Abstract
Background and objectives
Community-acquired infections are common, contributing to adverse outcomes and increased health care costs. We hypothesized that, with lower eGFR, the incidence of community-acquired infections increases, whereas the pattern of site-specific infections varies.
Design, setting, participants, & measurements
Among 1,139,470 health care users (mean age =52±18 years old, 53% women) from the Stockholm CREAtinine Measurements Project, we quantified the associations of eGFR with the risk of infections, overall and major types, over 12 months.
Results
A total of 106,807 counts of infections were recorded throughout 1,128,313 person-years. The incidence rate of all infections increased with lower eGFR from 74/1000 person-years for individuals with eGFR=90–104 ml/min per 1.73 m2 to 419/1000 person-years for individuals with eGFR<30 ml/min per 1.73 m2. Compared with eGFR of 90–104 ml/min per 1.73 m2, the adjusted incidence rate ratios of community-acquired infections were 1.08 (95% confidence interval, 1.01 to 1.14) for eGFR of 30–59 ml/min per 1.73 m2 and 1.53 (95% confidence interval, 1.39 to 1.69) for eGFR<30 ml/min per 1.73 m2. The relative proportions of lower respiratory tract infection, urinary tract infection, and sepsis became increasingly higher along with lower eGFR strata (e.g., low respiratory tract infection accounting for 25% versus 15% of community-acquired infections in eGFR<30 versus 90–104 ml/min per 1.73 m2, respectively). Differences in incidence associated with eGFR were in general consistent for most infection types, except for nervous system and upper respiratory tract infections, for which no association was observed.
Conclusions
This region-representative health care study finds an excess community-acquired infections incidence in individuals with mild to severe CKD. Lower respiratory tract infection, urinary tract infection, and sepsis are major infections in CKD.
Keywords: chronic kidney disease; Epidemiology and outcomes; renal function; risk factors; urinary tract infections; lower respiratory tract infection; sepsis; community; Adult; Aged; Communicable Diseases; Community-Acquired Infections; creatinine; Female; glomerular filtration rate; Health Care Costs; Humans; Incidence; Middle Aged; Nervous System; Renal Insufficiency, Chronic; Respiratory Tract Infections; Urinary Tract Infections
Introduction
CKD is common, with a population prevalence of 5%–15% in most developed countries (1,2), and it is associated with a markedly increased risk of death and hospitalizations (3,4). Infections are probably the most significant and serious noncardiovascular complications among persons with CKD (https://www.usrds.org/adr.aspx) (5). Decreased kidney function leads to retention of metabolic waste products and alteration of multiple pathways, including the immune system (6).
In patients undergoing dialysis, the risks of fatal and nonfatal infections are markedly high (7–9), and a few studies have shown that mildly to moderately decreased kidney function is also associated with increased risk of infections (10–15). However, almost all of these studies focus on mortality or hospitalization due to infections, including both nosocomial and community acquired (10–15).
Community-acquired infections account for considerable morbidity and mortality as well as substantial health care costs worldwide (16–18), but a comprehensive analysis on the risk of infections and possible differences in their proportions across the full spectrum of kidney function is lacking. Such an analysis would inform health care policymakers about appropriate prevention strategies and health service planning in the context of CKD. In this study, we hypothesized that, with lower eGFR, the incidence of community-acquired infections increases, whereas the pattern of specific infections varies.
Materials and Methods
Study Population
We used data from the Stockholm CREAtinine Measurements (SCREAM) Project (2,19). Briefly, the SCREAM Project is a health care utilization cohort from the region of Stockholm, Sweden, and it includes all residents who undertook at least one measurement of serum creatinine in ambulatory or hospital care during 2006–2011. Creatinine and other laboratory data were linked with regional and national administrative databases for information on health care utilization, dispensed drugs, validated RRT end points, and follow-up for death, with virtually no or minimal loss to follow-up. Given the commonness of creatinine testing, the SCREAM Project captured 66% of the complete population census of the region, including >75% of individuals above the age of 45 years old (19). For this study, index date was determined by the first available serum creatinine measurement of any adult (>18 years old) (Supplemental Figure 1). Exclusion criteria were creatinine measurement during a hospital stay, pregnancy (defined by the presence of an International Classification of Disease, 10th Revision, Clinical Modification [ICD-10] code among Z321, Z33–Z38, and any O code in the preceding 6 months), presence of chronic infections (including HIV; ICD-10 codes B15–B19, B20–B24, and A15–A19), or undergoing RRT (dialysis or history of kidney transplantation as ascertained by linkage with the Swedish Renal Registry (http://www.medscinet.net/snr/) (Supplemental Material). To avoid selecting creatinine values that may be determined by preexisting infections, we excluded serum creatinine measurements with a diagnosis of infection during the preceding 3 months (definitions of infection are in Supplemental Table 1).
Exposure and Covariates
The exposure was eGFR calculated from serum creatinine using the 2009 Chronic Kidney Disease Epidemiology Collaboration equation (20). All creatinine measurements were standardized to isotope dilution mass spectrometry standards. Although data for ethnicity were not available by law, misclassification of eGFR is expected to be minimal, because the vast majority of the residents of the Stockholm region are of white origin (21). Five categories of eGFR were studied: eGFR≥105, 90–104, 60–89, 30–59, and <30 ml/min per 1.73 m2, with eGFR of 90–104 ml/min per 1.73 m2 serving as the reference group for consistency with a previous publication in a comparable health care extraction from Canada (11) and because this range showed the lowest risk of the study outcome.
Other covariates were defined at index date of the first recorded serum creatinine measurement and included age, sex, and comorbidities on the basis of ICD-10 codes (cardiovascular disease [composite of myocardial infarction, congestive heart failure, peripheral vascular disease, and cerebrovascular disease], dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, liver disease, hemiplegia or paraplegia, cancer, and diabetes according to the domains of the Charlson comorbidity score [22,23] as well as the presence of hypertension). Comorbidities, such as diabetes and hypertension, were enriched with the current purchase of related medication (purchase of oral antidiabetics: Anatomic Therapeutic Chemical [ATC] code A10; or antihypertensives: ATC codes C03, C07, C08, and C09) up to 6 months before index date. We also included information on recent/current use of immunosuppressive drugs (ATC code L04), antibiotics (ATC codes J01, D06AA, and D06AX), antimycotic (ATC code J02), or antivirals (ATC code J05) in the 6 months preceding study start (i.e., precedes serum creatinine measurement/index date). Of note, the dispensation of these drugs in Swedish pharmacies is exclusively done via medical prescription (Supplemental Table 2).
Study Outcome
Participants were followed from index date to the end of follow-up (12 months, death, RRT, or migration from the county, whichever occurred first), and these events were taken into account when calculating individual time at risk. We chose this follow-up to reduce possible misclassification bias and assumed that eGFR remained stable during this short period. The primary outcome was the overall incidence of community-acquired infections, including upper and lower respiratory tract infections, gastrointestinal tract infections, urinary tract infections (UTIs), skin/soft tissue infections, nervous system infections, sepsis, musculoskeletal system infections, and cardiovascular system infections (Supplemental Table 1) diagnosed in health care (at primary care, outpatient specialist consultation, or primary hospitalization diagnosis). The secondary outcome was the incidence of type-specific infections. To avoid overestimation from repeated attendance for the same infection, repeated diagnostic codes recorded within 28 days of one another were attributed to a single episode of infection, and the date of appearance of the first code was selected as the event date. We further excluded infections likely to be acquired in hospital, which encompassed postsurgical infections, central line–associated bloodstream infections, catheter-associated UTIs, and ventilator-associated pneumonia ICD-10 diagnoses (a list of excluded codes is detailed in Supplemental Table 3); in addition, infections diagnosed in the 14 days after a hospital discharge were also considered hospital-acquired infections and excluded.
Data Analyses
We present descriptive values as mean and SD or count with proportion. We calculated crude incidence rates with 95% confidence intervals (95% CIs) using the exact method and adjusted incidence rates and incidence rates ratios (IRRs) using a zero-inflated negative binomial model to account for overdispersion and excess zero counts. We also included an offset term in the model to account for time at risk. Covariates included in the model were age in categories; sex; and use of immunosuppressive medication, antibiotics, antimycotics, and antivirals as well as the abovementioned comorbidities. As sensitivity analyses, we (1) modified the definition of the timeframe for counting infections to up to 3 months from inclusion date and to 3–12 months from inclusion date and (2) repeated the main analysis in specific subgroups defined by age (≥75, 65–74, 45–64, and <45 years old), sex, diabetes (yes/no), and history of cancer (yes/no). We performed the sensitivity analysis to explore whether unknown disease not captured by our ICD-10–based definitions may have influenced the serum creatinine levels (hence biasing the resulting GFR estimation) and at the same time, may be responsible for the infection risk observed. In that case, the pattern of 3 versus 3–12 months infection risk prediction would differ. All analyses were performed using R (https://www.r-project.org) and Stata, version 14.0 (StataCorp, College Station, TX).
Results
Baseline Characteristics
The study cohort consisted of 1,139,470 participants (Supplemental Figure 1) (53% women), with a mean age of 52±18 years old (Table 1). Mean eGFR was 94±21 ml/min per 1.73 m2, 31% of individuals had eGFR≥105 ml/min per 1.73 m2, 30% had eGFR of 90–104 ml/min per 1.73 m2, 32% had eGFR of 60–89 ml/min per 1.73 m2, 6% had eGFR of 30–59 ml/min per 1.73 m2, and 1% had eGFR<30 ml/min per 1.73 m2. The most common comorbidities were hypertension (present in 25% of the population), cardiovascular disease (7%), diabetes mellitus (6%), cancer (5%), and chronic obstructive pulmonary disease (4%). The remaining comorbidities (rheumatoid disease, dementia, peptic ulcer, liver disease, and hemiplegia paraplegia) were present in 1% or less of participants. Age, prevalence of comorbidities, and use of immunosuppressive and antibiotic medications were higher in lower eGFR categories; antimycotic and antiviral medications were more common in higher eGFR categories, and the proportion of women was similar (Table 1).
Table 1.
Baseline characteristics of the study cohort overall and by eGFR
| Characteristic | eGFR, ml/min per 1.73 m2 | Total, n=1,139,470 | ||||
|---|---|---|---|---|---|---|
| eGFR≥105, n=357,687 | eGFR=90–104, n=346,623 | eGFR=60–89, n=365,491 | eGFR=30–59, n=63,805 | eGFR<30, n=5864 | ||
| eGFR, ml/min per 1.73 m2, mean±SD | 117±9 | 97±4 | 79±8 | 50±8 | 23±6 | 94±21 |
| Age, yr, mean±SD | 34±10 | 51±13 | 63±15 | 78±11 | 79±14 | 52±18 |
| Women, no. (%) | 189,998 (53) | 170,910 (49) | 200,540 (55) | 39,151 (61) | 3219 (55) | 603,818 (53) |
| Recent or current use of medications, no. (%) | ||||||
| Immunosuppressives | 2120 (0.6) | 2244 (0.7) | 2604 (0.7) | 618 (1) | 61 (1) | 7647 (0.7) |
| Antibiotics | 63,236 (18) | 59,525 (17) | 64,699 (18) | 12,447 (20) | 1336 (23) | 201,243 (18) |
| Antimycotics | 5439 (2) | 3443 (1) | 2918 (0.8) | 307 (0.5) | 25 (0.4) | 12,132 (1) |
| Antivirals | 4827 (1) | 4620 (1) | 4246 (1) | 436 (0.7) | 31 (0.5) | 14,160 (1) |
| Medical history, no. (%) | ||||||
| Hypertension | 21,235 (6) | 75,650 (22) | 141,170 (39) | 46,093 (72) | 4885 (83) | 289,033 (25) |
| Cardiovascular disease | 3168 (0.9) | 12,992 (4) | 37,358 (10) | 20,968 (33) | 2928 (50) | 77,414 (7) |
| Myocardial infarction | 717 (0.2) | 4030 (1) | 10,238 (3) | 5812 (9) | 980 (17) | 21,777 (2) |
| Congestive heart failure | 652 (0.2) | 2940 (0.9) | 13,334 (4) | 11,779 (19) | 2061 (35) | 30,766 (3) |
| Peripheral vascular disease | 555 (0.2) | 2223 (0.6) | 6581 (2) | 3928 (6) | 600 (10) | 13,887 (1) |
| Cerebrovascular disease | 1558 (0.4) | 5835 (2) | 16,139 (4) | 7762 (12) | 954 (16) | 32,248 (3) |
| Diabetes | 10,336 (3) | 18,705 (5) | 27,590 (8) | 10,296 (16) | 1468 (25) | 68,395 (6) |
| Cancer | 4880 (1) | 14,414 (4) | 27,576 (8) | 8168 (13) | 870 (15) | 55,908 (5) |
| Chronic obstructive pulmonary disease | 7507 (2) | 10,461 (3) | 16,483 (5) | 5425 (9) | 632 (11) | 40,508 (4) |
| Rheumatoid disease | 1719 (0.5) | 3284 (1) | 6989 (2) | 2858 (5) | 284 (5) | 15,134 (1) |
| Dementia | 86 (0.02) | 880 (0.3) | 6051 (2) | 3530 (6) | 401 (7) | 10,948 (1) |
| Peptic ulcer | 1223 (0.3) | 1965 (0.6) | 3063 (0.8) | 1389 (2) | 250 (4) | 7890 (0.7) |
| Liver disease | 1120 (0.3) | 1550 (0.5) | 1679 (0.5) | 528 (0.8) | 62 (1) | 4939 (0.4) |
| Hemiplegia/paraplegia | 1119 (0.3) | 742 (0.2) | 794 (0.2) | 235 (0.4) | 42 (0.7) | 2932 (0.3) |
Recent or current use of medications considered any drug purchase at the time of or within the 6 mo proceeding the study start (index serum creatinine).
Crude Incidence Rates and Proportion of Type-Specific Infections
As many as 17,950 (1.6%) participants died, 211 (0.02%) initiated RRT, and 3900 (0.34%) migrated from the region before the end of follow-up. The remaining individuals (98.04%) completed 12 months of follow-up. Overall, 106,807 infectious events were recorded throughout 1,128,313 person-years. The majority of codes (68%) were identified in ambulatory care (primary care–issued codes, 25%; outpatient specialist–issued codes, 43%). The crude incidence rate of infections (any type) was 95/1000 person-years. Incidence rates were progressively higher across lower eGFR strata: from 74/1000 person-years for individuals with eGFR=90–104 ml/min per 1.73 m2 to 419/1000 person-years for individuals with eGFR<30 ml/min per 1.73 m2 (Table 2).
Table 2.
Numbers of infections (any type), incidence rates, and incident rate ratios by eGFR categories within 12 mo of follow-up
| Characteristic | No. of Participants with Multiple Infections, % in Row | Total Counts of Infections | Person-Years at Risk | Crude Incidence Rate per 1000 person-yr (95% CI) | Adjusted Incidence Ratea per 1000 person-yr (95% CI) | Adjusted Incidence Rate Ratioa (95% CI) | |||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | ≥3 | ||||||
| Overall (n=1,139,470) | 1,060,120 (93) | 61,082 (5.4) | 12,610 (1.1) | 5658 (0.5) | 106,807 | 1,128,313 | 95 (94 to 95) | 85 (84 to 86) | — |
| eGFR, ml/min per 1.73 m2 | |||||||||
| ≥105 (n=357,687) | 335,708 (93.9) | 17,825 (5) | 2974 (0.8) | 1180 (0.3) | 28,097 | 355,975 | 79 (78 to 80) | 92 (90 to 94) | 1.08 (1.03 to 1.14) |
| 90–104 (n=346,623) | 327,215 (94.4) | 15,452 (4.5) | 2756 (0.8) | 1200 (0.3) | 25,362 | 344,800 | 74 (73 to 74) | 80 (79 to 82) | 1 (Reference) |
| 60–89 (n=365,491) | 338,166 (92.5) | 20,585 (5.6) | 4641 (1.3) | 2099 (0.6) | 37,394 | 361,369 | 103 (102 to 105) | 78 (76 to 79) | 0.93 (0.89 to 0.98) |
| 30–59 (n=63,805) | 54,510 (85) | 6355 (10) | 1935 (3) | 1005 (2) | 13,850 | 61,141 | 227 (223 to 230) | 106 (102 to 110) | 1.08 (1.01 to 1.14) |
| <30 (n=5864) | 4521 (77) | 865 (15) | 304 (5) | 174 (3) | 2104 | 5026 | 419 (401 to 436) | 211 (194 to 229) | 1.53 (1.39 to 1.69) |
95% CI, 95% confidence interval.
The model was adjusted for age, sex, use of immunosuppressive drugs, prior anti-infection drugs (antibiotics, antimycotics, and antivirals), comorbidities (cardiovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, liver disease, hemiplegia/paraplegia, cancer, diabetes, and hypertension), and exposure time.
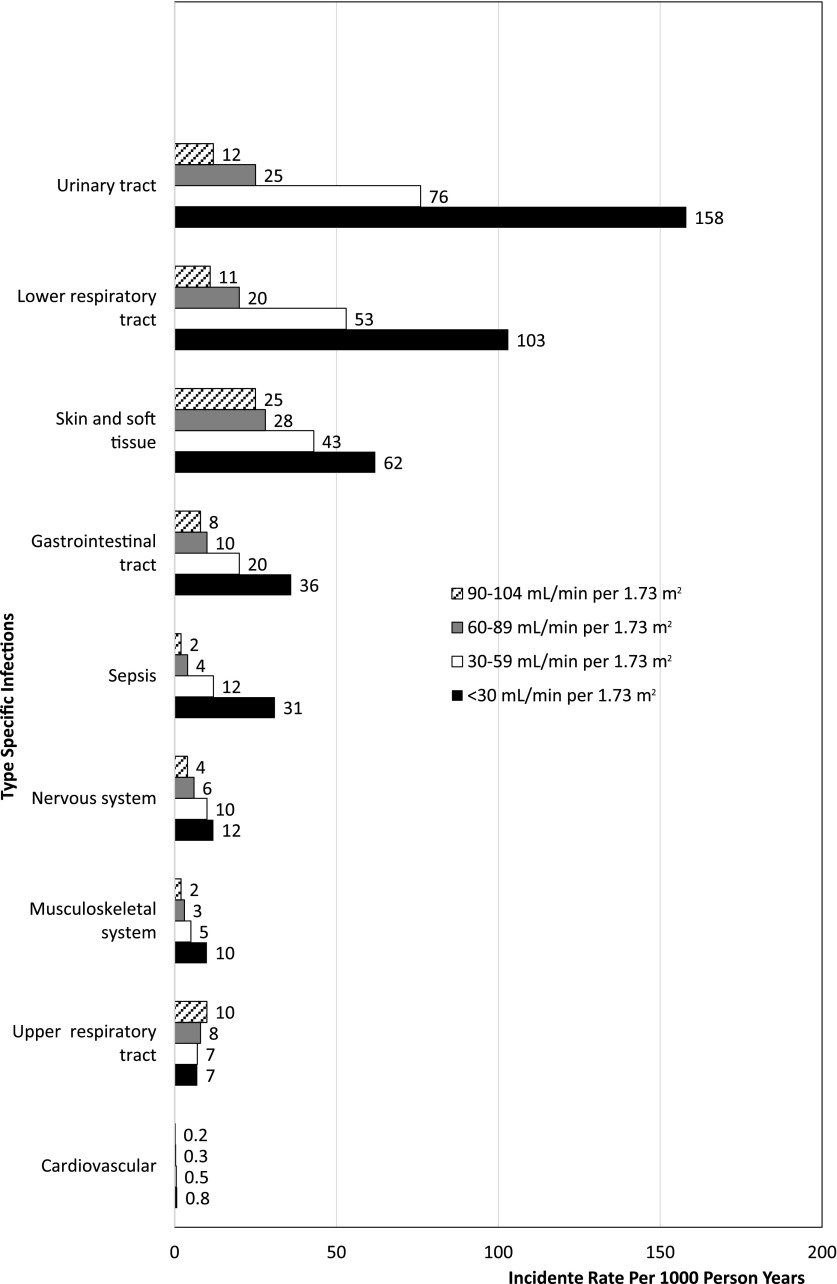
The most common infections observed were skin and soft tissue related (crude incidence rate of 28/1000 person-years) followed by UTIs (21/1000 person-years), lower respiratory tract infections (15/1000 person-years), upper respiratory tract infections (11/1000 person-years), and infections of the gastrointestinal tract (10/1000 person-years). The incidence rates of all single type–specific infections, with the exception of upper respiratory tract infections, also increased across lower eGFR categories (Figure 1, Supplemental Table 4).
Figure 1.
Increased incidence rate of type-specific infections across eGFR categories within 12 months of follow-up.
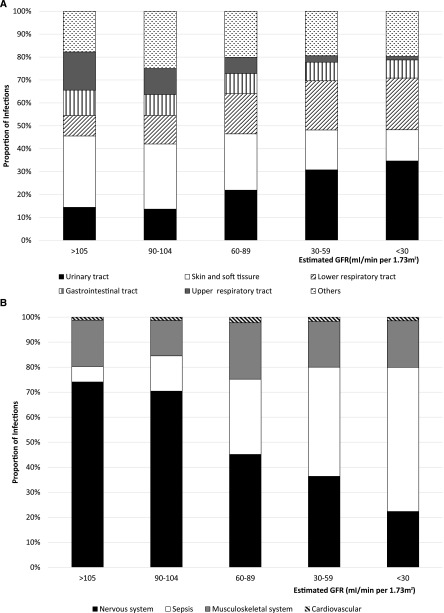
The pattern of type-specific infections, however, varied across eGFR categories (Figure 2A). Specifically, the proportions of UTIs and lower respiratory tract infections were higher along with lower eGFR categories (from 16% and 15% in individuals with eGFR=90–104 ml/min per 1.73 m2 to 38% and 25% in individuals with eGFR<30 ml/min per 1.73 m2, respectively), and skin/soft tissue–related and upper respiratory tract infections were less common in lower eGFR categories (from 34% and 14% in individuals with eGFR=90–104 ml/min per 1.73 m2 to 15% and 2% in individuals with eGFR<30 ml/min per 1.73 m2, respectively). For other less frequent infections (Figure 2B), a similar increasing pattern was observed for sepsis, and a decreasing proportion of nervous system infections emerged across lower eGFR strata.
Figure 2.
Different relative percentage for incidence rates of type-specific infections by eGFR categories within 12 months of follow-up. A shows the five most common infection types (those with an incidence rate ≥10/1000 person-years), and the remaining minority types were grouped under the category other. B expands the relative percentage of the less common infection types (those with an incidence rate <10/1000 person-years) grouped as other in A.
Adjusted Incidence Rates and IRRs
The majority of individuals did not acquire any infection (93%), 5% of individuals acquired one infection (any type), and the remaining 2% acquired two or more infections during follow-up (Table 2). After segregating by eGFR categories, the proportion of individuals with one infection increased from 4.5% to 15% across higher to lower eGFR strata, and the proportion of individuals with two or more infections likewise increased from <1% to 5%. We observed an overall adjusted incidence rate of 85 infections per 1000 person-years, which ranged from 78 to 211 infections per 1000 person-years when stratifying by eGFR strata.
IRRs were calculated against the reference category of eGFR=90–104 ml/min per 1.73 m2. Compared with those individuals, individuals with eGFR≥105 ml/min per 1.73 m2 had an 8% higher adjusted IRR (1.08; 95% CI, 1.03 to 1.14). Individuals with eGFR of 60–89 ml/min per 1.73 m2 did not have a significantly higher adjusted IRR, and those with eGFRs of 30–59 and <30 ml/min per 1.73 m2 had 8% (IRR, 1.08; 95% CI, 1.01 to 1.14) and 53% (IRR, 1.53; 95% CI, 1.39 to 1.69) higher IRRs of infections.
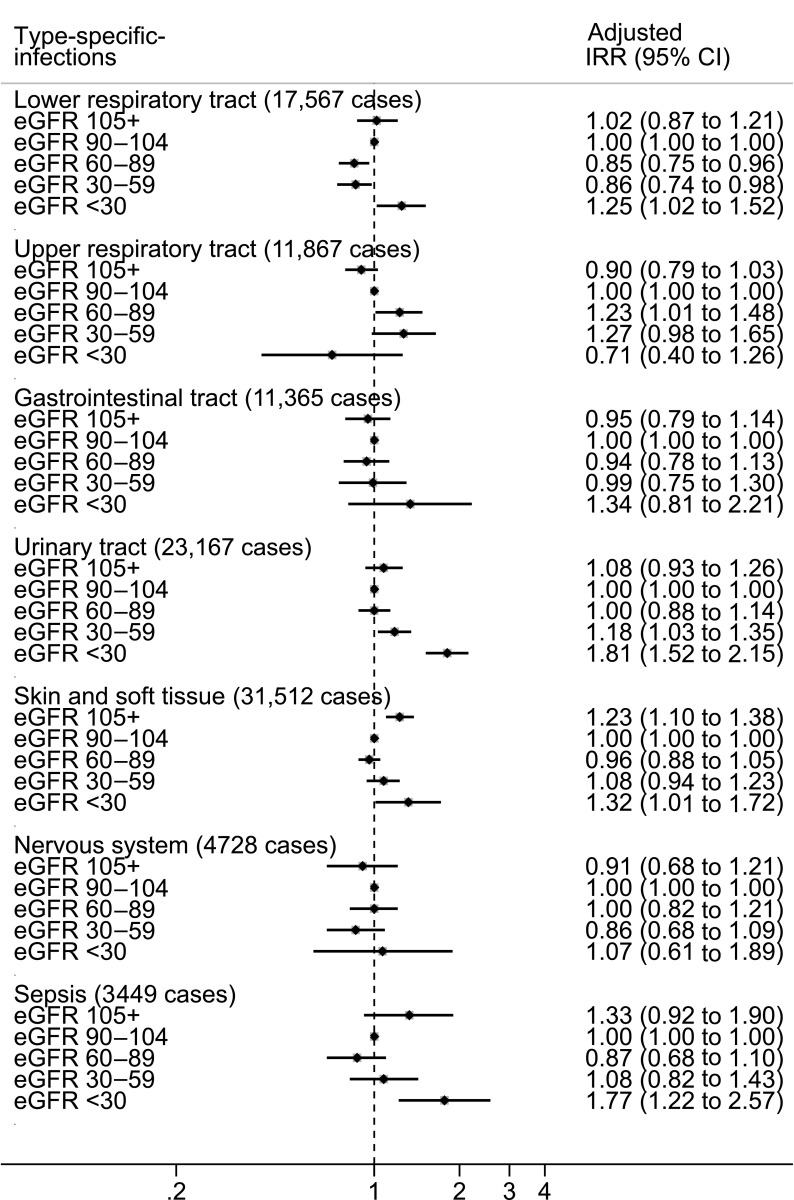
IRRs for single type–specific infections are shown in Figure 3. Because of low numbers of events, the categories of infections of the musculoskeletal and cardiovascular systems were not addressed. Compared with the category of eGFR=90–104 ml/min per 1.73 m2, participants with eGFR≥105 ml/min per 1.73 m2 were at a significantly increased rate of skin/soft tissue infections. For the other eGFR categories, we observed, in general, increased IRRs across lower eGFR strata for the risk of skin/soft tissue infections, sepsis, gastrointestinal infections, UTIs, and lower respiratory tract infections, particularly among individuals with eGFR<30 ml/min per 1.73 m2. No association was observed between eGFR strata and the risk of upper respiratory tract or nervous system infections.
Figure 3.
Increased incidence rate ratio (IRR) and 95% confidence intervals (CI) for type-specific community-acquired infections across eGFR categories within 12 months of follow-up. IRRs were adjusted for age, sex, use of immunosuppressive drugs, prior anti-infection drugs (antibiotics, antimycotics, and antivirals), comorbidities (cardiovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, liver disease, hemiplegia or paraplegia, cancer, diabetes, and hypertension), and exposure time.
Sensitivity Analyses
Time sequential analyses showed a similar pattern within the short (3 months of follow-up) and long term (between 3 and 12 months of follow-up) (Supplemental Figure 2). Age-, sex-, diabetes-, and cancer status–stratified analyses are shown in Supplemental Figure 3. Incidence rates were similar for both men and women but higher among those with (compared with without) diabetes or cancer. In all strata considered, a similar U-shape trend was noted across the broad eGFR spectrum. A higher incidence rate of infections was noted among the elderly, particularly among those in the highest eGFR category (≥105 ml/min per 1.73 m2).
Discussion
General Summary
Community-acquired infections are common and carry a large burden in terms of morbidity and health care costs. In a large health care utilization cohort of >1 million Swedes, we report that participants with reduced eGFR experienced increased risk of infections during 12 months of follow-up. The incidence rate of major infection types increased with more severe CKD, but the leading types of infections varied. Overall, this large and comprehensive description of changing infection patterns across eGFR can serve to increase patient and provider awareness, leading toward improved patient management and more effective vaccination strategies and health service planning.
Increased Incidence Rates and IRRs
Preceding literature suggests an overall direct relationship between reduced kidney function and infections requiring hospitalization (10–15). An analysis from the United Kingdom showed increased incidence of community-acquired infections for selected types of infections (lower respiratory tract infections and sepsis) among elderly patients with diabetes identified in primary care (12). Our study expands that evidence to the greater community by analyzing the whole spectrum of infections in ambulatory consultations.
We observe that the crude incidence of most infection types increased across lower eGFR strata. Compared with eGFR values of 90–104 ml/min per 1.73 m2, individuals with eGFR<30 ml/min per 1.73 m2 were at higher risk of most infections. This increased risk was observed for most infection types, with the exception of upper respiratory tract and central nervous system (CNS) infections, which did not clearly associate with eGFR. These are novel observations for which there is no comparison in the literature and warrant further confirmation.
The lack of association with upper respiratory tract infections may be due to patients with CKD being less prone to getting upper respiratory infections or being diagnosed with upper respiratory infections. For the former, high influenza vaccination rates among patients with CKD in Sweden may be indeed preventing upper respiratory infection (24). For the latter, it is possible that patients with CKD are more likely to present with a complex of symptoms and signs, and thus, the diagnosis by health care providers may lean toward lower respiratory infection (25).
For CNS infections, the crude incidence rate is higher in lower eGFR strata, but these differences were attenuated in multivariable analysis. CNS infections are rare but the most severe form of infections, and it is possible that our short observation period may have limited our power to observe an association. The risk of gastrointestinal tract infections was high in magnitude but did not reach statistical significance, something consistent with a previous study from the United States (11).
We do not observe a straightforward “dose relationship” between the risk of lower respiratory tract infections and the intermediate eGFR strata, which is at odds with two previous reports showing strong predictions for pneumonia hospitalizations (11,12). Differences in the severity of the event predicted, clinical practices, participant characteristics, and notably, follow-up (1 year in our study versus a median of 2.5 [11] and 4.6 [12] years of observation) may explain this discrepancy.
Finally, the associations between levels of eGFR of 105 ml/min per 1.73 m2 or greater and higher risk of some types of infections are also interesting and have been reported in previous studies (10–12). They are likely a consequence of inaccurate estimation of true GFR in these individuals due to low serum creatinine generation as a result of reduced muscle mass accompanying chronic illness. This is supported by our sensitivity analyses showing a markedly increased infection risk among the elderly in the highest eGFR category, presumably denoting frailty.
Differential Pattern of Infections and Clinical Implications
Disaggregating infection types across eGFR strata is potentially useful from a clinical perspective, because these subcategories may require different interventions and prevention strategies. We observed that UTIs, sepsis, and lower respiratory tract infections became increasingly common among individuals with more severe CKD, whereas the proportions of skin/soft tissue and upper respiratory tract infections were lower. This is a novel aspect of our report that may have clinical implications. Given the fact that CKD remains underdiagnosed and unrecognized in most societies, including our society (2), our findings may help clinicians become more aware of CKD and its complications. This in turn may be useful to identify patients at increased risk of infection and inform discussions about infection risk and vaccination strategies.
Infection prevention programs usually target patients on dialysis and individuals with severe kidney dysfunction (26–28), whereas our results suggest that these programs perhaps should be expanded to also include persons with less severe kidney dysfunction. Furthermore, because CKD associates with an increased risk of a number of adverse health outcomes, characterizing the relative distribution of specific infection types by eGFR category provides a comprehensive description of the type-specific infection burden of disease in CKD. This information may be helpful for prioritizing resource allocation to future interventions.
Plausible Mechanisms
Several health care–related factors and plausible mechanisms could explain our findings. It could be postulated that individuals with CKD might be monitored more closely and thus, are more likely to be diagnosed with infections compared with those without CKD. However, infections are, in general, acute conditions with unique symptoms and signs, and thus, they are less prone to such an ascertainment bias (15). Also, it is important that associations remained strong, despite adjustment for age, sex, and multiple comorbidities. More important is perhaps the fact that there is a biologic plausibility for the causality in the associations reported given the well known effect of uremic toxicity on T lymphocyte and antigen-presenting cells (6) and the generation of oxidative stress (29), factors that alter both cellular and humoral immunity. Because it is possible that additional mechanisms operate in the setting of moderate kidney disease, it may be worthwhile to pursue additional experimental studies in the search of underlying reasons.
Strengths and Limitations
The results should be interpreted in view of some unique strengths and limitations. The most important limitation is the reliance on ICD-10 diagnoses. The reliability of physician diagnosis codes may vary across different sites and severity of infection, raising a particular concern regarding the ascertainment of upper respiratory tract infections. We are not aware of any study validating outpatient infection diagnoses in Sweden. However, a previous study reported the validity of national inpatient diagnoses in Sweden to be 85%–95% (30). The validity of selected inpatient infections, namely infection, pneumonia, community-acquired sepsis, and tuberculosis codes, was found to be of low sensitivity (<50%) but excellent specificity (>95% in all of them) in Sweden (31).
This is the largest material so far addressing this topic in material with a large census population coverage of our region (19). However, this is a population accessing health care, and reasons for testing creatinine could also be confounders. Although the reasons for measurement are unknown and selected patients may differ from patients without creatinine measurements, this is unlikely to invalidate our findings, which are on the basis of a large proportion of patients from our source population and reflect standard outpatient clinical practices in a large health care region with individuals having universal access to health care. Additionally, our analytic design provides a careful quantification of community-acquired infections by excluding hospitalization-related codes and avoiding the overestimation from repeated attendances. We also studied a short 1-year follow-up, which allows us to assume that eGFR has been rather constant in that period. That said, our findings are restricted to the region of Stockholm, Sweden and may not be generalizable to other populations. Although we excluded participants with recent infections and controlled for the use of immunosuppressive and anti-infection medication, our records do not have reliable information on vaccination status or vaccine purchases, because they can be done in private practices/centers. In favor of our findings, however, Scandinavian countries have the lowest rates of antibiotic consumption and antibiotic resistance worldwide (32). Because information on proteinuria was lacking in most patients and we only used one serum creatinine to estimate GFR, we could not accurately stage CKD severity and therefore, refer to eGFR strata. Finally, because of the study design, we cannot assume causality in the associations reported, and residual confounding is inherent to all observational studies.
Among 1.1 million community-dwelling adults accessing health care in the region of Stockholm, an independent graded association was observed between reduced kidney function and overall incidence of community-acquired infections. Although associations were consistently seen for most separate infections, lower respiratory tract infections, UTIs, and sepsis were the infection types most markedly linked to a reduced kidney function, exhibiting the greatest difference in incidence rates and their relative proportions, in individuals with advanced CKD. Increasing patient and health care provider awareness of this differential pattern of risk could have benefits for patient management, prevention strategies, and health service planning.
Disclosures
B.L. is employed by Baxter Healthcare Corporation. None of the other authors declare any conflict of interest.
Supplementary Material
Acknowledgments
We thank the team from the London School of Hygiene & Tropical Medicine (Dorothea Nitsch, Sara Thomas, Elizabeth Millet, Jennifer Quint, and Helen McDonald) for sharing with us their International Classification of Disease, 10th Revision, Clinical Modification–based definitions of community-acquired infections.
This work was supported by the Stockholm County Council and the Swedish Heart and Lung Foundation. H.X. is partially supported by the Karolinska Institutet program for postgraduate education. Baxter Novum is the result of a grant from the Baxter Healthcare Corporation to Karolinska Institutet.
The funders of this study did not have a role in study design, collection, analysis, and interpretation of data; writing the report; or decision to submit the report for publication.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00250117/-/DCSupplemental.
References
- 1.Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Köttgen A, Levey AS, Levin A: Evolving importance of kidney disease: From subspecialty to global health burden. Lancet 382: 158–169, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Gasparini A, Evans M, Coresh J, Grams ME, Norin O, Qureshi AR, Runesson B, Barany P, Ärnlöv J, Jernberg T, Wettermark B, Elinder CG, Carrero JJ: Prevalence and recognition of chronic kidney disease in Stockholm healthcare. Nephrol Dial Transplant 31: 2086–2094, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried LF, Katz R, Sarnak MJ, Shlipak MG, Chaves PH, Jenny NS, Stehman-Breen C, Gillen D, Bleyer AJ, Hirsch C, Siscovick D, Newman AB: Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol 16: 3728–3735, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP: Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Cheikh Hassan HI, Tang M, Djurdjev O, Langsford D, Sood MM, Levin A: Infection in advanced chronic kidney disease leads to increased risk of cardiovascular events, end-stage kidney disease and mortality. Kidney Int 90: 897–904, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Eleftheriadis T, Antoniadi G, Liakopoulos V, Kartsios C, Stefanidis I: Disturbances of acquired immunity in hemodialysis patients. Semin Dial 20: 440–451, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Allon M, Radeva M, Bailey J, Beddhu S, Butterly D, Coyne DW, Depner TA, Gassman JJ, Kaufman AM, Kaysen GA, Lewis JA, Schwab SJ; HEMO Study Group : The spectrum of infection-related morbidity in hospitalized haemodialysis patients. Nephrol Dial Transplant 20: 1180–1186, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Slinin Y, Foley RN, Collins AJ: Clinical epidemiology of pneumonia in hemodialysis patients: The USRDS waves 1, 3, and 4 study. Kidney Int 70: 1135–1141, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Berman SJ, Johnson EW, Nakatsu C, Alkan M, Chen R, LeDuc J: Burden of infection in patients with end-stage renal disease requiring long-term dialysis. Clin Infect Dis 39: 1747–1753, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Dalrymple LS, Katz R, Kestenbaum B, de Boer IH, Fried L, Sarnak MJ, Shlipak MG: The risk of infection-related hospitalization with decreased kidney function. Am J Kidney Dis 59: 356–363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James MT, Quan H, Tonelli M, Manns BJ, Faris P, Laupland KB, Hemmelgarn BR; Alberta Kidney Disease Network : CKD and risk of hospitalization and death with pneumonia. Am J Kidney Dis 54: 24–32, 2009 [DOI] [PubMed] [Google Scholar]
- 12.McDonald HI, Thomas SL, Millett ER, Nitsch D: CKD and the risk of acute, community-acquired infections among older people with diabetes mellitus: A retrospective cohort study using electronic health records. Am J Kidney Dis 66: 60–68, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang HE, Gamboa C, Warnock DG, Muntner P: Chronic kidney disease and risk of death from infection. Am J Nephrol 34: 330–336, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James MT, Laupland KB, Tonelli M, Manns BJ, Culleton BF, Hemmelgarn BR; Alberta Kidney Disease Network : Risk of bloodstream infection in patients with chronic kidney disease not treated with dialysis. Arch Intern Med 168: 2333–2339, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Ishigami J, Grams ME, Chang AR, Carrero JJ, Coresh J, Matsushita K: CKD and risk for hospitalization with infection: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 69: 752–761, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman C, Anderson R: Community-acquired pneumonia: Still a major burden of disease. Curr Opin Crit Care 22: 477–484, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Tandogdu Z, Wagenlehner FM: Global epidemiology of urinary tract infections. Curr Opin Infect Dis 29: 73–79, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR: Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Runesson B, Gasparini A, Qureshi AR, Norin O, Evans M, Barany P, Wettermark B, Elinder CG, Carrero JJ: The Stockholm CREAtinine Measurements (SCREAM) project: Protocol overview and regional representativeness. Clin Kidney J 9: 119–127, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gasparini A, Evans M, Coresh J, Grams ME, Norin O, Qureshi AR, Runesson B, Barany P, Ärnlöv J, Jernberg T, Wettermark B, Elinder CG, Carrero JJ: Prevalence and recognition of chronic kidney disease in Stockholm healthcare. Nephrol Dial Transplant 31: 2086–2094, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA: Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43: 1130–1139, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Ethgen O, Cornier M, Chriv E, Baron-Papillon F: The cost of vaccination throughout life: A western European overview. Hum Vaccin Immunother 12: 2029–2037, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members : Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158: 825–830, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control: Recommendations for preventing transmission of infections among chronic hemodialysis patients. MMWR Recomm Rep 50: 1–43, 2001 [PubMed]
- 27.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, Saint S, Schaeffer AJ, Tambayh PA, Tenke P, Nicolle LE; Infectious Diseases Society of America : Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis 50: 625–663, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Ford DW, Goodwin AJ, Simpson AN, Johnson E, Nadig N, Simpson KN: A severe sepsis mortality prediction model and score for use with administrative data. Crit Care Med 44: 319–327, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Himmelfarb J: Uremic toxicity, oxidative stress, and hemodialysis as renal replacement therapy. Semin Dial 22: 636–643, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO: External review and validation of the Swedish national inpatient register. BMC Public Health 11: 450, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gedeborg R, Furebring M, Michaëlsson K: Diagnosis-dependent misclassification of infections using administrative data variably affected incidence and mortality estimates in ICU patients. J Clin Epidemiol 60: 155–162, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Hellen G, Molly M-P, Suraj P, Sumanth G, Jordan L, Devra B, Andrea W, Ramanan L: State of the World's Antibiotics, Center for Disease Dynamics, Economics & Policy: Washington, DC, 2015
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.