Abstract
Background and objectives
Vitamin D is implicated in vascular health in CKD. This study compared placebo, calcifediol, and calcitriol treatment with changes in vascular stiffness, BP, proteinuria, mineral metabolism parameters, C-reactive protein, and fibroblast growth factor 23 in patients with stable CKD.
Design, setting, participants, & measurements
We conducted a double-blind, randomized controlled trial in out-patient CKD clinics in Vancouver, Canada, from February of 2011 to August of 2014, enrolling 119 patients with an eGFR of 15–45 ml/min per 1.73 m2. Change in pulse wave velocity (PWV) was measured after 6 months of treatment with a fixed dose of oral calcifediol (5000 IU 25-hydroxyvitamin D3), calcitriol (0.5 µg 1,25-dihydroxyvitamin D3), or placebo, thrice weekly.
Results
Eighty-seven participants were evaluated. Mean age was 66 years, 71% were men, 40% were diabetic, and mean baseline PWV was 11.5 m/s (SD=3.9 m/s). After 6 months, the PWV decreased in the calcifediol group (mean change, −1.1; 95% confidence interval [95% CI], −2.2 to 0.1 m/s), remained unchanged in the calcitriol group (mean change, 0.2; 95% CI, −0.9 to 1.4 m/s), and increased in the placebo group (mean change, 1.1; 95% CI, −0.1 to 2.2 m/s). The overall P value for between-arm changes was 0.03. Absolute PWV change was significantly different between groups (P=0.04): the combined vitamin D treatment group saw decreased PWV (mean change, −0.4; 95% CI, −1.2 to 0.4 m/s) whereas the placebo group saw increased PWV (mean change, +1.1; 95% CI, −0.1 to 2.2 m/s). The treatment group demonstrated significantly decreased serum parathyroid hormone (mean difference, −0.5; 95% CI, −0.7 to −0.3 ln[pg/ml]; P<0.001) and increased calcium (mean difference, 0.4; 95% CI, −0.1 to 0.7 mg/dl; P=0.02). In observational analysis, participants in the highest 25-hydroxyvitamin D tertile at trial end had significant decreases in PWV (mean change, −1.0; 95% CI, −2.0 to 0.0 m/s) compared with the middle and lowest tertiles (P<0.01). Side effects were minor and rare.
Conclusions
Six months of supplemental vitamin D analogs at fixed doses may achieve a reduction of PWV in patients with advanced CKD. Because the treatment effect was attenuated when baseline PWV was included as a covariate, these findings should be replicated in larger populations and further studied.
Keywords: Vitamin D; vascular calcification; vascular disease; mineral metabolism; Aged; Arm; blood pressure; C-Reactive Protein; Calcifediol; Calcitriol; calcium; Canada; diabetes mellitus; Fibroblast Growth Factors; Humans; Male; Minerals; Outpatients; parathyroid hormone; proteinuria; Pulse Wave Analysis; Renal Insufficiency, Chronic; Vascular Stiffness; Vitamins; fibroblast growth factor 23
Introduction
Vitamin D acts on multiple aspects of the physiologic processes involved in the pathogenesis of cardiovascular (CV) disease in multiple populations, including those with CKD (1). Deficiency is common in CKD populations as they have reduced capacity to fully hydroxylate 25-hydroxyvitamin D [25(OH)D] into its active form, 1,25-dihydroxyvitamin D [1,25(OH)2D], and has been associated with endothelial dysfunction and high CV mortality (2–10). Previously, we have demonstrated that a decline in 1,25(OH)D precedes the fall in 25(OH)D in CKD populations, and occurs earlier than changes in parathyroid hormone (PTH) and phosphate (9). Clinical trials to date have compared different vitamin D compounds, in both CKD and non-CKD populations for CV risk reduction, and have shown variable results on the effect of supplementation on vascular health (11–13). Small short-term studies have suggested a benefit of paricalcitol supplementation on proteinuria (14) and reduction of PTH and CV hospitalizations (15). The value of replenishing deficiencies of both 25(OH)D and 1,25(OH)2D, with respect to its effect on important outcomes, needs to be further investigated.
This randomized control trial was designed to examine the hypothesis that mediation of vascular tone may be the physiologic mechanism by which vitamin D confers CV protection in CKD. In particular, we were interested in the biologic effect of vitamin D, irrespective of potential pathway, on vascular stiffness, measured by pulse wave velocity (PWV) and BP. The primary objective of this study is to test the effect of two currently used vitamin D formulations, calcifediol [25(OH)D] and calcitriol [1,25(OH)D], compared with placebo on objective measures of vascular stiffness in patients with stage 3 and 4 CKD already receiving conventional CV risk reduction medications. We hypothesized that supplementation with either of two different vitamin D analogs would result in greater changes in PWV measurements than placebo.
Materials and Methods
This is a randomized, double-blind, placebo-controlled, three-arm study examining the effect of calcifediol and calcitriol supplementation compared with placebo on vascular stiffness, as measured by PWV. The study was conducted from February of 2011 to August of 2014 (Clinicaltrials.gov identifier: NCT01247311).
Patients
Patients were randomized at a ratio of 1:1:1 by permuted variable block design to receive placebo, calcitriol, or calcifediol. A sample size of 105 (35 in the placebo arm and 70 in the combined treatment arms) was calculated to provide 85% power to demonstrate the mean PWV difference of 1 m/s between arms, with 0.05 target α for a two-sided test (16). The study was approved by the University of British Columbia Research Ethics Board and funded by the Kidney Foundation of Canada in collaboration with Pfizer Pharmaceuticals, through a peer-reviewed grant. All participants provided informed consent. Patients were enrolled from two tertiary care, established Kidney Care Clinics in Vancouver if they met the following inclusion criteria: a stable eGFR (between 15 and 45 ml/min per 1.73 m2, defined as <±5 ml/min per 1.73 m2 change in the previous 6 months) and on stable doses of renin-angiotensin-aldosterone system blockade (Table 1). For those already receiving vitamin D therapies, a 3-month washout period before randomization was mandatory. Participants were excluded if they had active infections or inflammatory diseases, terminal malignancies, a planned transplant, were likely to commence dialysis within the 6 months after enrollment, or were unable to provide informed consent.
Table 1.
Baseline characteristics of the randomized participants by study arm
| Variable | All Participants | Placebo | Vitamin D Treatment | ||
|---|---|---|---|---|---|
| Calcitriol | Calcifediol | Total Vitamin D Treatment | |||
| N | 119 | 40 | 39 | 40 | 79 |
| Age, mean (SD), yr | 65.8 (13.1) | 64.5 (12.2) | 66.9 (11.7) | 65.9 (15.3) | 66.4 (13.5) |
| Men, n (%) | 81 (71) | 29 (73) | 28 (72) | 28 (70) | 56 (71) |
| Race, n (%) | |||||
| White | 77 (65) | 27 (68) | 23 (59) | 27 (68) | 50 (63) |
| Asian | 22 (18) | 10 (25) | 6 (15) | 6 (15) | 12 (15) |
| South Asian | 10 (8) | 3 (8) | 4 (10) | 3 (8) | 7 (9) |
| Other | 10 (8) | — | 6 (15) | 4 (10) | 10 (13) |
| Diabetes, n (%) | 48 (40) | 15 (38) | 18 (46) | 15 (38) | 33 (42) |
| Cardiovascular disease, n (%) | 41 (34) | 30 (38) | 14 (36) | 16 (40) | 11 (28) |
| Peripheral vascular disease, n (%) | 8 (7) | 3 (8) | 3 (8) | 2 (5) | 5 (6) |
| Cerebral vascular disease, n (%) | 10 (8) | 4 (10) | 2 (5) | 4 (10) | 6 (8) |
| Primary kidney disease, n (%) | |||||
| Diabetic nephropathy | 27 (23) | 8 (20) | 11 (29) | 8 (22) | 19 (25) |
| Hypertensive nephropathy | 31 (27) | 12 (30) | 9 (24) | 10 (27) | 19 (25) |
| GN | 28 (24) | 12 (30) | 7 (18) | 9 (24) | 16 (21) |
| Polycystic | 6 (5) | 1 (3) | 4 (11) | 1 (3) | 5 (7) |
| Tubulo-interstitial disease | 2 (2) | — | 1 (3) | 1 (3) | 2 (3) |
| Other | 15 (13) | 4 (10) | 5 (13) | 6 (16) | 11 (15) |
| Unknown | 6 (5) | 3 (8) | 1 (3) | 2 (5) | 3 (4) |
| Medications, n (%) | |||||
| Phosphate binder (calcium-based) | 37 (31) | 12 (30) | 13 (33) | 12 (30) | 25 (32) |
| Statin | 92 (77) | 32 (80) | 32 (82) | 28 (70) | 60 (76) |
| Bisphosphonate | 3 (3) | 2 (5) | — | 1 (3) | 1 (1) |
| Warfarin | 9 (8) | 3 (8) | 4 (10) | 2 (5) | 6 (8) |
| BMI, mean (SD), kg/m2 | 28.7 (6.6) | 28.8 (5.3) | 29.7 (5.9) | 29.0 (5.0) | 28.6 (7.2) |
| Systolic BP, mean (SD), mmHg | 138.5 (17.1) | 140.5 (17.2) | 134.9 (12.5) | 140.0 (20.3) | 137.5 (17.1) |
| Diastolic BP, mean (SD), mmHg | 72.3 (11.6) | 74.2 (12.3) | 70.4 (9.7) | 72.1 (12.5) | 71.3 (11.2) |
| Heart rate, mean (SD), bpm | 66.7 (11.2) | 66.9 (12.9) | 67.9 (11) | 65.5 (9.9) | 66.6 (10.3) |
| Radial artery pulse pressure, mean (SD), mmHg | 15.0 (9.1) | 15.5 (10.6) | 14.6 (8.3) | 14.8 (8.3) | 14.7 (8.3) |
| Augmentation index, mean (SD), % | 24.4 (10.5) | 24.0 (11.0) | 25.4 (8.8) | 23.7 (11.6) | 24.6 (10.3) |
| Pulse wave velocity, mean (SD), m/s | 11.5 (3.9) | 10.7 (3.7) | 11.6 (3.8) | 12.1 (4.2) | 11.9 (4.0) |
| eGFR-MDRD, mean (SD), ml/min per 1.73 m2 | 28.9 (8.9) | 26.4 (7.4) | 29.1 (9.0) | 31.1 (9.7) | 30.1 (9.4) |
| Urine ACR, median [IQR], mg/g | 151.8 [36.3–1161.1] | 303.6 [27.4–1465.5] | 101.8 [32.8–841.2] | 168.2 [49.6–802.2] | 137.6 [36.8–841.1] |
| Calcium, mean (SD), mg/dl | 9.3 (0.5) | 9.3 (0.4) | 9.3 (0.6) | 9.2 (0.5) | 9.3 (0.5) |
| Phosphate, mean (SD), mg/dl | 3.5 (0.6) | 3.5 (0.7) | 3.5 (0.6) | 3.5 (0.7) | 3.45 (0.6) |
| PTH, median [IQR], pg/ml | 102.8 [60.4–188.1] | 150.0 [75.4–232.0] | 84.0 [58.5–153.7] | 90.1 [57.1–150.9] | 87.3 [57.5–152.8] |
| FGF-23, median [IQR], pg/ml | 103.0 [69.4–236] | 142.3 [87–292.2] | 89.2 [59.7–136.8] | 101.6 [66.5–160.4] | 93.9 [66.4–222.9] |
| CRP, median [IQR], mg/L | 1.5 [0.7–4.2] | 1.4 [0.6–3.6] | 1.1 [0.6–3.0] | 2.9 [0.9–5.3] | 1.5 [0.7–4.8] |
| 25(OH)D, mean (SD), ng/ml | 27.0 (11.0) | 29.4 (12.7) | 26.6 (10.7) | 25.2 (9.3) | 25.9 (10.0) |
| 1,25(OH)2D, mean (SD), pg/ml | 21.8 (9.2) | 20 (7.2) | 21.7 (9.5) | 23.7 (10.6) | 22.7 (10) |
SI conversion factors: to convert urine ACR to mg/mmol, multiply by 0.113; to convert calcium to mmol/L, multiply by 0.25; to convert phosphate to mmol/L, multiply by 0.323; to convert PTH to pmol/L, multiply by 0.106; to convert 25(OH)D to nmol/L, multiply by 2.496; to convert 1,25(OH)2D to pmol/L, multiply by 2.6. —, null; BMI, body mass index; bpm, beats per minute; eGFR-MDRD, eGFR calculated using the Modification of Diet in Renal Disease Study equation; ACR, albumin-to-creatinine ratio; IQR, interquartile range; PTH, parathyroid hormone; FGF-23, fibroblast growth factor 23; CRP, C-reactive protein; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH) 2D, 1,25-dihydroxyvitamin D.
Interventions
The active medications were prescribed in fixed doses, irrespective of serum levels. The study drug was prepared in tinted vials and distributed to patients as a 6-week supply by a third-party pharmacy, MacDonald’s Prescriptions Ltd. Study participants were instructed to take 0.5 ml orally (equal to 5000 IU calcifediol [1,25-dihydroxyvitamin D3] or 0.5 µg calcitriol [1,25-dihydroxyvitamin D3]) with a graduated syringe, three times a week for 6 months. For the purpose of blinding, the calcifediol was suspended in almond oil to match the calcitriol product (Rocaltrol; Hoffmann-La Roche Ltd); pure almond oil was used for the placebo. The number of medications dispensed per patient was reported by the pharmacy.
Outcome Definitions
The primary outcome measure was vascular stiffness, measured noninvasively by PWV, 6 months after randomization, by applanation tonometry (SphygmoCor Technology). Measurements were done in triplicate at each visit and taken with the participant in the supine position, rested for 10 minutes. Secondary outcomes included measures of BP, proteinuria, and blood levels of calcium, phosphate, PTH, fibroblast growth factor 23 (FGF-23), and C-reactive protein (CRP). BP was measured in duplicate after 5 minutes of supine rest. Kidney function was calculated by using the Modification of Diet in Renal Disease formula for eGFR (17). Serum creatinine, CRP, 25(OH)D, and 1,25(OH)D were analyzed centrally in an accredited Providence Healthcare Laboratory using a high-performance liquid chromatography tandem mass spectrometer (modified iMethod; Applied Biosystems). FGF-23 was measured in duplicate using a two-step ELISA with a quantitative range of 10–800 pg/ml (Kainos Laboratories Inc., Tokyo, Japan) in the Centre for Heart and Lung Innovation, University of British Columbia (Vancouver, Canada). Serial levels of calcium and phosphate were drawn for safety purposes and performed at the two accredited hospital laboratories.
Statistical Analyses
Normally distributed data were summarized using the mean (SD) and skewed data were summarized using the median (interquartile range [IQR]). Markers with highly skewed distributions (urine albumin-to-creatinine ratio, PTH, FGF-23, and CRP) were transformed to the natural logarithmic scale for analyses. Unadjusted 6-month changes of primary and secondary outcomes were compared between the three arms by ANOVA (Table 2), with P values and 95% confidence intervals (95% CIs) adjusted for multiple comparisons using the Tukey–Kramer method. The t test was used for two-group analysis of unadjusted 6-month changes, reported with 95% CIs (Table 3). In accordance with the analysis plan (18), analysis of covariance (ANCOVA) was performed with 6-month changes of primary and secondary outcome as the dependent variable, allocated treatment as the independent variable, and respective baseline value as the baseline covariate. Treatment by covariate interactions was assessed to ensure the validity of this technique. In the three-arm analysis, P values and 95% CIs were adjusted for multiple comparisons using the Tukey–Kramer method. We used a mixed model approach to confirm robustness of the ANCOVA results. To examine difference in ΔPWV in the treatment arm compared with the placebo, we modeled the treatment and baseline PWV as random effects. Analyses were considered statistically significant if the P value was <0.05 for allocated treatment. A similar analytical approach was taken for secondary and observational analyses. For the observational analysis, we divided the study data set by tertiles of achieved 25(OH)D and achieved 1,25(OH)2D, anticipating that higher achieved vitamin D levels would be associated with improved primary and secondary outcomes. The Spearman correlation was used to describe relationships between rate of change of the laboratory parameters, including vitamin D levels, and rate of PWV change over 6 months. The analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC).
Table 2.
The primary and secondary outcomes by treatment type
| Variable | Placebo | Calcitriol | Calcifediol | Calcitriol versus Placebo | Calcifediol versus Placebo | Calcifediol versus Calcitriol |
|---|---|---|---|---|---|---|
| Pulse wave velocity, m/s | n=30 | n=28 | n=29 | |||
| Unadjusted baseline, mean (SD) | 10.6 (3.6) | 12.2 (3.6) | 12.4 (4.4) | |||
| Unadjusted 6 mo, mean (SD) | 11.7 (3.5) | 12.4 (3.5) | 11.3 (3.9) | |||
| Unadjusted change (95% CI) at 6 moa | 1.1 (−0.1 to 2.2) | 0.2 (−0.9 to 1.4) | −1.1 (−2.2 to 0.1) | −0.8 (−2.8 to 1.1) | −2.1 (−4.1 to −0.2) | −1.3 (−3.2 to 0.7) |
| P valuea | 0.55 | 0.03 | 0.26 | |||
| Adjusted change (95% CI) at 6 mob | 0.7 (−0.3 to 1.7) | 0.4 (−0.6 to 1.4) | −0.8 (−1.8 to 0.2) | −0.3 (−2.0 to 1.5) | −1.5 (−3.2 to 0.3) | −1.2 (−2.9 to 0.5) |
| P valueb | 0.93 | 0.11 | 0.23 | |||
| Systolic BP, mmHg | n=35 | n=34 | n=33 | |||
| Unadjusted baseline, mean (SD) | 138.8 (16.6) | 135.2 (13.0) | 140.2 (21.3) | |||
| Unadjusted 6 mo, mean (SD) | 139.3 (10.8) | 135.7 (13.0) | 144.1 (23.6) | |||
| Unadjusted change (95% CI) at 6 moa | 0.5 (−5.0 to 6.0) | 0.4 (−5.1 to 6.0) | 4.0 (−1.7 to 9.6) | −0.1 (−9.4 to 9.3) | 3.4 (−6.0 to 12.9) | 3.5 (−6.0 to 13.0) |
| P valuea | 0.99 | 0.66 | 0.65 | |||
| Adjusted change (95% CI) at 6 mob | 0.9 (−3.9 to 5.6) | −0.9 (−5.7 to 3.9) | 5.0 (0.1 to 9.9) | −1.8 (−9.9 to 6.3) | 4.1 (−4.0 to 12.3) | 5.9 (−2.4 to 14.2) |
| P valueb | 0.60 | 0.45 | 0.21 | |||
| Diastolic BP, mmHg | n=35 | n=34 | n=33 | |||
| Unadjusted baseline, mean (SD) | 73.7 (11.8) | 70.0 (9.9) | 73.0 (13.1) | |||
| Unadjusted 6 mo, mean (SD) | 75.1 (10.1) | 70.7 (9.1) | 75.0 (13.8) | |||
| Unadjusted change (95% CI) at 6 moa | 1.4 (−1.6 to 4.4) | 0.7 (−2.3 to 3.8) | 2.0 (−1.1 to 5.2) | −0.7 (−5.8 to 4.5) | 0.6 (−4.6 to 5.8) | 1.3 (−4.0 to 6.5) |
| P valuea | 0.83 | 0.96 | 0.83 | |||
| Adjusted change (95% CI) at 6 mob | 1.9 (−0.8 to 4.6) | −0.0 (−2.8 to 2.8) | 2.3 (−0.5 to 5.1) | −1.9 (−6.6 to 2.8) | 0.4 (−4.3 to 5.1) | 2.3 (−2.4 to 7.1) |
| P valueb | 0.59 | 0.98 | 0.48 | |||
| Urine ACR, ln(mg/g) | n=35 | n=31 | n=32 | |||
| Unadjusted baseline, mean (SD) | 5.3 (2.1) | 5.1 (1.6) | 5.2 (2.0) | |||
| Unadjusted 6 mo, mean (SD) | 5.4 (2.1) | 5.0 (1.8) | 5.2 (2.1) | |||
| Unadjusted change (95% CI) at 6 moa | 0.1 (−0.1 to 0.3) | −0.1 (−0.3 to 0.1) | 0.0 (−0.2 to 0.2) | −0.2 (−0.5 to 0.2) | −0.1 (−0.5 to 0.3) | 0.8 (−0.3 to 0.5) |
| P valuea | 0.54 | 0.85 | 0.87 | |||
| Adjusted change (95% CI) at 6 mob | 0.1 (−0.1 to 0.3) | −0.1 (−0.3 to 0.1) | 0.0 (−0.2 to 0.2) | −0.2 (−0.5 to 0.2) | −0.1 (−0.5 to 0.3) | 0.8 (−0.3 to 0.5) |
| P valueb | 0.54 | 0.85 | 0.87 | |||
| Calcium, mg/dl | n=35 | n=34 | n=33 | |||
| Unadjusted baseline, mean (SD) | 9.3 (0.4) | 9.3 (0.5) | 9.2 (0.5) | |||
| Unadjusted 6 mo, mean (SD) | 9.0 (1.2) | 9.4 (0.5) | 9.3 (0.5) | |||
| Unadjusted change (95% CI) at 6 moa | −0.3 (−0.5 to −0.0) | 0.1 (−0.2 to 0.4) | 0.1 (−0.2 to 0.4) | 0.4 (−0.1 to 0.8) | 0.4 (−0.1 to 0.8) | −0.0 (−0.4 to 0.4) |
| P valuea | 0.10 | 0.11 | >0.99 | |||
| Adjusted change (95% CI) at 6 mob | −0.3 (−0.5 to −0.0) | 0.1 (−0.1 to 0.4) | 0.1 (−0.2 to 0.3) | 0.4 (−0.0 to 0.8) | 0.4 (−0.1 to 0.8) | −0.0 (−0.5 to 0.4) |
| P valueb | 0.09 | 0.12 | 0.99 | |||
| Phosphate, mg/dl | n=35 | n=34 | n=33 | |||
| Unadjusted baseline, mean (SD) | 3.5 (0.7) | 3.4 (0.6) | 3.5 (0.7) | |||
| Unadjusted 6 mo, mean (SD) | 3.6 (0.8) | 3.6 (0.8) | 3.6 (0.8) | |||
| Unadjusted change (95% CI) at 6 moa | 0.2 (−0.0 to 0.4) | 0.2 (−0.0 to 0.4) | 0.2 (−0.0 to 0.4) | 0.0 (−0.3 to 0.3) | −0.0 (−0.4 to 0.3) | −0.0 (−0.4 to 0.3) |
| P valuea | 0.99 | 0.99 | 0.97 | |||
| Adjusted change (95% CI) at 6 mob | 0.2 (−0.0 to 0.4) | 0.2 (−0.0 to 0.4) | 0.2 (−0.0 to 0.4) | 0.0 (−0.3 to 0.3) | −0.0 (−0.3 to 0.3) | −0.0 (−0.4 to 0.3) |
| P valueb | 0.99 | 0.99 | 0.97 | |||
| PTH, ln(pg/ml) | n=35 | n=33 | n=33 | |||
| Unadjusted baseline, mean (SD) | 4.9 (0.7) | 4.6 (0.7) | 4.6 (0.7) | |||
| Unadjusted 6 mo, mean (SD) | 5.0 (0.8) | 4.4 (0.8) | 4.1 (0.9) | |||
| Unadjusted change (95% CI) at 6 moa | 0.1 (−0.0 to 0.3) | −0.3 (−0.4 to −0.1) | −0.5 (−0.6 to −0.3) | −0.4 (−0.7 to −0.1) | −0.6 (−0.9 to −0.3) | −0.2 (−0.5 to 0.1) |
| P valuea | <0.01 | <0.001 | 0.24 | |||
| Adjusted change (95% CI) at 6 mob | 0.2 (−0.0 to 0.3) | −0.2 (−0.4 to −0.1) | −0.5 (−0.6 to −0.3) | −0.4 (−0.7 to −0.1) | −0.6 (−0.9 to −0.3) | −0.2 (−0.5 to 0.1) |
| P valueb | <0.01 | <0.001 | 0.22 | |||
| FGF-23, ln(pg/ml) | n=33 | n=32 | n=33 | |||
| Unadjusted baseline, mean (SD) | 5.0 (0.7) | 4.7 (0.9) | 4.7 (0.9) | |||
| Unadjusted 6 mo, mean (SD) | 5.0 (0.9) | 4.9 (1.0) | 4.9 (0.9) | |||
| Unadjusted change (95% CI) at 6 moa | 0.1 (−0.1 to 0.3) | 0.2 (0.0 to 0.3) | 0.2 (0.1 to 0.4) | 0.1 (−0.2 to 0.4) | 0.1 (−0.1 to 0.4) | 0.1 (−0.2 to 0.3) |
| P valuea | 0.70 | 0.41 | 0.89 | |||
| Adjusted change (95% CI) at 6 mob | 0.1 (−0.1 to 0.3) | 0.2 (0.0 to 0.3) | 0.2 (0.1 to 0.4) | 0.1 (−0.2 to 0.4) | 0.1 (−0.1 to 0.4) | 0.1 (−0.2 to 0.3) |
| P valueb | 0.71 | 0.43 | 0.89 | |||
| CRP, ln(mg/L) | n=34 | n=32 | n=33 | |||
| Unadjusted baseline, mean (SD) | 0.3 (1.0) | 0.4 (1.3) | 0.6 (1.2) | |||
| Unadjusted 6 mo, mean (SD) | 0.2 (1.0) | 0.5 (1.1) | 0.6 (1.1) | |||
| Unadjusted change (95% CI) at 6 moa | −0.1 (−0.4 to 0.2) | 0.1 (−0.2 to 0.3) | 0.0 (−0.2 to 0.3) | 0.1 (−0.3 to 0.6) | 0.1 (−0.4 to 0.6) | −0.0 (−0.5 to 0.4) |
| P valuea | 0.75 | 0.83 | 0.99 | |||
| Adjusted change (95% CI) at 6 mob | −0.1 (−0.4 to 0.1) | 0.1 (−0.2 to 0.3) | 0.1 (−0.2 to 0.3) | 0.2 (−0.2 to 0.6) | 0.2 (−0.2 to 0.6) | 0.0 (−0.4 to 0.4) |
| P valueb | 0.54 | 0.51 | >0.99 | |||
| 25(OH)D, ng/ml | n=33 | n=32 | n=32 | |||
| Unadjusted baseline, mean (SD) | 29.1 (11.4) | 26.7 (10.8) | 25.8 (9.6) | |||
| Unadjusted 6 mo, mean (SD) | 26.2 (11.6) | 23.8 (8.9) | 94.1 (51.9) | |||
| Unadjusted change (95% CI) at 6 moa | −2.9 (−13.0 to 7.2) | −2.9 (−13.2 to 7.3) | 68.3 (58.1 to 78.6) | −0.0 (−17.3 to 17.2) | 71.2 (54.0 to 88.5) | 71.3 (53.9 to 88.7) |
| P valuea | >0.99 | <0.001 | <0.001 | |||
| Adjusted change (95% CI) at 6 mob | −2.8 (−13.0 to 7.4) | −2.9 (−13.2 to 7.4) | 68.3 (58.0 to 78.6) | −0.1 (−17.5 to 17.3) | 71.1 (53.6 to 88.6) | 71.2 (53.7 to 88.7) |
| P valueb | >0.99 | <0.001 | <0.001 | |||
| 1,25(OH)2D, pg/ml | n=33 | n=31 | n=32 | |||
| Unadjusted baseline, mean (SD) | 20.3 (7.4) | 21.7 (10.2) | 23.9 (10.9) | |||
| Unadjusted 6 mo, mean (SD) | 21.7 (7.6) | 27.2 (10.9) | 27.5 (14.3) | |||
| Unadjusted change (95% CI) at 6 moa | 1.4 (−2.6 to 5.4) | 5.5 (1.4 to 9.7) | 3.5 (−0.5 to 7.6) | 4.2 (−2.7 to 11.0) | 2.2 (−4.7 to 9.0) | −2.0 (−8.9 to 5.0) |
| P valuea | 0.33 | 0.73 | 0.78 | |||
| Adjusted change (95% CI) at 6 mob | 0.5 (−3.1 to 4.1) | 5.4 (1.7 to 9.1) | 4.6 (0.9 to 8.3) | 4.9 (−1.3 to 11.1) | 4.1 (−2.1 to 10.4) | −0.8 (−7.1 to 5.5) |
| P valueb | 0.15 | 0.25 | 0.95 |
SI conversion factors: to convert urine ACR to mg/mmol, multiply by 0.113; to convert calcium to mmol/L, multiply by 0.25; to convert phosphate to mmol/L, multiply by 0.323; to convert PTH to pmol/L, multiply by 0.106; to convert 25(OH)D to nmol/L, multiply by 2.496; to convert 1,25(OH)2D to pmol/L, multiply by 2.6. 95% CI, 95% confidence interval; ACR, albumin-to-creatinine ratio; PTH, parathyroid hormone; FGF-23, fibroblast growth factor 23; CRP, C-reactive protein; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
P values and 95% CIs adjusted for multiple comparisons using the Tukey–Kramer method.
Analysis of covariance results: adjusted mean, 95% CI, and P value for treatment effects with the respective baseline value as covariate.
Table 3.
The primary and secondary outcomes (6-month changes) by treatment type
| Variable | Placebo | Vitamin D Treatment | Treatment versus Placeboa,b | Treatment versus Placeboc |
|---|---|---|---|---|
| Pulse wave velocity, m/s | n=30 | n=57 | ||
| Unadjusted baseline, mean (SD) | 10.6 (3.6) | 12.3 (4.0) | ||
| Unadjusted 6 mo, mean (SD) | 11.7 (3.5) | 11.8 (3.7) | ||
| Unadjusted change (95% CI) at 6 mo | 1.1 (−0.1 to 2.2) | −0.4 (−1.2 to 0.4) | −1.5 (−2.9 to −0.1) | |
| P valuea | 0.04 | |||
| Adjusted change (95% CI) at 6 mob | 0.7 (−0.3 to 1.7) | −0.2 (−0.9 to 0.5) | −0.9 (−2.1 to 0.4) | −0.9 (−1.9 to 0.2) |
| P valueb | 0.17 | 0.10 | ||
| Systolic BP, mmHg | n=35 | n=67 | ||
| Unadjusted baseline, mean (SD) | 138.8 (16.6) | 137.7 (17.6) | ||
| Unadjusted 6 mo, mean (SD) | 139.3 (10.8) | 139.8 (19.3) | ||
| Unadjusted change (95% CI) at 6 mo | 0.5 (−5.0 to 6.0) | 2.2 (−1.8 to 6.1) | 1.7 (−5.1 to 8.4) | |
| P valuea | 0.63 | |||
| Adjusted change (95% CI) at 6 mob | 0.9 (−3.9 to 5.6) | 2.0 (−1.5 to 5.4) | 1.1 (−4.8 to 7.0) | N/A |
| P valueb | 0.70 | |||
| Diastolic BP, mmHg | n=35 | n=67 | ||
| Unadjusted baseline, mean (SD) | 73.7 (11.8) | 71.4 (11.6) | ||
| Unadjusted 6 mo, mean (SD) | 75.1 (10.1) | 72.8 (11.8) | ||
| Unadjusted change (95% CI) at 6 mo | 1.4 (−1.6 to 4.4) | 1.4 (−0.8 to 3.6) | −0.0 (−3.8 to 3.7) | |
| P valuea | 0.99 | |||
| Adjusted change (95% CI) at 6 mob | 1.9 (−0.8 to 4.6) | 1.1 (−0.9 to 3.1) | −0.8 (−4.2 to 2.6) | −0.8 (−4.2 to 2.6) |
| P valueb | 0.65 | 0.65 | ||
| Urine ACR, ln(mg/g) | n=35 | n=63 | ||
| Unadjusted baseline, mean (SD) | 5.3 (2.1) | 5.2 (1.8) | ||
| Unadjusted 6 mo, mean (SD) | 5.4 (2.1) | 5.1 (2.0) | ||
| Unadjusted change (95% CI) at 6 mo | 0.1 (−0.1 to 0.3) | −0.0 (−0.2 to 0.1) | −0.1 (−0.4 to 0.1) | |
| P valuea | 0.35 | |||
| Adjusted change (95% CI) at 6 mob | 0.1 (−0.1 to 0.3) | −0.0 (−0.2 to 0.1) | −0.1 (−0.4 to 0.1) | −0.1 (−0.4 to 0.1) |
| P valueb | 0.35 | 0.35 | ||
| Calcium, mg/dl | n=35 | n=67 | ||
| Unadjusted baseline, mean (SD) | 9.3 (0.4) | 9.3 (0.5) | ||
| Unadjusted 6 mo, mean (SD) | 9.0 (1.2) | 9.4 (0.5) | ||
| Unadjusted change (95% CI) at 6 mo | −0.3 (−0.5 to −0.0) | 0.1 (−0.1 to 0.3) | 0.4 (−0.1 to 0.7) | |
| P valuea | 0.02 | |||
| Adjusted change (95% CI) at 6 mob | −0.3 (−0.5 to −0.0) | 0.1 (−0.1 to 0.3) | 0.4 (−0.1 to 0.7) | 0.4 (−0.1 to 0.7) |
| P valueb | 0.02 | 0.02 | ||
| Phosphate, mg/dl | n=35 | n=67 | ||
| Unadjusted baseline, mean (SD) | 3.5 (0.7) | 3.5 (0.7) | ||
| Unadjusted 6 mo, mean (SD) | 3.6 (0.8) | 3.6 (0.8) | ||
| Unadjusted change (95% CI) at 6 mo | 0.2 (−0.0 to 0.4) | 0.2 (0.0 to 0.3) | −0.0 (−0.2 to 0.2) | |
| P valuea | 0.99 | |||
| Adjusted change (95% CI) at 6 mob | 0.2 (−0.0 to 0.4) | 0.2 (0.0 to 0.3) | −0.0 (−0.2 to 0.2) | 0.0 (−0.2 to 0.2) |
| P valueb | 0.98 | 0.96 | ||
| PTH, ln(pg/ml) | n=35 | n=66 | ||
| Unadjusted baseline, mean (SD) | 4.9 (0.7) | 4.6 (0.7) | ||
| Unadjusted 6 mo, mean (SD) | 5.0 (0.8) | 4.3 (0.8) | ||
| Unadjusted change (95% CI) at 6 mo | 0.1 (−0.0 to 0.3) | −0.3 (−0.5 to −0.2) | −0.5 (−0.7 to −0.3) | |
| P valuea | <0.001 | |||
| Adjusted change (95% CI) at 6 mob | 0.2 (−0.0 to 0.3) | −0.4 (−0.5 to −0.2) | −0.5 (−0.7 to −0.3) | −0.5 (−0.7 to −0.4) |
| P valueb | <0.001 | <0.001 | ||
| FGF-23, ln(pg/ml) | n=33 | n=65 | ||
| Unadjusted baseline, mean (SD) | 5.0 (0.7) | 4.7 (0.9) | ||
| Unadjusted 6 mo, mean (SD) | 5.0 (0.9) | 4.9 (1.0) | ||
| Unadjusted change (95% CI) at 6 mo | 0.1 (−0.1 to 0.3) | 0.2 (0.1 to 0.3) | 0.1 (−0.1 to 0.3) | |
| P valuea | 0.23 | |||
| Adjusted change (95% CI) at 6 mob | 0.1 (−0.1 to 0.3) | 0.2 (0.1 to 0.3) | 0.1 (−0.1 to 0.3) | 0.1 (−0.1 to 0.3) |
| P valueb | 0.24 | 0.26 | ||
| CRP, ln(mg/L) | n=34 | n=65 | ||
| Unadjusted baseline, mean (SD) | 0.3 (1.0) | 0.5 (1.2) | ||
| Unadjusted 6 mo, mean (SD) | 0.2 (1.0) | 0.5 (1.1) | ||
| Unadjusted change (95% CI) at 6 mo | −0.1 (−0.4 to 0.2) | 0.0 (−0.2 to 0.2) | 0.1 (−0.2 to 0.5) | |
| P valuea | 0.45 | |||
| Adjusted change (95% CI) at 6 mob | −0.1 (−0.4 to 0.1) | 0.1 (−0.1 to 0.2) | 0.2 (−0.1 to 0.5) | 0.2 (−0.1 to 0.5) |
| P valueb | 0.21 | 0.23 | ||
| 25(OH)D, ng/ml | n=33 | n=64 | ||
| Unadjusted baseline, mean (SD) | 29.1 (11.4) | 26.3 (10.1) | ||
| Unadjusted 6 mo, mean (SD) | 26.2 (11.6) | 59.0 (51.2) | ||
| Unadjusted change (95% CI) at 6 mo | −2.9 (−17.1 to 11.4) | 32.7 (22.5 to 42.9) | 35.6 (18.1 to 53.1) | |
| P valuea | <0.001 | |||
| Adjusted change (95% CI) at 6 mob | −2.6 (−17.0 to 11.7) | 32.6 (22.3 to 42.9) | 35.2 (17.5 to 53.0) | 35.2 (17.5 to 53.0) |
| P valueb | <0.001 | <0.001 | ||
| 1,25(OH)2D, pg/ml | n=33 | n=63 | ||
| Unadjusted baseline, mean (SD) | 20.3 (7.4) | 22.8 (10.6) | ||
| Unadjusted 6 mo, mean (SD) | 21.7 (7.6) | 27.3 (12.6) | ||
| Unadjusted change (95% CI) at 6 mo | 1.4 (−2.6 to 5.4) | 4.5 (1.6 to 7.4) | 3.1 (−1.8 to 8.1) | |
| P valuea | 0.21 | |||
| Adjusted change (95% CI) at 6 mob,c | 0.5 (−3.1 to 4.1) | 5.0 (2.4 to 7.6) | 4.5 (0.1 to 9.0) | 4.9 (0.9 to 8.8) |
| P valueb,c | 0.05 | 0.02 |
SI conversion factors: to convert urine ACR to mg/mmol, multiply by 0.113; to convert calcium to mmol/L, multiply by 0.25; to convert phosphate to mmol/L, multiply by 0.323; to convert PTH to pmol/L, multiply by 0.106; to convert 25(OH)D to nmol/L, multiply by 2.496; to convert 1,25(OH)2D to pmol/L, multiply by 2.6. 95% CI, 95% confidence interval; ACR, albumin-to-creatinine ratio; ln, natural logarithm; PTH, parathyroid hormone; FGF-23, fibroblast growth factor 23; CRP, C-reactive protein; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
Unadjusted mean change, 95% CI, and P value for treatment effects compared using the t test.
Adjusted mean change, 95% CI, and P value for treatment effects, with the respective baseline value as covariate, compared using analysis of covariance.
Mixed model results: estimates, 95% CI, and P value for treatment effects reported. All models include the treatment effect and respective baseline variable. The participants and treatment were modeled as random effects.
Further details of the study protocol and rationale have been previously published (16).
Results
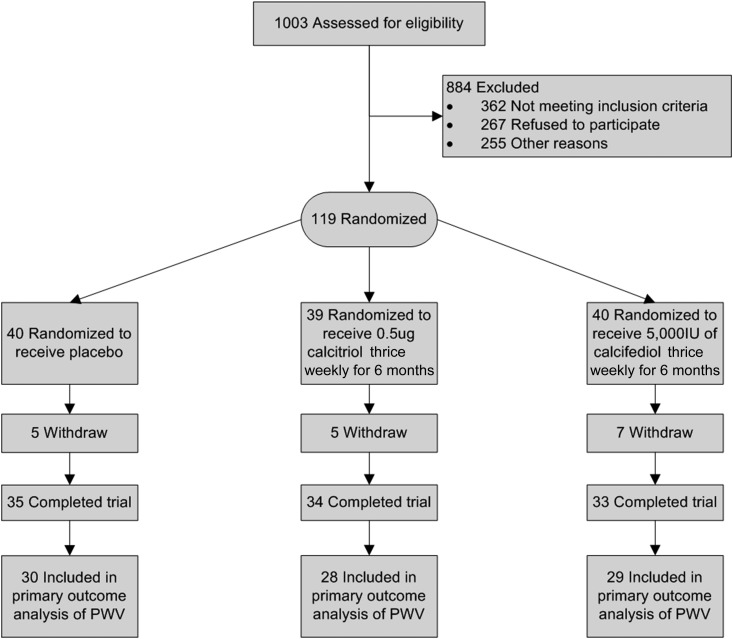
A total of 119 study participants were randomized to placebo (n=40), calcitriol (n=39), or calcifediol (n=40). Figure 1 describes the participant flow (19). There were no statistically significant differences in baseline characteristics between 102 patients who completed the 6-month follow-up and 17 patients who withdrew from study (n=15) or died (n=2). All patients with two evaluable measurements of PWV, at least 3 months apart, were included in the analysis of primary outcome according to their original randomization. Five patients in the placebo group, six patients in the calcitriol group, and four patients in the calcifediol group did not have evaluable 6-monthly PWV measurements.
Figure 1.
Participant flow diagram/CONSORT study diagram (19). Fifteen patients (five in the placebo group, six in the calcitriol group, and four in the calcifediol group) who completed the study but do not have the primary end point measure because of technical difficulties in measurement (difficulties obtaining good tracing because of body habitus, arrhythmias, or pacemakers that produced recordings that the SphygmoCor machine was unable to analyze). CONSORT, CONsolidated Standards Of Reporting Trials; PWV, pulse wave velocity.
Table 1 describes the baseline characteristics of the randomized participants in the placebo and specific treatment groups (calcitriol and calcifediol, respectively), the combined vitamin D treatment arm, and in total.
Mean age (SD) of randomized study participants was 66 years (13). The study participants were predominantly men (71%) and white (65%), 40% had diabetes, and 34% had a history of CV disease. The patients with CKD stage 3B and 4 had a mean (SD) eGFR of 28.9 (8.9) ml/min per 1.73 m2. The demographics and majority of clinical characteristics were balanced between the two groups. Sixty one percent of participants had moderate to mild (10–30 ng/ml) 25(OH)D deficiency and 63% of participants had 1,25(OH)2D deficiency (<24 pg/ml) at baseline.
Changes in Vitamin D Levels by Treatment Group
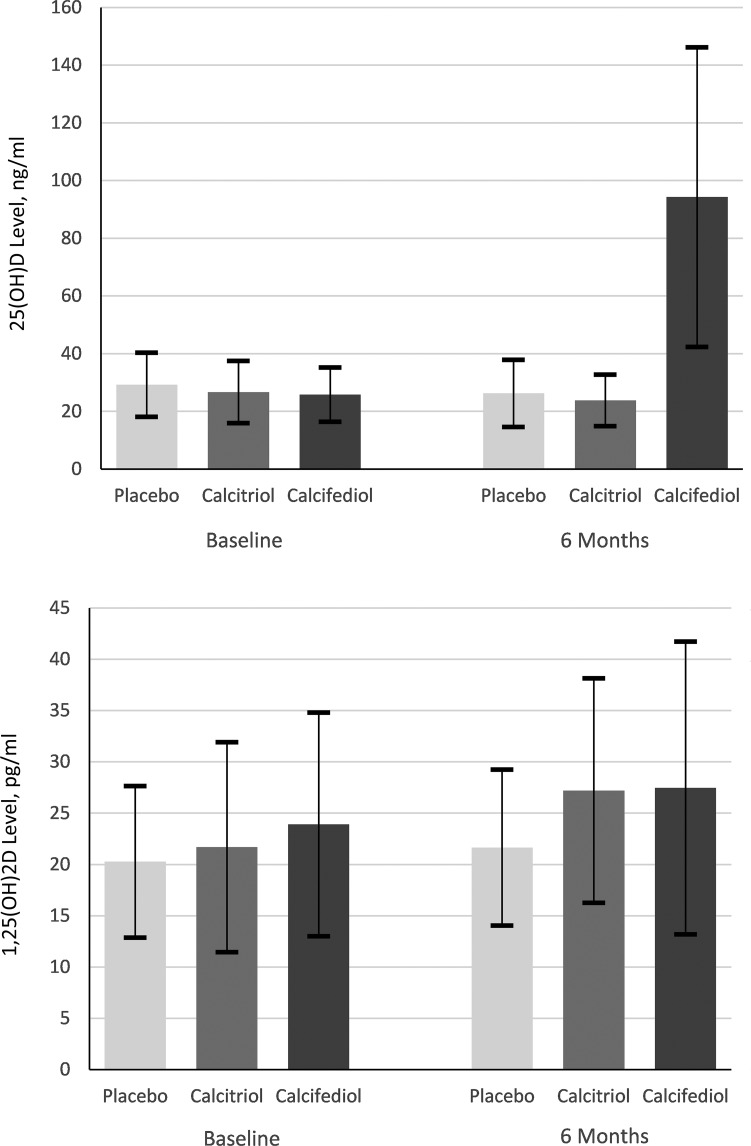
The median vitamin D study treatment time was 182 days (IQR 168–193). The calcitriol group received the mean (SD) 40.1 (3.1) µg of calcitriol and the calcifediol group received 387,772 (48,294) IU of calcifediol. Serum 25(OH)D and 1,25(OH)2D levels at baseline and 6 months are presented in Figure 2. Unadjusted 6-month changes in vitamin D levels and 6-month change adjusted for vitamin D baseline levels by treatment type are shown in Tables 2 and 3, with detailed information in Supplemental Material.
Figure 2.
25(OH)D and 1,25(OH)2D levels by treatment group at baseline and 6 months. Serum 25(OH)D and 1,25(OH)2D levels were summarized using mean (SD) and compared across treatment arms by analysis of covariance with baseline levels as covariate. P values are adjusted for multiple comparisons using the Tukey–Kramer method. The 25(OH)D and 1,25(OH)2D levels were balanced at the baseline. The 6-month change in the 25(OH)D levels in the calcifediol group was statistically significantly higher than both calcitriol (P<0.001) and placebo (P<0.001) group changes. The between-arm differences in the 6-month changes in the 1,25(OH)2D levels were not statistically significant. SI conversion factors: to convert 25(OH)D to nmol/L, multiply by 2.496; to convert 1,25(OH)2D to pmol/L, multiply by 2.6. 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
Primary Outcome: PWV Change during Study Period
Median time between the first and last PWV was 6.0 months (IQR, 5.9–6.7). The within-arm and between-arm 6-month changes in PWV are summarized in Table 2. The PWV decreased in the calcifediol group (mean change, −1.1; 95% CI, −2.2 to 0.1 m/s) over a 6-month period, remained unchanged in the calcitriol group (mean change, 0.2; 95% CI, −0.9 to 1.4 m/s), and increased in the placebo group (mean change, 1.1; 95% CI, −0.1 to 2.2 m/s). The overall P value for between-arm changes was 0.03. After accounting for the baseline PWV values using ANCOVA, the overall difference between three groups was not statistically significant (P=0.10). The analysis using mixed models also indicated statistically significant PWV decrease in the calcifediol group (estimate, −0.7; 95% CI, −1.6 to 0.3 m/s) compared with placebo group (estimate, 0.7; 95% CI, −0.2 to 1.6 m/s; P=0.03), with no significant difference in the calcitriol group (estimate, 0.3; 95% CI, −0.7 to 1.3 m/s), when compared with the placebo group (P=0.56) after adjusting for baseline PWV. However, there were no statistically significant differences between calcitriol and calcifediol groups (Table 2).
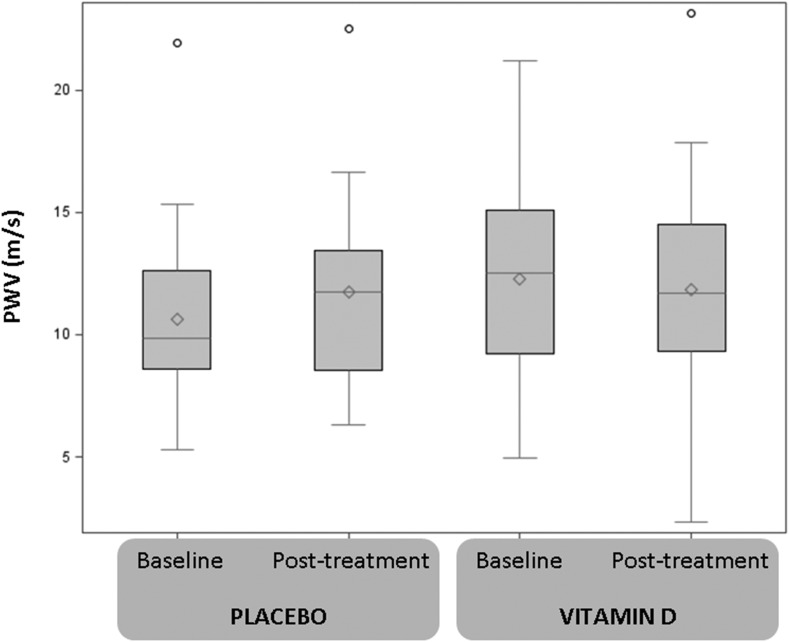
Figure 3 describes the change in PWV in the combined vitamin D treatment and the placebo group. Mean (SD) 6-month PWV values in the placebo and treatment arms were 11.7 (3.5) m/s and 11.8 (3.8) m/s, respectively. The treatment and placebo group differed in 6-month PWV change, with PWV decreasing in the treatment group (mean change, −0.4; 95% CI, 1.2 to 0.4 m/s) over a 6-month period, whereas it increased in the placebo group. The between-arm difference in the absolute PWV change was statistically significant (P=0.04).
Figure 3.
PWV levels by study arm at baseline and after 6 months of treatment. PWV levels were summarized using box-plots (mean, median, lower and upper quartile, 1.5 of interquartile range, and outliers) and compared between treatment arms using the t test. By chance, the vitamin D treatment arm had a higher baseline PWV. The 6-month change in PWV levels in the vitamin D treatment arm was statistically significantly different than the placebo group change (P=0.04). PWV, pulse wave velocity.
In accordance with the analysis plan (18), ANCOVA was performed with 6-month change of PWV as the dependent variable, allocated treatment as the independent variable, and baseline PWV as the baseline covariate comparing placebo versus combined vitamin D treatment arms. By chance, the vitamin D treatment arm had a higher baseline PWV (Table 3). In the model controlling for the baseline PWV, the between-arm (vitamin D versus placebo) difference was −0.9 m/s (95% CI, −2.1 to 0.4 m/s) and it was not statistically significant (type III F test, F=1.89; P=0.17). Treatment by covariate interactions was assessed to ensure the validity of this technique, and the interaction term was not statistically significant (P=0.98).
Similarly, the use of mixed model, with the baseline PWV as a baseline random effect covariate, resulted in consistent estimate of treatment effect. The estimate of difference between the vitamin D PWV change and the placebo PWV change was −0.9 m/s (95% CI, −1.9 to 0.2 m/s; P=0.10) (Table 3). Further planned adjustments for baseline covariates associated with outcome (age, sex, diabetes, preexisting CV disease, baseline kidney function, proteinuria, and BP level) did not significantly change the treatment effect estimates (data not shown).
Secondary Outcomes by Treatment Group
Table 2 summarizes baseline, 6-month, and Δ values for the secondary outcomes by three treatment groups for participants with available data. The analysis of secondary outcomes demonstrated statistically significant differences in 6-month changes between three groups in serum PTH. By chance, the vitamin D treatment arms had a higher baseline PWV. After adjustment for baseline PTH levels, both treatment groups had statistically significantly decreased PTH compared with the placebo group (Table 3); however, the difference between the calcifediol group and the calcitriol group was not statistically significant. None of the other tested biomarkers, nor BP, demonstrated statistically significantly different changes over 6 months between the three treatment arms.
The comparison of secondary outcomes between the combined vitamin D treatment group and the placebo group (Table 3) demonstrated statistically significant differences in 6-month changes in serum calcium and PTH. After accounting for the baseline calcium and PTH values using ANCOVA, the placebo group had a statistically significant decrease in calcium levels and an increase in PTH levels compared with the vitamin D group. The median PTH increased from 154.7 (IQR, 65.1–238.6) to 169.7 (IQR, 74.5–261.2) pg/ml in the placebo group and decreased from 90.5 (IQR, 58.5–153.7) to 68.4 (IQR, 39.6–116.9) pg/ml in the vitamin D group. The analysis using mixed models resulted in the consistent estimates of treatment effect (Table 3).
Treatment Effect Stratified by Baseline Vitamin D Levels
The results of subgroup analysis are presented in Tables 4 and 5. The change in PWV over the 6-month trial period differed by treatment group only among patients who were not 1,25(OH)2D–deficient at baseline. Similarly, the 6-month change in PWV was different by treatment group only among patients who were not 25(OH)D–deficient at baseline, although this difference only marginally missed statistical significance (P=0.05). The treatment effect did not differ by baseline vitamin D level for calcium and PTH; both among vitamin D–deficient and vitamin D nondeficient participants, calcium decreased in the placebo group and increased in the treatment group, whereas PTH increased in the placebo group and decreased in the treatment group.
Table 4.
Subgroup analysis: 6-month changes in primary and secondary outcomes by baseline vitamin 25(OH)D
| Variable | 25(OH)D=10–30 ng/ml (Mild-to-Moderate Deficiency), n=60 | 25(OH)D=31–100 ng/ml (Optimal Range), n=42 | ||||
|---|---|---|---|---|---|---|
| Placebo, n=17 | Treatment, n=43 | P Valuea | Placebo, n=18 | Treatment, n=24 | P Valuea | |
| Primary outcome | ||||||
| Pulse wave velocity, m/s | 0.3 (−1.1 to 1.6) | 0.2 (−0.6 to 1.1) | 0.97 | 1.1 (−0.5 to 2.7) | −1.1 (−2.4 to 0.3) | 0.04 |
| Secondary outcome | ||||||
| Systolic BP, mmHg | 1.4 (−5.2 to 7.9) | 1.8 (−2.3 to 5.9) | 0.92 | −0.9 (−8.4 to 6.5) | 3.3 (−3.1 to 9.7) | 0.40 |
| Diastolic BP, mmHg | 2.1 (−1.7 to 6.0) | 1.3 (−1.2 to 3.7) | 0.70 | 1.6 (−2.6 to 5.8) | 0.9 (−2.7 to 4.6) | 0.81 |
| Urine ACR, ln(mg/g) | −0.0 (−0.3 to 0.3) | −0.0 (−0.2 to 0.2) | 0.94 | 0.2 (−0.1 to 0.5) | −0.1 (−0.3 to 0.2) | 0.22 |
| Calcium, mg/dl | −0.4 (−0.9 to 0.0) | 0.1 (−0.2 to 0.4) | 0.07 | −0.1 (−0.3 to 0.0) | 0.2 (0.0 to 0.3) | 0.003 |
| Phosphate, mg/dl | 0.2 (−0.1 to 0.5) | 0.1 (−0.1 to 0.3) | 0.64 | 0.2 (−0.1 to 0.4) | 0.3 (0.1 to 0.6) | 0.35 |
| PTH, ln(pg/ml) | 0.2 (−0.0 to 0.5) | −0.3 (−0.5 to −0.2) | 0.002 | 0.1 (−0.1 to 0.4) | −0.4 (−0.6 to −0.2) | <0.001 |
| FGF-23, ln(pg/ml) | 0.1 (−0.2 to 0.3) | 0.1 (−0.0 to 0.3) | 0.58 | 0.1 (−0.1 to 0.4) | 0.4 (0.2 to 0.6) | 0.12 |
| CRP, ln(mg/l) | −0.1 (−0.4 to 0.3) | −0.0 (−0.2 to 0.2) | 0.81 | −0.2 (−0.5 to 0.2) | 0.2 (−0.1 to 0.5) | 0.10 |
| Serum vitamin D | ||||||
| 25(OH)D, ng/ml | 1.9 (−19.9 to 23.7) | 31.8 (18.1 to 45.3) | 0.02 | −6.4 (−25.8 to 13.0) | 33.7 (17.1 to 50.4) | 0.003 |
| 1,25(OH)2D, pg/ml | 2.1 (−2.1 to 6.2) | 4.2 (1.6 to 6.7) | 0.39 | −1.1 (−7.6 to 5.3) | 6.6 (0.9 to 12.3) | 0.08 |
Data are displayed as mean change and 95% CI. SI conversion factors: to convert urine ACR to mg/mmol, multiply by 0.113; to convert calcium to mmol/L, multiply by 0.25; to convert phosphate to mmol/L, multiply by 0.323; to convert PTH to pmol/L, multiply by 0.106; to convert 25(OH)D to nmol/L, multiply by 2.496; to convert 1,25(OH)2D to pmol/L, multiply by 2.6. 95% CI, 95% confidence interval; 25(OH)D, 25-hydroxyvitamin D; ACR, albumin-to-creatinine ratio; ln, natural logarithm; PTH, parathyroid hormone; FGF-23, fibroblast growth factor 23; CRP, C-reactive protein; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
Adjusted mean change, 95% CI, and P value for treatment effects, with the respective baseline value as covariate, compared using analysis of covariance.
Table 5.
Subgroup analysis: 6-month changes in primary and secondary outcomes by baseline vitamin 1,25(OH)2D levels
| Variable | 1,25(OH)2D<24 pg/ml (Deficiency), n=64 | 1,25(OH)2D=24–65 pg/ml (Reference Range), n=38 | ||||
|---|---|---|---|---|---|---|
| Placebo, n=25 | Treatment, n=39 | P Valuea | Placebo, n=10 | Treatment, n=28 | P Valuea | |
| Primary outcome | ||||||
| Pulse wave velocity, m/s | 0.1 (−1.4 to 1.4) | −0.1 (−1.0 to 1.2) | 0.98 | 1.7 (0.3 to 3.1) | −0.5 (−1.4 to 0.4) | 0.01 |
| Secondary outcome | ||||||
| Systolic BP, mmHg | −0.5 (−7.0 to 6.0) | 4.2 (−1.0 to 9.4) | 0.60 | 4.0 (−2.2 to 10.2) | −1.0 (−4.7 to 2.7) | 0.17 |
| Diastolic BP, mmHg | 1.9 (−1.9 to 5.8) | 0.6 (−2.4 to 3.7) | 0.59 | 2.7 (−1.0 to 6.5) | 1.5 (−0.7 to 3.7) | 0.58 |
| Urine ACR, ln(mg/g) | 0.2 (−0.1 to 0.5) | −0.1 (−0.3 to 0.1) | 0.13 | 0.0 (−0.4 to 0.4) | −0.1 (−0.3 to 0.2) | 0.71 |
| Calcium, mg/dl | −0.2 (−0.3 to −0.0) | 0.1 (0.0 to 0.3) | 0.003 | −0.6 (−1.3 to 0.2) | 0.0 (−0.4 to 0.5) | 0.16 |
| Phosphate, mg/dl | −0.2 (−0.0 to 0.4) | 0.2 (0.1 to 0.4) | 0.70 | 0.1 (−0.3 to 0.6) | 0.1 (−0.1 to 0.4) | 0.95 |
| PTH, ln(pg/ml) | 0.2 (−0.0 to 0.4) | −0.4 (−0.6 to −0.3) | <0.001 | 0.1 (−0.2 to 0.4) | −0.3 (−0.4 to −0.1) | 0.08 |
| FGF-23, ln(pg/ml) | 0.05 (−0.16 to 0.26) | 0.24 (0.08 to 0.40) | 0.15 | 0.20 (−0.07 to 0.47) | 0.16 (−0.01 to 0.32) | 0.79 |
| CRP, ln(mg/L) | −0.11 (−0.44 to 0.21) | 0.12 (−0.14 to 0.38) | 0.26 | −0.14 (−0.53 to 0.24) | −0.05 (−0.28 to 0.18) | 0.69 |
| Serum vitamin D | ||||||
| 25(OH)D, ng/ml | −0.4 (−17.8 to 17.0) | 35.6 (22.0 to 49.3) | 0.002 | −6.3 (−33.4 to 20.7) | 27.9 (11.4 to 44.3) | 0.03 |
| 1,25(OH)2D, pg/ml | 3.2 (−0.2 to 6.6) | 8.2 (5.5 to 10.9) | 0.03 | −4.4 (−13.3 to 4.5) | −0.1 (−5.5 to 5.4) | 0.41 |
Data are displayed as mean change and 95% CI. SI conversion factors: to convert urine ACR to mg/mmol, multiply by 0.113; to convert calcium to mmol/L, multiply by 0.25; to convert phosphate to mmol/L, multiply by 0.323; to convert PTH to pmol/L, multiply by 0.106; to convert 25(OH)D to nmol/L, multiply by 2.496; to convert 1,25(OH)2D to pmol/L, multiply by 2.6. 95% CI, 95% confidence interval; 1,25(OH)2D, 1,25-dihydroxyvitamin D; ACR, albumin-to-creatinine ratio; ln, natural logarithm; PTH, parathyroid hormone; FGF-23, fibroblast growth factor 23; CRP, C-reactive protein; 25(OH)D, 25-hydroxyvitamin D.
Adjusted mean change, 95% CI, and P value for treatment effects, with the respective baseline value as covariate, compared using analysis of covariance.
Observational Analysis by Tertiles of Achieved Vitamin D Levels
Participants who achieved the highest 25(OH)D tertile at the end of 6-month trial period had a statistically significant decrease in PWV, with a mean change of −1.0 m/s (95% CI, −2.0 to 0.0 m/s), compared with the middle and the lowest tertile (P<0.01). Similarly, the participants who achieved the highest 25(OH)D tertile had a decrease in PTH levels from baseline: the mean PTH change was −0.6 (95% CI, −0.8 to −0.4) ln(pg/ml). This decrease was statistically significantly different from the middle and the lowest tertile. See Supplemental Table 1, A and B for more details.
Overall, the PWV change had the highest correlation with log-transformed PTH change (r=0.26; P=0.02), 25(OH)D change (r=−0.24; P=0.03), and log-transformed albumin-to-creatinine ratio (r=0.24; P=0.03).
Biochemical and Clinical Safety
Predefined adverse events are summarized in Supplemental Table 2 and described in Supplemental Material. One patient in the calcitriol group experienced a transitory, asymptomatic, episode of hypercalcemia, identified on routine blood tests. We did not observe any other significant adverse outcomes related to the vitamin D treatment.
Discussion
We demonstrated that fixed dose supplementation with vitamin D analogs may result in a reduction in PWV, as compared with placebo. After 6 months, the PWV decreased in the calcifediol group, remained unchanged in the calcitriol group, and increased in the placebo group. In these patients with CKD stage 3B and 4, 25(OH)D and calcium levels increased and PTH decreased in those receiving supplements as compared with placebo. Because we observed correlation among the changes of PWV, PTH, and 25(OH)D, it is not possible to disentangle the PWV changes related to changes of PTH or vitamin D supplementation. There was no attempt to address deficiencies, given that optimal serum levels are not known, and in most regions, routine measurement of vitamin D is not done and compounds are prescribed. Thus, this study mimics current clinical practice. Those participants who achieved the highest 25(OH)D tertile demonstrated the greatest decrease in PWV, which was statistically significant. Interestingly, and perhaps further supporting our inattention to baseline serum levels of vitamin D, in those who were not deficient, the change in PWV was greatest, and was associated with the change in 25(OH)D levels. The reasons for this are not clear, and need to be further explored. We would submit that a critical assessment of current thresholds for 25(OH)D deficiency in CKD populations may be warranted, given these findings.
In keeping with our analytical plan, the baseline value was included as a covariate in the outcome analysis. Because the baseline PWV values were imbalanced between the groups (lowest in the placebo group), the treatment effect was attenuated when baseline PWV was included as a covariate. Because of this, we cannot exclude potential regression toward the mean effect. However, further exploratory analyses revealed that those who achieved the highest serum levels of vitamin D had the largest improvement in PWV. This is in keeping with our hypothesis that vascular stiffness does appear to be affected by vitamin D therapies. There are numerous studies in cell culture and animal models describing possible mechanisms by which vitamin D deficiency effects vascular tone, including altered vascular oxidative stress and smooth muscle cell function (20,21). These include modulation of response elements in the vascular endothelial growth factor promotor, reducing cytokine-induced expression of adhesion molecules and protecting against advanced glycation products. Furthermore, vitamin D has been implicated in matrix Gla-protein (inhibitor of vascular calcification) activation, which would add further credence to our observations (22).
The effect of vitamin D supplementation in patients with CKD on CV risk factors have been explored, although with different study designs, smaller numbers, different treatment regimens, and outcomes measures (15,23–29). In addition to studies in CKD populations, there are also randomized control trials examining arterial stiffness and vitamin D therapies in those with hypertension or diabetes (30–36), without impaired kidney function. The recent meta-analysis of ten randomized controlled trials (RCTs) (37) testing the effect of vitamin D supplementation on measures of arterial stiffness concluded that there is inconsistent evidence to suggest that vitamin D supplementation improves indicators of arterial stiffness. Vitamin D was associated with a nonsignificant reduction in PWV. An 8-week RCT of patients with CKD on cholecalciferol (40,000 IU, weekly) demonstrated a significant increase in 25(OH)D and 1,25(OH)2D, serum calcium, and FGF-23, as well as a lowering of PTH; however, there were no differential changes in PWV (35). Another RCT of nondiabetic patients with CKD stage 3–4 who received oral ergocalciferol (50,000 IU, weekly for 1 month, then monthly for up to 6 months) or placebo demonstrated improved microcirculatory endothelial function, but no significant changes in bone mineral parameters, BP, or aortic PWV (26). Both RCTs included only patients who were vitamin D–deficient, had other primary outcomes, and had small numbers. However, our study used consistent doses of vitamin D, given at the same interval (thrice weekly) for a 6-month period, in well treated patients with stable CKD already on RAS blockade (an agent known to affect PWV). Dosing was irrespective of blood levels of 25(OH)D or 1,25(OH)D, and irrespective of PTH or calcium levels. Thus, we address a different question in the context of previously published studies, and add to the current knowledge.
Our data are consistent with some previous findings and the Rodriguez meta-analysis conclusions (36), which show an inconsistent, nonsignificant reduction in PWV. Given the different durations, compounds used, comparators, and different selection criteria, it is difficult to draw firm conclusions. This study is one of the largest conducted to date, with novelty factors being fixed doses of supplementation and no consideration of baseline serum levels for treatment allocation or study enrolment. We mimic current clinical practice in many jurisdictions, thus allowing some practical translation of our findings to clinical care.
This study is of relatively short duration, although longer than most of the others cited above, and despite being larger than many of these studies, still has a relatively small sample size and, by chance, randomization did not balance a key variable of interest (PWV). Furthermore, the PWV values had higher variations than we had hypothesized: the observed SD was twice that of the hypothesized SD. Also, the attrition rate was even higher than expected. Thus, as is often the case with RCTs, with the relatively small sample size, smaller than anticipated effect size, and the a priori planned adjustments, we were not able to consistently demonstrate the statistical significance of the treatment effect. Note that many of the P values do approach significance, indicating that the study was underpowered. However, recent statements by the American Statistics Association (38) have questioned the strict adherence to P<0.05 for all studies and outcomes. We would suggest that, given the signals seen in this study and the supporting biologic data from other studies in humans and animals, our findings may be seen as suggestive and warrant further confirmation.
In patients with advanced CKD, the 6 months of supplemental administration of vitamin D analogs may reduce PWV in those who have higher PWV values. However, this positive effect was attenuated when adjustments for baseline PWV were made. There does not appear to be any adverse effect of this fixed dose regimen on patient outcomes, tolerance, or other parameters. Interestingly, there were no incidences of symptomatic hypercalcemia. Furthermore, despite a statistically significant difference in PTH values (lower in the treatment arm), the results trended toward improvement of vascular stiffness measurements with supplementation. This suggests that basing commencement of therapy on values of PTH may not be valid.
This study is important in that the findings suggest a potential mechanism of action of vitamin D analogs (reduction in vascular stiffness) that would help to explain outcomes in other studies (15) and inform future study design. We suggest a larger study with a similar design would be essential to validate these findings, and to facilitate the understanding of the effect of different vitamin D analogs.
Disclosures
None.
Supplementary Material
Acknowledgements
This study was sponsored by the Kidney Foundation of Canada and Pfizer Pharmaceuticals.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “What Is the Role of Vitamin D Supplementation on Vascular Health in CKD?,” on pages 1377–1379.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10791016/-/DCSupplemental.
References
- 1.Dusso AS, Tokumoto M: Defective renal maintenance of the vitamin D endocrine system impairs vitamin D renoprotection: A downward spiral in kidney disease. Kidney Int 79: 715–729, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Chitalia N, Recio-Mayoral A, Kaski JC, Banerjee D: Vitamin D deficiency and endothelial dysfunction in non-dialysis chronic kidney disease patients. Atherosclerosis 220: 265–268, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Pilz S, Tomaschitz A, Friedl C, Amrein K, Drechsler C, Ritz E, Boehm BO, Grammer TB, März W: Vitamin D status and mortality in chronic kidney disease. Nephrol Dial Transplant 26: 3603–3609, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Chitalia N, Ismail T, Tooth L, Boa F, Hampson G, Goldsmith D, Kaski JC, Banerjee D: Impact of vitamin D supplementation on arterial vasomotion, stiffness and endothelial biomarkers in chronic kidney disease patients. PLoS One 9: e91363, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Z, Shi H, Jia J, Li D, Lin S: Vitamin D supplementation and mortality risk in chronic kidney disease: A meta-analysis of 20 observational studies. BMC Nephrol 14: 199, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duranton F, Rodriguez-Ortiz ME, Duny Y, Rodriguez M, Daurès JP, Argilés A: Vitamin D treatment and mortality in chronic kidney disease: A systematic review and meta-analysis. Am J Nephrol 37: 239–248, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Navaneethan SD, Schold JD, Arrigain S, Jolly SE, Jain A, Schreiber MJ Jr, Simon JF, Srinivas TR, Nally JV Jr: Low 25-hydroxyvitamin D levels and mortality in non-dialysis-dependent CKD. Am J Kidney Dis 58: 536–543, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isakova T, Gutiérrez OM, Patel NM, Andress DL, Wolf M, Levin A: Vitamin D deficiency, inflammation, and albuminuria in chronic kidney disease: Complex interactions. J Ren Nutr 21: 295–302, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Levin A, Le Barbier M, Er L, Andress D, Sigrist MK, Djurdjev O: Incident isolated 1,25(OH)2D3 deficiency is more common than 25(OH)D deficiency in CKD. J Nephrol 25: 204–210, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Zoccali C, Mallamaci F: Moderator’s view: Vitamin D deficiency treatment in advanced chronic kidney disease: A close look at the emperor’s clothes. Nephrol Dial Transplant 31: 714–716, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Goldsmith DJ: Pro: Should we correct vitamin D deficiency/insufficiency in chronic kidney disease patients with inactive forms of vitamin D or just treat them with active vitamin D forms? Nephrol Dial Transplant 31: 698–705, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Agarwal R, Georgianos PI: Con: Nutritional vitamin D replacement in chronic kidney disease and end-stage renal disease. Nephrol Dial Transplant 31: 706–713, 2016 [DOI] [PubMed] [Google Scholar]
- 14.de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D: Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): A randomised controlled trial. Lancet 376: 1543–1551, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, Bhan I, Agarwal R, Zoccali C, Wanner C, Lloyd-Jones D, Cannata J, Thompson BT, Andress D, Zhang W, Packham D, Singh B, Zehnder D, Shah A, Pachika A, Manning WJ, Solomon SD: Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: The PRIMO randomized controlled trial. JAMA 307: 674–684, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Levin A, Perry T, De Zoysa P, Sigrist MK, Humphries K, Tang M, Djurdjev O: A randomized control trial to assess the impact of vitamin D supplementation compared to placebo on vascular stiffness in chronic kidney disease patients. BMC Cardiovasc Disord 14: 156, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Annals Intern Med 145: 247–254 [DOI] [PubMed] [Google Scholar]
- 18.Committee for Medicinal products for Human Use, European Medicines Agency Science Medicines Health: Guideline on adjustment for baseline covariates in clinical trials, 2015. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500184923.pdf. Accessed January 15, 2016
- 19.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, Elbourne D, Egger M, Altman DG; CONSORT : CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int J Surg 10: 28–55, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Kassi E, Adamopoulos C, Basdra EK, Papavassiliou AG: Role of vitamin D in atherosclerosis. Circulation 128: 2517–2531, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Argacha JF, Egrise D, Pochet S, Fontaine D, Lefort A, Libert F, Goldman S, van de Borne P, Berkenboom G, Moreno-Reyes R: Vitamin D deficiency-induced hypertension is associated with vascular oxidative stress and altered heart gene expression. J Cardiovasc Pharmacol 58: 65–71, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Proudfoot D, Shanahan CM: Molecular mechanisms mediating vascular calcification: Role of matrix Gla protein. Nephrology (Carlton) 11: 455–461, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Lundwall K, Jörneskog G, Jacobson SH, Spaak J: Paricalcitol, microvascular and endothelial function in non-diabetic chronic kidney disease: A randomized trial. Am J Nephrol 42: 265–273, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Zoccali C, Curatola G, Panuccio V, Tripepi R, Pizzini P, Versace M, Bolignano D, Cutrupi S, Politi R, Tripepi G, Ghiadoni L, Thadhani R, Mallamaci F: Paricalcitol and endothelial function in chronic kidney disease trial. Hypertension 64: 1005–1011, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Mose FH, Vase H, Larsen T, Kancir ASP, Kosierkiewic R, Jonczy B, Hansen AB, Oczachowska-Kulik AE, Thomsen IM, Bech JN, Pedersen EB: Cardiovascular effects of cholecalciferol treatment in dialysis patients--a randomized controlled trial. BMC Nephrol 15: 50, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dreyer G, Tucker AT, Harwood SM, Pearse RM, Raftery MJ, Yaqoob MM: Ergocalciferol and microcirculatory function in chronic kidney disease and concomitant vitamin d deficiency: An exploratory, double blind, randomised controlled trial. PLoS One 9: e99461, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hewitt NA, O’Connor AA, O’Shaughnessy DV, Elder GJ: Effects of cholecalciferol on functional, biochemical, vascular, and quality of life outcomes in hemodialysis patients. Clin J Am Soc Nephrol 8: 1143–1149, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thadhani R, Appelbaum E, Chang Y, Pritchett Y, Bhan I, Agarwal R, Zoccali C, Wanner C, Lloyd-Jones D, Cannata J, Thompson T, Audhya P, Andress D, Zhang W, Ye J, Packham D, Singh B, Zehnder D, Manning WJ, Pachika A, Solomon SD: Vitamin D receptor activation and left ventricular hypertrophy in advanced kidney disease. Am J Nephrol 33: 139–149, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, Light RP, Agarwal R: Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: A randomized double-blind pilot trial. Hypertension 52: 249–255, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Forouhi NG, Menon RK, Sharp SJ, Mannan N, Timms PM, Martineau AR, Rickard AP, Boucher BJ, Chowdhury TA, Griffiths CJ, Greenwald SE, Griffin SJ, Hitman GA: Effects of vitamin D2 or D3 supplementation on glycaemic control and cardiometabolic risk among people at risk of type 2 diabetes: Results of a randomized double-blind placebo-controlled trial. Diabetes Obes Metab 18: 392–400, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pilz S, Gaksch M, Kienreich K, Grübler M, Verheyen N, Fahrleitner-Pammer A, Treiber G, Drechsler C, Ó Hartaigh B, Obermayer-Pietsch B, Schwetz V, Aberer F, Mader J, Scharnagl H, Meinitzer A, Lerchbaum E, Dekker JM, Zittermann A, März W, Tomaschitz A: Effects of vitamin D on blood pressure and cardiovascular risk factors: A randomized controlled trial. Hypertension 65: 1195–1201, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Ryu O-H, Chung W, Lee S, Hong K-S, Choi M-G, Yoo HJ: The effect of high-dose vitamin D supplementation on insulin resistance and arterial stiffness in patients with type 2 diabetes. Korean J Intern Med 29: 620–629, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witham MD, Adams F, McSwiggan S, Kennedy G, Kabir G, Belch JJ, Khan F: Effect of intermittent vitamin D3 on vascular function and symptoms in chronic fatigue syndrome--a randomised controlled trial. Nutr Metab Cardiovasc Dis 25: 287–294, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Yiu YF, Yiu KH, Siu CW, Chan YH, Li SW, Wong LY, Lee SW, Tam S, Wong EW, Lau CP, Cheung BM, Tse HF: Randomized controlled trial of vitamin D supplement on endothelial function in patients with type 2 diabetes. Atherosclerosis 227: 140–146, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Marckmann P, Agerskov H, Thineshkumar S, Bladbjerg E-M, Sidelmann JJ, Jespersen J, Nybo M, Rasmussen LM, Hansen D, Scholze A: Randomized controlled trial of cholecalciferol supplementation in chronic kidney disease patients with hypovitaminosis D. Nephrol Dial Transplant 27: 3523–3531, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Witham MD, Dove FJ, Dryburgh M, Sugden JA, Morris AD, Struthers AD: The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: A randomised controlled trial. Diabetologia 53: 2112–2119, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez AJ, Scott D, Srikanth V, Ebeling P: Effect of vitamin D supplementation on measures of arterial stiffness: A systematic review and meta-analysis of randomized controlled trials. Clin Endocrinol (Oxf) 84: 645–657, 2016 [DOI] [PubMed] [Google Scholar]
- 38.Wasserstein RL, Lazar NA: The ASA’s statement on p-values: Context, process, and purpose. Am Stat 70: 129, 2016 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.