infected mice (Figure 4 and 9c). These studies confirm that MNV can infect macrophages and dendritic cells in a variety of tissues including liver, lung, mesenteric lymph node, peritoneum, small intestine, spleen and aorta, indicating that systemic spread of the virus may occur in both immunocompetent and immunodeficient mice (Table 6). In the liver, MNV antigen was detected in Kupffer cells38,47,50 and inflammatory cells including F4/80+ macrophages.49 MNV antigen was detected in mesenteric lymph node DC-like cells, F4/80+ cells in the medullary cords, and CD40+ paracortical cells in alveolar mononuclear cells and pleural inflammatory cells, peritoneal macrophages and mesothelium.49 Studies examining the spleen demonstrated MNV antigen in macrophages and macrophage-like cells,47 red pulp and marginal zone50 and non-lymphoid cells in the white pulp.50 Although the majority of MNV+ cells in the small intestine are inflammatory or antigen-presenting cells within the lamina propria or gastrointestinal-associated lymphoid tissues, enteric epithelium was also positive in some studies.3,33,49
Table 6.
Localization of MNV by immunohistochemistry, immunofluorescence, in situ hybridization, or in situ RT-PCR.a
| Reference | Mouse Strain | MNV Strain | Test Performed | Liver | Lung | MLN | Peritoneum | Small Intestine | Spleen | Aorta |
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | B6.129P2-Il10tm1Cgn/JZtm | MNV/Hannover1/2007/DEU | in situ RT-PCR | n/r | n/r | n/r | n/r | + | n/r | n/r |
| 4 | Atg16L1HM | MNV CR6 | immunohistochemistry | n/r | n/r | n/r | n/r | − | n/r | n/r |
| 12 | B6.129P2-Apoetm1Unc/J | MNV-4 | in situ hybridization | n/r | n/r | n/r | n/r | n/r | n/r | + |
| 33 | 129S6/SvEvTac | MNV-1.CW3 | immunofluorescence | n/r | n/r | n/r | n/r | + | n/r | n/r |
| 33 | 129S6/SvEv-Stat1tm1Rds | MNV-1.CW3 | immunofluorescence | n/r | n/r | n/r | n/r | + | n/r | n/r |
| 38 | Tac:SW | endemic | immunohistochemistry | + | n/r | + | n/r | n/r | n/r | n/r |
| 47 | Rag−/−/γc−/− | MNV-1.CW3 | immunohistochemistry | + | n/r | n/r | n/r | n/r | + | n/r |
| 49 | Rag1−/−/IFNγR−/− | endemic | immunohistochemistry | + | + | + | + | + | n/r | n/r |
| 49 | OT1Rag1−/−/IFNγR−/− | endemic | immunohistochemistry | + | + | n/r | − | − | + | n/r |
| 49 | OT2Rag1−/−/IFNγR−/− | endemic | immunohistochemistry | + | + | n/r | n/r | − | n/r | n/r |
| 49 | Rag1−/−/Stat1−/− | endemic | immunohistochemistry | + | + | + | + | + | n/r | n/r |
| 49 | Rag2−/− | endemic | immunohistochemistry | − | − | + | − | − | n/r | n/r |
| 50 | 129S6/SvEv-Stat1tm1Rds | MNV-1 | immunohistochemistry | + | n/r | n/r | n/r | n/r | + | n/r |
+ = MNV antigen detected, − = MNV antigen not detected, n/r = not reported, MLN= mesenteric lymph node
Conclusion
MNV infection in laboratory mice continues to be endemic in many research colonies. Therefore, determining the impact that MNV infection may have on various mouse models of disease is important in order to ensure that research results are valid. While it is impractical to test whether MNV infection alters all mouse models of disease, more and more studies are being performed to answer the question: “Will MNV infection alter and confound my research results?”. MNV is detected and may induce lesions in a wide variety of tissues, principally liver, spleen, and gastrointestinal tract, from immunocompetent and immunodeficient mice where it infects mononuclear cells including macrophages and dendritic cells. Importantly, MNV has a variable impact on mouse models, in some cases increasing lesions while in other cases having no impact. Consequently, it is important to recognize reported MNV lesions to help discern the possible influence of MNV infection on data generated in mouse models.
Supplementary Material
Acknowledgments
We thank Claudia Monaco from the University of Oxford for sharing ApoE/TLR3 double knockout mice. We thank Herbert “Skip” Virgin from Washington University and Jerrold Ward from Global VetPathology for sharing previously published figures for reprint. We also thank Jisun Paik, Thea Brabb, and Audrey Seamons from the University of Washington for their assistance with the unpublished studies.
Funding
This work was supported by the National Institutes of Health [grant numbers R21-OD011135, R01-OD011149]; and the University of Washington’s Nutrition Obesity Research Center [grant number P30-DK035816].
Footnotes
References
- 1.Ammann CG, Messer RJ, Varvel K, et al. Effects of acute and chronic murine norovirus infections on immune responses and recovery from Friend retrovirus infection. J Virol. 2009;83(24):13037–13041. doi: 10.1128/JVI.01445-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barron EL, Sosnovtsev SV, Bok K, et al. Diversity of murine norovirus strains isolated from asymptomatic mice of different genetic backgrounds within a single U.S. research institute. PLoS One. 2011;6(6):e21435. doi: 10.1371/journal.pone.0021435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basic M, Keubler LM, Buettner M, et al. Norovirus triggered microbiota-driven mucosal inflammation in interleukin 10-deficient mice. Inflamm Bowel Dis. 2014;20(3):431–443. doi: 10.1097/01.MIB.0000441346.86827.ed. [DOI] [PubMed] [Google Scholar]
- 4.Cadwell K, Patel KK, Maloney NS, et al. Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in intestine. Cell. 2010;141(7):1135–1145. doi: 10.1016/j.cell.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doom CM, Turula HM, Hill AB. Investigation of the impact of the common animal facility contaminant murine norovirus on experimental murine cytomegalovirus infection. Virology. 2009;392(2):153–161. doi: 10.1016/j.virol.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elena SF, Sanjuan R. Adaptive value of high mutation rates of RNA viruses: separating causes from consequences. J Virol. 2005;79(18):11555–11558. doi: 10.1128/JVI.79.18.11555-11558.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–276. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 8.Green KY. Caliciviridae: The Noroviruses. In: Knipe DM, Howley PM, editors. Fields virology. 6. Vol. 2013. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health; pp. 582–608. [Google Scholar]
- 9.Hanaki K, Ike F, Kajita A, et al. A broadly reactive one-step SYBR Green I real-time RT-PCR assay for rapid detection of murine norovirus. PLoS One. 2014;9(5):e98108. doi: 10.1371/journal.pone.0098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hensley SE, Pinto AK, Hickman HD, et al. Murine norovirus infection has no significant effect on adaptive immunity to vaccinia virus or influenza A virus. J Virol. 2009;83(14):7357–7360. doi: 10.1128/JVI.00623-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins PD, Johnson LA, Sauder K, et al. Transient or persistent norovirus infection does not alter the pathology of Salmonella typhimurium induced intestinal inflammation and fibrosis in mice. Comp Immunol Microbiol Infect Dis. 2011;34(3):247–257. doi: 10.1016/j.cimid.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu CC, Paik J, Brabb TL, et al. Murine norovirus infection variably alters atherosclerosis in mice lacking apolipoprotein E. Comparative Medicine. 2015 [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu CC, Paik J, Treuting PM, et al. Infection with murine norovirus 4 does not alter helicobacter-induced inflammatory bowel disease in il10−/− mice. Comp Med. 2014;64(4):256–263. [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu CC, Riley LK, Livingston RS. Molecular characterization of three novel murine noroviruses. Virus Genes. 2007;34(2):147–155. doi: 10.1007/s11262-006-0060-1. [DOI] [PubMed] [Google Scholar]
- 15.Hsu CC, Riley LK, Wills HM, Livingston RS. Persistent Infection and Serologic Cross-Reactivity of Three Novel Murine Noroviruses. Comp Med. 2006;56(4):247–251. [PubMed] [Google Scholar]
- 16.Hsu CC, Wobus CE, Steffen EK, Riley LK, Livingston RS. Development of a microsphere-based serologic multiplexed fluorescent immunoassay and a reverse transcriptase PCR assay to detect murine norovirus 1 infection in mice. Clin Diagn Lab Immunol. 2005;12(10):1145–1151. doi: 10.1128/CDLI.12.10.1145-1151.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen S, Thomsen AR. Sensing of RNA viruses: a review of innate immune receptors involved in recognizing RNA virus invasion. J Virol. 2012;86(6):2900–2910. doi: 10.1128/JVI.05738-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones MK, Watanabe M, Zhu S, et al. Enteric bacteria promote human and mouse norovirus infection of B cells. Science. 2014;346(6210):755–759. doi: 10.1126/science.1257147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahan SM, Liu G, Reinhard MK, Hsu CC, Livingston RS, Karst SM. Comparative murine norovirus studies reveal a lack of correlation between intestinal virus titers and enteric pathology. Virology. 2011;421(2):202–210. doi: 10.1016/j.virol.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karst SM. Pathogenesis of noroviruses, emerging RNA viruses. Viruses. 2010;2(3):748–781. doi: 10.3390/v2030748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karst SM, Wobus CE, Goodfellow IG, Green KY, Virgin HW. Advances in norovirus biology. Cell Host Microbe. 2014;15(6):668–680. doi: 10.1016/j.chom.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karst SM, Wobus CE, Lay M, Davidson J, Virgin HW. STAT1-dependent innate immunity to a Norwalk-like virus. Science. 2003;299(5612):1575–1578. doi: 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- 23.Karst SM, Zhu S, Goodfellow IG. The molecular pathology of noroviruses. J Pathol. 2015;235(2):206–216. doi: 10.1002/path.4463. [DOI] [PubMed] [Google Scholar]
- 24.Kelmenson JA, Pomerleau DP, Griffey S, Zhang W, Karolak MJ, Fahey JR. Kinetics of transmission, infectivity, and genome stability of two novel mouse norovirus isolates in breeding mice. Comp Med. 2009;59(1):27–36. [PMC free article] [PubMed] [Google Scholar]
- 25.Kernbauer E, Ding Y, Cadwell K. An enteric virus can replace the beneficial function of commensal bacteria. Nature. 2014;516(7529):94–98. doi: 10.1038/nature13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JR, Seok SH, Kim DJ, et al. Prevalence of murine norovirus infection in Korean laboratory animal facilities. J Vet Med Sci. 2011;73(5):687–691. doi: 10.1292/jvms.10-0226. [DOI] [PubMed] [Google Scholar]
- 27.Kitajima M, Oka T, Tohya Y, Katayama H, Takeda N, Katayama K. Development of a broadly reactive nested reverse transcription-PCR assay to detect murine noroviruses, and investigation of the prevalence of murine noroviruses in laboratory mice in Japan. Microbiol Immunol. 2009;53(9):531–534. doi: 10.1111/j.1348-0421.2009.00152.x. [DOI] [PubMed] [Google Scholar]
- 28.Lencioni KC, Drivdahl R, Seamons A, Treuting PM, Brabb T, Maggio-Price L. Lack of effect of murine norovirus infection on a mouse model of bacteria-induced colon cancer. Comp Med. 2011;61(3):219–226. [PMC free article] [PubMed] [Google Scholar]
- 29.Lencioni KC, Seamons A, Treuting PM, Maggio-Price L, Brabb T. Murine norovirus: an intercurrent variable in a mouse model of bacteria-induced inflammatory bowel disease. Comp Med. 2008;58(6):522–533. [PMC free article] [PubMed] [Google Scholar]
- 30.Martella V, Lorusso E, Decaro N, et al. Detection and molecular characterization of a canine norovirus. Emerg Infect Dis. 2008;14(8):1306–1308. doi: 10.3201/eid1408.080062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McFadden N, Bailey D, Carrara G, et al. Norovirus regulation of the innate immune response and apoptosis occurs via the product of the alternative open reading frame 4. PLoS Pathog. 2011;7(12):e1002413. doi: 10.1371/journal.ppat.1002413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller B, Klemm U, Mas Marques A, Schreier E. Genetic diversity and recombination of murine noroviruses in immunocompromised mice. Arch Virol. 2007;152(9):1709–1719. doi: 10.1007/s00705-007-0989-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mumphrey SM, Changotra H, Moore TN, et al. Murine norovirus 1 infection is associated with histopathological changes in immunocompetent hosts, but clinical disease is prevented by STAT1-dependent interferon responses. J Virol. 2007;81(7):3251–3263. doi: 10.1128/JVI.02096-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson AM, Elftman MD, Pinto AK, et al. Murine norovirus infection does not cause major disruptions in the murine intestinal microbiota. Microbiome. 2013;1(1):7. doi: 10.1186/2049-2618-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paik J, Fierce Y, Drivdahl R, et al. Effects of murine norovirus infection on a mouse model of diet-induced obesity and insulin resistance. Comp Med. 2010;60(3):189–195. [PMC free article] [PubMed] [Google Scholar]
- 36.Paik J, Fierce Y, Mai PO, et al. Murine norovirus increases atherosclerotic lesion size and macrophages in Ldlr−/− mice. Comp Med. 2011;61(4):330–338. [PMC free article] [PubMed] [Google Scholar]
- 37.Paik J, Kwok F, Seamons A, et al. Effects of murine norovirus on atherosclerosis in ldlr−/− mice depends on the timing of infection. Comp Med. 2015;65(2):114–122. [PMC free article] [PubMed] [Google Scholar]
- 38.Perdue KA, Green KY, Copeland M, et al. Naturally occurring murine norovirus infection in a large research institution. J Am Assoc Lab Anim Sci. 2007;46(4):39–45. [PubMed] [Google Scholar]
- 39.Pinto P, Wang Q, Chen N, et al. Discovery and genomic characterization of noroviruses from a gastroenteritis outbreak in domestic cats in the US. PLoS One. 2012;7(2):e32739. doi: 10.1371/journal.pone.0032739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pritchett-Corning KR, Cosentino J, Clifford CB. Contemporary prevalence of infectious agents in laboratory mice and rats. Lab Anim. 2009;43(2):165–173. doi: 10.1258/la.2008.008009. [DOI] [PubMed] [Google Scholar]
- 41.Randall RE, Goodbourn S. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J Gen Virol. 2008;89(Pt 1):1–47. doi: 10.1099/vir.0.83391-0. [DOI] [PubMed] [Google Scholar]
- 42.Rocha-Pereira J, Jochmans D, Debing Y, Verbeken E, Nascimento MS, Neyts J. The viral polymerase inhibitor 2′-C-methylcytidine inhibits Norwalk virus replication and protects against norovirus-induced diarrhea and mortality in a mouse model. J Virol. 2013;87(21):11798–11805. doi: 10.1128/JVI.02064-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenstiel P, Sina C, Franke A, Schreiber S. Towards a molecular risk map–recent advances on the etiology of inflammatory bowel disease. Semin Immunol. 2009;21(6):334–345. doi: 10.1016/j.smim.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Scipioni A, Mauroy A, Vinje J, Thiry E. Animal noroviruses. Vet J. 2008;178(1):32–45. doi: 10.1016/j.tvjl.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 45.Shortland A, Chettle J, Archer J, et al. Pathology caused by persistent murine norovirus infection. J Gen Virol. 2014;95(Pt 2):413–422. doi: 10.1099/vir.0.059188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strong DW, Thackray LB, Smith TJ, Virgin HW. Protruding domain of capsid protein is necessary and sufficient to determine murine norovirus replication and pathogenesis in vivo. J Virol. 2012;86(6):2950–2958. doi: 10.1128/JVI.07038-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taube S, Kolawole AO, Hohne M, et al. A mouse model for human norovirus. MBio. 2013;4(4) doi: 10.1128/mBio.00450-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tse H, Chan WM, Lam CS, Lau SK, Woo PC, Yuen KY. Complete genome sequences of novel rat noroviruses in Hong Kong. J Virol. 2012;86(22):12435–12436. doi: 10.1128/JVI.01976-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ward JM, Wobus CE, Thackray LB, et al. Pathology of immunodeficient mice with naturally occurring murine norovirus infection. Toxicol Pathol. 2006;34(6):708–715. doi: 10.1080/01926230600918876. [DOI] [PubMed] [Google Scholar]
- 50.Wobus CE, Karst SM, Thackray LB, et al. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biology. 2004;2(12):e432. doi: 10.1371/journal.pbio.0020432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wobus CE, Thackray LB, Virgin HW. Murine norovirus: a model system to study norovirus biology and pathogenesis. J Virol. 2006;80(11):5104–5112. doi: 10.1128/JVI.02346-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolf S, Williamson W, Hewitt J, et al. Molecular detection of norovirus in sheep and pigs in New Zealand farms. Vet Microbiol. 2009;133(1–2):184–189. doi: 10.1016/j.vetmic.2008.06.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


