Abstract
Background and objectives
Several drugs used in CKD can prolong electrocardiographic conduction. We examined the use of electrocardiogram QT-prolonging medications in predialysis CKD and their association with QT duration.
Design, setting, participants, & measurements
In total, 3252 Chronic Renal Insufficiency Cohort participants with at least one study electrocardiogram between 2003 and 2011 were included. QT-prolonging medications used in 100 or more visits (n=16,451 visits) along with diuretics and proton pump inhibitors, given their potential for electrolyte disturbances, were examined for QT interval prolongation.
Results
Mean QT interval corrected for heart rate was at 414±21 (±SD) milliseconds and prolonged (≥450 milliseconds) in 4.6% of electrocardiograms. QT interval corrected for heart rate was inversely related to serum potassium and calcium. Medications classified as QT prolonging were taken at 76% of visits, with two or more of these taken at 33% of visits. Of 30 medications examined, eight were associated with statistically significant QT interval corrected for heart rate prolongation after adjustment for comorbidities, potassium, and calcium, including amiodarone (+10±2 milliseconds), metolazone (+7±2 milliseconds), fluoxetine (+4±1 milliseconds), citalopram (+4±1 milliseconds), hydroxyzine (+4±1 milliseconds), escitalopram (+3±2 milliseconds), venlafaxine (+3±1 milliseconds), and furosemide (+3±0 milliseconds). Potassium-depleting diuretics were associated with minimal decrements in potassium (between 0.1 and 0.3 mEq/L) and smaller changes in calcium. Diuretics associated with a change in QT interval corrected for heart rate before adjustment for potassium and calcium were metolazone (+8±3 milliseconds), furosemide (+4±1 milliseconds), and spironolactone (−3±3 milliseconds). Most of the QT prolongation associated with metolazone and furosemide, but not spironolactone, remained after adjustment for potassium and calcium. Proton pump inhibitors were not associated with QT prolongation.
Conclusions
Use of medications associated with QT prolongation is common in CKD; the safety implications of these findings should be considered in these high-risk patients.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2017_08_09_CJASNPodcast_17_09_b.mp3
Keywords: Amiodarone; Arrhythmias, Cardiac; Brugada Syndrome; Citalopram; Diuretics; Electrocardiography; Electrolytes; Fluoxetine; Furosemide; Heart Conduction System; Humans; Hydroxyzine; Metolazone; Potassium; Proton Pump Inhibitors; Renal Dialysis; Renal Insufficiency, Chronic; Sodium Chloride Symporter Inhibitors; Spironolactone; Venlafaxine Hydrochloride; Cardiac Conduction Defect
Introduction
Patients with CKD have a high incidence of cardiovascular disease (CVD) and related mortality (1,2) from congestive heart failure (CHF), coronary disease, and sudden cardiac death (3). Abnormalities detectable on a resting electrocardiogram (ECG) are predictors of adverse CVD outcomes in CKD (4–6). Specifically, a prolonged QT interval corrected for heart rate (QTc), which is a predictor of the fatal arrhythmia torsade de pointes (TdP), is associated with increased CKD mortality (4). Some medications commonly used in CKD can cause QTc prolongation. The threshold for regulatory concern from the Food and Drug Administration (FDA) is for drugs prolonging the QT/QTc interval by 5 milliseconds or more (7).
This report sets out to identify potentially ECG QT-prolonging drugs taken by patients with predialysis CKD and quantify any QT prolongation associated with these drugs. In addition, because patients with CKD frequently are prescribed diuretics and proton pump inhibitors (PPIs), which potentially prolong QT via potassium and calcium imbalance for the former and magnesium depletion for the latter, we included these in the analysis.
Materials and Methods
Cohort and ECG Ascertainment
We examined the National Institute of Diabetes and Digestive and Kidney Diseases–funded prospective, multicenter Chronic Renal Insufficiency Cohort (CRIC) Study. The methods and participant characteristics were described previously (8). Briefly, the CRIC Study phase 1/2 recruitment was from 2003 to 2008. Eligible subjects were ages 21–74 years old with mild to moderate CKD (GFR between 20 and 70 ml/min per 1.73 m2). By design, approximately one half of those recruited had diabetes, and one half were women.
Study participants had annual visits with resting ECGs obtained using a GE MAC 1200 electrocardiograph (GE Healthcare, Wauwatosa, WI) with a standardized protocol that were transmitted to the CRIC ECG Reading Center (Wake Forest School of Medicine, Winston-Salem, NC). The ECGs were processed, after visual inspection for quality, using the 2011 version of the GE Marquette 12-SL program (GE Healthcare). Enrollment included 3939 individuals who completed 21,303 in-center visits with medication data at time of data lock for analysis. We stepwise excluded visits including those after ESRD onset (n=1549 visits), missing an ECG (n=857 visits), with ECG findings indeterminate for QT duration due to atrial fibrillation or flutter (n=333 visits) or missing a code for atrial fibrillation or flutter (n=373 visits), or with QRS duration ≥120 milliseconds (n=1740 visits). The final visit number was 16,451 in 3252 participants.
Medication Ascertainment
Participants were interviewed for medication usage annually. All prescription and nonprescription medications, herbal and dietary supplements, and vitamins from the preceding 30 days were recorded at the time of the participant visit. Participants maintained a list of medications or brought them to facilitate recall. Individual drug codes and route of administration were recorded using the dynamically updated CRIC Medication Reference Tool, which contains a dictionary of drug codes applied to medications and supplements available over the counter.
In total, 180 QT-prolonging drugs were identified by CredibleMeds.org (9). We included the following three categories as defined by CredibleMeds.org. (1) Known risk of TdP (n=48 drugs): these drugs prolong the QT interval and have a known risk of TdP, even when taken as recommended. (2) Possible risk of TdP (n=72): these drugs can cause QT prolongation but lack evidence for a risk of TdP when taken as recommended. (3) Conditional risk of TdP (n=32): these drugs are associated with TdP but only under certain circumstances of use (e.g., with excessive dose, hypokalemia, or interacting drugs) or by creating conditions that induce TdP (e.g., by inhibiting metabolism of a QT-prolonging drug or causing an electrolyte disturbance that induces TdP). A fourth category, drugs to avoid in congenital long QT, including drugs that do not prolong the QT interval but have a risk of causing arrhythmia on the basis of their cardiac stimulant actions (e.g., β-adrenoceptor agonists), were disregarded because they are not relevant to this project.
Given that diuretic use is common in CKD and that associated electrolyte derangements, especially hypokalemia, can cause QT prolongation (10–13), we examined hydrochlorothiazide, furosemide, and torsemide, which are included by CredibleMeds.org, and all other thiazide, loop, and potassium-sparing diuretics—hypothesizing that the latter would shorten QT duration. Likewise, because PPIs are postulated to cause QT prolongation due to magnesium depletion (14), we examined all PPIs in addition to pantoprazole, which is the only PPI at CredibleMeds.org.
The frequency of usage for the listed drugs ranged widely. The most common, furosemide, was recorded at almost 30% of visits, whereas others were not recorded at all. We restricted our analysis to drugs recorded at ≥100 visits.
Data Preparation
In agreement with the American Heart Association, the American College of Cardiology, and the Heart Rhythm Society (15), we used linear regression to correct QT duration for heart rate rather than Bazett or other historically used equations. QTc was calculated by regressing observed QT duration on RR duration using the general estimating equation (GEE) to adjust for within-patient clustering as described in Statistical Methods. The resulting equation was QTc = QT+0.175×(1000−RR), where QT was observed QT in milliseconds, and RR was RR duration in milliseconds. For perspective, the coefficient derived by Sagie et al. (16) in the Framingham Study participants aged 28–62 years old was 0.154 (rather than 0.175). In agreement with the FDA Guidance for Industry (7), we used the same cutoff for prolonged QTc in men and women: ≥450 milliseconds.
Measurement of Covariates
At each visit, staff collected demographic variables, clinical characteristics, and self-reported history of diabetes, hypertension, heart failure, CVD (prior myocardial infarction [MI] or coronary resvascularization), and stroke. Body mass index (BMI) was calculated with measured height and weight. eGFR was calculated from serum creatinine and cystatin C using the CRIC-based equation (17). The CRIC laboratory at the University of Pennsylvania performed all laboratory assays. All participants underwent a transthoracic echocardiogram at years 1 and 4 until 2008, after which participants with only a year 1 echocardiogram underwent their final echocardiogram ≥1 year after the first study. All studies underwent standardized central readings at University of Pennsylvania.
Statistical Methods
Descriptive analyses examined group differences by t tests for continuous variables and chi-squared tests for categorical variables. The associations between medication use and QTc duration were examined with multivariable models, which simultaneously included as independent variables all 30 drugs recorded at ≥100 visits. The demographic and clinical variables collected at each visit used as covariates in all multivariable analyses and retaining nominal significance (P<0.05) included (except where noted) serum potassium, serum calcium, systolic BP, age, sex, race/ethnicity, eGFR, BMI, current smoking, history of MI/revascularization, hypertension, chronic heart failure, and stroke. To account for possible correlations within patients, we used the GEE as implemented in SAS PROC GENMOD (SAS Institute, Cary, NC). Logistic regression with a specified binomial distribution and logit link in GEE evaluated the association between drug use and QTc prolongation as a binary outcome.
To examine the QTc changes associated with diuretics and the extent to which they seem to be mediated by serum potassium and calcium, we first examined whether taking a particular diuretic was associated with electrolyte changes. We also included potassium-sparing diuretics in the analysis. Next, we quantified the association of each drug with QTc duration in a GEE model as described above that included all 30 eligible drugs plus three potassium-sparing diuretics and all covariates with the exception of serum potassium and calcium. Lastly, to assess whether electrolyte shifts account for associations between QTc and use of a given drug, we examined whether associations between drugs and QTc duration were sustained after adjustment for the electrolytes.
As a confirmatory analysis, we examined the association between QTc duration and drug use in a subset of visits selected for their reported first use of a particular drug by a study participant. We used the immediately preceding visit without use of the drug in the same patient as reference (off-on) and determined the mean difference in QTc duration between the two visits. Lastly, we used GEE to examine whether the associations with the use of diuretics in our main analysis persisted with adjustment for left ventricular mass (corrected for body surface area) and ejection fraction measured by echocardiography at a subset of 4743 visits. All analyses used SAS 9.3, with each participant visit as the unit of observation.
Results
Characteristics of the Subjects
The mean (±SD) number of visits completed by each participant was 5±2, with a median of six (interquartile range of 3–7). Table 1 shows the demographic, comorbidity, and clinical characteristics of participant visits classified by ECG QTc prolongation. At the 757 (4.6%) study visits where QTc was prolonged, participants were older, were more likely to be non-Hispanic black, currently smoked, and were more likely to have a history of MI/revascularization, chronic heart failure, diabetes, hypertension, or stroke. At visits with prolonged QTc, participants had a higher BMI and lower eGFR. The average serum potassium was also lower at visits with QTc prolongation.
Table 1.
Participant characteristics by QT interval corrected for heart rate duration at each person visit (N=16,451)
| Characteristics | All | QTc<450 ms | QTc≥450 ms |
|---|---|---|---|
| N visits (%) | 16,451 | 15,694 (95.4) | 757 (4.6) |
| Age, yr | 60±11 | 60±11a | 62±9 |
| Men, % | 53 | 54a | 40 |
| Race/ethnicity, % | |||
| Non-Hispanic white | 46 | 47a | 34 |
| Non-Hispanic black | 40 | 40a | 52 |
| Hispanic | 9 | 9b | 7 |
| Other race/ethnicity | 4 | 4a | 7 |
| Self-reported comorbidity, % | |||
| MI/revascularization | 23 | 22a | 36 |
| Congestive heart failure | 8 | 7a | 20 |
| Diabetes | 47 | 47a | 56 |
| Hypertension | 90 | 89a | 95 |
| Stroke | 10 | 10a | 17 |
| Current smoker, % | 11 | 11a | 15 |
| Body mass index, kg/m2 | 31.9±7.8 | 31.8±7.7a | 33.9±8.4 |
| eGFR, ml/min per 1.73 m2 | 42±15 | 42.6±15.1a | 37±16 |
| Serum potassium, mmol/l | 4.4±0.5 | 4.4±0.5a | 4.1±0.6 |
| Serum calcium, mg/dl | 9.3±0.5 | 9.3±0.5 | 9.1±0.7 |
| Serum magnesium, mg/dl | 2.0±0.3 | 2.0±0.3c | 2.0±0.3 |
| Systolic BP, mm Hg | 127±21 | 126±21a | 135±27 |
| Diastolic BP, mm Hg | 70±13 | 70±12 | 70±15 |
| Heart rate, min−1 | 65±11 | 65±11a | 63±10 |
| QTc duration, ms | 414±21 | 413±17d | 462±16 |
Values expressed as mean±SD. Footnotes refer to comparisons according to QTc group. QTc, QT interval corrected for heart rate; MI, myocardial infarction.
P<0.001.
P<0.05.
Serum magnesium available only at baseline visit (n=3252 visits).
P value irrelevant (grouping variable).
Serum Electrolytes
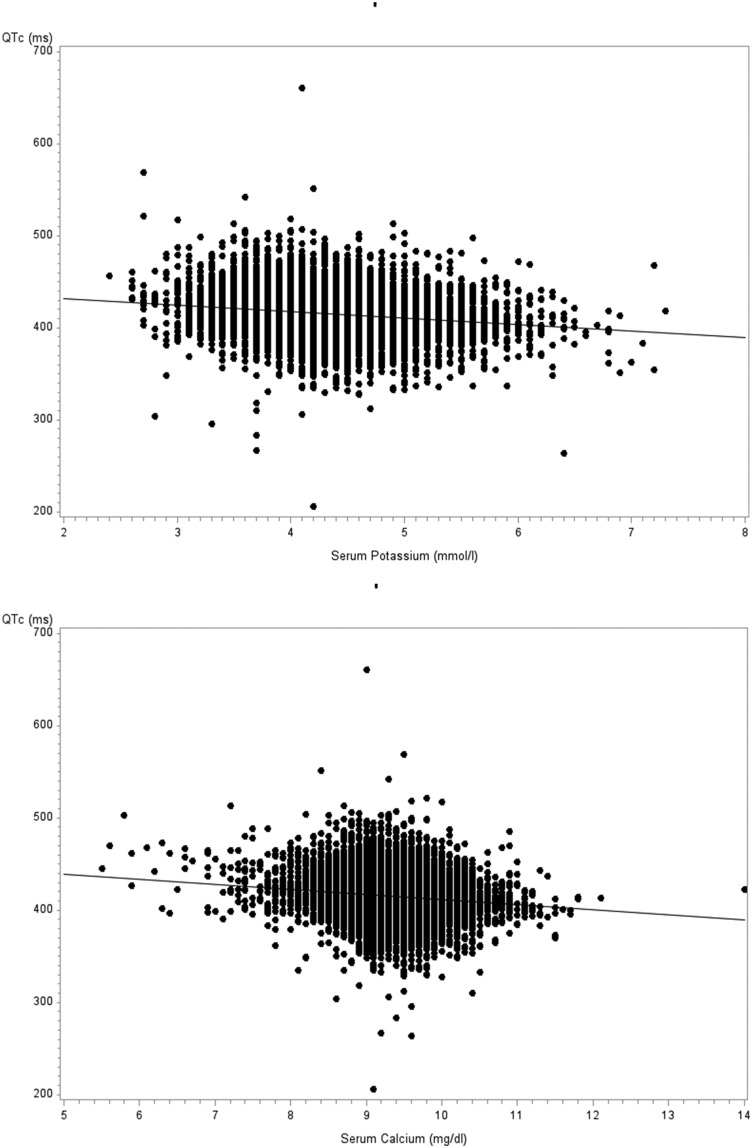
QTc was inversely and weakly correlated with serum potassium and calcium (r=−0.18 and −0.14, respectively; both P<0.001) as shown in Figure 1, but it was not correlated with serum magnesium (measured at baseline visit only and not shown).
Figure 1.
QT interval corrected for heart rate (QTc) is inversely correlated with simultaneously measured serum potassium (upper panel) and calcium (lower panel).
Frequency of Drug Use and Its Association with QTc
One or more potentially QTc-prolonging drugs from the three selected CredibleMeds.org categories were reported at 11,544 (70%) of visits. When including the drugs on the expanded list of drugs that were not on CredibleMeds.org, this number increased to 12,459 (76%) visits. At 6996 (43%) visits, the patient reported taking only one QTc-prolonging drug, whereas two drugs were reported taken concomitantly at 3507 (21%) visits, and three or more were reported at 1956 (12%) visits.
Table 2 includes all eligible drugs, except diuretics, and lists QTc prolongation for each drug as a continuous and discrete outcome (QTc≥450 milliseconds). Six drugs were associated with QTc prolongation as a continuous variable, including fluoxetine, citalopram, escitalopram, venlafaxine, hydroxyzine, and amiodarone. The same six drugs were associated with increased odds ratio of (QTc≥450 milliseconds), except venlafaxine. Nortriptyline was associated with QTc shortening rather than prolongation but was not associated with a lower odds ratio of a QTc≥450 milliseconds.
Table 2.
Association between medications reported and QT interval corrected for heart rate prolongation on electrocardiogram obtained at annual study visits (n=16,451)
| Drug | CMO Category | N, Visits | Adjusted Difference in QTc, ms | Adjusted OR (QTc≥450) | ||
|---|---|---|---|---|---|---|
| Estimate [95% CI] | P Value | Estimate [95% CI] | P Value | |||
| Selective serotonin reuptake inhibitors | ||||||
| Sertraline | C | 516 | 1 [−1 to 3] | 0.15 | 1.3 [0.8 to 2.2] | 0.26 |
| Fluoxetine | C | 495 | 4 [2 to 6] | <0.001 | 1.7 [1.1 to 2.7] | 0.02 |
| Citalopram | K | 306 | 4 [2 to 7] | 0.002 | 1.8 [1.0 to 3.1] | 0.04 |
| Paroxetine | C | 222 | 1 [−2 to 4] | 0.44 | 1.2 [0.7 to 2.1] | 0.58 |
| Escitalopram | K | 203 | 3 [0 to 6] | 0.03 | 2.2 [1.1 to 4.2] | 0.02 |
| Other antidepressants | ||||||
| Amitriptyline | C | 476 | 2 [0 to 4] | 0.14 | 1.3 [0.8 to 2.2] | 0.24 |
| Trazodone | C | 356 | 0 [−2 to 2] | 0.95 | 0.8 [0.4 to 1.5] | 0.50 |
| Venlafaxine | P | 219 | 3 [0 to 5] | 0.05 | 1.1 [0.5 to 2.7] | 0.84 |
| Nortriptyline | P | 165 | −3 [6 to 0] | 0.03 | 0.9 [0.4 to 1.9] | 0.80 |
| Mirtazapine | P | 106 | −3 [−7 to 1] | 0.24 | 0.6 [0.2 to 1.7] | 0.34 |
| Other central nervous system drugs | ||||||
| Hydroxyzine | C | 213 | 4 [1 to 6] | 0.004 | 2.1 [1.2 to 3.7] | <0.01 |
| Quetiapine | C | 151 | −1 [−5 to 2] | 0.43 | 1.8 [0.8 to 4.0] | 0.12 |
| Proton pump inhibitors | ||||||
| Omeprazole | — | 1626 | 1 [0 to 2] | 0.13 | 1.1 [0.9 to 1.6] | 0.21 |
| Esomeprazole | — | 834 | −1 [−3 to 0] | 0.13 | 0.6 [0.4 to 1.1] | 0.09 |
| Lansoprazole | — | 525 | −2 [−3 to 0] | 0.09 | 0.7 [0.4 to 1.2] | 0.24 |
| Pantoprazole | C | 447 | −1 [−3 to 1] | 0.30 | 0.9 [0.5 to 1.3] | 0.50 |
| Rabeprazole | — | 143 | 0 [−4 to 3] | 0.75 | 1.6 [0.8 to 3.2] | 0.17 |
| Other drugs | ||||||
| Diphenhydramine | C | 695 | 0 [−2 to 1] | 0.82 | 1.1 [0.8 to 1.6] | 0.51 |
| Famotidine | P | 342 | 1 [−1 to 3] | 0.49 | 1.0 [0.6 to 1.9] | 0.88 |
| Metoclopramide | C | 274 | 0 [−2 to 2] | 0.97 | 0.8 [0.4 to 1.5] | 0.50 |
| Quinine | C | 132 | 1 [−1 to 4] | 0.43 | 0.9 [0.4 to 1.9] | 0.71 |
| Amiodarone | K | 121 | 10 [5 to 14] | <0.001 | 3.9 [2.1 to 7.2] | <0.001 |
| Cilostazol | K | 108 | −3 [−6 to 1] | 0.18 | 0.6 [0.2 to 1.7] | 0.38 |
| Tolterodine | P | 106 | −1 [−5 to 4] | 0.79 | 1.2 [0.5 to 3.1] | 0.63 |
This table includes all drugs taken at ≥100 visits, except diuretics. All estimates were adjusted for serum potassium, serum calcium, systolic BP, age, sex, race/ethnicity, eGFR, body mass index, current smoking, history of myocardial infarction/revascularization, hypertension, chronic heart failure, and stroke. CMO category, the CredibleMeds.org category; QTc, QT interval corrected for heart rate; 95% CI, 95% confidence interval; OR, odds ratio; C, conditional risk of torsade de pointes; K, known risk of torsade de pointes; P, possible risk of torsade de pointes; —, not on CredibleMeds.org list.
Diuretics
Table 3 reveals that most loop and thiazide diuretics were associated with a significant decrement in serum potassium but of a magnitude of ≤0.3 mEq/L, whereas spironolactone was associated with 0.2 mEq/L higher serum potassium. Table 3 also shows that furosemide was associated with a 0.1 mg/dl lower serum calcium and that hydrochlorothiazide was associated with 0.1 mg/dl higher serum calcium. Consistent with their associations with serum potassium, both metolazone and furosemide were associated with mean QTc prolongations of 8 and 4 milliseconds, respectively, without adjustment for electrolytes. Spironolactone was associated with a mean QTc shortening of −3 milliseconds, except when adjusted for serum potassium and calcium. The associations of QTc with metolazone and furosemide remained statistically significant at 6 and 3 milliseconds, respectively, with adjustment for serum potassium and calcium. Metolazone and furosemid were also statistically significantly associated with a QTc≥450 milliseconds, with odds ratios of 1.6 (95% confidence interval [95% CI], 1.0 to 2.5; P=0.04) for metolazone and 1.5 (95% CI, 1.2 to 1.9; P<0.001) for furosemide.
Table 3.
Relationship of diuretics with serum potassium and calcium and QT interval corrected for heart rate with and without adjustment for potassium and calcium
| Drug | N, Visits | Model 1 | Model 2 | Model 3: Not Adjusted for Serum Potassium and Serum Calcium | Model 4: Adjusted for Serum Potassium and Serum Calcium | ||||
|---|---|---|---|---|---|---|---|---|---|
| Difference in Serum Potassium, mmol/L | P Value | Difference in Serum Calcium, mg/dl | P Value | Difference in QTc, ms | P Value | Difference in QTc, ms | P Value | ||
| Loop diuretics | |||||||||
| Furosemide | 4738 | −0.1 [−0.2 to −0.1] | <0.001 | −0.1 [−0.1 to −0.1] | <0.001 | 4 [3 to 4] | <0.001 | 3 [2 to 3] | <0.001 |
| Torsemide | 287 | −0.1 [−0.2 to −0.1] | <0.001 | −0.1 [−0.1 to 0.0] | 0.05 | 3 [0 to 6] | 0.06 | 2 [1 to 5] | 0.28 |
| Bumetanide | 169 | −0.1 [−0.2 to 0.0] | 0.10 | −0.0 [−0.2 to 0.1] | 0.52 | 2 [−3 to 7] | 0.38 | 1 [−3 to 6] | 0.56 |
| Thiazide diuretics and thiazide-like diuretics | |||||||||
| Hydrochlorothiazide | 4556 | −0.1 [−0.2 to −0.1] | <0.001 | 0.1 [0.0 to 0.1] | <0.001 | 1 [0 to 1] | 0.25 | 0 [0 to 1] | 0.56 |
| Metolazone | 284 | −0.3 [−0.4 to −0.2] | <0.001 | 0.0 [−0.1 to 0.1] | 0.74 | 8 [5 to 11] | <0.001 | 7 [4 to 10] | <0.001 |
| Chlorthalidone | 164 | −0.2 [−0.3 to −0.1] | <0.001 | 0.1 [0.0 to 0.2] | 0.11 | 0 [−2 to 3] | 0.79 | −1 [−4 to 2] | 0.48 |
| Potassium-sparing diuretics | |||||||||
| Spironolactone | 558 | 0.2 [0.1 to 0.3] | <0.001 | 0.1 [0.1 to 0.2] | <0.001 | −3 [−5 to −0] | 0.03 | 1 [−3 to 2] | 0.49 |
| Triamterene | 544 | 0.0 [−0.1 to 0.0] | 0.59 | 0.0 [−0.1 to 0.0] | 0.38 | 1 [−2 to 3] | 0.74 | 1 [−2 to 3] | 0.62 |
| Amiloride | 155 | 0.0 [0.1 to 0.2] | 0.95 | 0.1 [−0.2 to −0.1] | 0.34 | 0 [−6 to 5] | 0.90 | 0 [−5 to 6] | 0.98 |
Association between reported diuretic use, serum electrolytes, and QTc. Models 1 and 2 determine the increase or decrease in serum potassium and calcium, respectively, associated with taking a given diuretic compared with not taking that diuretic after adjustment for all other drugs, systolic BP, age, sex, race/ethnicity, eGFR, body mass index, current smoking, history of myocardial infarction/revascularization, hypertension, chronic heart failure, and stroke. Model 3 determines the increase or decrease in QTc associated with a given diuretic compared with not taking that diuretic (same adjustments). Model 4 is identical to model 3, except that the covariates also include serum potassium and serum calcium. Potassium-sparing diuretics were included solely for comparison and are not suspected of causing QTc prolongation. Furosemide, torsemide, and hydrochlorothiazide are listed by CredibleMeds.org in the category conditional risk of torsade de pointes. No other drug in the table is listed by CredibleMeds.org. The 95% confidence intervals are in brackets. QTc, QT interval corrected for heart rate.
PPIs
PPI use was not associated with a lower serum magnesium, except for omeprazole in the amount of −0.1 mg/dl (95% CI, −0.1 to 0.1); however, no PPI was associated with QTc duration.
Off-On Drug Effect on QTc Prolongation
Table 4 displays the mean difference in ECG QTc obtained at a visit where one of the drugs found to be QT prolonging was initiated and compared with the prior visit. Point estimates from this subset analysis were largely concordant with those of the GEE model analysis that involved all visits but had wider CIs, as expected, with the attendant reduction in number of visits where an eligible drug was initiated.
Table 4.
Mean difference in electrocardiogram QT interval corrected for heart rate between visit with initiation of QT prolonging medication and visit before initiation
| Drug | First Visit with Drug Use versus Preceding Visit, t Test | ||
|---|---|---|---|
| N, Visits | QTc Mean Difference, msa | P Value | |
| Amiodarone | 14 | 10 [−3.0 to 22.0] | 0.11 |
| Metolazone | 69 | 8 [4 to 13] | 0.001 |
| Citalopram | 71 | 4 [−1 to 8] | 0.11 |
| Fluoxetine | 61 | 3 [−3 to 9] | 0.28 |
| Hydroxyzine | 58 | 4 [−2 to 10] | 0.22 |
| Furosemide | 427 | 3 [1 to 4] | <0.01 |
| Escitalopram | 46 | 0 [−5 to 5] | 0.95 |
| Venlafaxine | 27 | 6 [−1 to 12] | 0.11 |
QTc mean difference resulting from a comparison of the visit with first use of drug found to significantly prolong QTc with the preceding visit as determined by paired t tests. QTc, QT interval corrected for heart rate.
Mean value of QTc with the drug minus QTc without the drug [95% confidence interval].
Sensitivity Analyses Related to Echocardiographic Measures of Heart Failure
When additionally adjusting for left ventricular mass and ejection fraction in the 4743 visits where an echocardiogram was available, the estimated QTc prolongations associated with metolazone and furosemide were 5 milliseconds (95% CI, 0 to 9; P=0.03) and 2 milliseconds (95% CI, 0 to 3; P=0.01), respectively (i.e., somewhat lower than when not making these adjustments but still reaching nominal statistical significance).
Drug Interactions
Patients took one or more of the eight drugs with statistically significant associations with QTc prolongation as a continuous trait at 7593 (38%) visits. For these eight drugs (as well as spironolactone), we examined pairwise drug × drug interactions. Only furosemide plus venlafaxine and furosemide plus spironolactone were taken at 100 visits or more. Neither combination gave rise to a statistically significant drug × drug interaction. Notably, because spironolactone was associated with QTc shortening, it negated some of the QTc prolongation associated with furosemide.
Discussion
Use of medications with potential to prolong the ECG QT interval was prevalent in this CKD cohort. Of the potentially QT-prolonging medications, the most common were diuretics, which are a cornerstone of CKD pharmacotherapy. Of 30 drugs that were taken frequently enough to warrant consideration, eight were associated with significant QT prolongation. Among these were three members of the antidepressant class of selective serotonin reuptake inhibitors, namely fluoxetine, citalopram, and escitalopram. Other drugs associated with QTc prolongation included the antidepressant venlafaxine; hydroxyzine, which is often prescribed as an antiemetic in CKD; and the antiarrhythmic amiodarone, which was associated with the greatest QTc prolongation.
We confirmed the inverse relationships between QT and both serum potassium and calcium, corroborating that electrolyte shifts are associated with ECG QT prolongation. However, we found only small differences in these serum electrolyte concentrations between visits where diuretics were and were not used. Both furosemide and metolazone were associated with significant QT prolongation, and most of the prolongation observed persisted after adjustment for serum potassium and calcium, suggesting that these drugs have inherent effects on cardiac conduction independent of electrolyte balance. This finding has important implications for the clinical surveillance for iatrogenic effects of diuretics, which is typically limited to electrolyte assessment.
Although only two drugs (amiodarone and metolazone) exhibited a mean QTc prolongation of >5 milliseconds, the threshold for FDA regulatory concern (7), one third of the cohort took two or more QT-prolonging drugs concomitantly. While most of the resulting drug pairs were taken too infrequently to be evaluable, furosemide plus venlafaxine and furosemide plus spironolactone were each taken at >100 visits and demonstrated additivity of the associated QTc prolongations. Extending this observation to other drugs, many subjects with CKD have the potential for QTc prolongation of >5 milliseconds due to additive effects of multiple drugs.
Prior evaluation of the CRIC has shown a significant relationship between several ECG interval abnormalities, notably QT prolongation, and cardiovascular mortality, with similar findings shown in patients with CKD enrolled in the Cardiovascular Health Study (4–6). An analogous relationship between ECG QT prolongation and mortality was reported in 60- to 79-year-old British community dwellers (18). Several additional studies in non-CKD populations also show the influence of medications on ECG intervals (18–20). Oregon residents who had sudden cardiac death compared with comparable controls with coronary artery disease showed a higher frequency of QT prolongation in ECGs in the former relative to the latter and an association between QT-prolonging medications and QT prolongation (19). The British cohort of community dwellers showed a higher frequency of diuretic, β-blocker, and calcium channel blocker use among participants with prolonged QT intervals. A sample of 1270 asymptomatic patients surveyed in Argentina (20) for drug toxicity before and after administration of new drugs revealed that β-lactam antibiotics and furosemide were associated with QT prolongation. Although potassium also was associated inversely with a prolonged QT, the effect of furosemide was independent of potassium as shown in this study.
Animal models have shown the inverse relationship between serum potassium and QT interval prolongation via furosemide-induced hypokalemia (21,22). However, these experiments did not report on an independent association between the use of furosemide or any other diuretic and the QT interval beyond its influence on serum potassium. Similar inverse relationships between serum potassium and calcium with QT interval on resting ECGs were shown in humans in the British cohort described above. No relationship was shown with serum magnesium and the QT interval (18).
This study adds to the literature by quantifying the use of potentially cardiotoxic drugs in CKD and reporting on their association with the ECG QT interval obtained in nonclinical settings. However, several factors must be taken into account when interpreting findings of this cross-sectional visit-level analysis. It is not possible to exclude the possibility of further confounding by unmeasured variables, particularly cardiovascular-related factors. The study did not include several candidate drugs, such as antibiotics, because their use was uncommon in the CRIC. The size of the study sample limits our ability to examine interactions between drugs with cardiotoxic potential. Moreover, diuretics are frequently prescribed for CHF, a disease that is associated with QT prolongation. To examine whether this circumstance could lead to confounding by indication, we performed a sensitivity analysis on a subset of the visits with echocardiograms, adjusting for left ventricular mass and ejection fraction. This analysis attenuated the associations between diuretics and QT prolongation, but they remained statistically significant. For drugs prescribed for indications other than CHF, such as depression and emesis, this type of confounding is less likely.
Although the study examined more than one hypothesis, we did not adjust for multiple comparisons by reducing α below the conventional 0.05, because this would increase the risk of type 2 error, which is a particular concern in drug safety. It is thus conceivable that the study of multiple exposures and outcomes may have increased the possibility of false discovery. However, we were not testing random hypotheses but using a list of drugs flagged for their previously documented effect on the QT interval on the basis of multiple lines of evidence; additionally, the two end points (discrete and continuous) were, by design, of a mutually supportive nature. The fact that our findings (drugs with strongest association) agree across both end points provides reassurance of the reliability of our results.
In conclusion, we found that patients with CKD, as represented in the CRIC, report taking many medications for which there is potential and evidence for QT prolongation. This notable association of commonly used drugs with cardiac electroconduction illustrates the relatively ubiquitous but often underappreciated iatrogenic influence of pharmacotherapy in CKD. Discriminating underlying cardiovascular pathology from ECG abnormalities conferred by medications is critical for CKD management and points to the possibility of identifying potentially reversible causes of the adverse cardiac outcomes characteristic of this disease.
Disclosures
At the time of acceptance for publication, S.S. was an employee of Novo Nordisk A/S. There are no other potential conflicts to disclose.
Supplementary Material
Acknowledgments
S.S., R.M.D., M.Z., and J.C.F. were supported by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01 DK090008. Funding for the Chronic Renal Insufficiency Cohort (CRIC) Study was obtained under a cooperative agreement from the NIDDK (grants U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported, in part, by Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science NIH/National Center for Advancing Translational Sciences (NCATS) award UL1TR000003, Johns Hopkins University grant UL1 TR-000424, University of Maryland General Clinical Research Center grant M01 RR-16500, the Clinical and Translational Science Collaborative of Cleveland, grant UL1TR000439 from the NCATS component of the NIH and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research grant V 2014.07.28 UL1TR000433, University of Illinois at Chicago Clinical and Translational Science Award grant UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology grant P30GM103337, and Kaiser Permanente NIH/National Center for Research Resources University of California, San Francisco-Clinical and Translational Science Institute grant UL1 RR-024131.
The CRIC Study investigators include Lawrence J. Appel (The Johns Hopkins University), Harold I. Feldman (University of Pennsylvania), Alan S. Go (Kaiser Permanente of Northern California), Jiang He (Tulane University), John W. Kusek (National Institute of Diabetes, and Digestive, and Kidney Diseases), Mahboob Rahman (Case Western Reserve University School of Medicine), Panduranga Rao (University of Michigan), and Raymond R. Townsend (University of Pennsylvania).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12991216/-/DCSupplemental.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Herzog CA, Asinger RW, Berger AK, Charytan DM, Díez J, Hart RG, Eckardt KU, Kasiske BL, McCullough PA, Passman RS, DeLoach SS, Pun PH, Ritz E: Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 80: 572–586, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Deo R, Shou H, Soliman EZ, Yang W, Arkin JM, Zhang X, Townsend RR, Go AS, Shlipak MG, Feldman HI: Electrocardiographic measures and prediction of cardiovascular and noncardiovascular death in CKD. J Am Soc Nephrol 27: 559–569, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kestenbaum B, Rudser KD, Shlipak MG, Fried LF, Newman AB, Katz R, Sarnak MJ, Seliger S, Stehman-Breen C, Prineas R, Siscovick DS: Kidney function, electrocardiographic findings, and cardiovascular events among older adults. Clin J Am Soc Nephrol 2: 501–508, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Dobre M, Brateanu A, Rashidi A, Rahman M: Electrocardiogram abnormalities and cardiovascular mortality in elderly patients with CKD. Clin J Am Soc Nephrol 7: 949–956, 2012 [DOI] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER): Guidance for Industry: E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs, 2001. Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073153.pdf. Accessed December 1, 2016
- 8.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic Renal Insufficiency Cohort (CRIC) study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woosley RL, Romero KA: QTdrugs List. AZCERT, Inc. Available at: www.Crediblemeds.org. Accessed June 30, 2016
- 10.Schwartz AB: Diuretic-induced hypokalemia. Am Fam Physician 11: 101–104, 1975 [PubMed] [Google Scholar]
- 11.Seller RH, Banach S, Namey T, Neff M, Swartz C: Cardiac effect of diuretic drugs. Am Heart J 89: 493–500, 1975 [DOI] [PubMed] [Google Scholar]
- 12.Knochel JP: Diuretic-induced hypokalemia. Am J Med 77: 18–27, 1984 [DOI] [PubMed] [Google Scholar]
- 13.Kuller LH, Hulley SB, Cohen JD, Neaton J: Unexpected effects of treating hypertension in men with electrocardiographic abnormalities: A critical analysis. Circulation 73: 114–123, 1986 [DOI] [PubMed] [Google Scholar]
- 14.Kieboom BC, Kiefte-de Jong JC, Eijgelsheim M, Franco OH, Kuipers EJ, Hofman A, Zietse R, Stricker BH, Hoorn EJ: Proton pump inhibitors and hypomagnesemia in the general population: A population-based cohort study. Am J Kidney Dis 66: 775–782, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJ, Gorgels A, Hancock EW, Josephson M, Kligfield P, Kors JA, Macfarlane P, Mason JW, Mirvis DM, Okin P, Pahlm O, van Herpen G, Wagner GS, Wellens H; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology, American College of Cardiology Foundation, Heart Rhythm Society, Endorsed by the International Society for Computerized Electrocardiology : AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part IV: The ST segment, T and U waves, and the QT interval: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. J Am Coll Cardiol 53: 982–991, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Sagie A, Larson MG, Goldberg RJ, Bengtson JR, Levy D: An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study). Am J Cardiol 70: 797–801, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI; CRIC Study Investigators : Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 60: 250–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sohaib SM, Papacosta O, Morris RW, Macfarlane PW, Whincup PH: Length of the QT interval: Determinants and prognostic implications in a population-based prospective study of older men. J Electrocardiol 41: 704–710, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Chugh SS, Reinier K, Singh T, Uy-Evanado A, Socoteanu C, Peters D, Mariani R, Gunson K, Jui J: Determinants of prolonged QT interval and their contribution to sudden death risk in coronary artery disease: The Oregon sudden unexpected death study. Circulation 119: 663–670, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keller GA, Alvarez PA, Ponte ML, Belloso WH, Bagnes C, Sparanochia C, Gonzalez CD, Villa Etchegoyen MC, Diez RA, Di Girolamo G: Drug-induced QTc interval prolongation: A multicenter study to detect drugs and clinical factors involved in every day practice. Curr Drug Saf 11: 86–98, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Hanton G, Yvon A, Provost JP, Racaud A, Doubovetzky M: Quantitative relationship between plasma potassium levels and QT interval in beagle dogs. Lab Anim 41: 204–217, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Akita M, Kuwahara M, Tsubone H, Sugano S: ECG changes during furosemide-induced hypokalemia in the rat. J Electrocardiol 31: 45–49, 1998 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.