Abstract
Background and objectives
Dose-dependent clearing of podocyte globotriaosylceramide has previously been shown in patients with classic Fabry disease treated with enzyme replacement. Our study evaluates the dose-dependent effects of agalsidase therapy in serial kidney biopsies of patients treated for up to 14 years.
Design, setting, participants, & measurements
Twenty patients with classic Fabry disease (12 men) started enzyme replacement therapy at a median age of 21 (range =7–62) years old. Agalsidase-α or -β was prescribed for a median of 9.4 (range =5–14) years. The lower fixed dose group received agalsidase 0.2 mg/kg every other week throughout the follow-up period. The higher dose group received a range of agalsidase doses (0.2–1.0 mg/kg every other week). Dose changes were made due to disease progression, suboptimal effect, or agalsidase-β shortage. Serial kidney biopsies were performed along with clinical assessment and biomarkers and scored according to recommendations from the International Study Group of Fabry Nephropathy.
Results
No statistical differences were found in baseline or final GFR or albuminuria. Kidney biopsies showed significant reduction of podocyte globotriaosylceramide in both the lower fixed dose group (−1.39 [SD=1.04]; P=0.004) and the higher dose group (−3.16 [SD=2.39]; P=0.002). Podocyte globotriaosylceramide (Gb3) reduction correlated with cumulative agalsidase dose (r=0.69; P=0.001). Arterial/arteriolar intima Gb3 cleared significantly in the higher dose group, all seven patients with baseline intimal Gb3 cleared the intima, one patient gained intimal Gb3 inclusions (P=0.03), and medial Gb3 did not change statistically in either group. Residual plasma globotriaosylsphingosine levels remained higher in the lower fixed dose group (20.1 nmol/L [SD=11.9]) compared with the higher dose group (10.4 nmol/L [SD=8.4]) and correlated with cumulative agalsidase dose in men (r=0.71; P=0.01).
Conclusions
Reduction of podocyte globotriaosylceramide was found in patients with classic Fabry disease treated with long-term agalsidase on different dosing regimens, correlating with cumulative dose. Limited clearing of arterial/arteriolar globotriaosylceramide raises concerns regarding long-term vascular effects of current therapy. Residual plasma globotriaosylsphingosine correlated with cumulative dose in men.
Keywords: genetic renal disease, chronic kidney disease, podocyte, Adolescent, Adult, albuminuria, Biomarkers, Biopsy, Child, Disease Progression, Enzyme Replacement Therapy, Fabry Disease, Follow-Up Studies, glomerular filtration rate, Humans, Isoenzymes, Male, Middle Aged, Podocytes, Trihexosylceramides, Young Adult, alpha-Galactosidas
Introduction
Enzyme replacement therapy has been available since 2001 as a treatment for the alfa-galactosidase deficiency underlying the X-linked Fabry disease. The enzyme deficiency induces lysosomal substrate accumulation of predominantly globotriaosylceramide (Gb3), causing dysfunction in many cell types (1). Several hundred mutations in the GLA gene have been described, some of which are variants of unknown significance. Severe mutations in the GLA gene manifest as classic Fabry disease in men and some women, with acroparesthesia, angiokeratomas, cornea verticillata, progressive multiorgan damage, and premature death (2,3). Agalsidase-α 0.2 mg/kg and agalsidase-β 1.0 mg/kg every other week are licensed in Europe as equipotent treatments for Fabry disease (4,5). Subsequent studies have suggested that the two products have similar biochemical properties per milligram of protein (6,7). However, comparative clinical studies have been underpowered or biased by differences in phenotypes (8–11), and additional comparative studies are needed (12).
Plasma Gb3 and particularly, globotriaosylsphingosine (lysoGb3) are biomarkers that have proved useful in risk stratification and distinguishing classic from nonclassic Fabry disease (13). The multifactorial pathophysiology is, however, poorly understood but likely includes vascular damage (14), podocyte dysfunction, foot process effacement (15,16), glomerular sclerosis, fibrosis, and loss of renal function. Enzyme dose-dependent clearing of podocyte Gb3 deposits in young patients has been shown in a cohort of patients with Fabry disease treated for 5 years (8), whereas the mesangium and glomerular endothelium were cleared irrespective of dose, in line with previous findings (4,5,17). Changes in clinical parameters (18) and/or histology have also been shown to correlate with agalsidase dose changes in a clinical trial and a case series (17,19). This study has expanded previous findings (8), investigating clinical and histologic findings in a cohort of 20 patients with classic Fabry disease treated with agalsidase for up to 14 years.
Materials and Methods
This observational, single-center cohort study was approved by the Regional Ethics Committee. Baseline kidney biopsies were accrued from September of 2003 to November of 2010, and one biopsy was performed in 1990. From November of 2001 to November of 2016, 40 agalsidase-treated patients were followed at Haukeland University Hospital: 37 had at least one kidney biopsy, and of those, 22 patients were treated for ≥4 years. Two patients were included in a clinical trial, rendering their biopsies unavailable for this study. All remaining 20 patients were included. Confirmation of classic disease was performed on the basis of previously published criteria for phenotypic classification (13). Four missense mutations (c.800T>G, c.56T>C, c.334C>T, and c.108G>C) and three truncating mutations (c.1212_1214delAAG, c679C>T, and c.963delG) were found (Table 1). Patient 18 received a kidney transplant, and Fabry disease was diagnosed in the patient and his living kidney donor (a woman) shortly after the transplantation. Baseline podocyte Gb3 scores were comparable in recipient and donor.
Table 1.
Characteristics of 20 patients with classic Fabry disease before start of enzyme replacement therapy with agalsidase
| No. | Age at Agalsidase Start, yr | Sex | A-Gal Variant | Treatment Group | Alfa Gal Activity | pGb3, μmol/L | Albumin-to-Creatinine Ratio, mg/mmol Creatinine | Protein-to-Creatinine Ratio, mg/mmol Creatinine | eGFR, ml/min per 1.73 m2 | Measured GFR, ml/min per 1.73 m2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 | M | Missense | Higher dose | 2.40 | 7.5 | 4.8 | 28.3 | 113 | 106 |
| 2 | 11 | M | Truncating | Lower fixed dose | 2.70 | 9.3 | 3.8 | 13.0 | 109 | 120 |
| 3 | 11 | W | Missense | Higher dose | 16.80 | 2.6 | 6.0 | <0.15 g/L | 126 | 105 |
| 4 | 12 | M | Truncating | Lower fixed dose | 3.80 | 8.1 | 0.6 | 9.0 | 98 | 103 |
| 5 | 12 | W | Missense | Higher dose | 16.5 | 4.3 | 1.5 | 19.2 | 154 | 103 |
| 6a | 13 | M | Truncating | Higher dose | 0.65 | 13.3 | 6.4 | 11.8 | 113 | 107 |
| 7a | 15 | M | Truncating | Higher dose | 2.00 | 9.8 | 3.0 | 10.5 | 111 | 112 |
| 8 | 16 | M | Truncating | Lower fixed dose | 8.60 | 13.4 | 1.3 | 10.3 | 95 | 112 |
| 9 | 17 | M | Missense | Lower fixed dose | 2.20 | 9.0 | 2.6 | 8.5 | 71 | 99 |
| 10 | 18 | M | Missense | Higher dose | 3.30 | 4.8 | 11.8 | 28.4 | 126 | 96 |
| 11 | 23 | M | Truncating | Lower fixed dose | 2.50 | 8.5 | 13.6 | 27.5 | 124 | 113 |
| 12 | 30 | M | Truncating | Lower fixed dose | 4.90 | 10.2 | 7.2 | 25.1 | 118 | 86 |
| 13a | 30 | M | Truncating | Higher dose | 2.30 | 13.1 | 3.6 | 15.4 | 123 | 111 |
| 14 | 42 | W | Truncating | Higher dose | 20.2b | 3 | 7.7 | <0.15 g/L | 97 | 118 |
| 15 | 44 | W | Truncating | Higher dose | 2.2c | 3.7 | 4.4 | <0.15 g/L | 79 | 92 |
| 16 | 49 | W | Truncating | Lower fixed dose | 17.4 | 2.3 | 5.9 | 31.8 | 111 | 120 |
| 17 | 51 | W | Truncating | Lower fixed dose | 13.1d | 3.5 | 1.2 | 14.2 | 97 | 72 |
| 18 | 53 | M | Missense | Higher dose | 2.1 | 7 | 66.4 | 111.4 | 75 | 47 |
| 19 | 57 | W | Missense | Lower fixed dose | 12.9 | 1.8 | 22 | 45 | 73 | 70 |
| 20a | 62 | W | Truncating | Lower fixed dose | 26.8 | 4.5 | 12.3 | 32.2 | 81 | 60 |
GFR was measured by iohexol clearance. Alfa Gal activity reference: 17.7–26.4 μkat/kg per protein (without inhibitor). pGb3 reference: 1.6–3.3 μmol/L. Albumin-to-creatinine ratio reference: <2.5 mg/mmol creatinine. Protein-to-creatinine ratio reference: <20 mg/mmol creatinine. GLA mutations: four missense mutations (c.800T>G, c.56T>C, c.334C>T, and c.108G>C) and three truncating mutations (c.1212_1214delAAG, c.679C>T, and c.963delG). A-Gal variant, alfa galactosidase genotype; Alfa Gal activity, alfa galactosidase activity; pGb3, plasma globotriaosylceramide; M, man; W, woman.
Start of enzyme replacement therapy 2–3 years before baseline kidney biopsy.
After kidney transplantation.
Reference: 19.5–39.0 μkat/kg per protein (without inhibitor).
Reference: 23–38 μkat/kg per protein (with inhibitor).
Patients were included consecutively and arbitrarily prescribed either agalsidase-α or -β; family members were usually prescribed the same product. All patients were intended for fixed dose agalsidase. Two treatment groups were defined on the basis of received enzyme dose (Table 1). The low fixed dose group (n=10) received agalsidase 0.2 mg/kg every other week throughout the study period. The higher dose group (n=10) received a range of agalsidase doses. Two patients received agalsidase-β 1.0 mg/kg every other week during the entire study period. Eight patients received variable doses of agalsidase-α or -β every other week during follow-up; three patients were switched from agalsidase-β to -α due to the global shortage of agalsidase-β during 2009–2012, and five patients were switched from agalsidase-α to -β due to signs of progressive nephropathy, cardiomyopathy, or suboptimal treatment effect. Baseline kidney biopsies were performed before initiating agalsidase therapy in 16 of 20 patients. Patients 6, 7, and 20 underwent baseline kidney biopsies after 2 years of agalsidase-α, and patient 13 had a baseline kidney biopsy after 3 years of agalsidase-α. All patients underwent routine follow-up kidney biopsies to evaluate kidney disease progression and treatment effects. The biopsies were scored according to the scoring system of the International Study Group of Fabry Nephropathy by two experienced nephropathologists (20). Podocyte Gb3 inclusions were scored zero to four in toluidine blue–stained semithin sections, and podocyte vacuolizations were scored zero to three in sections stained with periodic acid–Schiff. These scores were combined to a composite score, accounting for all glomeruli that were scored. The intima and media of arteries and/or arterioles were examined for Gb3 inclusions in toluidine blue–stained semithin sections using a binary system—Gb3 inclusions present or not present.
Urine albumin-to-creatinine ratio and protein-to-creatinine ratio were measured in three consecutive early morning voids or 24-hour urine samples. Median albumin-to-creatinine ratio <2.5 mg/mmol and protein-to-creatinine ratio <20 mg/mmol were considered normal. GFR were measured by iohexol plasma clearance (21) normalized to 1.73 m2 body surface (22). eGFR was calculated on the basis of serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration formula in adults (23) and Schwartz bedside formula in children (24).
Baseline and/or 5-year biopsy data for patients 1–13 have previously been published (8,19,25,26).
LysoGb3 and antibody analyses were performed at the Academic Medical Center. Antibody analyses were performed as previously published by Linthorst et al. (27). Different dilutions of patient sera were incubated with a standard amount of recombinant agalsidase to determine the serum dilution resulting in 50% enzyme activity reduction. A titer of greater than or equal to six was considered antibody positive. Plasma lysoGb3 levels were measured by UPLC tandem mass spectrometry using N-glycine lysoGb3 as the internal standard. Blood samples were stored at Haukeland University Hospital at −80°C until shipment. Plasma Gb3 was measured at Sahlgrenska Hospital.
General disease load was evaluated using the Fabry disease severity scoring system (28). Clinical events were defined as new-onset albuminuria or increase from low-range albuminuria (2.5–20 mg/mmol creatinine) to levels of >20 mg/mmol creatinine; measured GFR loss of >5 ml/min per 1.73 m2 per year or RRT; new-onset or progressive left ventricular hypertrophy; initiation of antiarrhythmic drugs, pacemaker, or ICD implantation; transitory ischemic attack/stroke; new cerebral white matter lesions; sudden deafness; or death. Statistics were performed using SPSS v.23. The Mann–Whitney test was used for non-normally distributed data, the t test was used for normally distributed data, and P<0.05 was considered statistically significant. Linear regression was used for correlation between podocyte Gb3 score and cumulative agalsidase dose. Adjusted analyses were performed using mixed model regression.
Results
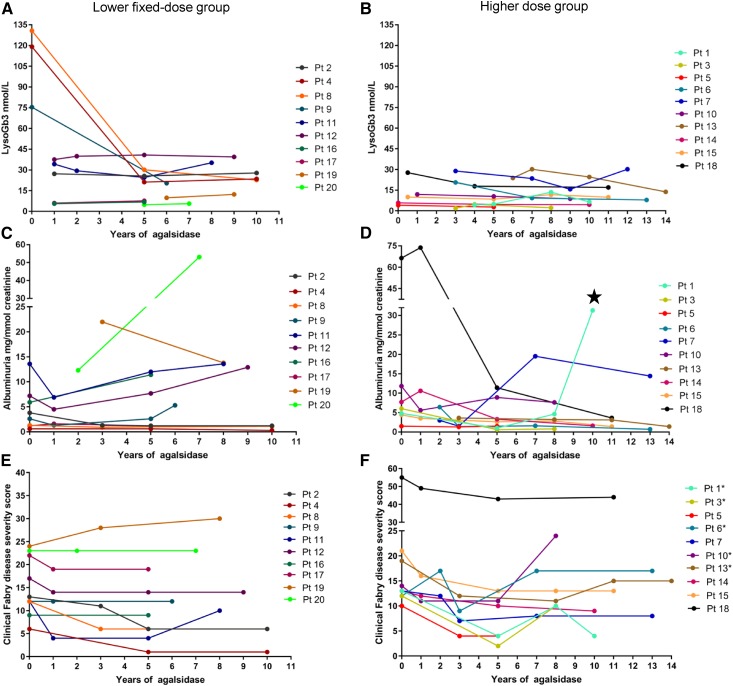
Median age at start of enzyme replacement therapy was 21 (range =7–62) years old, and median duration of agalsidase therapy was 9.4 (range =4.8–13.8) years (Table 2). Five patients had CKD stages 2–3 at baseline, four of whom were 50 years old or older (Table 1). Median measured GFR at baseline was 104 (range =47–120) ml/min per 1.73 m2. Baseline albuminuria >2.5 mg/mmol creatinine was found in sixteen patients, baseline proteinuria above 20 mg/mmol creatinine was found in eight patients (Figure 1, Table 1). Three women (patients 14, 15, and 17) received RAAS blockade for the entire follow-up period. Eight patients (patients 2–5 and 8–11) did not receive RAAS blockade. The remaining nine patients received RAAS blockade for some but not all of the follow-up period. At baseline, there were no statistical differences between the two groups with respect to GFR, albuminuria, and plasma Gb3 (Table 2). The higher dose group was a median of 10 years younger than the lower fixed dose group (P=0.28) at the start of agalsidase therapy.
Table 2.
Mean baseline data for the lower fixed dose and higher dose groups
| Variable | Total Cohort | Lower Fixed Dose Group | Higher Dose Group | P Value |
|---|---|---|---|---|
| Patients, N | 20 | 10 | 10 | |
| Men, N (%) | 12 (60) | 6 (60) | 6 (60) | >0.99 |
| Median age, yr (range) | 21 (7–62) | 27 (11–62) | 17 (7–53) | 0.28 |
| pGb3, μmol/L (95% CI) | 6.99 (5.21 to 8.77) | 7.06 (4.33 to 9.80) | 6.91 (4.06 to 9.80) | 0.93 |
| Albumin-to-creatinine ratio, mg/mmol (95% CI) | 9.31 (2.56 to 16.05) | 7.05 (2.08 to 12.02) | 11.56 (−2.37 to 25.49) | 0.63 |
| Measured GFR, ml/min per 1.73 m2 (95% CI) | 98 (88 to 107) | 96 (80 to 111) | 100 (85 to 114) | 0.66 |
| eGFR, ml/min per 1.73 m2 (95% CI) | 105 (95 to 115) | 98 (85 to 111) | 112 (95 to 129) | 0.15 |
| Composite podocyte Gb3 score (95% CI) | 6.83 (6.63 to 7.03) | 6.97 (6.93 to 7.00) | 6.71 (6.31 to 7.11) | 0.55 |
| pLysoGb3 (95% CI)a | 16.9 (10.2 to 23.6) | 17.8 (4.5 to 31.1) | 16.1 (7.3 to 24.9) | 0.80 |
pGb3 reference: 1.6–3.3 μmol/L. Urine albumin-to-creatinine ratio reference: <2.5 mg/mmol creatinine. GFR was measured by iohexol clearance. pGb3, plasma globotriaosylceramide; 95% CI, 95% confidence interval; Gb3, globotriaosylceramide; pLysoGb3, plasma globotriaosylsphingosine.
Analyses of the 15 patients without true baseline levels.
Figure 1.
The course of biochemical and clinical parameters in patients during enzyme replacement therapy. Plasma globotriaosylsphingosine (lysoGb3), albumin-to-creatinine ratio, and clinical Fabry disease severity scores for the lower fixed dose and higher dose groups during enzyme replacement therapy. True baseline plasma lysoGb3 values are only available for three men in the lower fixed dose group and two women in the higher dose group. (A, C, and E) The lower fixed dose group. (B, D, and F) The higher dose group. Albuminuria due to de novo GN. The numeration of the patients corresponds to the numeration in Table 1. The same color corresponds to the same patient throughout the panels for each treatment group (i.e., the lower fixed dose and higher dose groups). *Patient in whom the clinical Fabry disease severity scores increased on agalsidase dose <1.0 mg/kg every other week. ⋆, denotes de novo GN.
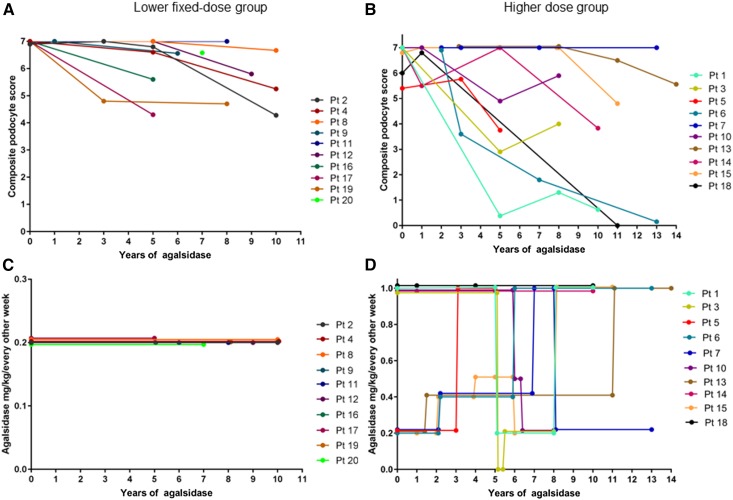
Figure 2.
Changes in podocyte globotriaosylceramide burden related to agalsidase dose. (A and C) The lower fixed dose group. (B and D) The higher dose group. Lower fixed-dose group: Agalsidase-α 0.2 mg/kg every other week, patients 11 and 17 received agalsidase-β 0.2 mg/kg every other week. Higher fixed dose group: Agalsidase-α 0.2 or 0.4 mg/kg every other week, agalsidase-β 0.5 or 1.0 mg/kg every other week, pt 13 received agalsidase-α 0.2 mg/kg every week for 6 years. Each line represents a single patient, and the color code is explained in the right margin. The numeration of the patients corresponds to the numeration in Table 1.
At the time of the baseline kidney biopsy, 11 patients (55%) had evidence of Fabry-related complications. Clinical Fabry disease severity scores are summarized in Figure 1. Thirteen clinical events occurred in seven patients (Table 3). Seven events (54%) occurred in the three oldest patients included in the cohort. No progress in Fabry disease severity score was seen in patients on agalsidase-β 1.0 mg/kg every other week (Figure 1, Table 3).
Table 3.
Clinical events on enzyme replacement therapy observed in a group of 20 patients with classic Fabry disease with serial kidney biopsies
| Agalsidase 0.2 mg/kg Every Other Week | Agalsidase 0.4–1.0 mg/kg Every Other Week |
|---|---|
| Patient 6: de novo albuminuria (2 yr of agalsidase-α; later normalized after dose increase to 0.4 mg/kg every other week) | Patient 1: sudden deafness (4 yr of agalsidase-β) |
| Patient 6: left ventricular hypertrophy (2 yr of agalsidase-α) | Patient 6: arrhythmia, pacemaker (6 yr of agalsidase-α: 2 yr of 0.2 mg/kg every other week and 4 yr of 0.4 mg/kg every other week |
| Patient 7: de novo albuminuria (2 yr of agalsidase-α) | Patient 18: cerebral infarction (1 yr of agalsidase-β 1.0 mg/kg every other week) |
| Patient 10: GFR loss >5 ml/min per 1.73 m2 per 1 yr (3 yr of agalsidase-α; switch due to shortage after 5 yr of agalsidase-β 1.0 mg/kg every other week) | Patient 18: cerebral infarction (3 yr of agalsidase-β 1.0 mg/kg every other week) |
| Patient 19: TIA (2 yr of agalsidase-α) | Patient 18: arrhythmia, implantable cardioverter defibrillator (7 yr of agalsidase-β 1.0 mg/kg every other week) |
| Patient 19: TIA (4 yr of agalsidase-α) | |
| Patient 19: arrhythmia medication (4 yr of agalsidase-α) | |
| Patient 20: albuminuria progression (8 yr of agalsidase-α) |
TIA, transitory ischemic attack.
Median time from first to last kidney biopsy was 9.4 (range =4.4–11.7) years, during which measured GFR decreased by means of −3 ml/min per 1.73 m2 (SD=13) and −8 ml/min per 1.73 m2 (SD=10) in the lower fixed dose and higher dose groups, respectively (P=0.34) (Table 4). The higher dose group had a statistically significant reduction in measured GFR during follow-up (P=0.02). However, measured GFR fell within the normal range in four patients, and one patient had a significant fall in measured GFR after agalsidase dose was changed from 1.0 to 0.2 mg/kg every other week.
Table 4.
Changes in clinical parameters and podocyte scores of 20 patients with Fabry disease treated with lower fixed dose agalsidase or higher dose agalsidase
| Variable | Total Cohort | Lower Fixed Dose Group | Higher Dose Group | P Value |
|---|---|---|---|---|
| Patients | 20 | 10 | 10 | |
| pGb3, μmol/L (95% CI) | −2.98 (−4.95 to −1.01) | −2.95 (−6.25 to 0.35) | −3.01 (−5.89 to −0.13) | 0.98 |
| Albumin-to-creatinine ratio, mg/mmol (95% CI)a | −1.81 (−10.57 to 6.95) | 4.34 (−5.26 to 13.94) | −8.64 (−24.79 to 7.51) | 0.05 |
| Albumin-to-creatinine ratio, mg/mmol (95% CI)b | −2.26 (−13.66 to 9.14) | 6.27 (−8.51 to 21.06) | −9.73 (−28.27 to 8.82) | 0.09 |
| Measured GFR, ml/min per 1.73 m2 (95% CI)a | −5 (−11 to 0) | −3 (−12 to 6) | −9 (−15 to 0) | 0.34 |
| pLysoGb3 (95% CI)c | −1.5 (−4.4 to 1.4) | 1.4 (0.7 to 2.1) | −4.1 (−9.2 to 1.0) | 0.03 |
| Median agalsidase (range), yr | 9.4 (4.8–13.8) | 7.8 (4.9–9.9) | 10.5 (4.8–13.8) | 0.04 |
| Median (range) from first to last biopsy,d yr | 9.8 (4.4–11.7) | 7.8 (4.4–9.9) | 9.9 (4.8–11.7) | 0.05 |
| Composite podocyte Gb3 score (95% CI) | −2.32 (−3.31 to −1.34) | −1.39 (−2.19 to −0.59) | −3.16 (−4.87 to −1.45) | 0.06 |
pGb3, plasma globotriaosylceramide; 95% CI, 95% confidence interval; pLysoGb3, plasma globotriaosylsphingosine; Gb3, globotriaosylceramide.
Patient 1 was excluded from analyses due to de novo GN during follow-up.
Patients who were normoalbuminuric at baseline plus end of follow-up were excluded (lower fixed dose group: three patients; higher dose group: one patient).
Five patients (three in the lower fixed dose group and two in the higher dose group) with true baseline globotriaosylsphingosine were excluded from the analyses. Where first biopsy was taken before the start of enzyme replacement therapy, the time point of the start of enzyme replacement therapy was used as the start time.
Patient 19 was excluded, because baseline biopsy was taken 19 years before the start of enzyme replacement therapy.
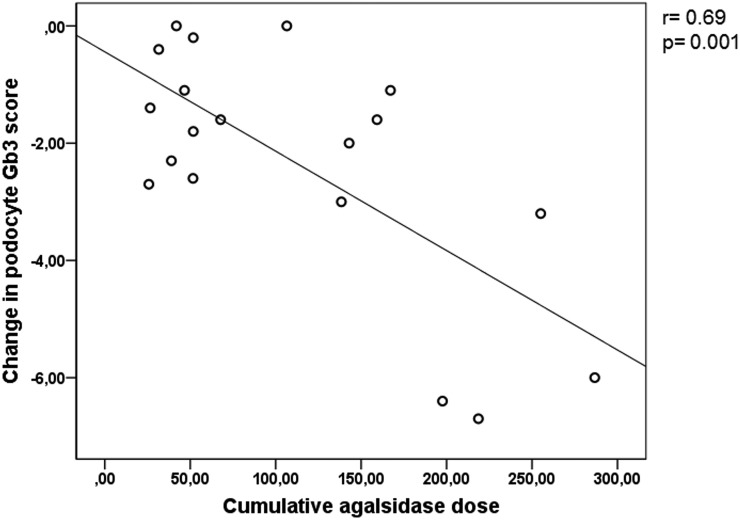
Four patients started agalsidase-α therapy before the baseline kidney biopsy, three had maximum composite podocyte Gb3 scores of 7.0 in the first biopsy (patients 6, 7, and 13), and only periodic acid–Schiff sections (scored 3.0 of 3.0) were available for patient 20. Mean baseline composite podocyte Gb3 scores were 6.97 (SD=0.05) and 6.71 (SD 0.55) for the lower fixed dose and higher dose groups, respectively. At the final biopsy, mean composite podocyte Gb3 score was 5.68 (SD=1.03) in the lower fixed dose group and 3.55 (SD=2.48) in the higher dose group. Mean reduction in composite podocyte Gb3 score from first to final biopsy was −1.39 (SD=1.04; P=0.004) in the lower fixed dose group and −3.16 (SD=2.39; P=0.002) in the higher dose group (Table 4). The difference in clearing of podocyte Gb3 burden between the groups was −1.77 (P=0.06). Podocyte Gb3 load was reduced by −3.74 (SD=2.13) in analyses including only patients who received agalsidase-β 1.0 mg/kg every other week for at least 2 years preceding the kidney biopsy, and the reduction was statistically greater than that of the lower fixed dose group (P=0.01). Decrease in podocyte composite score correlated with increasing cumulative agalsidase dose (r=0.69; P=0.001) (Figure 3). The correlation did not alter significantly when patient 18 (recipient of a Fabry kidney transplant from a woman donor) was excluded (r=0.61; P<0.01).
Figure 3.
Change in podocyte globotriaosylceramide correlates with cumulative agalsidase dose.
In the lower fixed dose group, seven of eight patients with available information had baseline Gb3 inclusions in the arterial/arteriolar intima (endothelium) and media (smooth muscle), whereas seven of ten and ten of ten patients in the higher dose group had baseline arterial/arteriolar intima and media Gb3 inclusions, respectively. In the final biopsy, six of eight patients in the lower fixed dose group had arterial/arteriolar intima Gb3 inclusions compared with one of ten patients in the higher dose group (P=0.02). In the final biopsy, medial Gb3 inclusions were seen in eight of eight and six of ten patients in the lower fixed dose and higher dose groups, respectively (P=0.17). Sixteen patients had combined information on arteries/arterioles in the first and final biopsies. No statistical change in the number of patients with intimal Gb3 inclusions in the lower fixed dose group was observed. In the higher dose group, fewer patients had intimal Gb3 inclusions in the final biopsy (one of ten) compared with baseline (seven of ten; P=0.03). No statistical change in medial inclusions was observed in either group.
The lower fixed dose group received agalsidase for a median of 7.8 (range =4.9–9.9) years compared with 10.5 (range =4.8–13.8) years in the higher dose group. Median treatment duration was significantly different between the two groups (P=0.04). However, in mixed model regression, adjustments for time, sex, and genotype did not significantly alter the correlation between cumulative agalsidase dose and reduction of podocyte Gb3. The statistical power was insufficient with respect to effect of genotype on podocyte Gb3 clearance.
True baseline plasma lysoGb3 values were only available for five patients: patients 4, 8, and 9 (men) in the lower fixed dose group (range =75.4–130.7 nmol/L) and patients 5 and 14 (women) in the higher dose group (4.1 and 5.8 nmol/L). The median reduction in lysoGb3 for the three men (lower fixed dose group) was 80%, whereas the two women (higher dose group) had lysoGb3 reductions of 32% and 24%. Median treatment time at the first lysoGb3 measurement was 1.1 (range =0.2–6.3) years for individuals without true baseline values. Mean lysoGb3 at the first available time point was not statistically different between groups, excluding those with true baseline levels. LysoGb3 did not normalize in any patients (Figure 1). Residual lysoGb3 at the time of the final kidney biopsy was significantly lower in the higher dose group (10.4 nmol/L [SD=8.4]) compared with in the lower fixed dose group (20.1 nmol/L [SD=11.9]; P=0.04), and residual lysoGb3 levels were found to correlate with cumulative agalsidase dose in men (r=0.71; P=0.01) but not women (r=0.29; P=0.49).
Inhibitory antibody analyses were available for all patients. None developed antibody titers greater than or equal to six, except patient 13, who developed an antibody titer of 16 after 10 years of agalsidase-α, dosed as 0.2 mg/kg every other week, 0.2 mg/kg weekly, or 0.4 mg/kg every other week. He was switched to agalsidase-β 1.0 mg/kg every other week after 11 years of agalsidase-α due to inadequate symptom relief. The antibody titer transiently doubled after starting agalsidase-β before decreasing to nine.
Discussion
This study shows sustained dose-dependent clearing of podocyte Gb3 in a cohort of patients with classic Fabry disease followed by clinical assessments and kidney biopsies for up to 14 years. This cohort is younger, with less burden of pretreatment comorbidity and subsequent events, compared with other long-term treatment reports (29,30). The age span is, however, greater than in the 5-year biopsy cohort previously published from our center (8). This cohort consists of children and adults with classic Fabry disease using strict phenotypic criteria (13,31). For the first time, podocyte Gb3 was significantly reduced in both treatment groups; however, the reduction of podocyte Gb3 was found to increase with increasing agalsidase dose (r=0.69; P=0.001). Moreover, the effect was significantly higher in the group that received agalsidase-β 1.0 mg/kg every other week as opposed to agalsidase 0.2 mg/kg every other week leading up to the final kidney biopsy (P=0.01), extending findings previously published from our group (8,19).
Increasing density of podocyte Gb3 inclusions assessed by unbiased stereologic methods has previously been linked to podocyte damage, increased podocyte volume, and albuminuria, with concomitant volume reduction as podocyte Gb3 is cleared by enzyme replacement therapy (15,32). In this cohort, albuminuria did not change significantly in either treatment group when patients with baseline and final normoalbuminuria were excluded (Table 4), and no correlation between albuminuria at the final time point and change in podocyte Gb3 burden was found. Twelve patients (60%) were prescribed RAAS blockade for some of the follow-up period, which may have contributed to the small change in albuminuria levels observed.
There is a lack of sensitive early nephropathy biomarkers in Fabry disease. Podocyturia has recently been linked to progressive Fabry nephropathy (33), and optimal treatment of the podocyte is thus likely important to maintain long-term kidney function in Fabry disease. The dose-dependent clearing of podocyte Gb3 shown in serial kidney biopsies in this Fabry cohort indicates improved podocyte health and should be considered a marker for individual tailoring of enzyme replacement therapy along with clinical assessment of individual patient risk and treatment effects.
Baseline lysoGb3 has been shown to differ significantly between men and women with classic Fabry disease and in classic versus nonclassic disease (13). LysoGb3 was increased in all patients; however, true baseline levels were only available for five patients. A considerable drop was seen in the five patients with true baseline values, in line with earlier studies (34,35). The most prominent agalsidase effect on lysoGb3 can be found during the first year of treatment (35). Follow-up samples showed a further decline in mean lysoGb3 for the higher dose group in contrast to the lower fixed dose group (P=0.03), and lysoGb3 at the final time point was found to be significantly lower in men who had received a higher cumulative agalsidase dose (P=0.01). This may indicate potential dose-dependent effects on lysoGb3. A new observation was the significant reduction of arterial/arteriolar intimal Gb3 inclusions from baseline to final biopsy in the higher dose group, which was not found in the lower fixed dose group. Medial Gb3 inclusions did not change significantly in either treatment group, indicating that the vascular myocyte is more difficult to reach than the endothelium, irrespective of dose (17,36). Individual differences were, however, seen in the higher dose group. As previously shown, glomerular endothelium was cleared of Gb3, irrespective of cumulative dose (8). Persistently elevated lysoGb3 has been linked to progressive vascular damage, independent of age and sex (37). LysoGb3 is related to disease severity (13) and has been found to be an independent risk factor for white matter lesions and left ventricular hypertrophy in men and women, respectively (38). Its usefulness in predicting risk of further complications on the basis of residual levels is uncertain, and plasma lysoGb3 levels have been correlated with total disease severity scores in women but not men (38). Whether lysoGb3 is a marker of disease or causally linked to Fabry complications has not been fully elucidated. Mechanical allodynia was induced by lysoGb3 but not normal saline when injected into paws of healthy mice. High levels of lysoGb3 were found to increase intracellular Ca2+ in peripheral sensory neurons (39), indicating that lysoGb3 may play a direct role in acroparesthesia. LysoGb3 has been shown to promote secondary mediators of glomerular injury similar to diabetic nephropathy (40) as well as profibrotic and proinflammatory cytokines in cultured human podocytes (41), and it may thus be implicated in the processes causing glomerular damage in Fabry disease. In clinical practice, decline of lysoGb3 is expected in response to effective treatment, and increasing levels of lysoGb3 occur after agalsidase dose reduction, after noncompliance, or in the presence of inhibitory antibodies (42,43).
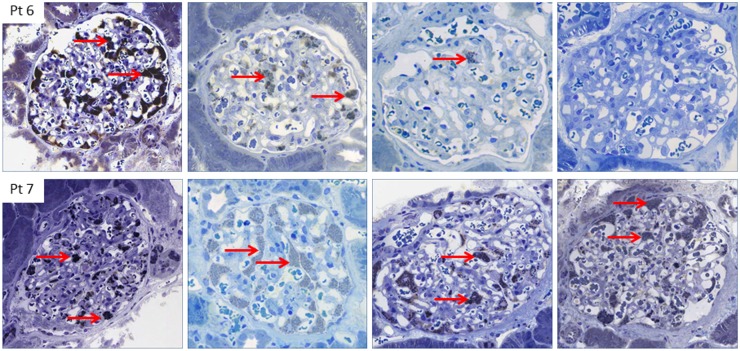
The need for individual risk and therapy assessments has been highlighted (12,44). The clinical spectrum of Fabry disease is heterogeneous, and symptoms may differ greatly in patients within the same family and from classic to nonclassic disease. Consequently, treatment should be tailored accordingly. Patients 6 and 7, who started enzyme replacement therapy at the ages of 13 and 15 years, respectively, underscore this point (Figures 1 and 4). Despite similar ages at the start of therapy, the same agalsidase dose for the first years, and the same GLA mutation, the younger boy developed a cardiac complication necessitating a pacemaker after 6 years of enzyme replacement therapy, at which time he was switched to agalsidase-β 1.0 mg/kg every other week. After the switch, the podocytes were virtually completely cleared of Gb3, whereas his older brother (patient 7), who received a lower cumulative agalsidase dose, continued to have a full podocyte score after a total of 13 years of agalsidase (Figures 3 and 4). Interestingly, lysoGb3 doubled in patient 7 when he was switched from agalsidase-β 1.0 to agalsidase-α 0.2 mg/kg every other week due to the global shortage of agalsidase-β, and lysoGb3 at the final time point was comparable with that in patients in the lower fixed dose group.
Figure 4.
Different reduction of podocyte globotriaosylceramide in two brothers after 13 years of enzyme replacement therapy, with increased clearing on higher dose. Baseline and follow-up biopsies for patients 6 and 7 (sections stained with toluidine blue). Column 1 shows 2 years of agalsidase. Podocyte globotriaosylceramide (Gb3) scores are 6.9 and 7.0 for patients 6 and 7, respectively. Column 2 shows 3 years of agalsidase. Podocyte Gb3 scores are 3.6 and 7.0 for patients 6 and 7, respectively. Column 3 shows 7 years of agalsidase. Podocyte Gb3 scores are 1.8 and 7.0 for patients 6 and 7, respectively. Column 4 shows 13 years of agalsidase. Podocyte Gb3 scores are 0.15 and 7.0 for patients 6 and 7, respectively. The images show inclusions of Gb3 in podocytes (red arrows). All images in the upper panel are from patient 6. All images in the lower panel are from patient 7.
Patient 10 also exemplifies the need for individually tailored enzyme replacement therapy. Measured GFR fell from 97 to 79 ml/min per 1.73 m2 after switching from agalsidase 1.0 to 0.2 mg/kg every other week (19). (Figures 1 and 3). After reinstating agalsidase-β 1.0 mg/kg every other week, GFR improved, indicating that agalsidase dose increase may have a beneficial effect on early decline of GFR in individual patients in contrast to the progressive loss of GFR reported in patients with more advanced CKD (30).
This study is limited by the observational design. Although a classic phenotype was confirmed in all patients, the cohort was small and heterogeneous, consisting of men and women who started agalsidase at various ages. The long follow-up period and clinical evaluations, including measured GFR, albuminuria, lysoGb3, and serial kidney biopsies, are the main strengths of the study. In conclusion, this study has expanded on previous findings of dose-dependent clearing of Gb3 from podocytes and clinical benefit in individual patients by confirming it in older patients as well as children treated over a longer time period. For the first time, a statistically significant reduction in podocyte Gb3 score is also shown in patients receiving long-term agalsidase 0.2 mg/kg every other week. Only the higher dose group showed significant clearing of arterial/arteriolar intimal Gb3 inclusions. Medial Gb3 inclusions remained statistically unchanged in both groups, although four patients in the higher dose group completely cleared medial Gb3. This raises questions regarding the long-term effects of enzyme replacement therapy on the vasculature. Residual lysoGb3 levels were found to correlate with cumulative dose in men. Repeated individual clinical and histologic assessments are necessary when tailoring the optimal treatment for patients with Fabry disease.
Disclosures
R.S. and S.L. received travel grants and speaker fees from Sanofi-Genzyme. C.T., G.H., and E.S. received travel grants and speaker fees from Shire and Sanofi-Genzyme. K.K.L. received travel grants from Sanofi-Genzyme. E.S.D. received travel grants from Sanofi-Genzyme. C.H. acts as an investigator in premarketing studies in the field of lysosomal storage disorders with Sanofi-Genzyme and Protalix and is an advisor to the College ter Beoordeling van Geneesmiddelen-Medicines Evaluation Board (CBG-MEB). The Academic Medical Center receives financial support to submit patient data from Genzyme and Shire for Gaucher disease (Genzyme) and Fabry disease (Sanofi-Genzyme and Shire). This study was supported by grants from the Western Norway Regional Health Authority. A.B.P.v.K. and F.M.V. have no conflicts of interest to declare.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Desnick RJ, Ioannou YA, Eng CM: Alpha-galactosidase A deficiency: Fabry disease. In: The Metabolic and Molecular Bases of Inherited Disease, edited by Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, New York, McGraw Hill, 2001, pp 3733–3774 [Google Scholar]
- 2.Desnick RJ, Wasserstein MP, Banikazemi M: Fabry disease (alpha-galactosidase A deficiency): Renal involvement and enzyme replacement therapy. Contrib Nephrol 136: 174–192, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Branton MH, Schiffmann R, Sabnis SG, Murray GJ, Quirk JM, Altarescu G, Goldfarb L, Brady RO, Balow JE, Austin Iii HA, Kopp JB: Natural history of Fabry renal disease: Influence of alpha-galactosidase A activity and genetic mutations on clinical course. Medicine (Baltimore) 81: 122–138, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, Caplan L, Linthorst GE, Desnick RJ; International Collaborative Fabry Disease Study Group : Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry’s disease. N Engl J Med 345: 9–16, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Schiffmann R, Kopp JB, Austin HA 3rd, Sabnis S, Moore DF, Weibel T, Balow JE, Brady RO: Enzyme replacement therapy in Fabry disease: A randomized controlled trial. JAMA 285: 2743–2749, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Sakuraba H, Murata-Ohsawa M, Kawashima I, Tajima Y, Kotani M, Ohshima T, Chiba Y, Takashiba M, Jigami Y, Fukushige T, Kanzaki T, Itoh K: Comparison of the effects of agalsidase alfa and agalsidase beta on cultured human Fabry fibroblasts and Fabry mice. J Hum Genet 51: 180–188, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Lee K, Jin X, Zhang K, Copertino L, Andrews L, Baker-Malcolm J, Geagan L, Qiu H, Seiger K, Barngrover D, McPherson JM, Edmunds T: A biochemical and pharmacological comparison of enzyme replacement therapies for the glycolipid storage disorder Fabry disease. Glycobiology 13: 305–313, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Tøndel C, Bostad L, Larsen KK, Hirth A, Vikse BE, Houge G, Svarstad E: Agalsidase benefits renal histology in young patients with Fabry disease. J Am Soc Nephrol 24: 137–148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linthorst GE, Burlina AP, Cecchi F, Cox TM, Fletcher JM, Feldt-Rasmussen U, Giugliani R, Hollak CE, Houge G, Hughes D: Recommendations on reintroduction of agalsidase Beta for patients with fabry disease in Europe, following a period of shortage. In: JIMD Reports-Case and Research Reports, 2012/5, edited by Zschocke J, Gibson KM, Brown G, Morava E, Peters V, Berlin, Springer, 2013, pp 51–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenders M, Canaan-Kühl S, Krämer J, Duning T, Reiermann S, Sommer C, Stypmann J, Blaschke D, Üçeyler N, Hense H-W, Brand S-M, Wanner C, Weidemann F, Brand E: Patients with Fabry disease after enzyme replacement therapy dose reduction and switch-2-year follow-up. J Am Soc Nephrol 27: 952–962, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vedder AC, Linthorst GE, Houge G, Groener JE, Ormel EE, Bouma BJ, Aerts JM, Hirth A, Hollak CE: Treatment of Fabry disease: Outcome of a comparative trial with agalsidase alfa or beta at a dose of 0.2 mg/kg. PLoS One 2: e598, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiffmann R, Hughes DA, Linthorst GE, Ortiz A, Svarstad E, Warnock DG, West ML, Wanner C; Conference Participants : Screening, diagnosis, and management of patients with Fabry disease: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) controversies conference. Kidney Int 91: 284–293, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Smid BE, van der Tol L, Biegstraaten M, Linthorst GE, Hollak CE, Poorthuis BJ: Plasma globotriaosylsphingosine in relation to phenotypes of Fabry disease. J Med Genet 52: 262–268, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Rombach SM, Twickler TB, Aerts JMFG, Linthorst GE, Wijburg FA, Hollak CEM: Vasculopathy in patients with Fabry disease: Current controversies and research directions. Mol Genet Metab 99: 99–108, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Najafian B, Svarstad E, Bostad L, Gubler MC, Tøndel C, Whitley C, Mauer M: Progressive podocyte injury and globotriaosylceramide (GL-3) accumulation in young patients with Fabry disease. Kidney Int 79: 663–670, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kriz W, Shirato I, Nagata M, LeHir M, Lemley KV: The podocyte’s response to stress: The enigma of foot process effacement. Am J Physiol Renal Physiol 304: F333–F347, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Lubanda JC, Anijalg E, Bzduch V, Thurberg BL, Benichou B, Tylki-Szymanska A: Evaluation of a low dose, after a standard therapeutic dose, of agalsidase beta during enzyme replacement therapy in patients with Fabry disease. Genet Med 11: 256–264, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Weidemann F, Krämer J, Duning T, Lenders M, Canaan-Kühl S, Krebs A, Guerrero González H, Sommer C, Üçeyler N, Niemann M, Störk S, Schelleckes M, Reiermann S, Stypmann J, Brand SM, Wanner C, Brand E: Patients with Fabry disease after enzyme replacement therapy dose reduction versus treatment switch. J Am Soc Nephrol 25: 837–849, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skrunes R, Svarstad E, Kampevold Larsen K, Leh S, Tøndel C: Reaccumulation of globotriaosylceramide in podocytes after agalsidase dose reduction in young Fabry patients. Nephrol Dial Transplant 32: 807–813, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Fogo AB, Bostad L, Svarstad E, Cook WJ, Moll S, Barbey F, Geldenhuys L, West M, Ferluga D, Vujkovac B, Howie AJ, Burns A, Reeve R, Waldek S, Noël LH, Grünfeld JP, Valbuena C, Oliveira JP, Müller J, Breunig F, Zhang X, Warnock DG; all members of the International Study Group of Fabry Nephropathy (ISGFN) : Scoring system for renal pathology in Fabry disease: Report of the International Study Group of Fabry Nephropathy (ISGFN). Nephrol Dial Transplant 25: 2168–2177, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobsson L: A method for the calculation of renal clearance based on a single plasma sample. Clin Physiol 3: 297–305, 1983 [DOI] [PubMed] [Google Scholar]
- 22.Haycock GB, Schwartz GJ, Wisotsky DH: Geometric method for measuring body surface area: A height-weight formula validated in infants, children, and adults. J Pediatr 93: 62–66, 1978 [DOI] [PubMed] [Google Scholar]
- 23.Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT: Performance of the cockcroft-gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol 5: 1003–1009, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tøndel C, Bostad L, Hirth A, Svarstad E: Renal biopsy findings in children and adolescents with Fabry disease and minimal albuminuria. Am J Kidney Dis 51: 767–776, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Tøndel C, Kanai T, Larsen KK, Ito S, Politei JM, Warnock DG, Svarstad E: Foot process effacement is an early marker of nephropathy in young classic Fabry patients without albuminuria. Nephron 129: 16–21, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Linthorst GE, Hollak CE, Donker-Koopman WE, Strijland A, Aerts JM: Enzyme therapy for Fabry disease: Neutralizing antibodies toward agalsidase alpha and beta. Kidney Int 66: 1589–1595, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Giannini EH, Mehta AB, Hilz MJ, Beck M, Bichet DG, Brady RO, West M, Germain DP, Wanner C, Waldek S, Clarke JT, Mengel E, Strotmann JM, Warnock DG, Linhart A: A validated disease severity scoring system for Fabry disease. Mol Genet Metab 99: 283–290, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Germain DP, Charrow J, Desnick RJ, Guffon N, Kempf J, Lachmann RH, Lemay R, Linthorst GE, Packman S, Scott CR, Waldek S, Warnock DG, Weinreb NJ, Wilcox WR: Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease. J Med Genet 52: 353–358, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ortiz A, Abiose A, Bichet DG, Cabrera G, Charrow J, Germain DP, Hopkin RJ, Jovanovic A, Linhart A, Maruti SS, Mauer M, Oliveira JP, Patel MR, Politei J, Waldek S, Wanner C, Yoo HW, Warnock DG: Time to treatment benefit for adult patients with Fabry disease receiving agalsidase β: Data from the Fabry registry. J Med Genet 53: 495–502, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arends M, Wanner C, Hughes D, Mehta A, Oder D, Watkinson OT, Elliott PM, Linthorst GE, Wijburg FA, Biegstraaten M, Hollak CE: Characterization of classical and nonclassical Fabry disease: A multicenter study. J Am Soc Nephrol 28: 1631–1641, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Najafian B, Tøndel C, Svarstad E, Sokolovkiy A, Smith K, Mauer M: One year of enzyme replacement therapy reduces globotriaosylceramide inclusions in podocytes in male adult patients with Fabry disease. PLoS One 11: e0152812, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fall B, Scott CR, Mauer M, Shankland S, Pippin J, Jefferson JA, Wallace E, Warnock D, Najafian B: Urinary podocyte loss is increased in patients with Fabry disease and correlates with clinical severity of Fabry nephropathy. PLoS One 11: e0168346, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu HC, Lin HY, Yang CF, Liao HC, Hsu TR, Lo CW, Chang FP, Huang CK, Lu YH, Lin SP, Yu WC, Niu DM: Globotriaosylsphingosine (lyso-Gb3) might not be a reliable marker for monitoring the long-term therapeutic outcomes of enzyme replacement therapy for late-onset Fabry patients with the Chinese hotspot mutation (IVS4+919G>A). Orphanet J Rare Dis 9: 111, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Breemen MJ, Rombach SM, Dekker N, Poorthuis BJ, Linthorst GE, Zwinderman AH, Breunig F, Wanner C, Aerts JM, Hollak CE: Reduction of elevated plasma globotriaosylsphingosine in patients with classic Fabry disease following enzyme replacement therapy. Biochim Biophys Acta 1812: 70–76, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Germain DP, Waldek S, Banikazemi M, Bushinsky DA, Charrow J, Desnick RJ, Lee P, Loew T, Vedder AC, Abichandani R, Wilcox WR, Guffon N: Sustained, long-term renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. J Am Soc Nephrol 18: 1547–1557, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Rombach SM, van den Bogaard B, de Groot E, Groener JE, Poorthuis BJ, Linthorst GE, van den Born BJ, Hollak CE, Aerts JM: Vascular aspects of Fabry disease in relation to clinical manifestations and elevations in plasma globotriaosylsphingosine. Hypertension 60: 998–1005, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Rombach SM, Dekker N, Bouwman MG, Linthorst GE, Zwinderman AH, Wijburg FA, Kuiper S, vd Bergh Weerman MA, Groener JE, Poorthuis BJ, Hollak CE, Aerts JM: Plasma globotriaosylsphingosine: Diagnostic value and relation to clinical manifestations of Fabry disease. Biochim Biophys Acta 1802: 741–748, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Choi L, Vernon J, Kopach O, Minett MS, Mills K, Clayton PT, Meert T, Wood JN: The Fabry disease-associated lipid Lyso-Gb3 enhances voltage-gated calcium currents in sensory neurons and causes pain. Neurosci Lett 594: 163–168, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez-Niño MD, Sanz AB, Carrasco S, Saleem MA, Mathieson PW, Valdivielso JM, Ruiz-Ortega M, Egido J, Ortiz A: Globotriaosylsphingosine actions on human glomerular podocytes: Implications for Fabry nephropathy. Nephrol Dial Transplant 26: 1797–1802, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Sanchez-Niño MD, Carpio D, Sanz AB, Ruiz-Ortega M, Mezzano S, Ortiz A: Lyso-Gb3 activates Notch1 in human podocytes. Hum Mol Genet 24: 5720–5732, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Rombach SM, Aerts JMFG, Poorthuis BJHM, Groener JEM, Donker-Koopman W, Hendriks E, Mirzaian M, Kuiper S, Wijburg FA, Hollak CEM, Linthorst GE: Long-term effect of antibodies against infused alpha-galactosidase A in Fabry disease on plasma and urinary (lyso)Gb3 reduction and treatment outcome. PLoS One 7: e47805, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bénichou B, Goyal S, Sung C, Norfleet AM, O’Brien F: A retrospective analysis of the potential impact of IgG antibodies to agalsidase β on efficacy during enzyme replacement therapy for Fabry disease. Mol Genet Metab 96: 4–12, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Basic-Jukic N, Kes P, Mokos I, Coric M: Do we need more intensive enzyme replacement therapy for Anderson-Fabry disease? Med Hypotheses 72: 476–477, 2009 [DOI] [PubMed] [Google Scholar]