Abstract
Background and objectives
The literature on strategies to increase the number of potential living kidney donors is extensive and has yet to be characterized. Scoping reviews are a novel methodology for systematically assessing a wide breadth of a given body of literature and may be done before conducting a more targeted systematic review.
Design, setting, participants, & measurements
We performed a scoping review and summarized the evidence for existing strategies to increase living kidney donation.
Results
Our review identified seven studies that tested interventions using rigorous methods (i.e., randomized, controlled trials) and outcome measures, all of which focused on using education targeted at potential recipients to increase living donation. Of these, two studies that targeted the potential recipients’ close social network reported statistically significant results. Other interventions were identified, but their effect was assessed through quasiexperimental or observational study designs.
Conclusions
We identified an important gap in the literature for evidence-based strategies to increase living kidney donation. From the limited data available, strategies directed at potential recipients and their social networks are the most promising. These results can inform transplant programs that are considering strategies to increase living kidney donation and highlight the need for conduct of high-quality study to increase living donation.
Keywords: end stage kidney disease, kidney donation, kidney transplantation, kidney, Living Donors, Nephrectomy, Outcome Assessment (Health Care), Randomized Controlled Trials as Topic, Social Support, Tissue and Organ Harvesting
Introduction
ESRD is a global health challenge and expected to reach epidemic proportions over the coming years (1–3). Kidney transplantation in eligible potential recipients is the preferred treatment for ESRD given improved patient outcomes and reduced health care costs compared with dialysis (4–6). However, transplantation is limited by the availability of donor kidneys (7,8). Many jurisdictions and programs have either implemented or are considering a variety of strategies to increase rates of kidney donation to meet the growing demand for kidney transplantation. Unfortunately, there is limited evidence and guidance on what effective strategies to increase kidney donation have the most promise.
Although living kidney donation (LKD) has superior outcomes for patient and graft survival (9) along with the potential to provide more kidneys for donation than deceased donation, it is underused in many kidney transplant programs. Furthermore, rates of LKD have become stagnant over the last 10 years in many countries, including the United States and Canada. Various strategies to increase the rate of LKD have been reported in the literature, including tax policies, cost reimbursement, educational interventions, and public campaigns. Despite the breadth of literature reporting on these various strategies to increase donation, there is uncertainty about the effectiveness and feasibility of these options. A summary of strategies and their effect is needed by programs to inform deployment of strategies that are most likely to be effective.
A scoping review is a form of qualitative research methodology that facilitates the description of a diverse and complex body of literature (Table 1) (10). Scoping reviews outline what is known in existing research, while mapping the evidence in areas that are not well described, and they can be particularly useful in instances where not all possible interventions are known. Furthermore, scoping reviews explore the source, range, and nature of studies and may serve as the foundation for further systematic review (11). Given the current context of inadequate supply of kidneys for transplantation, stagnation in growth of LKD, and programs seeking to deploy strategies to increase LKD, we undertook a scoping review. Our objectives were to (1) outline the strategies that have been evaluated to increase the rates number of LKD, (2) map and describe the evidence available and the quality of the evidence, and (3) identify and guide future research priorities.
Table 1.
Characteristics of scoping and systematic reviews
| Systematic Review | Scoping Review |
|---|---|
| A single research question with narrow focus | Encompasses broad research area or question(s) |
| Inclusion/exclusion criteria explicitly defined at outset | Inclusion/exclusion can be developed post hoc |
| Quality of identified studies important | Quality not an initial priority |
| Detailed data abstraction defined a priori | May or may not involve data extraction |
| Formal quantitative synthesis often performed | Synthesis descriptive and typically not quantitative |
| Results in a formal conclusion to the stated research question | Identifies gaps in a body of literature |
Modified from ref. 10, with permission.
Materials and Methods
Search Strategy
We performed a scoping review of the literature to identify studies evaluating strategies to increase LKD. The search strategy was developed with the aid of a librarian (D.L.) experienced in both systematic and scoping reviews (Supplemental Material). Searches were conducted in PubMed, MEDLINE, EMBASE, PsychINFO, and CINAHL (the search strategy is in Supplemental Material); the gray literature was not systematically searched. Works published in peer-reviewed academic journals, doctoral dissertations, research reports, and conference papers were considered. Reference lists of included papers were searched to identify papers missed in electronic searches. The search was kept purposely broad to capture all possible strategies. To be included, studies had to report original data and investigate a strategy for increasing LKD. The scope of publications was narrowed to those reporting one or more of the following outcomes: number of living donors or donation rate, number of living donor evaluations, number of contacts with transplant center from potential living donors, number of potential donors identified, number of potential donors asked, number of potential recipients evaluated, discussion of living donation, stated intent to engage in living donation, and consideration of living donation. We excluded (1) nonhuman studies, (2) non-English studies, and (3) editorials/letters, with no date limit. Because strategies to increase LKD and deceased kidney donation differ in their approach, we also excluded studies on deceased kidney donation; a scoping review of deceased donation strategies will be presented separately.
Study Selection
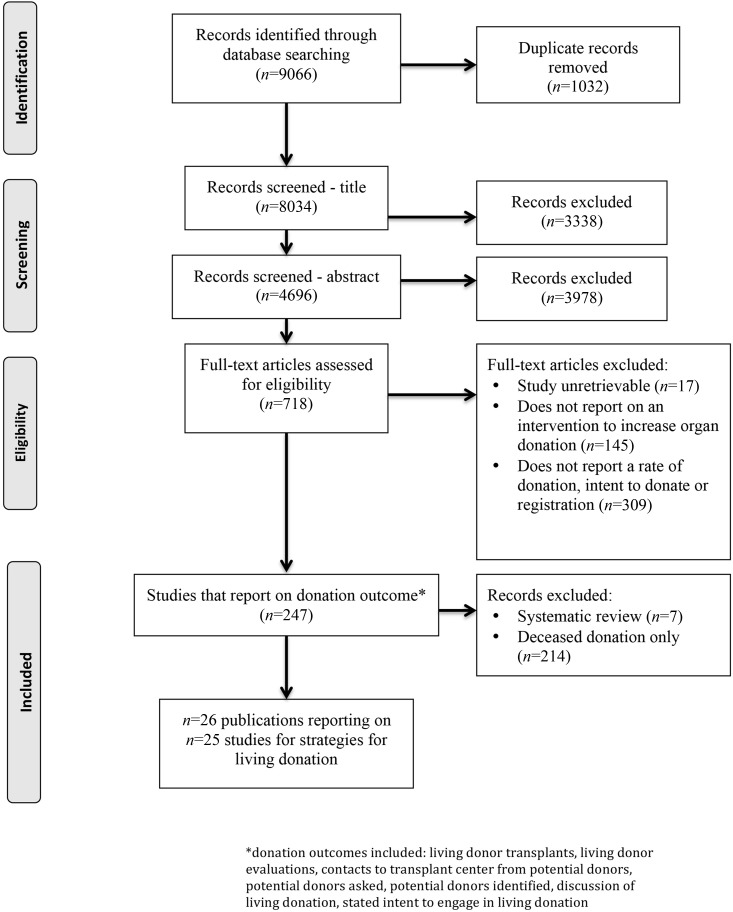
Two reviewers (L.B. and D.C.) screened the titles of all identified papers first by title and then by abstract followed by the screening of full-text papers (Figure 1). Discrepancies between the two reviewers were resolved by discussion with a third reviewer (S.K.).
Figure 1.
Flow of studies.
Data Extraction
The following information was extracted from all included papers: author, country of publication, year of publication, study design, population setting and type (potential recipients versus potential donors), intervention type, and outcome measure. All studies were assessed by hierarchy of directness and relevance of the study outcome(s). In addition to the above, the following results were extracted: number of participants, results, direction of effect, and significance of results. An assessment of the quality of the paper was also done for all randomized, controlled trials (RCTs), non-RCTs, and interrupted time series using the Cochrane risk of bias tools as a guide (12,13).
Results
The search was conducted up to March of 2016. A total of 8032 records were screened, of which 26 were found to report on strategies to increase living donation and included one or more of the prespecified outcomes (Supplemental Table 1 shows a description of the studies). Of these 26 publications, two reported on the same study, and thus, 25 studies (in 26 publications) were included in this review (Figure 1).
RCTs
Of the 26 publications identified, seven studies were RCTs that reported across nine outcomes (Table 2). All RCTs examined education-based interventions, with two of the studies targeting potential donors (14,15) and five of the studies targeting primarily potential recipients (16–20). Results from the RCTs varied across outcomes, despite examining similar education-based interventions.
Table 2.
Results and risk of bias by outcome for RCTs (all education intervention strategies)
| Ref. | No. of Participants | Outcomes | Direction of Effect | P Value | Risk of Bias | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Random Sequence Generation | Allocation Concealment | Blinding of Outcome Assessment | No Incomplete Outcome Data | No Selective Reporting | |||
| No. of living donor transplants | |||||||||||
| Rodrigue et al. (16) | 63 | 69 | 33 | 21 | + | 0.01 | ? | ? | ? | X | ? |
| Ismail et al. (19) | 39b | 41b | 17 | 4 | + | 0.003 | ✓ | ✓ | ✓ | ✓ | ✓ |
| No. of living donor evaluations | |||||||||||
| Rodrigue et al. (16) | 63 | 69 | 38 | 24 | + | 0.005 | ? | ? | ? | X | ? |
| Ismail et al. (19) | 39 | 41 | 25 | 7 | + | <0.001 | ✓ | ✓ | ✓ | ✓ | ✓ |
| No. of contacts with transplant center from potential living donors | |||||||||||
| Barnieh et al. (18) | 50 | 50 | 4 | 2 | + | >0.05 | ✓ | ✓ | ✓ | ✓ | ✓ |
| Rodrigue et al. (16) | 63 | 69 | 52 | 44 | + | 0.02 | ? | ? | ? | X | ? |
| Ismail et al. (19) | 39b | 41b | 29 | 13 | + | <0.001 | ✓ | ✓ | ✓ | ✓ | ✓ |
| No. of potential donors asked | |||||||||||
| Pradel et al. (17) | 107 | 107 | 26 | 35 | — | NR | ✓ | ? | ? | ✓ | ? |
| No. of potential donors identified | |||||||||||
| Boulware et al. (20)c | 43 | 44 | 12 | 10 | + | >0.05 | ✓ | ✓ | ? | ✓ | ✓ |
| Discussion of living donation | |||||||||||
| Pradel et al. (17) | 107 | 107 | 57 | 53 | + | NR | ✓ | ? | ? | ✓ | ? |
| Boulware et al. (20) | 43 | 44 | 20 | 11 | + | 0.05 | ✓ | ✓ | ? | ✓ | ✓ |
| Stated intent to engage in living donation | |||||||||||
| Piccoli et al. (14) | 808 | 659 | 595 | 485 | = | NR | ? | ✓ | ? | X | ? |
| Thornton et al. (15) | 443 | 509 | 96%a | 97%a | = | NR | ? | ✓ | ? | ? | ? |
+, positive direction of effect in favor of the intervention; ?, unclear risk of bias; X, high risk of bias; ✓, low risk of bias; —, negative direction of effect in favor of the intervention; NR, not reported; =, means that there is no difference in the direction of effect between the two groups.
Only proportion reported.
Population after censoring for death and deceased donor transplant.
Intervention without social worker.
Rodrigue et al. (16) and Ismail et al. (19) reported statistically significant increases in the number of living donors and the number of living donor evaluations (Table 2) (16,19). These two studies included an element of home-based education targeting the recipient and participants of their choice, such as family members or friends; furthermore, they were conducted on a directed individual level, providing individualized (one on one) rather than group education. In the work by Rodrigue et al. (16), clinic- plus home-based education resulted in 52% (33 of 63) and 30% (21 of 69) of patients on dialysis acquiring living donors in the intervention and control groups, respectively. Furthermore, 60% (38 of 63) and 35% (24 of 69) of intervention and control participants, respectively, had a living donor evaluated (16). The study by Ismail et al. (19) evaluated the delivery of a home-based educational intervention including two home visits, which resulted in 13 more living donors (intervention: 17 of 39, control: four of 41) and 18 more living donor evaluations (intervention: 25 of 39, control: seven of 41) (19).
The number of contacts with a transplant center from potential living donors varied among studies: two studies (Rodrigue et al. [21] and Ismail et al. [19]) reported a statistically significant increase, and one study (Barnieh et al. [18]) reported a positive increase, although it was not statistically significant.
Of the remaining outcomes (potential donors asked, potential donors identified, discussion of living donation, and stated intent to engage in living donation), none reported statistically significant findings, although there were positive trends toward the number of potential donors identified, the number of potential recipients evaluated, and discussion of living donation. In the work by Pradel et al. (17), an educational intervention aimed at potential recipients had a negative effect on number of potential donors asked by the potential recipient, although this result was not statistically significant.
Although the assessment of quality of studies is not an explicit objective in scoping reviews, we explored the variation in quality across outcomes in the included RCTs (Table 2). The risk of bias was highest for studies that reported the number of living donors (n=2) and the number of living donor evaluations (n=2; same studies as previous studies); it was lowest for the number of potential donors identified (n=1) and the number of recipients evaluated (n=1). It was difficult to assess the quality across the studies reporting on remaining outcomes given key missing information within the reporting of the studies.
Quasiexperimental Studies
Quasiexperimental studies were studies that were experimental in nature but lacking random assignment. Six articles (Table 3) were quasiexperimental studies, including controlled before and after or interrupted time series (22–27). Unlike RCTs, the strategies to increase donation varied and included education (n=1), campaign (n=1), removal of disincentives (donor reimbursement, leave policies, and tax benefits; n=3), and an intervention that examined the effect of a web-based survey to enable screening of potential donors by providing an immediate response regarding the potential donor’s candidacy. These interventions were aimed at the general population, potential donors or recipients, or the health system.
Table 3.
Results and risk of bias by outcome for quasiexperimental studies
| Study Intervention | No. of Participants | Outcomes | Direction of Effect | P Value | Selection of Participants | Comparability of Groups | Exposure | Assessment of Outcome | Follow-Up | Summary Risk of Bias | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | Control | Experimental | Control | |||||||||
| No. or rate of living donors | ||||||||||||
| Boulware et al. (24): remove disincentives | 27 States | 2.39a | 1.68a | + | >0.05 | ✓ | ✓ | ✓ | ✓ | ✓ | Unclear | |
| Venkataramani et al. (25): remove disincentives | 16 States | 2.64b | 2.47b | + | 0.65 | ✓ | ✓ | ✓ | ✓ | X | ||
| Chatterjee et al. (26): education | 42 Statesc | 5%d | NR | + | 0.07 | ✓ | X | ✓ | ✓ | ? | ||
| Chatterjee et al. (26): leave, public employer | 42 Statesc | 8%d | NR | + | 0.06 | ✓ | X | ✓ | ✓ | ? | ||
| Chatterjee et al. (26): leave, private employer | 42 Statesc | 8%d | NR | + | 0.91 | ✓ | X | ✓ | ✓ | ? | ||
| Chatterjee et al. (26): tax benefit | 42 Statesc | 4%d | NR | + | 0.17 | ✓ | X | ✓ | ✓ | ? | ||
| Moore et al. (27): web-based screening | 1200 | 76 | 54 | + | NR | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Schweitzer et al. (22): education | NR | NR | NR | NR | + | 0.02 | ✓ | ? | ✓ | ✓ | ✓ | |
| No. of living donor evaluations | ||||||||||||
| Moore et al. (27): web-based screening | 1200 | 249 | 186 | + | NR | ✓ | ✓ | ✓ | ✓ | ✓ | Low | |
| Schweitzer et al. (22): education | NR | NR | 39.4% | 33.4% | + | 0.03 | ✓ | ? | ✓ | ✓ | ✓ | |
| No. of contacts with transplant center from potential living donors | ||||||||||||
| Moore et al. (27): web-based screening | 1200 | 116e | 61e | + | <0.001 | ✓ | ✓ | ✓ | ✓ | ✓ | Low | |
| Stated intent to engage in living donation | ||||||||||||
| Alvaro et al. (23): campaign | 405 | 419 | NR | NR | = | NR | ? | ? | ? | ? | ✓ | Unclear |
✓, low risk of bias; X, high risk of bias; NR, not reported; ?, unclear; +, =, means that there is no difference in the direction of effect between the two groups.
Annual increase in donations per 100,000.
Number of living donations per 100,000.
Includes the District of Columbia.
Change in living donors per capita.
Number of contacts per month.
Outcomes reported for quasiexperimental studies included the number or rate of living donors (n=5), the number of living donor evaluations (n=2), the number of contacts with the transplant center from a potential donor (n=1), and a stated intent to engage in living donation (n=1) (Table 3). The only intervention that resulted in a significant increase in the number or rate of living donors was a structured educational program for potential recipients and their families (22).
Among the other interventions identified in quasiexperimental studies, three examined removal of disincentives (24–26); although all three found a positive trend toward increasing living donors, none of the studies reported statistically significant differences. One study reported on a novel web-based intervention for potential donors to self-screen themselves as candidates (27). They found that a web-based tool significantly increased the number of contacts with the transplant center; the study also found an increase in the number of living donors and living donor evaluations but did not report whether these findings were significant.
Quality across outcomes was mixed. It was difficult to determine the risk of bias in quasiexperimental studies for the outcome of number or rate of living donors due to missing information in one study that reported on several interventions. For the number of living donor evaluations and the number of contacts with the transplant center from living donors, the risk of bias was assessed to be low.
Observational Studies
Of the 26 publications (25 studies) identified in the scoping review, the remaining 13 studies were observational studies (Table 4). Strategies in the observational studies included living donor pool exchange programs (28–31) (n=4), education (32,33) (n=2), creation of an interdisciplinary team (34,35) (n=2), prohibiting the reimbursement for transplantations performed in countries contravening the Declaration of Istanbul (36,37) (n=2), use of a donor champion to facilitate the donation process (38) (n=1), presumed consent (39) (n=1), and establishment of a living donor ABO-incompatible program (40) (n=1).
Table 4.
Results by outcome for living donor observational studies
| Reference | Description of Results | Direction of Effect | P Value |
|---|---|---|---|
| No. or rate of living donors | |||
| Kwak et al. (28): exchange program | Over the study period of 6 yr, an increase of 61 living donors (n=411) with introduction of exchange program | + | NR |
| Park et al. (29): exchange program | Over the study period, an increase from 2.2% (four of 180) to 31% (38 of 86) of living donor transplants with introduction of exchange program | + | NR |
| Roodnat et al. (30): exchange program | Over the study period, an increase of 35 donors (n=581) with the introduction of exchange program | + | NR |
| Cole et al. (31): exchange program | Over the study period, an increase of 158 donors (n=2219) with the introduction of exchange program | + | NR |
| Fonouni et al. (34): multidisciplinary team | Over the study period, an increase of 48% in living donors (from 18 to 42) with multidisciplinary team | + | NR |
| González Monte et al. (32): promotion program | An increase in the rate of donation from 0.8% (16 of 1964) to 4.4% (45 of 1022) through a living donor promotion program | + | <0.01 |
| Cankaya et al. (33): education | More preemptive living donations in the education group (26 of 61) than the noneducation group (five of 27) | + | <0.001 |
| Bendorf et al. (39): presumed consent | A decrease in living donor kidney rates of 8.7 pmp in countries with presumed consent | − | 0.003 |
| Cardinal et al. (35): multidisciplinary team | An increase from 50 to 73 in number of living donors (incident rate difference, 0.029; 95% CI, 0.00 to 0.06) with the creation of a multidisciplinary team | + | NR |
| Garonzik-Wang et al. (38): living donor champion | More living donors in the living donor champion group (four of 15) than in the control group (zero of 15) | + | <0.001 |
| Lavee et al. (36): law to prevent transplant tourism | An increase in the number of living donors from 71 to 117 after enactment of law | + | 0.003 |
| Boas et al. (37): law to prevent transplant tourism | An increase in the number of living donors from 211 (prelaw) to 364 (postlaw) | + | <0.001 |
| No. of living donor evaluations | |||
| Garonzik-Wang et al. (38): living donor champion | More living donor evaluations in the living donor champion group (three of 15) than in the control group (zero of 15) | + | <0.001 |
| Romagnoli et al. (40): ABO-incompatible program | An increase of 15% (12 of 78) in living donor evaluations with introduction of ABO-incompatible program | + | NR |
| No. of contacts with transplant center from potential living donors | |||
| Cardinal et al. (35): multidisciplinary team | An increase in the number of contacts from 191 to 304 (incident rate difference, 0.143; 95% CI, 0.09 to 0.20) with the creation of a multidisciplinary team | + | NR |
| Discussion of living donation | |||
| Garonzik-Wang et al. (38): living donor champion | Increased comfort of approaching a family member in the living donor champion group | + | 0.07 |
+, indicates a positive direction of effect in favour of the intervention; NR, not reported; pmp, per million population; 95% CI, 95% confidence interval.
Of the 13 observational studies, 12 reported a number or rate of living donors. Of these, five reported a significantly positive increase in the number or rate of living donors, six reported a trend toward a positive increase (significance testing not reported), and one reported a negative statistically significant outcome. This latter study examined the effect of presumed consent (a policy for deceased donation) on living donation rates across 53 countries (39). Studies that reported significant increases in the number or rate of living donors included the interventions examining living donor promotion programs, education for preemptive transplantation, use of a live donor champion—a friend, family member, or community member who advocates for the transplant candidate in the living donation process, and laws that prohibited reimbursement for recipients getting transplanted in countries that did not adhere to the Declaration of Istanbul.
Other outcomes that reported significant results included the effect on the number of living donor evaluations using a live donor champion. These evaluations were ongoing at time of data analysis and could have potentially turned into living donor transplants, which would have increased the total number of living donor transplants to seven (of 15 participants) in those with a living donor champion compared with zero in the control group.
Assessment of the quality of studies in observational studies was not done, because these study designs have inherently high risk of bias and because quality is difficult to assess objectively.
Discussion
This scoping review highlights gaps in knowledge on the effect of various strategies to increase LKD. We found that, of the various strategies that have been considered to increased LKD, very few strategies have been evaluated using a high-quality study design (such as RCT; even quasiexperimental studies were limited); many also used surrogate or intermediate outcomes to assess the effect on increasing donation. The only strategy formally tested (i.e., using an RCT design) was education, and within this, outcomes varied, with only two of the seven studies reporting statistically significant increases in the number of living donors and living donor evaluations. It should be noted that both of these studies targeted not only the recipient but also, the recipient’s close social network, such as family members and friends, and they were done on an individual basis, indicating a more direct approach to potential donors.
Although living kidney donor transplantation is cost saving compared with dialysis therapy, deploying finite resources to interventions that have minimal or no effect on the rate of living donation is unwise. As programs implement strategies to increase donation, given the lack of evidence, these strategies should be implemented within a robust evaluation framework to determine effectiveness. Many of the interventions in this review seem promising; however, the sample sizes were small, and further evidence is needed.
This scoping review can guide decision making on policies and strategies that should be considered by outlining the scope and general effectiveness of candidate strategies. However, the most promising strategy of education by a health care professional that targets the recipient and their social network is relatively resource intensive compared with more commonly used passive education or group learning. Although likely cost effective, given the significant resources required, conducting a formal cost-effectiveness analysis would be prudent.
Quasiexperimental studies included more varied interventions, including removing disincentives, which has been discussed widely elsewhere in the literature (41–43). Removing disincentives was not found to significantly increase the number of living donors, although these studies largely examined primary tax benefits at a state-wide level and not at an individual level.
Observational studies encompassed the most varied of strategies, but the interpretation of these study designs is challenged by the low quality inherent in this study design and minimal reporting of factors that could potentially affect study quality. Some novel interventions show promise and should be further tested: they include the use of a multidisciplinary team and the use of a live donor champion to help the patient navigate through the process of living donation. This latter intervention is another example of a directed approach at the recipient toward increasing living donors.
It is worth mentioning that some novel interventions were identified in this review. One such intervention was the use of a web-based self-screen tool that significantly increased the number of contacts with the transplant center. This study, however, did not find that these contacts led to an increase in the number of living donor transplantations.
Our review has identified a gap in the literature of evidence-based strategies to increase living donation. Despite the necessity to increase the number of living donors through any given strategy, the number of studies identified is relatively small. Furthermore, only education was assessed using the most unbiased study design of an RCT. Although an RCT may not be feasible to study all interventions, it could be applied to study donor champions or multidisciplinary teams—two interventions identified as increasing the number of donors. The lack of clear evidence of effectiveness of most of these strategies should be an impetus to rigorously study new initiatives to increase living donation, which may allow higher-quality study designs (cluster RCT, stepped wedge, etc.) than post hoc observational studies.
Our study has limitations. It is possible that our review missed some relevant studies due to the broad scope of the search strategy and the expectation to retrieve and screen all relevant studies. Furthermore, we limited our included studies to those that reported a rate or percentage increase in any of our included outcomes (inclusion of a denominator). Many studies were excluded that reported absolute numbers under a given strategy with no reference to the relative increase of the given strategy. Excluding these studies allowed us to focus on studies in which the true effect of a strategy could be assessed. We also excluded non-English studies. Strategies being used in non-English–speaking countries may not have been reported in the English literature. A scoping review is not intended to be as exhaustive or comprehensive as a systematic review: it is a challenge in a scoping review to strike a balance between the breadth and depth of analysis. Although other strategies to potentially increase living donation were reported in the literature and identified through our review, we limited included studies to those with a measurable increase in one of the specific a priori outcomes deemed to be a reasonable indicator of effectiveness; arguably, excluded studies with less relevant outcome measures would not inform decision making. A limitation of scoping reviews is that there is no quantitative summary of the pooling of the results or formal summary of the quality of included studies. Given the heterogeneous results and the limited findings, pooling the results as a systematic review would not have been possible.
In conclusion, recognizing that the limitations of the evidence base that we identified in this scoping review preclude firm conclusions on optimal strategies to increase LKD, we believe that further research is urgently required. Strategies to increase living donation, whether implemented at a local, regional, or national level, should include a rigorous evaluative component embedded into the deployment with attempts to minimize bias and account for temporal trends; at a minimum, quasiexperimental study designs reporting on living donor outcomes should be considered. Our review excluded a significant number of interventional studies that did not report on living donor rates (n=309). Because many of the commonly used outcome measures are quality metrics that most programs already capture, determining the effectiveness of deployed strategies should be feasible, even in busy transplant programs.
Specific interventions in living organ donation that may be promising and warrant further study are those that facilitate donor identification and engagement. Targeted strategies that overcome barriers faced by recipients in approaching or finding potential donors should be tested and may include elements of interventions identified in this scoping review, including educational initiatives that specifically target recipient family and friends, donor champions, patient navigators, and novel approaches to donor identification, such as social media strategies. It is possible that patients with factors that are difficult to modify that may negatively affect donor availability, identification, and engagement, such as cultural differences, lack of secure employment, poor supportive social networks, low socioeconomic status, and low health literacy, may require more intensive strategies to have an effect. The effect of these barriers on the conversion from potential to actual donors should also be examined.
LKD is the best option for eligible transplant candidates, and transplant programs and jurisdictions are seeking strategies to increase the currently stagnant living donation rate. Our scoping review indicates a paucity of high-quality studies showing effectiveness of various strategies to address this shortage. Recipient-based education that reaches family and friends has the best evidence of being effective. Implementation of unproven strategies should be performed with an evaluative component to assess their effect. There is a critical need for high-quality studies to advance the evidence base and provide guidance to policymakers to achieve this goal; we outlined a few possible suggestions that would guide research and program evaluation. Our scoping review may be particularly useful to guide regional, provincial, or national organizations and decision makers in determining which, of the many strategies that may be used to increase living donation, should be considered.
Disclosures
None.
Supplementary Material
Acknowledgments
B.M. and S.K. are supported by a joint initiative between Alberta Health and the University of Alberta. B.M. is supported by the Svare Chair in Health Economics, an Alberta Innovates Health Scholar award, and a Canadian Institutes for Health Research Foundation award. N.N.L. was supported by a Kidney Research Scientist Core Education and National Training Program New Investigator award. S.K. is supported by the Kidney Health Research Chair and the Division of Nephrology at the University of Alberta. This work is part of the Canadian National Transplant Research Program (CNTRP) and was supported by the CIHR and partners (Grant Number TFU 127880)”; S.K. is a member of the CNTRP.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Moving from Intuition to Data: Building the Evidence to Support and Increase Living Donor Kidney Transplantation,” on pages 1383–1385.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01470217/-/DCSupplemental.
References
- 1.Meguid El Nahas A, Bello AK: Chronic kidney disease: The global challenge. Lancet 365: 331–340, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Atkins RC: The epidemiology of chronic kidney disease. Kidney Int Suppl 94: S14–S18, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Perico N, Remuzzi G: Chronic kidney disease: A research and public health priority. Nephrol Dial Transplant 27[Suppl 3]: iii19–iii26, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K: Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 270: 1339–1343, 1993 [PubMed] [Google Scholar]
- 5.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Laupacis A, Keown P, Pus N, Krueger H, Ferguson B, Wong C, Muirhead N: A study of the quality of life and cost-utility of renal transplantation. Kidney Int 50: 235–242, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Meier-Kriesche HU, Ojo AO, Port FK, Arndorfer JA, Cibrik DM, Kaplan B: Survival improvement among patients with end-stage renal disease: Trends over time for transplant recipients and wait-listed patients. J Am Soc Nephrol 12: 1293–1296, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Schold J, Srinivas TR, Sehgal AR, Meier-Kriesche HU: Half of kidney transplant candidates who are older than 60 years now placed on the waiting list will die before receiving a deceased-donor transplant. Clin J Am Soc Nephrol 4: 1239–1245, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medin C, Elinder CG, Hylander B, Blom B, Wilczek H: Survival of patients who have been on a waiting list for renal transplantation. Nephrol Dial Transplant 15: 701–704, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Brien SE, Lorenzetti DL, Lewis S, Kennedy J, Ghali WA: Overview of a formal scoping review on health system report cards. Implement Sci 5: 2, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arksey H, O’Malley L: Scoping studies: Towards a methodological framework. Soc Res Methodol 8: 19–32, 2005 [Google Scholar]
- 12.Bilandzic A, Fitzpatrick T, Rosella L, Henry D: Risk of bias in systematic reviews of non-randomized studies of adverse cardiovascular effects of Thiazolidinediones and Cyclooxygenase-2 inhibitors: Application of a new cochrane risk of bias tool. PLoS Med 13: e1001987, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group : The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343: d5928, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piccoli GB, Soragna G, Putaggio S, Mezza E, Burdese M, Vespertino E, Bonetto A, Jeantet A, Segoloni GP, Piccoli G: Efficacy of an educational programme for secondary school students on opinions on renal transplantation and organ donation: A randomized controlled trial. Nephrol Dial Transplant 21: 499–509, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Thornton JD, Alejandro-Rodriguez M, León JB, Albert JM, Baldeon EL, De Jesus LM, Gallardo A, Hossain S, Perez EA, Martin JY, Lasalvia S, Wong KA, Allen MD, Robinson M, Heald C, Bowen G, Sehgal AR: Effect of an iPod video intervention on consent to donate organs: A randomized trial. Ann Intern Med 156: 483–490, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigue JR, Cornell DL, Kaplan B, Howard RJ: A randomized trial of a home-based educational approach to increase live donor kidney transplantation: Effects in blacks and whites. Am J Kidney Dis 51: 663–670, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Pradel FG, Suwannaprom P, Mullins CD, Sadler J, Bartlett ST: Short-term impact of an educational program promoting live donor kidney transplantation in dialysis centers. Prog Transplant 18: 263–272, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Barnieh L, McLaughlin K, Manns BJ, Klarenbach S, Yilmaz S, Taub K, Hemmelgarn BR; Alberta Kidney Disease Network : Evaluation of an education intervention to increase the pursuit of living kidney donation: A randomized controlled trial. Prog Transplant 21: 36–42, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Ismail SY, Luchtenburg AE, Timman R, Zuidema WC, Boonstra C, Weimar W, Busschbach JJ, Massey EK: Home-based family intervention increases knowledge, communication and living donation rates: A randomized controlled trial. Am J Transplant 14: 1862–1869, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Boulware LE, Hill-Briggs F, Kraus ES, Melancon JK, Falcone B, Ephraim PL, Jaar BG, Gimenez L, Choi M, Senga M, Kolotos M, Lewis-Boyer L, Cook C, Light L, DePasquale N, Noletto T, Powe NR: Effectiveness of educational and social worker interventions to activate patients’ discussion and pursuit of preemptive living donor kidney transplantation: A randomized controlled trial. Am J Kidney Dis 61: 476–486, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodrigue JR, Cornell DL, Lin JK, Kaplan B, Howard RJ: Increasing live donor kidney transplantation: A randomized controlled trial of a home-based educational intervention. Am J Transplant 7: 394–401, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Schweitzer EJ, Yoon S, Hart J, Anderson L, Barnes R, Evans D, Hartman K, Jaekels J, Johnson LB, Kuo PC, Hoehn-Saric E, Klassen DK, Weir MR, Bartlett ST: Increased living donor volunteer rates with a formal recipient family education program. Am J Kidney Dis 29: 739–745, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Alvaro EM, Siegel JT, Crano WD, Dominick A: A mass mediated intervention on Hispanic live kidney donation. J Health Commun 15: 374–387, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Boulware LE, Troll MU, Plantinga LC, Powe NR: The association of state and national legislation with living kidney donation rates in the United States: A national study. Am J Transplant 8: 1451–1470, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venkataramani AS, Martin EG, Vijayan A, Wellen JR: The impact of tax policies on living organ donations in the United States. Am J Transplant 12: 2133–2140, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Chatterjee P, Venkataramani AS, Vijayan A, Wellen JR, Martin EG: The effect of state policies on organ donation and transplantation in the United States. JAMA Intern Med 175: 1323–1329, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Moore DR, Feurer ID, Zavala EY, Shaffer D, Karp S, Hoy H, Moore DE: A web-based application for initial screening of living kidney donors: Development, implementation and evaluation. Am J Transplant 13: 450–457, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Kwak JY, Kwon OJ, Lee KS, Kang CM, Park HY, Kim JH: Exchange-donor program in renal transplantation: A single-center experience. Transplant Proc 31: 344–345, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Park K, Moon JI, Kim SI, Kim YS: Exchange-donor program in kidney transplantation. Transplant Proc 31: 356–357, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Roodnat JI, Kal-van Gestel JA, Zuidema W, van Noord MA, van de Wetering J, IJzermans JN, Weimar W: Successful expansion of the living donor pool by alternative living donation programs. Am J Transplant 9: 2150–2156, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Cole EH, Nickerson P, Campbell P, Yetzer K, Lahaie N, Zaltzman J, Gill JS: The Canadian kidney paired donation program: A national program to increase living donor transplantation. Transplantation 99: 985–990, 2015 [DOI] [PubMed] [Google Scholar]
- 32.González Monte E, Delgado I, Polanco N, Hernández E, Dipalma T, Hernández A, Castillo M, Morales E, Praga M, Morales JM, Andrés A: Results of a living donor kidney promotion program. Transplant Proc 42: 2837–2838, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Cankaya E, Cetinkaya R, Keles M, Gulcan E, Uyanik A, Kisaoglu A, Ozogul B, Ozturk G, Aydinli B: Does a predialysis education program increase the number of pre-emptive renal transplantations? Transplant Proc 45: 887–889, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Fonouni H, Golriz M, Mehrabi A, Oweira H, Schmied BM, Müller SA, Jarahian P, Tahmasbi Rad M, Esmaeilzadeh M, Tönshoff B, Weitz J, Büchler MW, Zeier M, Schmidt J: The role of an interdisciplinary transplant team on living donation kidney transplantation program. Transplant Proc 42: 137–140, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Cardinal H, Durand C, Larrivee S, Verhave J, Paquet MR, Fortin MC: Strategies to increase living kidney donation: A retrospective cohort study. Can J Kidney Health Dis 2: 15, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lavee J, Ashkenazi T, Stoler A, Cohen J, Beyar R: Preliminary marked increase in the national organ donation rate in Israel following implementation of a new organ transplantation law. Am J Transplant 13: 780–785, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Boas H, Mor E, Michowitz R, Rozen-Zvi B, Rahamimov R: The impact of the israeli transplantation law on the socio-demographic profile of living kidney donors. Am J Transplant 15: 1076–1080, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Garonzik-Wang JM, Berger JC, Ros RL, Kucirka LM, Deshpande NA, Boyarsky BJ, Montgomery RA, Hall EC, James NT, Segev DL: Live donor champion: Finding live kidney donors by separating the advocate from the patient. Transplantation 93: 1147–1150, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bendorf A, Pussell BA, Kelly PJ, Kerridge IH: Socioeconomic, demographic and policy comparisons of living and deceased kidney transplantation rates across 53 countries. Nephrology (Carlton) 18: 633–640, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Romagnoli J, Salerno MP, Mamode N, Calia R, Spagnoletti G, Bianchi V, Maresca M, Piccirillo N, Putzulu R, Piselli P, Cola E, Zini G, Citterio F: Expanding the living donor pool “Second Act”: Laparoscopic donor nephrectomy and ABO-incompatible kidney transplantation improve donor recruitment. Transplant Proc 47: 2126–2129, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Delmonico FL, Martin D, Domínguez-Gil B, Muller E, Jha V, Levin A, Danovitch GM, Capron AM: Living and deceased organ donation should be financially neutral acts. Am J Transplant 15: 1187–1191, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Salomon DR, Langnas AN, Reed AI, Bloom RD, Magee JC, Gaston RS; AST/ASTS Incentives Workshop Group (IWG) : AST/ASTS workshop on increasing organ donation in the United States: Creating an “arc of change” from removing disincentives to testing incentives. Am J Transplant 15: 1173–1179, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Klarenbach S, Garg AX, Vlaicu S: Living organ donors face financial barriers: A national reimbursement policy is needed. CMAJ 174: 797–798, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.