Abstract
Background and objectives
Depression in patients with nondialysis-dependent CKD is often undiagnosed, empirically overlooked, and associated with higher risk of death, progression to ESRD, and hospitalization. However, there is a paucity of evidence on the association between the presence of depression in patients with advanced nondialysis-dependent CKD and post-ESRD mortality, particularly among those in the transition period from late-stage nondialysis-dependent CKD to maintenance dialysis.
Design, setting, participants, & measurements
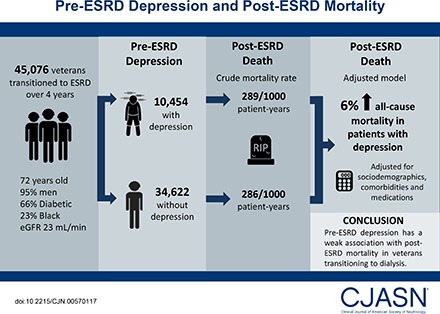
From a nation-wide cohort of 45,076 United States veterans who transitioned to ESRD over 4 contemporary years (November of 2007 to September of 2011), we identified 10,454 (23%) patients with a depression diagnosis during the predialysis period. We examined the association of pre-ESRD depression with all-cause mortality after transition to dialysis using Cox proportional hazards models adjusted for sociodemographics, comorbidities, and medications.
Results
Patients were 72±11 years old (mean±SD) and included 95% men, 66% patients with diabetes, and 23% blacks. The crude mortality rate was similar in patients with depression (289/1000 patient-years; 95% confidence interval, 282 to 297) versus patients without depression (286/1000 patient-years; 95% confidence interval, 282 to 290). Compared with patients without depression, patients with depression had a 6% higher all-cause mortality risk in the adjusted model (hazard ratio, 1.06; 95% confidence interval, 1.03 to 1.09). Similar results were found across all selected subgroups as well as in sensitivity analyses using alternate definitions of depression.
Conclusion
Pre-ESRD depression has a weak association with post-ESRD mortality in veterans transitioning to dialysis.
Keywords: mortality; chronic kidney disease; end stage kidney disease; transition; African Americans; Comorbidity; Confidence Intervals; depression; diabetes mellitus; Disease Progression; hospitalization; Humans; Kidney Failure, Chronic; Male; Proportional Hazards Models; quality of life; renal dialysis; Renal Insufficiency, Chronic; Risk; Veterans
Introduction
Previous studies have indicated that approximately 20%–40% of patients with nondialysis-dependent CKD (NDD-CKD) as well as patients on maintenance dialysis and kidney transplant recipients suffer from depression (1–6). Depression is known to negatively and severely affect patients’ quality of life (7,8), and has also been associated with higher rates of hospitalization (9–12) and mortality (9,13,14) in patients with NDD-CKD, patients on dialysis, and patients with kidney transplants (15). In a cohort of 568 patients with NDD-CKD in Taiwan, Tsai et al. (16) showed that the presence of depression is associated with a higher risk of death, a faster progression to ESRD, a faster time to first hospitalization, and a more rapid decline in eGFR. We previously showed strong association between presence of depression and antidepressant use and higher mortality risk in almost 600,000 patients with NDD-CKD (17). In addition, we recently also showed that depression was associated with a higher risk of incident NDD-CKD as well as a higher risk of incident cardiovascular disease in a cohort of >900,000 diabetic United States veterans without NDD-CKD at baseline (18). Furthermore, a recent meta-analysis, which included over 80,000 patients with NDD-CKD, confirmed the association between the presence of depression and a higher risk of death in patients with NDD-CKD (19). However, the association between depression in patients with advanced NDD-CKD and mortality outcomes post-ESRD is still unknown. To address this knowledge gap, we aimed to investigate the association of depression in the pre-ESRD transition period with post-ESRD all-cause mortality using a large nationally representative cohort of United States veterans with advanced NDD-CKD transitioning to RRT. We hypothesized that the presence of depression in patients before transition is associated with higher risk of death after transition to ESRD.
Materials and Methods
Study Population
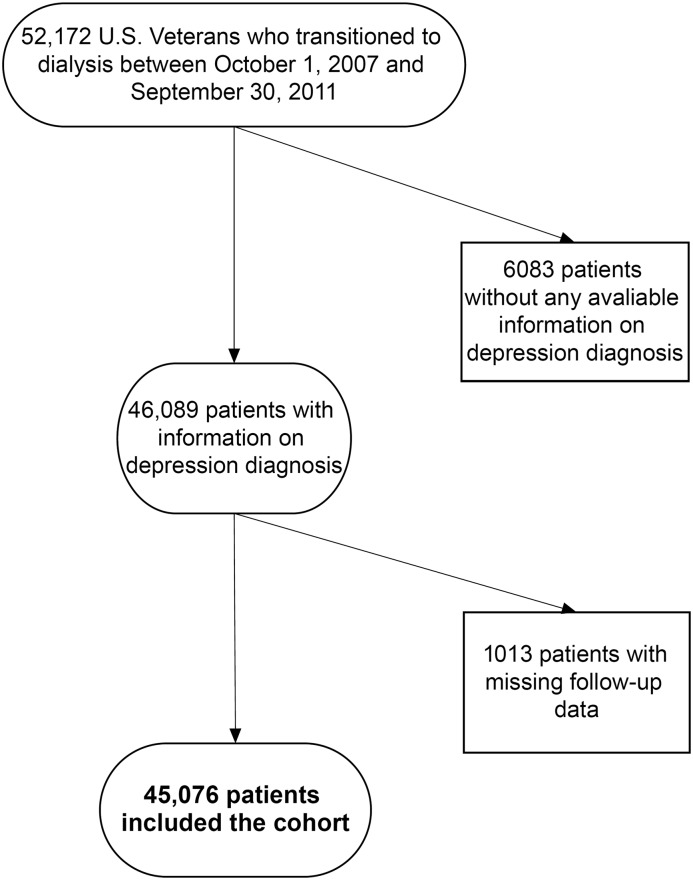
We analyzed longitudinal data from the Transition of Care in CKD Study, a retrospective cohort study examining United States veterans with late-stage NDD-CKD transitioning to RRT from October 1, 2007 to September 30, 2011 (20–22). A total of 52,172 United States veterans were identified from the US Renal Data System (USRDS) as a source population. Only individuals who transitioned to receive RRT were included in the source population. The algorithm for the cohort definition is shown in Figure 1. We excluded patients without any available information on comorbid conditions, including depression diagnoses (n=6083). We also excluded those who were missing follow-up data (n=1013 who died or received a kidney transplant on the date of transition to ESRD), resulting in a study population of 45,076 patients.
Figure 1.
Flow chart of the study population.
Exposure Variable
We used the validated algorithm described by Frayne et al. (23) to define depression using outpatient or inpatient medical record before transition to dialysis. In sensitivity analyses, we also examined associations according to depression defined by Frayne et al. (23) for depression and/or being on antidepressant medication(s) 6 months before transition to ESRD (baseline). Because antidepressant medications often have other indications, such as pain syndrome and post-traumatic stress disorders, we used the definition solely on the basis of the algorithm of Frayne et al. (23) for our main analysis. In a second sensitivity analysis, we examined the combined effect of both having a depression diagnosis according to Frayne et al. (23) and using antidepressant medications (Supplemental Table 1) by creating three groups of exposure as follows: absence of depression (no diagnosis and not on medication), depression with absence of pharmacotherapy (has diagnosis and not on medication), and depression treated with pharmacotherapy (has diagnosis and on medication or no diagnosis and on medication).
Covariates
Data from the USRDS Patient and Medical Evidence files were used to determine patients’ baseline demographic characteristics and type of vascular access at the time of dialysis initiation. Information on comorbidities at the time of dialysis initiation was extracted from the Veterans Affairs (VA) Inpatient and Outpatient Medical SAS Datasets using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic and Current Procedural Terminology codes as well as from the VA/Centers for Medicare and Medicaid Services data. Medication data were collected from both the Centers for Medicare and Medicaid Services Data (Medicare Part D) and the VA pharmacy dispensation records. Patients who received at least one dispensation of medications within the 6-month predialysis period immediately preceding ESRD transition were recorded as having been treated with these medications. Cardiovascular medication adherence was defined as the proportion of days covered by a drug during the 6-month predialysis period capped at 100% (22). Laboratory data were obtained from the VA research databases as previously described (24,25), and their baseline values were defined as the average of each covariate during the 6-month predialysis period preceding dialysis initiation. eGFR was calculated by the CKD Epidemiology Collaboration equation (26).
Outcome Assessment
The primary outcome of interest was all-cause mortality after dialysis initiation. All-cause mortality data, censoring events, and associated dates were obtained from the VA and the USRDS data sources. The start of the follow-up period was the date of dialysis initiation, and patients were followed up until death or other censoring events, including kidney transplantation, loss to follow-up, or end of the follow-up period (3, 6, and 12 months after dialysis initiation or December 27, 2012 for the entire follow-up period) (20–22). The primary analysis used the entire follow-up time.
Statistical Analyses
Baseline patient characteristics were summarized according to the presence or absence of depression before ESRD and presented as percentage for categorical variables and mean±SD for continuous variables. Differences between categories were assessed using t tests and chi-squared tests for continuous and categorical variables, respectively.
The association between the presence of depression and mortality was estimated using the Kaplan–Meier method and Cox proportional hazards models. Models were incrementally adjusted for the following potential confounders on the basis of theoretical considerations and their availability in this study: unadjusted; model 1 adjusted for age, sex, race/ethnicity, and marital status; model 2 additionally accounted for comorbidities (dementia, myocardial infarction, congestive heart failure, peripheral vascular disease, connective tissue disease, lung disease, peptic ulcer disease, HIV, diabetes mellitus, stroke/paraplegia, liver disease, malignancy, and hypertension), type of vascular access (arteriovenous fistula, arteriovenous graft, or catheter), eGFR slope before ESRD initiation, post-traumatic stress disorder, substance abuse, and numbers of mental health care and emergency department visits in the last year; model 3 (main model) additionally accounted for medications (phosphorous binder, active vitamin D, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, bicarbonate, β-blockers, calcium channel blockers, vasodilators, diuretics, statins, and erythropoietin stimulating agents); and model 4 additionally accounted for blood hemoglobin, serum albumin, income, and cardiovascular medication adherence.
We conducted several sensitivity analyses to evaluate the robustness of our main findings. To compare the effect of depression on outcomes during different follow-up periods, we repeated our analyses separately using additional short-term definitions (3, 6, and 12 post-transition months).
The associations of depression with outcomes were examined in subgroups of patients stratified by sex, age, race, marital status, and presence/absence of select comorbidities using multivariable adjusted model 3. Potential interactions were formally tested by including relevant interaction terms.
Of the 45,076 patients in our study population, 41,582 (92%) had complete data available for the main adjusted multivariable model (model 3). Because it is possible that missingness was not at random and that the proportion of patients with missingness was acceptable in our main analyses, imputation was not used. We used only these patients with complete cases in our unadjusted model as well as our models 1 and 2; >50% of the laboratory markers were missing (Supplemental Table 2), and therefore, model 4 was performed as an additional sensitivity analysis. However, we also performed sensitivity analysis using multiple imputation procedures. Missing values were replaced by multiple imputations with a multivariate normal regression method with data augmentation by an iterative Markov chain Monte Carlo procedure (27,28). Five imputed datasets were generated; primary analyses were performed on each imputed dataset, and the combination rules of Rubin (29) were used to form one set of results.
Reported P values were two sided and reported as significant at <0.05 for all analyses. All analyses were conducted using STATA/MP, Version 14 (StataCorp, College Station, TX). The study was approved by the institutional review boards of the Memphis and Long Beach VA Medical Centers, with exemption from informed consent.
Results
Baseline Characteristics
Patients’ baseline characteristics in the overall cohort and stratified by the depression status are presented in Table 1. The overall mean±SD age at baseline was 72±11 years old; 95% were men, 23% were black, and 66% were diabetic. The mean±SD of the last measured eGFR before ESRD initiation was 23±19 ml/min per 1.73 m2. We identified 10,454 (23%) patients with depression. The median time from the last ICD-9-CM code and initiation to the dialysis was 355 days (interquartile range, 90–973 days). Compared with patients without a depression diagnosis, those with depression were younger, were more likely to be women and black, and had a higher prevalence of myocardial infarction, diabetes, cerebrovascular disease, peripheral vascular disease, dementia, and peptic ulcer disease at baseline. They were also more likely to use antidepressant medications within 6 months before transition and less likely to use statins and antihypertensive medications. The available baseline laboratory parameters were clinically similar in patients with and without depression.
Table 1.
Baseline patient characteristics overall and according to pre-ESRD presence of depression
| Total Cohort | n=45,076 | Depression on the Basis of Only Algorithm | |
|---|---|---|---|
| No, n=34,622 | Yes, n=10,454 | ||
| Age, yr | 72±11 | 72±11 | 68±11 |
| Men, % | 95 | 95 | 94 |
| Black race, % | 23 | 23 | 25 |
| Marital status, married, % | 58 | 59 | 52 |
| Body mass index, kg/m2 | 29.9±6.6 | 29.6±6.4 | 30.4±7.0 |
| Vascular access type, catheter, % | 78 | 78 | 78 |
| Comorbid conditions, % | |||
| Myocardial infarction | 29 | 28 | 32 |
| Congestive heart failure | 58 | 56 | 62 |
| Peripheral vascular disease | 40 | 39 | 45 |
| Cerebrovascular disease | 32 | 29 | 38 |
| Dementia | 3 | 2 | 6 |
| Chronic obstructive pulmonary disease | 45 | 42 | 54 |
| Rheumatic disease | 5 | 4 | 6 |
| Peptic ulcer disease | 8 | 8 | 10 |
| Hemiplegia | 4 | 3 | 6 |
| HIV/AIDS | <1 | <1 | <1 |
| Diabetes mellitus | 66 | 63 | 74 |
| Liver disease | 12 | 10 | 18 |
| Cancera | 26 | 26 | 25 |
| Hypertension | 45 | 46 | 44 |
| Medications, % | |||
| ACEIs/ARBs | 35 | 33 | 41 |
| Antidepressants | 20 | 10 | 52 |
| β-Blockers | 53 | 50 | 63 |
| Calcium channel blockers | 47 | 44 | 56 |
| Diuretics | 56 | 53 | 65 |
| Statins | 47 | 44 | 55 |
| Vasodilators | 3 | 3 | 4 |
| Vitamin D analogs | 22 | 21 | 25 |
| ESAs | 17 | 15 | 23 |
| Laboratory parameters | |||
| Serum albumin, g/dl | 3.3±0.6 | 3.3±0.6 | 3.2±0.6 |
| Serum AST, U/La | 26±39 | 26±42 | 26±30 |
| Serum ALT, U/La | 24±34 | 23±33 | 24±26 |
| Serum BUN, mg/dl | 61±23 | 62±23 | 58±21 |
| Serum creatinine, mg/dl | 4.6±2.4 | 4.7±2.5 | 4.5±2.2 |
| First eGFR in the cohort, ml/min per 1.73 m2 | 44±24 | 41±23 | 50±26 |
| Last eGFR before ESRD,a ml/min per 1.73 m2 | 23±19 | 23±19 | 23±20 |
| Serum phosphorus, mg/dl | 5.1±1.3 | 5.1±1.3 | 5.0±1.2 |
| Serum calcium, mg/dl | 8.8±0.7 | 8.8±0.8 | 8.7±0.7 |
| Alkaline phosphatase, IU/L | 98±66 | 97±66 | 102±61 |
| Blood hemoglobin, g/dla | 10.3±1.5 | 10.3±1.5 | 10.4±1.5 |
| Serum bicarbonate, mg/dla | 23±4 | 23±4 | 23±4 |
| Cholesterol, mg/dl | 155±50 | 153±49 | 158±51 |
| Serum potassium, mEq/L | 4.5±0.6 | 4.5±0.6 | 4.5±0.5 |
| WBC, 109/L | 8±3 | 8±3 | 8±3 |
Data are presented as number (percentage) or mean±SD. All laboratory results were averaged over the 6-month predialysis period. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ESA, erythropoietin stimulating agent; AST, aspartate aminotransferase; ALT, alanine aminotransferase; WBC, white blood cell.
No statistically significant difference between patients with and without depression.
Association of Pre-ESRD Presence of Depression with Post-ESRD All-Cause Mortality in the Entire Follow-Up Period after Dialysis Initiation
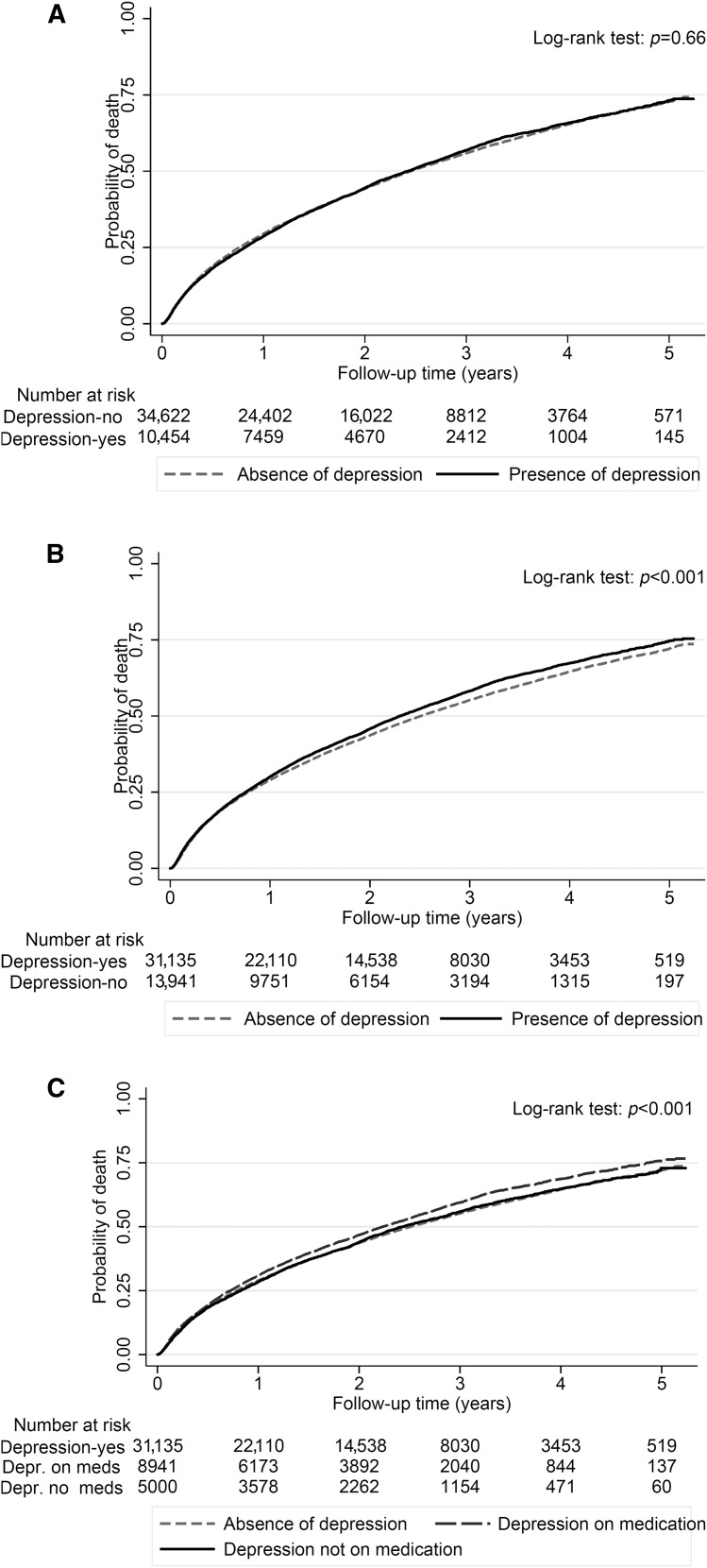
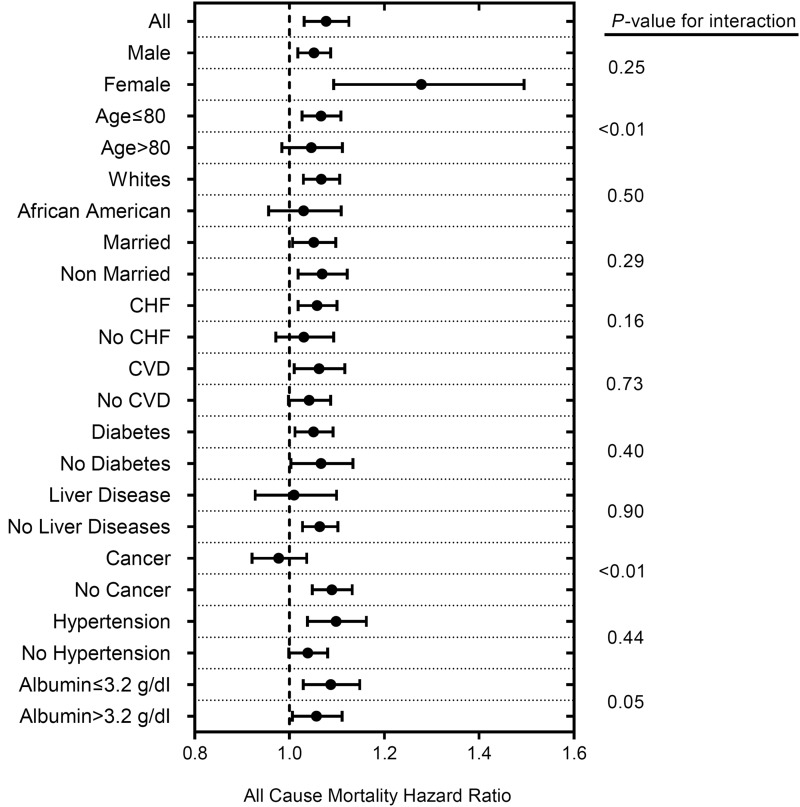
During the entire follow-up period after dialysis initiation, a total of 25,901 (57%) all-cause deaths occurred (crude incidence rate, 287/1000 patient-years; 95% confidence interval [95% CI], 283 to 290). The crude mortality rate was similar in patients with depression (5953 [57%] deaths; 289/1000 patient-years; 95% CI, 282 to 297) versus patients without depression (19,948 [58%] deaths; 286/1000 patient-years; 95% CI, 282 to 290) as shown in the Kaplan–Meier survival curve in Figure 2A. Compared with patients without a depression diagnosis, patients with depression had similar mortality risk in the unadjusted model (hazard ratio [HR], 1.00; 95% CI, 0.97 to 1.03). On further adjustment for sociodemographics, comorbidities, and medications, patients with depression had 6% higher mortality risk (HR, 1.06; 95% CI, 1.03 to 1.09) (Table 2). A similar result was found after further adjustment for laboratory parameters and a marker of adherence (model 4) (Table 2). In subgroup analyses, compared with patients without depression, patients with depression had higher all-cause mortality risk overall and across almost all subgroups (Figure 3). Statistically significant interactions were present for age and cancer, with stronger associations between depression and all-cause mortality risk among younger patients and those without cancer.
Figure 2.
Probability of all-cause mortality of patients with and without depression using different definitions of depression. (A) Depression defined only using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code is not associated with mortality. (B) Depression defined on the basis of the ICD-9-CM code and/or antidepressant medication, or on the basis of the ICD-9-CM code and/or antidepressant medication separated by treatment (C) is associated with higher mortality.
Table 2.
Adjusted hazard ratios (95% confidence intervals) for all-cause mortality initiation using different definitions of depression
| Definition of Depression | |||||||
|---|---|---|---|---|---|---|---|
| On the Basis of Algorithm (Main Model) | On the Basis of Algorithm and/or Antidepressant Medication | On the Basis of Algorithm and/or Antidepressant Medication | |||||
| Absence of Depression | Presence of Depression | Absence of Depression | Presence of Depression | Absence of Depression | Depression with Absence of Pharmacotherapy | Depression Treated with Pharmacotherapy | |
| Patients | 34,622 | 10,454 | 31,135 | 13,941 | 31,135 | 5000 | 8941 |
| Events | 19,948 (58) | 5953 (57) | 17,711 (57) | 8190 (59) | 17,711 (57) | 2798 (56) | 5392 (60) |
| Crude incident rate | 286 (282 to 290) | 289 (282 to 297) | 280 (276 to 284) | 302 (296 to 309) | 280 (276 to 284) | 283 (273 to 294) | 313 (304 to 321) |
| Unadjusted, n=41,582 | 1 (Reference) | 1.00 (0.97 to 1.03) | 1 (Reference) | 1.06 (1.04 to 1.09) | 1 (Reference) | 1.00 (0.97 to 1.06) | 1.10 (1.06 to 1.13) |
| Model 1, n=41,582 | 1 (Reference) | 1.20 (1.17 to 1.24) | 1 (Reference) | 1.24 (1.21 to 1.28) | 1 (Reference) | 1.18 (1.13 to 1.23) | 1.29 (1.25 to 1.33) |
| Model 2, n=41,582 | 1 (Reference) | 1.04 (1.01 to 1.07) | 1 (Reference) | 1.06 (1.03 to 1.09) | 1 (Reference) | 1.04 (1.00 to 1.09) | 1.07 (1.04 to 1.11) |
| Model 3, n=41,582 | 1 (Reference) | 1.06 (1.03 to 1.09) | 1 (Reference) | 1.10 (1.07 to 1.13) | 1 (Reference) | 1.03 (0.99 to 1.07) | 1.14 (1.11 to 1.18) |
| Model 4, n=20,542 | 1 (Reference) | 1.08 (1.03 to 1.13) | 1 (Reference) | 1.12 (1.07 to 1.16) | 1 (Reference) | 1.06 (0.99 to 1.13) | 1.14 (1.09 to 1.19) |
Data are presented as number (percentage) or hazard ratio (95% confidence interval) unless otherwise specified. The crude incident rate presented is in per 1000 patient-years. Models are as follows. Unadjusted model: only exposure variable included. Model 1 adjusted for age, sex, race/ethnicity, and marital status. Model 2 additionally accounted for comorbidities (dementia, myocardial infarction, congestive heart failure, peripheral vascular disease, connective tissue disease, lung disease, peptic ulcer disease, HIV, diabetes mellitus, stroke/paraplegia, liver disease, malignancy, and hypertension), type of vascular access (arteriovenous fistula, arteriovenous graft, or catheter), eGFR slope before ESRD initiation, post-traumatic stress disorder, substance abuse, and numbers of mental health care and emergency department visits. Model 3 additionally accounted for medications (phosphorous binder, active vitamin D, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, bicarbonate, β-blockers, calcium channel blockers, vasodilators, diuretics, statins, and erythropoietin stimulating agents). Model 4 additionally accounted for blood hemoglobin, serum albumin, income, and cardiovascular medication adherence.
Figure 3.
Patients with depression experienced higher all-cause mortality risk across most examined subgroups. Model is adjusted for age, sex, race/ethnicity, marital status, comorbidities (dementia, myocardial infarction, congestive heart failure, peripheral vascular disease, connective tissue disease, lung disease, peptic ulcer disease, HIV, diabetes mellitus, stroke/paraplegia, liver disease, malignancy, and hypertension), type of vascular access (arteriovenous fistula, arteriovenous graft, or catheter), eGFR slope before ESRD initiation, post-traumatic stress disorder, substance abuse, numbers of mental health care and emergency department visits, and medications (phosphorous binder, active vitamin D, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, bicarbonate, β-blockers, calcium channel blockers, vasodilators, diuretics, statins, and erythropoietin stimulating agents). CHF, congestive heart failure; CVD, cerebrovascular disease.
Different results were observed in sensitivity analysis when antidepressant medication was taken into account in the definition of depression (Figure 2B). Compared with patients without a depression diagnosis, patients with depression had higher mortality risk in the unadjusted model. Moreover, compared with those without depression, patients with depression had a 10% higher mortality risk in the main multivariable adjusted model (model 3; HR, 1.10; 95% CI, 1.07 to 1.13) (Table 2). Finally, depression was associated with higher mortality risk in both the presence and absence of pharmacotherapy (Figure 2C). Compared with patients without depression (neither diagnosis nor antidepressant treatment), patients with depression treated with pharmacotherapy had a 14% higher multivariable adjusted mortality risk (HR, 1.14; 95% CI, 1.11 to 1.18), whereas patients with depression in the absence of pharmacotherapy had a 4% higher multivariable adjusted mortality risk (HR, 1.04; 95% CI, 1.00 to 1.09) (Table 2). Results did not differ when multiple imputations were used (HR, 1.06; 95% CI, 1.03 to 1.09).
Association of Pre-ESRD Presence of Depression with Post-ESRD All-Cause Mortality in the 3, 6, and 12 Months after Dialysis Initiation
Supplemental Figure 1 shows the association between presence of depression and 12 months all-cause mortality using the three definitions of depression. Compared with those without a depression diagnosis, patients with depression had a nominally higher mortality risk in the adjusted model, which did not reach statistical significance (HR, 1.02; 95% CI, 0.98 to 1.07) (Supplemental Table 3). Qualitatively similar results were found in short-term follow-up periods, such as 3 (Supplemental Table 4) and 6 months (Supplemental Table 5).
Discussion
In this large national cohort of United States veterans with late-stage NDD-CKD transitioning to dialysis, we found a weak association between pre-ESRD diagnosis of depression and all-cause mortality after dialysis initiation, independent of demographics, comorbidities, medications, and type of vascular access.
Several previous studies indicated strong associations between depression and higher risk of mortality in patients with kidney disease (15,17,18,30–34) and patients without kidney disease (35). However, the most recent analyses showed associations only between moderate and severe symptoms (Beck Depression Inventory >19) of depression and mortality in patients with ESRD (36). The underlying mechanisms linking depression to higher risk of mortality are likely to be multifactorial. Comorbid depression was shown to impair the ability to perform self-care in patients with diabetes (37), and it is also reportedly associated with obesity, persistence of smoking, and lack of physical exercise (38–40). Depression is also strongly associated with medication nonadherence, which has been shown to be an independent predictor of mortality after dialysis initiation (22). In our sensitivity analysis, we did adjust for medication nonadherence, and the association remained significant, which indicates that there are other mechanisms that may explain this association. These explanations may include a higher level of low-grade systemic inflammation, activation of the hypothalamic-pituitary-adrenal axis (increased cortisol secretion), and activation of the sympathetic nervous system, which have all been frequently reported in association with depression (41–43). In an earlier study, we also showed that the presence of depression is associated with severity of inflammation in prevalent kidney transplant recipients (44). Moreover, the presence of depression is also associated with less adherence with fluid restriction in patients on dialysis (45). Another potential explanation is that more severe symptoms of depression may develop as a result of a greater disease burden, and therefore, depression could be a mediator of the association between comorbid conditions and mortality (46). The converse can also be true, with depression resulting in more severe comorbidity, such as malnutrition due to anorexia, with these comorbidities mediating the relationship between depression and mortality (44,46).
Regardless of the underlying mechanisms, the association between depression and mortality in patients with NDD-CKD is clinically important, because it points to the possibility of improving outcomes through appropriate and successful management of depression. Antidepressant medication is only one treatment of choice for these patients. We did not find clinically significant survival difference between patients in the absence or presence of pharmacotherapy. This finding is seemingly unexpected; however, we do not have data about antidepressant medication adherence of these patients, and we could not ascertain the successfulness of the applied antidepressant medications in alleviating depression. In addition, there are other, potentially more effective treatment options for depression in patients with CKD, such as cognitive behavioral therapy, for which data were not available for this analysis. Cukor et al. (47) performed a small randomized, controlled trial, which indicated that cognitive behavioral therapy led to significant improvement in depression symptoms as well as medication adherence in patients with ESRD. Exercise training program is also a potential treatment for patients with depression (48).
One significant challenge in everyday clinical practice is the recognition of depression in patients with NDD-CKD. The main reason why physicians may fail to recognize depressive symptoms is related to the considerable overlap between somatic depressive symptoms and the burden of uremic symptoms of patients with NDD-CKD, such as sleep disturbance, lack of energy, decreased appetite, and concentrating difficulties (34,49). A multidisciplinary approach for recognizing and treating depression has shown promising results in patients with coronary artery disease (50) and could be considered in patients with NDD-CKD.
Our study is notable for its large sample size and event numbers and being representative of veterans who received care in the VA system across the entire United States. In addition, we used a validated method to make the depression diagnosis using an administrative dataset (23). To our knowledge, this is the first study to assess the associations between a diagnosis of comorbid depression before dialysis initiation and all-cause mortality after dialysis initiation.
This study also has several limitations that need to be acknowledged. First, because this was an observational study, only associations, but no cause-effect relationships, can be established. Second, most of our patients were men who were United States veterans; hence, the results may not be generalizable to women or other patient populations, in particular those outside the United States. Third, our study is also limited by the use of an administrative database and antidepressants to define depression. However, the significant correlates of baseline depression in our study were similar to those found in previous studies. In addition, some antidepressants may have been prescribed for diagnoses other than depression. However, we used only the definition on the basis of algorithm of Frayne et al. (23) as our main analysis to eliminate this potential bias. We did not have access to metrics quantifying the success rate of depression therapy; hence, we cannot determine if successful management of depression over time might alleviate some of the observed adverse effects. Moreover, there was a significant amount of missing laboratory data. However, the results remained qualitatively similar after multiple imputations in our final model. Finally, as with all observational studies, we were not able to eliminate the possibility of unmeasured confounders, such as proteinuria.
In conclusion, in this large national cohort of United States veterans with late-stage NDD-CKD transitioning to dialysis, we found an independent but weak association between pre-ESRD diagnosis of depression and all-cause mortality after dialysis initiation. Depression may be a potential modifiable factor before and after dialysis initiation. Although pre-ESRD treatment of depression may still be warranted to improve patients’ quality of life, such treatment may not have a benefit on post-ESRD mortality.
Disclosures
K.K.-Z. has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, the American Society of Nephrology, Astra-Zeneca, AVEO, Chugai, DaVita, Fresenius, Genentech, Haymarket Media, Hospira, Kabi, Keryx, National Institutes of Health, National Kidney Foundation, Relypsa, Resverlogix, Sanofi, Shire, Vifor, and ZS-Pharma. E.S. is supported by a career development award from the Office of Research and Development of the Department of Veterans Affairs (IK2- CX 001266-01). M.M.Z. has received honorarium from Merck.
Supplementary Material
Acknowledgments
This study is supported by grant 5U01DK102163 (to K.K.-Z. and C.P.K.) from the National Institutes of Health and resources from the US Department of Veterans Affairs. The data reported here have been supplied in part by the US Renal Data System. Support for Veterans Affairs/Centers for Medicare and Medicaid Services data is provided by Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, Veterans Affairs Information Resource Center projects SDR 02-237 and 98-004.
The results of this paper have not been published previously in whole or part. This work was presented as an oral presentation at the American Society of Nephrology Kidney Week 2016.
C.P.K. and K.K.-Z. are employees of the Department of Veterans Affairs. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the Department of Veterans Affairs or the US Government. Funders of this study had no role in study design, collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00570117/-/DCSupplemental.
References
- 1.Cukor D, Cohen SD, Peterson RA, Kimmel PL: Psychosocial aspects of chronic disease: ESRD as a paradigmatic illness. J Am Soc Nephrol 18: 3042–3055, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Watnick S, Wang PL, Demadura T, Ganzini L: Validation of 2 depression screening tools in dialysis patients. Am J Kidney Dis 46: 919–924, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Kalender B, Ozdemir AC, Koroglu G: Association of depression with markers of nutrition and inflammation in chronic kidney disease and end-stage renal disease. Nephron Clin Pract 102: c115–c121, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Dervisoglu E, Kir HM, Kalender B, Eraldemir C, Caglayan C: Depressive symptoms and proinflammatory cytokine levels in chronic renal failure patients. Nephron Clin Pract 108: c272–c277, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Palmer S, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, Pellegrini F, Saglimbene V, Logroscino G, Fishbane S, Strippoli GF: Prevalence of depression in chronic kidney disease: Systematic review and meta-analysis of observational studies. Kidney Int 84: 179–191, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Szeifert L, Molnar MZ, Ambrus C, Koczy AB, Kovacs AZ, Vamos EP, Keszei A, Mucsi I, Novak M: Symptoms of depression in kidney transplant recipients: A cross-sectional study. Am J Kidney Dis 55: 132–140, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Shidler NR, Peterson RA, Kimmel PL: Quality of life and psychosocial relationships in patients with chronic renal insufficiency. Am J Kidney Dis 32: 557–566, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Valderrábano F, Jofre R, López-Gómez JM: Quality of life in end-stage renal disease patients. Am J Kidney Dis 38: 443–464, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Hedayati SS, Bosworth HB, Briley LP, Sloane RJ, Pieper CF, Kimmel PL, Szczech LA: Death or hospitalization of patients on chronic hemodialysis is associated with a physician-based diagnosis of depression. Kidney Int 74: 930–936, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Lopes AA, Bragg J, Young E, Goodkin D, Mapes D, Combe C, Piera L, Held P, Gillespie B, Port FK; Dialysis Outcomes and Practice Patterns Study (DOPPS) : Depression as a predictor of mortality and hospitalization among hemodialysis patients in the United States and Europe. Kidney Int 62: 199–207, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Kimmel PL, Peterson RA, Weihs KL, Simmens SJ, Alleyne S, Cruz I, Veis JH: Multiple measurements of depression predict mortality in a longitudinal study of chronic hemodialysis outpatients. Kidney Int 57: 2093–2098, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Lacson E Jr, Bruce L, Li NC, Mooney A, Maddux FW: Depressive affect and hospitalization risk in incident hemodialysis patients. Clin J Am Soc Nephrol 9: 1713–1719, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diefenthaeler EC, Wagner MB, Poli-de-Figueiredo CE, Zimmermann PR, Saitovitch D: Is depression a risk factor for mortality in chronic hemodialysis patients? Rev Bras Psiquiatr 30: 99–103, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Dobbels F, Skeans MA, Snyder JJ, Tuomari AV, Maclean JR, Kasiske BL: Depressive disorder in renal transplantation: An analysis of medicare claims. Am J Kidney Dis 51: 819–828, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Novak M, Molnar MZ, Szeifert L, Kovacs AZ, Vamos EP, Zoller R, Keszei A, Mucsi I: Depressive symptoms and mortality in patients after kidney transplantation: A prospective prevalent cohort study. Psychosom Med 72: 527–534, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Tsai YC, Chiu YW, Hung CC, Hwang SJ, Tsai JC, Wang SL, Lin MY, Chen HC: Association of symptoms of depression with progression of CKD. Am J Kidney Dis 60: 54–61, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Balogun RA, Abdel-Rahman EM, Balogun SA, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Kalantar-Zadeh K, Kovesdy CP: Association of depression and antidepressant use with mortality in a large cohort of patients with nondialysis-dependent CKD. Clin J Am Soc Nephrol 7: 1793–1800, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novak M, Mucsi I, Rhee CM, Streja E, Lu JL, Kalantar-Zadeh K, Molnar MZ, Kovesdy CP: Increased risk of incident chronic kidney disease, cardiovascular disease, and mortality in diabetic patients with comorbid depression. Diabetes Care 39: 1940–1947, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer SC, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, Pellegrini F, Saglimbene V, Logroscino G, Hedayati SS, Strippoli GF: Association between depression and death in people with CKD: A meta-analysis of cohort studies. Am J Kidney Dis 62: 493–505, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Sumida K, Molnar MZ, Potukuchi PK, Thomas F, Lu JL, Jing J, Ravel VA, Soohoo M, Rhee CM, Streja E, Kalantar-Zadeh K, Kovesdy CP: Association of slopes of estimated glomerular filtration rate with post-end-stage renal disease mortality in patients with advanced chronic kidney disease transitioning to dialysis. Mayo Clin Proc 91: 196–207, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sumida K, Molnar MZ, Potukuchi PK, Thomas F, Lu JL, Ravel VA, Soohoo M, Rhee CM, Streja E, Yamagata K, Kalantar-Zadeh K, Kovesdy CP: Association between vascular access creation and deceleration of estimated glomerular filtration rate decline in late-stage chronic kidney disease patients transitioning to end-stage renal disease [published online ahead of print May 30, 2016]. Nephrol Dial Transplant doi:10.1093/ndt/gfw220https://doi.org/10.1093/ndt/gfw220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molnar MZ, Gosmanova EO, Sumida K, Potukuchi PK, Lu JL, Jing J, Ravel VA, Soohoo M, Rhee CM, Streja E, Kalantar-Zadeh K, Kovesdy CP: Predialysis cardiovascular disease medication adherence and mortality after transition to dialysis. Am J Kidney Dis 68: 609–618, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frayne SM, Berg E, Holmes TH, Laungani K, Berlowitz DR, Miller DR, Pogach L, Jackson VW, Moos R: Mental illness-related disparities in length of stay: Algorithm choice influences results. J Rehabil Res Dev 47: 709–718, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Kovesdy CP, Norris KC, Boulware LE, Lu JL, Ma JZ, Streja E, Molnar MZ, Kalantar-Zadeh K: Association of race with mortality and cardiovascular events in a large cohort of US veterans. Circulation 132: 1538–1548, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kovesdy CP, Alrifai A, Gosmanova EO, Lu JL, Canada RB, Wall BM, Hung AM, Molnar MZ, Kalantar-Zadeh K: Age and outcomes associated with BP in patients with incident CKD. Clin J Am Soc Nephrol 11: 821–831, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li KH: Imputation using Markov chains. J Stat Comput Simul 30: 57–79, 1988 [Google Scholar]
- 28.Tanner MA, Wong WH: The calculation of posterior distributions by data augmentation. J Am Stat Assoc 82: 528–550, 1987 [Google Scholar]
- 29.Rubin DB: Multiple Imputation for Nonresponse in Surveys, New York, Wiley & Sons, 1987 [Google Scholar]
- 30.Dew MA, Rosenberger EM, Myaskovsky L, DiMartini AF, DeVito Dabbs AJ, Posluszny DM, Steel J, Switzer GE, Shellmer DA, Greenhouse JB: Depression and anxiety as risk factors for morbidity and mortality after organ transplantation: A systematic review and meta-analysis. Transplantation 100: 988–1003, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loosman WL, Rottier MA, Honig A, Siegert CE: Association of depressive and anxiety symptoms with adverse events in Dutch chronic kidney disease patients: A prospective cohort study. BMC Nephrol 16: 155, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiang HH, Guo HR, Livneh H, Lu MC, Yen ML, Tsai TY: Increased risk of progression to dialysis or death in CKD patients with depressive symptoms: A prospective 3-year follow-up cohort study. J Psychosom Res 79: 228–232, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Farrokhi F, Abedi N, Beyene J, Kurdyak P, Jassal SV: Association between depression and mortality in patients receiving long-term dialysis: A systematic review and meta-analysis. Am J Kidney Dis 63: 623–635, 2014 [DOI] [PubMed] [Google Scholar]
- 34.van Dijk S, van den Beukel TO, Kaptein AA, Honig A, le Cessie S, Siegert CE, Boeschoten EW, Krediet RT, Dekker FW; NECOSAD Study Group : How baseline, new-onset, and persistent depressive symptoms are associated with cardiovascular and non-cardiovascular mortality in incident patients on chronic dialysis. J Psychosom Res 74: 511–517, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Mykletun A, Bjerkeset O, Overland S, Prince M, Dewey M, Stewart R: Levels of anxiety and depression as predictors of mortality: The HUNT study. Br J Psychiatry 195: 118–125, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Saglimbene V, Palmer S, Scardapane M, Craig JC, Ruospo M, Natale P, Gargano L, Leal M, Bednarek-Skublewska A, Dulawa J, Ecder T, Stroumza P, Marco Murgo A, Schön S, Wollheim C, Hegbrant J, Strippoli GF: Depression and all-cause and cardiovascular mortality in patients on haemodialysis: A multinational cohort study [published online ahead of print March 22, 2016]. Nephrol Dial Transplant [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez JS, Peyrot M, McCarl LA, Collins EM, Serpa L, Mimiaga MJ, Safren SA: Depression and diabetes treatment nonadherence: A meta-analysis. Diabetes Care 31: 2398–2403, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strine TW, Mokdad AH, Dube SR, Balluz LS, Gonzalez O, Berry JT, Manderscheid R, Kroenke K: The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. Gen Hosp Psychiatry 30: 127–137, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Pasco JA, Williams LJ, Jacka FN, Ng F, Henry MJ, Nicholson GC, Kotowicz MA, Berk M: Tobacco smoking as a risk factor for major depressive disorder: Population-based study. Br J Psychiatry 193: 322–326, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Østhus TB, Dammen T, Sandvik L, Bruun CM, Nordhus IH, Os I: Health-related quality of life and depression in dialysis patients: Associations with current smoking. Scand J Urol Nephrol 44: 46–55, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Hood KK, Lawrence JM, Anderson A, Bell R, Dabelea D, Daniels S, Rodriguez B, Dolan LM; SEARCH for Diabetes in Youth Study Group : Metabolic and inflammatory links to depression in youth with diabetes. Diabetes Care 35: 2443–2446, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holt RIG, de Groot M, Lucki I, Hunter CM, Sartorius N, Golden SH: NIDDK international conference report on diabetes and depression: Current understanding and future directions. Diabetes Care 37: 2067–2077, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayashino Y, Mashitani T, Tsujii S, Ishii H; Diabetes Distress and Care Registry at Tenri Study Group : Elevated levels of hs-CRP are associated with high prevalence of depression in Japanese patients with type 2 diabetes: The Diabetes Distress and Care Registry at Tenri (DDCRT 6). Diabetes Care 37: 2459–2465, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Czira ME, Lindner AV, Szeifert L, Molnar MZ, Fornadi K, Kelemen A, Laszlo G, Mucsi I, Keszei AP, Kennedy SH, Novak M: Association between the malnutrition-inflammation score and depressive symptoms in kidney transplanted patients. Gen Hosp Psychiatry 33: 157–165, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Khalil AA, Frazier SK, Lennie TA, Sawaya BP: Depressive symptoms and dietary adherence in patients with end-stage renal disease. J Ren Care 37: 30–39, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan L, Sarnak MJ, Tighiouart H, Drew DA, Kantor AL, Lou KV, Shaffi K, Scott TM, Weiner DE: Depression and all-cause mortality in hemodialysis patients. Am J Nephrol 40: 12–18, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cukor D, Ver Halen N, Asher DR, Coplan JD, Weedon J, Wyka KE, Saggi SJ, Kimmel PL: Psychosocial intervention improves depression, quality of life, and fluid adherence in hemodialysis. J Am Soc Nephrol 25: 196–206, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitrou GI, Grigoriou SS, Konstantopoulou E, Theofilou P, Giannaki CD, Stefanidis I, Karatzaferi C, Sakkas GK: Exercise training and depression in ESRD: A review. Semin Dial 26: 604–613, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Halen NV, Cukor D, Constantiner M, Kimmel PL: Depression and mortality in end-stage renal disease. Curr Psychiatry Rep 14: 36–44, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Davidson KW, Rieckmann N, Clemow L, Schwartz JE, Shimbo D, Medina V, Albanese G, Kronish I, Hegel M, Burg MM: Enhanced depression care for patients with acute coronary syndrome and persistent depressive symptoms: Coronary psychosocial evaluation studies randomized controlled trial. Arch Intern Med 170: 600–608, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.