Abstract
Cisplatin is currently one of the most widely-used chemotherapeutic agents against various malignancies. Its clinical application is limited, however, by inherent renal and cardiac toxicities and other side effects, of which the underlying mechanisms are only partly understood. Experimental studies show cisplatin generates reactive oxygen species, which impair the cell’s antioxidant defense system, causing oxidative stress and potentiating injury, thereby culminating in kidney and heart failure. Understanding the molecular mechanisms of cisplatin-induced renal and cardiac toxicities may allow clinicians to prevent or treat this problem better and may also provide a model for investigating drug-induced organ toxicity in general. This review discusses some of the major molecular mechanisms of cisplatin-induced renal and cardiac toxicities including disruption of ionic homeostasis and energy status of the cell leading to cell injury and cell death. We highlight clinical manifestations of both toxicities as well as (novel)biomarkers such as kidney injury molecule-1 (KIM-1), tissue inhibitor of metalloproteinase-1 (TIMP-1) and N-terminal pro-B-type natriuretic peptide (NT-proBNP). We also present some current treatment challenges and propose potential protective strategies with novel pharmacological compounds that might mitigate or prevent these toxicities, which include the use of hydrogen sulfide.
Keywords: Cancer, Cisplatin, Cisplatin-induced renal and cardiac toxicities, Reactive oxygen species
1. Introduction: Cisplatin, a potent anticancer drug
Cisplatin (cis-diamminediachloroplatinum [II], CDDP) is one of the most widely used drugs to treat various human malignancies and highly effective (Paolicchi et al., 2002; Townsend et al., 2009). In combination chemotherapy regimens, cisplatin is a potent anticancer agent. Cisplatin was accidentally discovered to inhibit cell division in 1965 by American biophysicist and chemist Barnett Rosenberg (Rosenberg et al., 1965). By 1969, the anticancer property of cisplatin was demonstrated in animal models (Rosenberg et al., 1969). Since its accidental discovery, cisplatin has been widely used to treat several cancers including cancers of the head, neck, esophagus, lung, bladder, ovary, cervix, breast, testis, penis, endometrium, mesothelium and many more (Paolicchi et al., 2002; Townsend et al., 2009). In testicular cancer, for example, cisplatin combination chemotherapy has been demonstrated to produce remission rates > 90% (Loehrer et al., 1998; Einhorn, 2002).
The anticancer property of cisplatin is not completely understood. Increasing evidence, however, indicates that cisplatin binds to DNA, resulting in the formation of inter- and intrastrand cross-links, which leads to defective DNA templates and subsequent inhibition of DNA synthesis and replication (Dzagnidze et al., 2007; Townsend et al., 2009). The replication of rapidly dividing cancer cells is inhibited by the cisplatin-DNA complex, and these cells are subsequently destroyed by the cross-links (Dzagnidze et al., 2007; Townsend et al., 2009).
Despite its effectiveness, the use of cisplatin in high-dose therapy has been reported to be limited by renal and cardiac toxicities, sharing similar cellular and molecular mechanisms (Schrier et al., 2004; Yao et al., 2007; El-Awady et al., 2011; Shen et al., 2012). Other side effects including ototoxicity, neurotoxicity, gastrotoxicity, myelosuppression, severe nausea and emetic effects have been reported (Hartmann and Lipp, 2003; McWhinney et al., 2009; Brock et al., 2012;). About 30% of patients receiving high-dose cisplatin have experienced severe renal dysfunction (Shiraishi et al., 2000). Skinner et al. (1998) also reported renal dysfunction in over 70% pediatric patients receiving cisplatin treatment. Understanding the mechanisms of cisplatin-induced renal and cardiac toxicities may help provide better treatment and preventive strategies. While several reviews have covered cisplatin-induced renal toxicity and very few on cardiac toxicity, none discusses the mechanisms of both toxicities. This review discusses some of the major molecular mechanisms underlying cisplatin-induced renal and cardiac toxicities. We also highlight clinical characteristics of both toxicities, (novel)biomarkers, current treatment challenges and propose measures with novel pharmacological compounds that could prevent or ameliorate these toxicities.
2. Renal injury by cisplatin administration
The major excretion route of cisplatin is renal. Like other drugs, cisplatin is filtered freely in the glomerular compartment of the nephron and retrieved in urine. However, only a small fraction of the total cisplatin dose is seen in urine after the first few days of treatment, as much of the drug is irreversibly bound to protein (Speer et al., 1975). Cisplatin has been reported to injure several renal structures including blood vessels, glomeruli, and most commonly the renal tubules (Pabla and Dong, 2008). Reports show cisplatin causes renal vasoconstriction in perfused pig kidneys following high-dose and continuous low-dose treatment, which directly affects renal vasculature (Miura et al., 1987; Daugaard et al., 1988; Robbins et al., 1990). In the glomeruli, cisplatin causes histological and functional injuries such as thickening of the glomerular basement membrane (GBM) and proliferation of capsular epithelial cells as well as damage to glomerular capillaries including endothelial and mesangial cells (Robbins et al., 1990; Kohn et al., 2002). Both animal and human studies have shown that cisplatin administration leads to a decline in renal blood flow (RBF), reduced single-nephron glomerular filtration rate (GFR), and reduced renal clearance in a time-dependent and dose-dependent manner (Winston and Safirstein, 1985; Miura et al., 187; Sanchez-Gonzalez et al., 2011). The cisplatin-induced vasoconstriction as well as the fall in RBF and GMB thickening could, at least in part, explain the underlying mechanism mediating the reduced GFR, which may potentiate further renal injury. Renal clearance studies in humans have shown that the clearance of free platinum surpasses GFR, suggesting that the renal tubules may actively secrete cisplatin or platinum metabolites (Weiner and Jacobs, 1983), which may result in a high concentration of cisplatin metabolites and accumulation in the renal tubular cells. Studies show that renal proximal tubular cells of the inner cortex and the outer medulla (S3 segment) accumulate cisplatin to a greater degree, and that cisplatin concentration in the proximal tubular epithelial cells is five times higher than the concentration in serum (Vickers et al., 2004). Such an uneven concentration of cisplatin in renal tissue contributes markedly to cisplatin renal toxicity. Functionally, this accumulation impairs proximal tubular salt and water reabsorption rates and also affects distal tubular function (Daugaard et al., 1988; Lajer et al., 2005). All these events culminate in the observed decline in GFR, tubular injury, and loss of renal function (figure 2).
3. Clinical features of cisplatin-induced renal toxicity
Cisplatin was first reported to induce renal toxicity in animal studies in 1971 (Kociba et al., 1971), in which the authors observed tubular necrosis and high serum creatinine, as well as elevated levels of urea and other nitrogen-rich compounds in the blood. Cisplatin-induced renal toxicity was reported in initial clinical trials of cisplatin chemotherapy, showing acute renal failure in 14–100% of patients in high dose (Goldstein and Mayor, 1983). Clinical reports indicate cisplatin-induced renal toxicity is often seen following 10 days of cisplatin treatment and ultimately results in acute renal failure, which is characterized by declines in RBF within 3 hours of cisplatin administration and decreased GFR (Sanchez-Gonzalez et al., 2011). Electrolyte abnormalities such as hypomagnesemia, hypokalemia, hyponatremia and hypocalcemia have been reported after repeated dose of cisplatin (Antunes et al., 2001; Goren, 2003; Ali et al., 2008). Other complications such as proteinuria, enzymuria, glucosuria, increased urinary electrolyte excretion as well as elevated level of serum creatinine and blood urea nitrogen, have also been reported in both acute and chronic cisplatin-induced renal toxicity (Groth et al., 1986; Daugaard et al., 1988; Akcay et al., 2010). A number of factors have been identified to be associated with increased risk for cisplatin-induced renal toxicity. These include cisplatin dose, frequency, and cumulative dose. Old age, female gender, and lifestyle such as smoking are also identified risk factors (de Jongh et al., 2001; de Jongh et al., 2003). Hypoalbuminemia and pre-existing renal insufficiency are also risk factors for cisplatin-induced renal toxicity (de Jongh et al., 2001; de Jongh et al., 2003). Animal studies suggest diabetic condition may reduce the risk of cisplatin-induced renal toxicity. However, human data linking diabetes to cisplatin-induced renal toxicity are lacking (Scott et al., 1989; Gogas et al., 1996).
4. Clinical manifestations of cisplatin-induced cardiac toxicity
Cardiovascular complications associated with conventional cancer chemotherapy are well-known issues in oncology studies. Recently, cardiac toxicity resulting from cisplatin treatment has been described (Pai and Nahata, 2000). Cisplatin-induced cardiotoxic effects have been reported to manifest through changes in electrocardiography (Al-Majed et al., 2006; Raja et al., 2013) and arrhythmias, which include ventricular arrhythmias, supraventricular tachycardia, atrial fibrillation, occasional sinus bradycardia and occasional complete atrioventricular block (Yavas et al., 2008; Guglin et al., 2009; Ozcan et al., 2011). Myocarditis, pericarditis, and angina have also been reported in cisplatin-induced cardiac toxicity (Khan et al., 2012; Bano et al., 2013). Other authors also observed acute myocardial infarction and autonomic cardiovascular dysfunction (Moore et al., 2011; Ryberg, 2012) as well as hypertension and hypotension (Dolci et al., 2008; Amit et al., 2012). Induction of coronary vasospasm (which could lead to coronary artery disease) and vascular endothelial injury due to increased von Willebrand factor (blood glycoprotein released by damaged endothelial cells) are other cardiovascular effects associated with cisplatin-induced cardiac toxicity (Guglin et al., 2009; Yeh et al., 2009). Taken together, these cardiotoxic (and other potentially unknown) events ultimately result in congestive heart failure and sudden cardiac death.
5. Molecular mechanisms underlying cisplatin-induced toxicity
The pathway of cisplatin-induced toxicity is complex and not completely understood. However, results from several experimental studies suggest a sequential injury pathway, which includes (i) role of membrane transporters (ii) cisplatin conversion to toxic metabolites; (iii) induction of nuclear and mitochondrial DNA damage; (iv) disruption of ionic homeostasis (v) role of oxidative stress and mitochondrial dysfunction; (vi) induction of inflammation; and (vii) activation of apoptotic machinery.
5.1. Role of membrane transporters
Recent studies have identified two different membrane transporters capable of transporting cisplatin. In the kidney, cisplatin is transported into renal tubular cells by facilitated diffusion, leading to disproportionate cisplatin accumulation and consequent renal toxicity. Several authors have identified a basolateral organic cation transporter (OCT), which is associated with cisplatin uptake into proximal tubular epithelial cells (Kolb et al., 2003; Ciarimboli et al., 2005). So far, three isoforms of OCT (OCT1, OCT2 and OCT3) have been identified in humans. However, OCT2 is the major organic transporter associated with cisplatin uptake and cytotoxicity in proximal tubules in both animals and humans (Kolb et al., 2003; Ciarimboli et al., 2005). Interestingly, although OCT2 is not expressed in human heart tissue, a number of other OCTs have been identified in cardiac drug uptake in human and animal studies but there are so far no studies describing the role of these OCTs in cisplatin uptake by cardiomyocytes (Grube et al., 2011). Apart from OCT2, a high-affinity copper transporter, CTR1, has also been identified to mediate cisplatin uptake into mammalian cells, although to a lesser extent (Pabla et al., 2009). Unlike OCT2, which is expressed only in renal tubular cells, CTR1 is expressed in both proximal tubular cells of adult kidney as well as in cardiac tissue (Pabla et al., 2009; Kim et al., 2010). Although there is currently no in vivo report on the specific role of CTR1 in cisplatin-induced toxicity, in vitro studies demonstrated that downregulation of CTR1 leads to reduced cisplatin uptake and cytotoxicity (Pabla et al., 2009), suggesting that CTR1 plays an important role in cisplatin uptake in proximal tubular cells and/or cardiomyocytes. Taken together, transporter-mediated uptake is the known mechanism of cisplatin uptake into renal tubular epithelial cells.
5.2. Biotransformation of cisplatin to toxic metabolites
Animal studies indicate that cisplatin is a protoxin that undergoes metabolic transformation to a more potent nephrotoxin when it is transported into the renal tubular epithelial cells. The process of metabolic transformation of cisplatin begins when cisplatin binds to glutathione, a naturally occurring antioxidant in the renal tubular cells, and forms glutathione conjugates, catalyzed by the enzyme glutathione-S-transferase Pi (GSTP) (Townsend et al., 2009). The glutathione conjugates then pass through the tubular cells and are cleaved to cysteinyl-glycine-conjugates and cysteine-conjugates respectively by gamma-glutamyl transpeptidase (GGT) and aminotransferase N (APN), which are abundantly expressed on the surface of the plasma membrane of the proximal tubular cells (Paolicchi et al., 2002; Townsend et al., 2009). The cysteine-conjugates are transported into the proximal tubular cells, where they undergo further metabolic conversion by cysteine-S-conjugate beta-lyase (CCBL) to highly reactive cysteine thiols (Paolicchi et al., 2002; Townsend et al., 2009). Binding of the reactive cysteine thiol to essential proteins within the proximal tubular cells leads to toxicity. Although the occurrence of metabolic toxification of cisplatin by GGT has not been studied in the heart, it is not very likely that this pathway plays a role in observed cardiotoxicity of cisplatin, since no GGT expression was detected in human heart tissue (Hanigan and Frierson, 1996) Thus in the kidney but probably not in the heart, cisplatin undergoes metabolic transformation into a reactive metabolite leading to cytotoxicity.
5.3. Induction of nuclear and mitochondrial DNA damage
Cisplatin forms intrastrand and interstrand cross-links with DNA strands. The DNA adduct formed triggers a cascade of cellular events, culminating in mitochondrial and nuclear DNA damage and cell death in renal proximal tubule (Nishikawa et al., 2001; Dzagnidze et al., 2007; Townsend et al., 2009). Also, the DNA adduct inhibits further DNA synthesis, cell cycle and replication, thus resulting in impaired replication and transcription and ultimately apoptosis of renal tubular cells (Deng et al., 2001; Dzagnidze et al., 2007; Townsend et al., 2009). In a rat model of cisplatin-induced cardiac toxicity, El-Awady et al. (2011) observed significant damage in both nuclear and mitochondrial DNA. As rapidly dividing cells, including cancer cells, exhibit high sensitivity to DNA damage, some argue that the anticancer property of cisplatin is largely due to the formation of DNA adducts (Dzagnidze et al., 2007). Others suggested mitochondrial DNA or other mitochondrial targets as the most common binding target for cisplatin due to its poor repair capabilities (Deng et al., 2001; Cullen et al., 2007). Apart from nuclear and mitochondrial DNA, studies have also highlighted the effect of cisplatin on RNA, proteins, and phospholipids (Wang and Lippard, 2005). In summary, cisplatin administration leads to nuclear and mitochondrial DNA damage, which is what makes cisplatin an effective antineoplastic agent.
5.4. Disruption of ionic homeostasis
Some studies have shown that cisplatin interrupts ionic homeostasis and water transport in renal tubular cells, leading to reduced ion reabsorption rates along the nephron and ultimately increased levels of these ions in the urine (Goren, 2003; Lajer et al., 2005; Ali et al., 2008). The pattern of expression of aquaporin 1 and 2 as well as Na+/K+/2Cl− co-transporter and type III Na+/H+ exchanger in the outer medulla region of the nephron decreased following cisplatin treatment (Lajer et al., 2005). Both in vitro and in vivo models of cisplatin renal toxicity have demonstrated reduced Na+/K+ ATPase (α-subunit) activity in the brush border following cisplatin treatment, leading to renal tubular dysfunction (Lajer et al., 2005). Thus, cisplatin disrupts ionic homeostasis in renal tubular cells leading to renal tubular cell injury.
5.5. Role of oxidative stress and mitochondrial dysfunction
Oxidative stress reflects an imbalance between generation of reactive oxygen species (ROS) and the ability of a biological system to readily counteract the effect of the pro-oxidants. Any shift in the normal redox state of a cell causes oxidative stress, which can be detrimental to cells. Cisplatin is able to shift the redox balance in cells by conjugation and thereby depletion of the antioxidant glutathione and as well as impairment of mitochondrial respiration, leading to ROS production (ref). Other authors have also observed significant reduction in plasma concentrations and activity of various antioxidants including glutathione during cisplatin-based chemotherapy in cancer patients (Mattson et al., 2009; Nakhaee et al., 2010). Lipid peroxidation, due to excessive ROS formation reflects oxidative stress, and has been associated with cisplatin-induced renal toxicity (Antunes et al., 2000; Nishikawa et al., 2001; Kadikoylu et al., 2004; Chirino et al., 2008) (figure 1). Oxidative stress is the main mechanism underlying cisplatin-induced cardiac toxicity (Wozniak et al., 2004; Karthikeyan et al., 2007; Demkow et al., 2011; El-Awady et al., 2011; Rosic et al. 2016). Rosic et al. (2016) have shown that in the isolated heart of rats treated with cisplatin, a decrease in coronary flow and increase in leakage of cardiac enzymes was accompanied by increased ROS concentrations and lipid peroxidation. The addition of n-acetyl cysteine, a precursor of glutathione with antioxidant activities, could attenuate these effects. In addition, El-Awady et al. (2011) showed that glutathione and SOD levels were reduced in cardiac tissue of cisplatin treated rats. Cisplatin-induced cardiac dysfunction is associated with mitochondrial membrane depolarization as well as mitochondrial ultrastructural abnormalities. Both mitochondrial and nuclear DNA were damaged and significant increases in the levels of cardiac enzymes were observed in serum samples. All these effects could be brought back to levels of untreated rats, when antioxidants were provided together with cisplatin. One of these antioxidants, l-carnitine, was also able to reduce lipid peroxidation and mitochondrial damage in proximal tubules of cisplatin treated rats. Taken together, oxidative stress plays a crucial role in both renal and cardiac toxicities of cisplatin therapy.
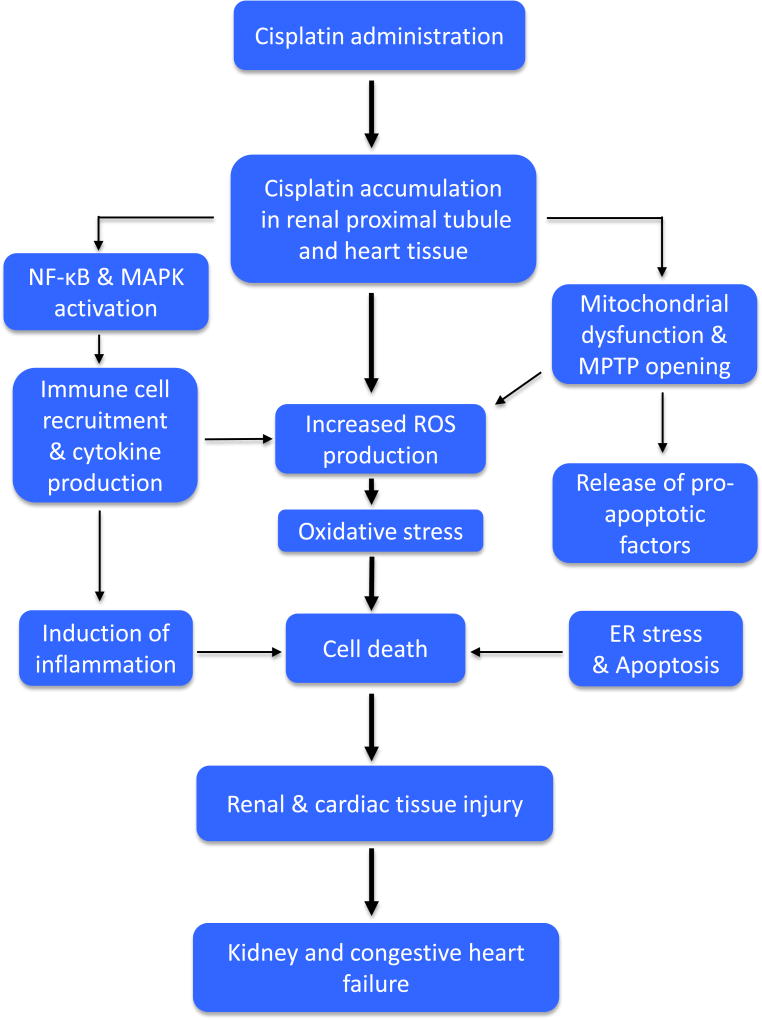
Figure 1. Schematic representation of pathophysiological events in cisplatin-induced renal and cardiac toxicities.
Cisplatin accumulates in renal proximal tubules and cardiac tissue. This accumulation activates the redox-sensitive transcription factor nuclear factor kappa-B (NF-κB) and mitogen-activated protein kinase (MAPK) leading to infiltration of immune cells such as macrophages and neutrophils and production of proinflammatory cytokines, which altogether induces inflammation and consequently cell death and tissue injury. Cisplatin also causes mitochondrial dysfunction, excessive production of reactive oxygen species (ROS) and opening of mitochondrial permeability transition pores (MPTPs), which allows the release of pro-apoptotic factors from the mitochondria into the cytosol and thereby activating signs of endoplasmic reticulum (ER) stress and apoptotic cell death. The increased mitochondrial ROS generation as well as production by infiltrated immune cells leads to oxidative stress and cell death. Activation of these pathological pathways (oxidative stress, inflammation and apoptosis) culminates in tissue and ultimately renal and congestive cardiac failure.
A plethora of evidence indicates that cisplatin accumulates in the mitochondrial matrix where it disturbs mitochondrial respiration and causes excessive ROS production (Kruidering et al., 1997; Davis et al., 2001; Nishikawa et al., 2001; Townsend et al., 2009). The mitochondrial oxidative stress, in turn, induces a cascade of events leading to mitochondrial dysfunction and activation of signaling molecules and transcription of pro-apoptotic genes and ultimately cell death (Yin et al., 2007; Jiang et al., 2009; Townsend et al., 2009). In the kidney, this cascade of events taken together may result in induction of secondary malignancies (Wozniak et al., 2004). The large amount of mitochondria in heart tissue and the fact that the function of this organ is highly dependent functional mitochondria may explain the predisposition of the heart to cisplatin toxicity. In summary, oxidative stress induced by cisplatin glutathione depletion and accumulation in the mitochondria may be partly responsible for the activation of various signaling pathways, which ultimately leads to cell death in both renal tubular and cardiac tissue. Moreover, cisplatin forms cross-links with mitochondrial DNA and other mitochondrial targets leading to mitochondrial DNA damage (Wozniak et al., 2004; Dzagnidze et al., 2007; Townsend et al., 2009).
Cisplatin may also disrupt the energy status of renal tubular cells and cardiomyocytes. As mitochondria are the powerhouses of cells, mitochondrial dysfunction induced by cisplatin may result in ATP depletion and consequently to a decrease in cellular energy production. The mechanism underlying cisplatin-induced energy depletion in the mitochondria was unraveled in in vitro studies which showed that cisplatin is a potent inhibitor of fatty acid oxidation, the major energy source for cells (Chang et al., 2002; Portilla et al., 2002; Dzagnidze et al., 2007). The same authors also reported that cisplatin affects cytochrome c oxidase, a key enzyme involved in the mitochondrial respiratory chain, leading to reduction in intracellular ATP levels and decrease in mitochondrial respiratory function. Thus, cisplatin accumulation causes mitochondrial dysfunction, depletes intracellular energy levels, and consequently leads to cell injury and cell death.
5.6. Induction of inflammation
An inflammatory response is an inevitable event often induced secondary after cell or tissue damage due to a toxic insult (Khan and McLean, 2012). Increasing evidence indicates that cisplatin induces a myriad of inflammatory cytokines and chemokines including translocation of the redox-sensitive transcription factor nuclear factor kappa B (NF-κB) from the cytosol to the nucleus, which leads to production of tumor necrosis factor alpha (TNF-α) in kidney tubular cells and cardiomyocytes, a pro-inflammatory cytokine that is actively involved in cisplatin-induced inflammation (Ramesh and Reeves, 2002; El-Sawalhi et al., 2014; Chowdhury et al. 2016) (figure 1). In the kidney, for example, cisplatin in the renal tubular epithelial cells leads to release of damage-associated molecular pattern molecules (DAMPs), leading to activation of toll-like receptor 4 (TLR4) and consequent TNF-α production by both immune and non-immune cells (Ramesh et al., 2007). Accumulation of cisplatin in the renal tubular epithelial cells promotes the binding of TNF-α to its receptors (TNFR1 and TNFR2), which are expressed on the surface of these epithelial cells. This triggers the induction of inflammatory factors and recruitment of immune cells such as neutrophils and macrophages. Cytokines and chemokines as well as ROS produced by these immune cells enhance the cytotoxic effect of cisplatin and eventually cause the loss of kidney function (Faubel et al., 2007; Ramesh et al., 2007). In addition, pharmacological inhibition of mitogen-activated protein kinases (MAPK), another key inflammatory signaling pathway, results in renal protection through suppression of TNF-α production in renal tubular cells (Ramesh and Reeves, 2005). Cisplatin has also been reported to induce significant production of myocardial TNF-α and myocardial myeloperoxidase activity in cisplatin-treated rats and mice (El-Sawalhi et al., 2014; Chowdhury et al. 2016). Although cisplatin-induced cardiac inflammation has not been described extensively, it’s likely that myocardial inflammation induced by cisplatin treatment results from similar molecular mechanisms described in the kidney. In summary, cisplatin activates pro-inflammatory factors that promote inflammation and subsequent cell injury in cisplatin-induced renal and cardiac toxicities.
5.7. Activation of apoptotic machinery
Apoptosis in renal and cardiac tissues has been demonstrated in in vivo and in vitro models of cisplatin-induced renal and cardiac toxicities respectively. Multiple pathways of apoptosis have been implicated, of which the major one is the intrinsic pathway, involving the mitochondria (Deng et al., 2001; Jiang et al., 2009; El-Awady et al. 2011). Cisplatin induces cellular stress, which activates Bax and Bak (pro-apoptotic proteins of Bcl-2 family), causing the opening of mitochondrial permeability transition pores (MPTPs), leading to the release of pro-apoptotic factors such as cytochrome c, endonuclease G, and apoptosis-inducing factor (AIF) from the mitochondria into the cytosol (Lee et al., 2001; Jiang et al., 2009) (figure 1). Cytochrome c activates caspase 9, leading to activation of several downstream caspases and induces apoptosis in caspase-dependent manner (Lee et al., 2001; Jiang et al., 2009). On the other hand, endonuclease G and AIF translocate and accumulate in the nucleus after their release from the mitochondria, leading to apoptosis in a caspase-independent manner (Yin et al., 2007). Recent studies have implicated the tumor suppressor protein, p53, in the induction of apoptosis in cisplatin-induced renal toxicity, in which DNA damage induced by cisplatin leads to phosphorylation and subsequent activation of p53, resulting in the induction of apoptotic genes including PUMA-α (p53-upregulated modulator of apoptosis) and PIDD (p53-induced protein with death domain). Activation of PUMA-α by cisplatin neutralizes the anti-apoptotic effect of Bcl-XL (a member of the Bcl-2 family), allowing Bax to become free to open more MPTPs, leading to apoptosis as described above. Activation of PIDD, in turn, activates caspase 2 resulting in the release of AIF from the mitochondria and inducing caspase-independent apoptosis (Jiang et al., 2006; Jiang et al., 2009). Cisplatin-induced apoptosis in renal tubular cells may also involve the endoplasmic reticulum (ER) pathway. Caspase-12 (in the cytosolic face of ER) is the key initiator caspase of ER stress, which induces apoptosis in a caspase-dependent manner (Liu and Baliga, 2005; Boyce and Yuan, 2006). Liu and Baliga (2005) observed activation of caspases 12 and induction of apoptosis in LLC-PK1 cells (epithelial cell line derived from porcine renal proximal tubule) following cisplatin treatment, which was attenuated upon anticaspase 12 antibody transfection. Also in cardiac tissue of mice, cisplatin induced several biomarkers indicative of ER stress in a recent study by Chowdhury et al. (2016). Cardiomyocytes showed signs of ER stress, increased caspase-3 activity and apoptosis in a mouse model of cisplatin-induced cardiac toxicity (Ma et al., 2010). El-Sawalhi et al. (2014) also demonstrated that mitochondria in rat heart treated with cisplatin exhibited significant loss of mitochondrial transmembrane potential, suggesting early apoptotic event. This observation was confirmed by markedly increased caspase-3 activity and significantly reduced cardiomyocyte cross-sectional area. Taken together, cisplatin treatment is associated with induction of apoptosis through both caspase-dependent and –independent pathways.
6. Novel biomarkers of cisplatin-induced renal toxicity
Evaluation of renal toxicity has traditionally depended on elevated levels of serum creatinine and blood urea nitrogen and urinary albumin excretion in clinical and laboratory studies as well as in routine clinical care (Bonventre et al., 2010; Marrer and Dieterle, 2010; George et al., 2015). However, these biomarkers may give a delayed signal since a significant degree of renal injury is required in order to produce elevated levels (Bonventre et al., 2010; George et al., 2015). Thus, the use of these biomarkers of renal toxicity is severely affected by sensitivity and specificity limitations, and therefore, they are not suitable to evaluate renal toxicity. It has become imperative to identify and develop new biomarkers for earlier and more accurate detection and assessment of renal toxicity as an alternative to the traditional renal biomarkers. In an in vitro study using HK-2 cells derived from human kidney proximal tubule epithelial cells, Sohn et al. (2013) observed a dose-dependent increase in the levels of proteins such as KIM-1 (kidney injury molecule-1), TIMP-1 (tissue inhibitor of metalloproteinase-1), and calbindin in conditioned media of HK-2 cells following 24 hours of cisplatin treatment. To confirm their in vitro observation, the authors also reported elevated levels of these nephrotoxic biomarkers in the urine of rats following 24 hours and 72 hours of cisplatin treatment. Further, cisplatin treatment significantly increased mRNA levels of KIM-1, TIMP-1 and calbindin, suggesting involvement of transcriptional activation (Sohn et al. 2013). Other authors also suggested pyruvate kinase M2 (PKM2) and eukaryotic translation elongation factor 1 gamma (EF-1γ) as biomarker candidates for detection and evaluation of cisplatin-induced renal toxicity and nephrotoxicity in general (Kim et al., 2014). They reported high levels of PKM2 and EF-1γ in conditioned media of HK-2 cells and in the urine and kidney tissue of rats upon 24 hours and 72 hours of cisplatin administration (Kim et al., 2014). These may not have been the best study designs, as HK-2 cells are cancer cells and are not contact inhibited and thus keep proliferating. In a more ideal model to study cisplatin-induced renal toxicity, Wilmes et al. (2015) used RPTEC/TERT1, a human proximal renal cell line in which sub-cytotoxic concentration of cisplatin at specific time intervals showed decreased metabolites of the enzymes choline dehydrogenase and butaine, and l-Carnitine, a metabolite of fatty acid metabolism in the cell lysates on day 1, 3 and 14 of cisplatin treatment. Such low concentration of cisplatin treatment was also associated with decreased glutathione and increased lactate levels in the cell supernatant from day 5 to day 10 while TEER (trans-epithelial electrical resistance; an indicator of barrier function of kidney epithelial cells or membrane perturbation by toxicants on kidney epithelial cell lines) remained unaffected after 10 days and increased slightly at day 14, suggesting that the barrier function was preserved throughout the duration of the experiment. All these indicators together may serve as novel biomarkers of cisplatin-induced renal toxicity. In a clinical study of thirty-nine cancer patients receiving cisplatin therapy, 2- and 4-fold increases in urinary KIM-1 were observed after 3 and 10 days of cisplatin infusion respectively. Also, baseline KIM-1 levels increased by 2-fold in patients who previously received cisplatin treatment (George et al., 2015). Taken together, these novel biomarkers offer an effective and more rapid and accurate detection of cisplatin-induced renal toxicity and may be used to evaluate drug-induced renal toxicity in general. However, additional research is needed to validate these novel biomarkers.
6.1. (Novel) biomarkers of cisplatin-induced cardiac toxicity
Chemotherapeutic agents have the ability to disrupt cell membranes leading to the release of intracellular proteins such as cardiac troponin (cTnT), lactate dehydrogenase (LDH), creatine kinase (CK), creatine kinase-myocardial band (CK-MB). These are biomarkers used to detect and evaluate the presence and extent myocardial damage. In a rat model of cisplatin-induced cardiac toxicity, El-Awady et al. (2011) reported significant increases in the activities of serum LDH, CK, CK-MB as well as plasma cTnI (cardiac troponin I) following a single dose of cisplatin compared to a control group. These observations suggest myocardial injury due to cisplatin cardiotoxicity. In addition, Demkow et al. (2011) reported occasional elevations in cTNT and NT-proBNP (N-terminal pro-B-type natriuretic peptide), another cardiac biomarker, increased significantly following cisplatin administration in a lung cancer patient, which may indicate cardiac insufficiency. In conclusion, cisplatin-induced cardiac toxicity can cause myocardial injury evidenced by increases in the levels and activities of cardiac biomarkers. More research is required before these markers are used clinically.
7. Current clinical approaches and challenges in treatment of cisplatin-induced renal and cardiac toxicities
Currently, hydration with the use of mannitol or hypertonic saline have been the primary approaches to treat renal insufficiency associated with cisplatin toxicity (Cornelison and Reed, 1993). Although the hydration regimens reduce the toxicities and enhance urine output, there is no substantial evidence in the literature on improvement in GFR or additional benefits of diuretics (Uchino et al., 2005). Also, there have been conflicting reports on the use of furosemide and its ability to affect renal toxicity associated with cisplatin treatment (Pera and Harder, 1978; Pera et al., 1979). More recently, amifostine has been used to reduce renal toxicity in patients with ovarian cancer due to its ability to reverse the platinum-DNA adducts and bind to ROS (Capizzi, 1999; Castiglione et al., 1999; Hensley et al., 2009). However, there is currently no available data on its use in other cancer sites. Clinical attempts to ameliorate cisplatin-induced cardiac toxicity have also met very little success (Kalam and Marwick, 2013; van Laar et al., 2014). Therefore, it has become imperative to develop a therapy to limit these toxicities associated with cisplatin treatment. In summary, treatments of cisplatin-induced renal and cardiac toxicities still remain major challenges despite several clinical interventions.
8. Potential protective strategies with novel pharmacological agents against cisplatin-induced renal and cardiac toxicities
Several pharmacological compounds are currently being tested to enhance protection during cisplatin treatment. Considering the principal targets of cisplatin as discussed above, one could propose the following measures to limit cisplatin-induced toxicity. Thus (a) Uptake of cisplatin by renal tubular cells. As OCT2 is the principal cisplatin transport mediator into renal tubular cells, pharmacological inhibition of OCT2 may offer renoprotective effects. OCT2 inhibitors such as cimetidine have been shown to protect against cisplatin-induced renal toxicity in mice without interfering with the antineoplastic actions of cisplatin (Franke et al., 2010; Sprowl et al., 2014). More recently, Kim et al. (2015) reported that administration of glutamine, a substrate for glutathione synthesis, reduced cisplatin-associated pathological changes in HK-2 cells and in rats via reduced expression of OCT2. Whereas these findings are promising, one possible drawback of pharmacological inhibition of OCT2 may be inhibition of cisplatin uptake by cancer cells, which are the target of cisplatin treatment, and hence, this may reduce the antineoplastic action of cisplatin. In addition, cardiotoxicity induced by cisplatin is not likely to be reduced by this strategy since OCT2 is not expressed in heart (b) Cisplatin biotransformation to toxic metabolites. Since GSTP, GGT, APN and CCBL are key enzymes involved in the metabolic conversion of cisplatin into a nephrotoxin, inhibition of any of these enzymes could disrupt the cisplatin biotransformation pathway, thereby limiting cisplatin toxicity in the kidney. This may also limit off-target effects of cisplatin and further enhance its anticancer action. Townsend et al. (2009) showed that mice deficient in GSTP (via non-pharmacological approach) had significantly less renal damage compared to wild type mice following 5 days of cisplatin treatment. In addition, Hannigan et al. (1994) showed that co-administration of cisplatin with the GGT inhibitor, acivicin, attenuated the toxic effects of cisplatin on the kidney of rats. On the other hand, Maroun et al. (1990) reported that co-administration of cisplatin and acivicin in a phase I study in lung cancer patients led to renal toxicity at lower doses than expected, indicating potentiation of cisplatin nephrotoxicity. However, no control group of cisplatin alone was included in this study. These finding suggests that inhibition or deficiency in any of the key enzymes involved in the bioconversion of cisplatin to nephrotoxin could protect against cisplatin-induced toxicity but needs further exploration. However, since GGT is not expressed in heart tissue, it is not likely that this strategy could prevent cardiotoxicity. (c) Oxidative stress. Increasing antioxidant levels in the kidney and heart through exogenous administration of antioxidants could boost the antioxidant defense system and hence reduce the amount of ROS generation in renal and cardiac tissues. This may help protect the kidney and heart against cisplatin toxicity. In a rat model of cisplatin-induced cardiac toxicity, administration of silymarin, a potent antioxidant, antagonized the depletion of glutathione and superoxide dismutase and consequently reduced the concentration of cardiac damage marker enzymes induced by cisplatin, towards normal level, resulting in cardiac protection (El-Awady et al., 2011). Also, treatment with the hydrogen sulfide (H2S) donor sodium hydrosulfide (NaHS) and garlic-derived diallyl disulfide (a natural source of H2S) has been reported to enhance the activity of renal antioxidant enzymes and reduced oxidative stress thereby attenuating cisplatin-induced renal toxicity in rats (Chiarandini et al., 2008; Fard et al., 2015). Furthermore, combination treatment of tumor-bearing mice and rats with vitamin C and cisplatin significantly increased endogenous antioxidant levels, decreased tissue lipid peroxidation and well protected the kidney, liver and testes against cisplatin-induced toxicity compared to cisplatin treatment alone (Fatima et al., 2007; Tarladacalisir et al., 2008; Longchar and Prasad, 2015). (d) Apoptotic pathways. Pharmacological or genetic disruption of the apoptotic pathway in mitochondria could inhibit the opening of MPTPs and prevent the release of pro-apoptotic factors and the activation of caspases. This would prevent mitochondrial and nuclear damage as well as cisplatin-induced apoptotic cell injury and cell death. In a rat model of cisplatin-induced renal toxicity, Molitoris et al. (2009) observed that administration of siRNA targeted to p53, a key protein in the apoptotic pathway, prevented the opening of MPTPs and attenuated renal injury associated with cisplatin treatment. Although there is currently no available data on the effect of H2S on MPTP in cisplatin-induced toxicity, An important point worthy of note is to target therapies aiming at eliminating the cause of enhanced apoptosis (for example, by reducing oxidative stress or mitochondrial damage), which are more likely to be clinically successful than therapies aiming at inhibiting apoptosis itself since inhibition of apoptosis would increase the risk of secondary tumors. In line with this, H2S related therapies could be interesting, since those would be aiming more at increasing adaptive responses to cellular stress. NaHS administration activated endogenous antioxidant defense system and protected proximal tubular cells from apoptosis in a rat model of cisplatin-induced renal toxicity (Ahangarpour et al., 2014). Recently, H2S related therapy has been proven successful in treating other forms of cellular stress due to myocardial ischemia-reperfusion injury and acute kidney injury (Ahmad et al., 2016; Ansari and Kurian, 2016). Moreover, Strutynska et al. (2013) observed that H2S treatment prevented opening of the MPTPs in a rat model of spontaneous hypertension, suggesting its application in cisplatin-induced toxicity. (e) Inflammatory response. Inflammation plays a critical role in cisplatin-induced toxicity. Therefore, inhibition or suppression of TNF-α during cisplatin treatment could prevent the influx of macrophages and neutrophils, and could limit the inflammatory process and hence protect the kidney and heart against cisplatin toxicity. Also, blocking specific pathways within the MAPK signaling pathway with specific inhibitors could attenuate the observed cisplatin-induced toxicity. Inhibitors of TNF-α have been shown to reduce cisplatin-associated toxicity in vivo (Ramesh et al., 2002) and may therefore be administered to cancer patients undergoing cisplatin treatment. Recently, Fard et al. (2013) observed that intraperitoneal administration of NaHS reduced TNF-α production and consequently reduced inflammation and thereby limited the progression of cisplatin-induced toxicity in rat kidney. Also, administration of GYY3147, a slow-release H2S donor, has been reported to inhibit activation of NF-κB and MAPK signaling and consequently inhibited inflammation in cardiomyocytes (Meng et al., 2015). Combination therapy of cisplatin with compounds such as apocynin, taurine, rosiglitazone and anti-4-1BB have been tested and proven to be cardio- and renoprotective against cisplatin-induced inflammation by inhibiting NF-κB activation and suppressing proinflammatory cytokine and chemokine production as well as adhesion molecules while synergizing with the antineoplastic activity of cisplatin in animals compared to cisplatin therapy alone (Kim et al., 2008; Tikoo et al., 2009; El-Sawalhi et al., 2014; Chowdhury et al., 2016). (f) Other protective measures. Other protective pharmacological agents that may not interfere with the anticancer action of cisplatin could be used if they are tested and proven to ameliorate cisplatin-induced toxicity. For example, following six hours of cisplatin treatment of human neuroblastoma cell lines, administration of thiosulfate in the form of sodium thiosulfate (an oxidation product of H2S), which is also a drug currently used in the clinic for the treatment of cyanide poisoning and calciphylaxis, protected against cisplatin-induced cytotoxicity without compromising the antineuroblastoma activity of cisplatin (Harned et al., 2008).
9. Concluding remarks
Renal and cardiac toxicities are major side effects of cisplatin administration, and worsen quality of life as well as survival of cancer patients undergoing cisplatin therapy. These toxicities are the net result of cisplatin uptake into renal and cardiac tissues, oxidative stress and mitochondrial dysfunction, nuclear and mitochondrial DNA damage, activation of apoptotic pathways as well as induction of inflammation. Despite several pre-clinical and clinical interventions to limit these toxicities, renal and cardiac toxicities remain a major concern in cancer patients receiving cisplatin treatment. Therefore, combinatorial strategies such as cisplatin plus H2S-related therapy or cisplatin with taurine, which target ROS generation, inflammatory and apoptotic pathways in cisplatin-induced toxicity may offter the best chance of clinically meaningful prevention. However, there are very few clinical trials reporting on combinatorial therapies to prevent these side effects, and hence require additional research. More importantly, there is the need to test pharmacological (and genetic) approaches to renal and cardiac protection in experimental or natural tumor-bearing animals to enhance their application to cisplatin treatment in oncology. Identification of novel interventions aimed at minimizing cisplatin-induced renal and cardiac toxicities while enhancing its antineoplastic efficacy would open new avenues to enhance cisplatin-based cancer therapy.
Acknowledgments
We would like to thank Dr. Susan Rosenthal for critical reading of the manuscript.
Footnotes
Conflict of interest
None
References
- Ahangarpour A, Fard AA, Gharibnaseri MK, Jalali T, Rashidi I. Hydrogen sulfide ameliorates the kidney dysfunction and damage in cisplatininduced nephrotoxicity in rat. Vet Res Forum. 2014;5:121–7. [PMC free article] [PubMed] [Google Scholar]
- Ahmad A, Olah G, Szczesny B, Wood ME, Whiteman M, Szabo C. AP39, A mitochondrially targeted hydrogen sulfide donor, exerts protective effects in renal epithelial cells subjected to oxidative stress in vitro and in acute renal injury in vivo. Shock. 2016;45:88–97. doi: 10.1097/SHK.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akcay A, Turkmen K, Lee D, Edelstein CL. Update on the diagnosis and management of acute kidney injury. Int J Nephrol Renovasc Dis. 2010;3:129–140. doi: 10.2147/IJNRD.S8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Majed AA, Sayed-Ahmed MM, Al-Yahya AA, Aleisa MA, Al-Rejaie SS, Al-Shabanah OA. Propionyl-l-Carnitine prevents the progression of cisplatin-induced cardiotoxicity in a carnitine-depleted rat model. Pharmacol Res. 2006;53:278–286. doi: 10.1016/j.phrs.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Ali BH, Al-Moundhri M, Tageldin M, et al. Ontogenic aspects of cisplatin-induced nephrotoxicity in rats. Food Chem Toxicol. 2008;46:3355–3359. doi: 10.1016/j.fct.2008.07.030. [DOI] [PubMed] [Google Scholar]
- Amit L, Ben-Aharon I, TIchler T, Inbar E, Sulkes A, Stemmer S. Cisplatin-induced posterior reversible encephalopathy syndrome – a brief report and review of the literature. J Behav Brain Sci. 2012;2:97–101. [Google Scholar]
- Ansari SB, Kurian GA. Hydrogen sulfide modulates sub-cellular susceptibility to oxidative stress induced by myocardial ischemic reperfusion injury. Chem Biol Interact. 2016;252:28–35. doi: 10.1016/j.cbi.2016.03.036. [DOI] [PubMed] [Google Scholar]
- Antunes LM, Darin JD, Bianchi Nde L. Effects of the antioxidant curcumin or selenium on cisplatin-induced nephrotoxicity and lipid peroxidation in rats. Pharm Res. 2001;43:145–150. doi: 10.1006/phrs.2000.0724. [DOI] [PubMed] [Google Scholar]
- Antunes LMG, Darin JDC, Bianchi MLP. Protective effects of Vitamin C against cisplatin-induced nephrotoxicity and lipid peroxidation in adult rats: a dose-dependent study. Pharmacol Res. 2000;41:405–411. doi: 10.1006/phrs.1999.0600. [DOI] [PubMed] [Google Scholar]
- Bano N, Najam R, Qazi F. Adverse cardiac manifestations of cisplatin- a review. Int J Pharm Sci Rev Res. 2013;18:80–85. [Google Scholar]
- Bonventre JV, Vaidya VS, Schmouder R, Feig P, Dieterle F. Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol. 2010;28:436–440. doi: 10.1038/nbt0510-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–373. doi: 10.1038/sj.cdd.4401817. [DOI] [PubMed] [Google Scholar]
- Brock PR, Knight KR, Freyer DR, et al. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol. 2012;30:2408–2417. doi: 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglione F, Mola AD, Porcile G. Protection of normal tissues from radiation and cytotoxic therapy: The development of amifostine. Tumori. 1999;85:85–91. [PubMed] [Google Scholar]
- Capizzi RL. Amifostine reduces the incidence of cumulative nephrotoxicity from cisplatin: Laboratory and clinical aspects. Semin Oncol. 1999;26:72–81. [PubMed] [Google Scholar]
- Chang BJ, Nishikawa M, Sato E, Utsumi K, Inoue M. l-Carnitine inhibits cisplatin-induced injury of the kidney and small intestine. Arch Biochem Biophys. 2002;405:55–64. doi: 10.1016/s0003-9861(02)00342-9. [DOI] [PubMed] [Google Scholar]
- Chiarandini Fiore JP, Fanelli SL, de Ferreyra EC, Castro JA. Diallyl disulfide prevention of cis-Diamminedichloroplatinum-induced nephrotoxicity and leukopenia in rats: potential adjuvant effects. Nutr Cancer. 2008;60:784–791. doi: 10.1080/01635580802100869. [DOI] [PubMed] [Google Scholar]
- Chirino YI, Sanchez-Gonzalez DJ, Martinez-Martinez CM, Cruz C, Pedraza-Chaverri J. Protective effects of apocynin against cisplatin-induced oxidative stress and nephrotoxicity. Toxicology. 2008;245:18–23. doi: 10.1016/j.tox.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Ciarimboli G, Ludwig T, Lang D, et al. Cisplatin nephrotoxicity is critically mediated via human organ cation transporter 2. Am J Pathol. 2005;167:1477–84. doi: 10.1016/S0002-9440(10)61234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelison TL, Reed E. Nephrotoxicity and hydration management for cisplatin, carboplatin and ormaplatin. Gynecol Oncol. 1993;50:147–158. doi: 10.1006/gyno.1993.1184. [DOI] [PubMed] [Google Scholar]
- Cullen KJ, Yang Z, Schumaker L, Guo Z. Mitochondria as a critical target of the therapeutic agent cisplatin in head and neck cancer. J Bioenerg Biomembr. 2007;39:43–50. doi: 10.1007/s10863-006-9059-5. [DOI] [PubMed] [Google Scholar]
- Daugaard G, Abildgaard U, Holstein-Rathlou NH, et al. Renal tubular function in patients treated with high-dose cisplatin. Clin Pharmacol Ther. 1988;44:164–172. doi: 10.1038/clpt.1988.132. [DOI] [PubMed] [Google Scholar]
- Davis CA, Nick HS, Agarwal A. Manganese superoxide dismutase attenuates cisplatin-induced renal injury: importance of superoxide. J Am Soc Nephrol. 2001;12:2683–2690. doi: 10.1681/ASN.V12122683. [DOI] [PubMed] [Google Scholar]
- de Jongh FE, Verweij J, Loos WJ, et al. Body-surface area-based dosing does not increase accuracy of predicting cisplatin exposure. J Clin Oncol. 2001;19:3733–3739. doi: 10.1200/JCO.2001.19.17.3733. [DOI] [PubMed] [Google Scholar]
- de Jongh FE, van Veen RN, Veltman SJ, et al. Weekly high-dose cisplatin is a feasible treatment option: Analysis on prognostic factors for toxicity in 400 patients. Br J Cancer. 2003;88:1199–1206. doi: 10.1038/sj.bjc.6600884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demkow U, Bialas-Chromiec B, Stelmaszczyk-Emmel, et al. The cardiac markers and oxidative stress parameters in advanced non-small cell lung cancer patients receiving cisplatin-based chemotherapy. eJIFCC. 2011 www.ifcc.org/media/58796/ejifcc_v22_01_02.pdf. [PMC free article] [PubMed]
- Deng J, Kohda Y, Chiao H, et al. Interleuikin-10 inhibits ischemic and cisplatin-acute renal injury. Kid Int. 2001;60:2118–2128. doi: 10.1046/j.1523-1755.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- Dhillon AS, Hagan S, Rath O, et al. MAP kinase signaling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- Dolci A, Dominici R, Cardinale D, Sandri MT, Panteghini M. Biochemical markers for prediction of chemotherapy-based cardiotoxicity: systematic review of the literature and recommendations for use. Am J Clin Pathol. 2008;130:688–695. doi: 10.1309/AJCPB66LRIIVMQDR. [DOI] [PubMed] [Google Scholar]
- Dzagnidze A, Katsarava Z, Makhalova J, Liedert B, et al. Repair capacity for platinum-DNA adducts determines the severity of cisplatin-induced peripheral neuropathy. J Nuero. 2007;27:9451–9457. doi: 10.1523/JNEUROSCI.0523-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn LH. Curing metastatic testicular cancer. Proc Natl Acad Sci. 2002;99:4592–4595. doi: 10.1073/pnas.072067999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Awady el-SE, Moustafa YM, Abo-Elmatty DM, Radwan A. Cisplatin-induced cardiotoxicity: mechanisms and cardioprotective strategies. Eur J Pharmacol. 2011;650:335–41. doi: 10.1016/j.ejphar.2010.09.085. [DOI] [PubMed] [Google Scholar]
- Fard AA, Ahangarpour A, Gharibnaseri KM, Ahmadizadeh M, Rashidi I, Jalali T. Effects of exogenous and endogenous hydrogen sulfide on plasma renin and erythropoietin in cisplatin-induced nephrotoxicity in rats. URMIAMJ. 2015;26:459–466. [Google Scholar]
- Fard AA, Ahangarpour A, Gharibnaseri MK, Jalali T, Rashidi I, Ahmadzadeh M. Effects of hydrogen sulfide on oxidative stress, tnf-α level and kidney histological changes in cisplatin nephrotoxicity in rat. J Phys Pharm Adv. 2013;3:57–65. [Google Scholar]
- Faubel S, Lewis EC, Reznikov L, et al. Cisplatin-induced acute renal failure is associate with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J Pharmacol Exp Ther. 2007;322:8–15. doi: 10.1124/jpet.107.119792. [DOI] [PubMed] [Google Scholar]
- Franke RM, Kosloske AM, Lancaster CS, et al. Influence of Oct1/Oct2-deficiency on cisplatin-induced changes in urinary N-Acetyl-{beta}-D-Glucosaminidase. Clin Cancer Res. 2010;16:4198–4206. doi: 10.1158/1078-0432.CCR-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George B, Wen X, Ellison L, Joy M, Aleksunes L. Urinary KIM-1 is a novel biomarker for cisplatin-induced subclinical nephrotoxicity in oncology patients. FASEB. 2015;29 www.fasebj.org/content/29/1_Supplement/938.6.short. [Google Scholar]
- Gogas H, Shapiro F, Aghaijanian C, et al. The impact of diabetes mellitus on the toxicity of therapy for advanced ovarian cancer. Gyenecol. Oncol. 1996;61:22–26. doi: 10.1006/gyno.1996.0090. [DOI] [PubMed] [Google Scholar]
- Goldstein RS, Mayor GH. Minireview. The nephrotoxicity of cisplatin. Life Sci. 1983;32:685–690. doi: 10.1016/0024-3205(83)90299-0. [DOI] [PubMed] [Google Scholar]
- Goren MP. Cisplatin nephrotoxicity affects magnesium and calcium metabolism. Med Ped Oncol. 2003;41:186–189. doi: 10.1002/mpo.10335. [DOI] [PubMed] [Google Scholar]
- Groth S, Nielson H, Sorensen JB, et al. Acute and long-term nephrotoxicity of cis-platinum in man. J Pharmacol Exp Ther. 1986;17:191–196. doi: 10.1007/BF00306754. [DOI] [PubMed] [Google Scholar]
- Grube M, Ameling S, Noutsias M, et al. Selective regulation of cardiac organic cation transporter novel type 2 (OCTN2) in dilated cardiomyopathy. Am J Pathol. 2011;178:2547–59. doi: 10.1016/j.ajpath.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglin M, Aljayeh M, Saiyad S, Ali R, Curtis AB. Introducing a new entity: chemotherapy-induced arrhythmia. Europace. 2009;11:1579–1586. doi: 10.1093/europace/eup300. [DOI] [PubMed] [Google Scholar]
- Harned TM, Kalous O, Nuewelt A, et al. Sodium thiosulfate administered six hours after cisplatin does not compromise antineuroblastoma activity. Clin Cancer Res. 2008;14:533–540. doi: 10.1158/1078-0432.CCR-06-2289. [DOI] [PubMed] [Google Scholar]
- Hartmann JT, Lipp HP. Toxicity of platinum compounds. Expert Opin Pharmacother. 2003;4:889–901. doi: 10.1517/14656566.4.6.889. [DOI] [PubMed] [Google Scholar]
- Hensley ML, Hagerty KL, Kewalramani T, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: Use of chemotherapy and radiation therapy protectants. J Clin Oncol. 2009;27:127–145. doi: 10.1200/JCO.2008.17.2627. [DOI] [PubMed] [Google Scholar]
- Jiang M, Wang CY, Huang S, Yang T, Dong Z. Cisplatin-induced apoptosis in p53-deficient renal cells via the intrinsic mitochondrial pathway. Am J Physiol Renal Physiol. 2009:F983–F993. doi: 10.1152/ajprenal.90579.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Wei Q, Wang J, Du Q, Yu J, Zhang L, Dong Z. Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene. 2006;25:4056–4066. doi: 10.1038/sj.onc.1209440. [DOI] [PubMed] [Google Scholar]
- Kadikoylu G, Bolaman Z, Demir S, Balkaya M, Akalin N, Enli Y. The effects of desferrioxamine on cisplatin-induced lip peroxidation and the activities of antioxidant enzymes in rat kidneys. Hum Exp Toxicol. 2004;23:29–34. doi: 10.1191/0960327104ht413oa. [DOI] [PubMed] [Google Scholar]
- Kalam K, Marwick TH. Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. Eur J Cancer. 2013;49:2900–2909. doi: 10.1016/j.ejca.2013.04.030. [DOI] [PubMed] [Google Scholar]
- Karthikeyan K, Saralabai-Bai BR, Niranjali-Devaraj S. Cardioprotective effects of grape seed proanthocyanidins on isoproterenol-induced myocardial injury in rats. Int J Cardiol. 2007;115:326–333. doi: 10.1016/j.ijcard.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Khan S, Chen CL, Brady MS, et al. Unstable angina associated with cisplatin and carboplatin in a patient with advanced melanoma. J Clin Oncol. 2012;30:e163–4. doi: 10.1200/JCO.2011.38.7852. [DOI] [PubMed] [Google Scholar]
- Khan SA, McLean MK. Toxicology of frequently endcountered nonsteroid anti-inflammatory drugs in dogs and cats. Vet Clin North Am Small Anim Pract. 2012;42:289–306. doi: 10.1016/j.cvsm.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Kim BE, Turski ML, Nose Y, Casad M, Rockman HA, Thiele DJ. Cardiac copper deficiency activates a systemic signaling mechanism that communicates with the copper acquisition and storage organs. Cell Metab. 2010;11:353–363. doi: 10.1016/j.cmet.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Park DJ, Kim JH, et al. Glutamine protects against cisplatin-induced nephrotoxicity by decreasing cisplatin accumulation. J Pharmacol Sci. 2015;127:117–26. doi: 10.1016/j.jphs.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Kim SY, Sohn SJ, Won AJ, Kim HS, Moon A. Identification of noninvasive biomarkers of nephrotoxicity using HK-2 human kidney epithelial cells. Toxicol Sci. 2014;140:247–258. doi: 10.1093/toxsci/kfu096. [DOI] [PubMed] [Google Scholar]
- Kociba RJ, Sleight SD. Acute toxicologic and pathologic effects of cis-diamminedichloroplatinum (NSC-119875) in the male rat. Cancer Chemother Rep. 1971;55:1–8. [PubMed] [Google Scholar]
- Kohn S, Fradis M, Ben-David J, Zidan J, Robinson E. Nephrotoxicity of combined treatment with cisplatin and gentamicin in the guinea pig: glomerular injury findings. Ultrastruct Pathol. 2002;26:371–782. doi: 10.1080/01913120290104683. [DOI] [PubMed] [Google Scholar]
- Kolb R, Ghazi M, Barfuss D. Inhibition of basolateral transport and cellular accumulation of cDDP and N-acetyl-l-cysteine-cDDP by TEA and PAH in the renal proximal tubule. Cancer Chemother. Pharmacol. 2003;51:132–138. doi: 10.1007/s00280-002-0537-0. [DOI] [PubMed] [Google Scholar]
- Kruidering M, Van de Water B, de Heer E, et al. Cisplatin-induced nephrotoxicity in porcine proximal tubular cells: mitochondrial dysfunction by inhibition of complexes I to IV of the respiratory chain. J Pharmacol Exp Ther. 1997;280:638–649. [PubMed] [Google Scholar]
- Lajer H, Kristensen M, Hansen HH, Nielsen S, et al. Magnesium depletion enhances cisplatin-induced nephrotoxicity. Cancer Chemother Pharmacol. 2005;56:535–542. doi: 10.1007/s00280-005-1010-7. [DOI] [PubMed] [Google Scholar]
- Lee RH, Song JM, Park MY, et al. Cisplatin-induced apoptosis by translocation of endogenous Bax in mouse collecting duct cells. Biochem Pharmacol. 2001;62:1013–1023. doi: 10.1016/s0006-2952(01)00748-1. [DOI] [PubMed] [Google Scholar]
- Liu H, Baliga R. Endoplasmic reticulum stress-associated caspase 12 mediates cisplatin-induced LLC-PK1 cell apoptosis. J Am Soc Nephrol. 2005;16:1985–1992. doi: 10.1681/ASN.2004090768. [DOI] [PubMed] [Google Scholar]
- Loehrer PJ, Gonin R, Nichols CR, Weathers T, Einhorn LH. Vinblastine plus ifosphamide plus cisplatin as initial salvage therapy in recurrent germ cell tumor. J Clin Oncol. 1998;16:2500–2504. doi: 10.1200/JCO.1998.16.7.2500. [DOI] [PubMed] [Google Scholar]
- Marrer E, Dieterle F. Impact of biomarker development on drug safety assessment. Toxicol Appl Pharmacol. 2010;243:167–179. doi: 10.1016/j.taap.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Mattson DM, Ahmad IM, Dayal D, et al. Cisplatin combined with zidovudine enhances cytoxicity and oxidative stress in human head and neck cancer cells via a thiol-dependent mechanism. Free Radic Biol Med. 2009;46:232–237. doi: 10.1016/j.freeradbiomed.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 2009;8:10–16. doi: 10.1158/1535-7163.MCT-08-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng G, Wang J, Xiao Y, Bai W, Xie L, Shan L, Moore PK, Ji Y. GYY4137 protects against myocardial ischemia and reperfusion injury by attenuating oxidative stress and apoptosis in rats. J Biomed Res. 2015;29:203–213. doi: 10.7555/JBR.28.20140037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Goldstein RS, Pasino DA, Hook J. Cisplatin nephrotoxicity: role of filtration and tubular transport of cisplatin in isolated perfused kidneys. Toxicology. 1987;44:147–158. doi: 10.1016/0300-483x(87)90145-4. [DOI] [PubMed] [Google Scholar]
- Molitoris BA, Dagher PC, Sandoval RM, et al. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J AM Soc Nephrol. 2009;20:1754–1764. doi: 10.1681/ASN.2008111204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RA, Adel N, Riedel E, et al. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol. 2011;29:3466–3473. doi: 10.1200/JCO.2011.35.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhaee A, Bokaeian M, Noori S, Mahboob T. Antioxidant effect of carnosine pretreatment on cisplatin-induced renal oxidative stress in rats. Indian J Clin Biochem. 2010;25:86–91. doi: 10.1007/s12291-010-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa M, Nagatomi H, Chang BJ, Sato E, Inoue M. Targeting superoxide dismutase to renal proximal tubule cells inhibits mitochondrial injury and renal dysfunction induced by cisplatin. Arch Biochem Biophys. 2001;387:78–84. doi: 10.1006/abbi.2000.2237. [DOI] [PubMed] [Google Scholar]
- Ozcan T, Cirit A, Kiykim A. Recurrent complete atrioventricular block during cisplatin infusion: a case report. J Clin Exp Cardiol. 2011;2:151. [Google Scholar]
- Pabla N, Dong Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- Pabla N, Murphy RF, Liu K, Dong Z. The copper transporter Ctrl contributes to cisplatin uptake by renal tubular cells during cisplatin-induced nephrotoxicity. Am J Physiol Renal Physiol. 2009;296:F505–F511. doi: 10.1152/ajprenal.90545.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000;22:263–302. doi: 10.2165/00002018-200022040-00002. [DOI] [PubMed] [Google Scholar]
- Paolicchi A, Sotiropuolou M, Perego P, et al. γ–Glutamyl transpeptidase catalyses the extracelular detoxification of cisplatin in a human cell line derived from the proximal convoluted tubule of the kidney. Eur J Cancer. 2002;39:996–1003. doi: 10.1016/s0959-8049(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Pera MF, Harder HC. Effects of furosemide- and mannitol-induced diuresis on the nephrotoxicity and physiologic disposition of cis-dichlorodiamminoplatinum in rats. Proc Am Assoc Cancer Res. 1978;19:100. [PubMed] [Google Scholar]
- Pera MF, Zook BC, Harder HC. Effects of mannitol or furosemide diuresis on nephrotoxicity and physiological disposition of cis-dicholorodiammineplatinum-(II) in rats. Cancer Res. 1979;39:1269–78. [PubMed] [Google Scholar]
- Portilla D, Dai G, McClure T, Bates L, et al. Alterations of PPARalpha and its coactivator PGC-1 in cisplatin-induced acute renal failure. Kidney Int. 2002;62:1208–19. doi: 10.1111/j.1523-1755.2002.kid553.x. [DOI] [PubMed] [Google Scholar]
- Raja W, Mir MH, Dar I, Banday MA, Ahmad I. cisplatin induced paroxysmal supraventricular tachycardia. Indian J Med Paediatr Oncol. 2013;34:330–2. doi: 10.4103/0971-5851.125262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh G, Kimball SR, Jefferson LS, Reeves WB. Endotoxin and cisplatin synergistically stimulate TNF-α production by renal epithelial cells. Am J Physiol. 2007;292:812–819. doi: 10.1152/ajprenal.00277.2006. [DOI] [PubMed] [Google Scholar]
- Ramesh G, Reeves WB. TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J Clin Invest. 2002;110:835–842. doi: 10.1172/JCI15606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh G, Reeves WB. P38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am J Physiol Renal Physiol. 2005;289:166–174. doi: 10.1152/ajprenal.00401.2004. [DOI] [PubMed] [Google Scholar]
- Robbins ME, Campling D, Whitehouse E, Hopewell JW, Michalowski A. Cisplatin-induced reduction in renal functional reserve uncovered by unilateral nephrectomy: an experimental study in the pig. Cancer Chemother Pharmacol. 1990;27:211–218. doi: 10.1007/BF00685715. [DOI] [PubMed] [Google Scholar]
- Rosenberg B, Vancamp L, Krigas T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature. 1965;205:698–699. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg B, Vancamp L, Trosko JE, Mansour VH. Platinum compounds: A new class of potent antitumour agents. Nature. 1969;222:385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- Ryberg M. Recent advances in cardiotoxicity of anticancer therapies. Am Soc Clin Oncol Educ Book. 2012;32:555–559. doi: 10.14694/EdBook_AM.2012.32.40. [DOI] [PubMed] [Google Scholar]
- Sanches-Gonzalez PD, Lopez-Hernandez FJ, Lopez-Novoa JM, Morales AI. An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Crit Rev Toxicol. 2011;41:803–821. doi: 10.3109/10408444.2011.602662. [DOI] [PubMed] [Google Scholar]
- Schrier RW, Wang W, Poole B, et al. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114:5–14. doi: 10.1172/JCI22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LA, Madan E, Valentovic MA. Attenuation of cisplatin nephrotoxicity by streptozotocin-induced diabetes. Fundam. Appl. Toxicol. 1989;12:530–539. doi: 10.1016/0272-0590(89)90026-2. [DOI] [PubMed] [Google Scholar]
- Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev. 2012;64:706–721. doi: 10.1124/pr.111.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi F, Curtis LM, Truong L, et al. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am J Physiol Renal Physiol. 2000;5:726–736. doi: 10.1152/ajprenal.2000.278.5.F726. [DOI] [PubMed] [Google Scholar]
- Skinner R, Pearson AD, English MW, et al. Cisplatin dose rate as a risk factor for nephroxicity in children. Br J Cancer. 1998;77:1677–1682. doi: 10.1038/bjc.1998.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn SJ, Kim SY, Kim HS, Chun YJ, Han SY, Kim SH, Moon A. In vitroevaluation of biomarkers for cisplatin-induced nephrotoxicity using HK-2 human kidney epithelial cells. Toxicol Lett. 2013;217:235–242. doi: 10.1016/j.toxlet.2012.12.015. [DOI] [PubMed] [Google Scholar]
- Speer RJ, Ridgway H, Hall LM. Coordination complexes of platinum as antitumor agents. Cancer Chemother Rep. 1979;59:629–41. [PubMed] [Google Scholar]
- Strutynska NA, Dorofeieva NO, Vavilova HL, Sahach VF. Hydrogen sulfide inhibits Ca(2+)-induced mitochondrial permeability transition pore opening in spontaneously hypertensive rats. Fiziol Zh. 2013;59:3–10. [PubMed] [Google Scholar]
- Townsend DM, Tew KD, Lin H, King JB, Hanigan MH. Role of glutathione-S-transferase Pi in cisplatin-induced nephrotoxicity. Biomed & Pharmacother. 2009;63:79–85. doi: 10.1016/j.biopha.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino H, Matsumura Y, Negishi T, et al. Cisplatin-incorporating polymeric micelles (NC-6004) can reduce nephrotoxicity and neurotoxicity of cisplatin in rats. Br J Cancer. 2005;93:678–687. doi: 10.1038/sj.bjc.6602772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Laar M, Fetbower RG, Gale CP, Bowen DT, Oliver SE, Glaser A. Cardiovascular sequelae in long-term survivors of young people’s cancer: a linked cohort study. Br J Cancer. 2014;110:1338–1341. doi: 10.1038/bjc.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers AE, Rose K, Fisher R, et al. Kidney slices of human and rat to characterize cisplatin-induced injury on cellular pathways and morphology. Toxicol Pathol. 2004;32:577–90. doi: 10.1080/01926230490508821. [DOI] [PubMed] [Google Scholar]
- Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- Weiner MW, Jacobs C. Mechanism of cisplatin nephrotoxicity. Fed Proc. 1983;42:2974–2978. [PubMed] [Google Scholar]
- Winston JA, Safirstein R. Reduced renal blood flow in early cisplatin-induced acute renal failure in the rat. Am J Physiol. 1985;249:F490–F496. doi: 10.1152/ajprenal.1985.249.4.F490. [DOI] [PubMed] [Google Scholar]
- Wozniak K, Czechowska A, Blasiak J. Cisplatin-evoked DNA fragmentation in normal and cancer cells and its modulation by free radical scavengers and the tyrosine kinase inhibitors STI571. Chem Biol Interact. 2004;147:309–318. doi: 10.1016/j.cbi.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334:115–124. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- Yavas O, Aytemir K, Celik I. The prevalence of silent arrhythmia in patients receiving cisplatin-based chemotherapy. Turk J Cancer. 2008;38:1. [Google Scholar]
- Yeh ET, Brickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis and management. J Am Coll Cardiol. 2009;53:2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- Yin X, Apostolov EO, Shah SV, et al. Induction of renal endonuclease G by cisplatin is reduced in DNase I-deficient mice. J Am Soc Nephrol. 2007;18:2544–2553. doi: 10.1681/ASN.2006080896. [DOI] [PubMed] [Google Scholar]