Abstract
Objectives
Chyluria is a medical condition with presence of chyle in urine. The disease is most prevalent in South East Asian countries mostly caused by parasitic (Wuchereria bancrofti) infections. Our objective was to investigate the spontaneous remission of non-parasitic chyluria.
Design and methods
The spontaneous remission of non-parasitic chyluria cases were worked out with diagnostic investigations, clinical assessment and studied with details of all aspects with evolutionary totality.
Results
We present two patients who presented to nephrology clinic with symptoms of milky urine and painless hematuria. Midnight blood smear was negative for filarial parasites. Urine culture was without mycobacteria. Urine cytology and IgG western blot for cysticercus were negative. Imaging for a lymphatic leak by lymphoscintigraphy was unrevealing. Chyluria resolved spontaneously in both patients.
Conclusions
In our cases, radiologic visualization via lymphoscintigraphy was unrevealing. The patients was managed conservatively and fortunately underwent spontaneous remission marked by the disappearance of chyluria within several months of her initial diagnosis. In our opinion this spontaneous remission could be due to unrevealed lymphatico-renal fistula collapse or sclerosis of lymphatics caused by contrast media.
Introduction
Chyle, a milky substance composed of lymphatic fluid and chylomicrons, is formed in the small intestine during the digestion of fatty foods. Once the emulsion is constituted, it is taken up by lymph vessels. Chyluria, the presence of chyle in the urine, occurs as a result of a pathologic communication between the lymphatic and urinary system [1]. Recorded cases of chyluria date back to time of Hippocrates who described the presence of “oily urine” in a post-partum woman. Thereafter, the French scientist Jean Pecquet identified the lymphatic system in 1651 leading to an eventual anatomic explanation of chyluria. [2]. Clinically, chyluria presents as a cloudy milk-colored urine accompanied by systemic symptoms such as weight loss, fatigue, and rarely, flank pain from retained clots. Herein we describe two cases of self-limited non-parasitic chyluria.
Case reports
Case One
A 72 year old Chinese woman without relevant comorbidities was referred to our institution for further work up of hematuria and chyluria (Figure 1). Two months prior to referral, the patient developed intermittent painless hematuria with clots. Then one month prior to referral, the patient noted the onset of foamy, milk-like urine. A CT urogram was performed indicating a bladder lesion at the level of the right ureteral orifice with subsequent cystoscopy revealing only a blood clot and the absence of malignant cells. In light of the patient's persistent symptoms, she was referred to the division of nephrology at our institution for further evaluation and management.
Figure 1. Patient 1 Urine Sample before (A) and after remission (B).
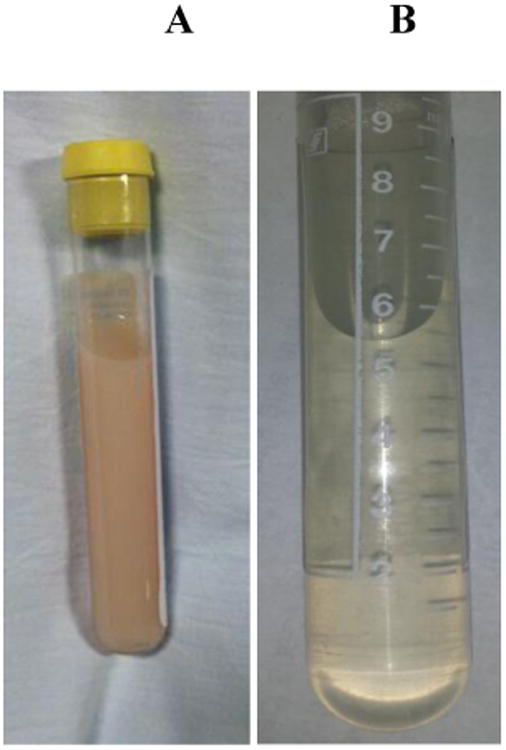
Upon interview, the patient reported emigrating from rural China to the United States thirty years prior to presentation. In the years preceding her arrival, she worked as a farmer raising both agriculture and livestock. Over the last two months, the patient reported a twenty-pound unintentional weight loss. She was otherwise asymptomatic. She denied a history of tuberculosis and reported a negative tuberculin test ten years prior to presentation.
On examination, the patient was normotensive and afebrile. Her weight was 39kg; her BMI was 17. Physical exam revealed a slender but well appearing woman without lymphadenopathy or edema. Table 1 shows the patient's initial laboratory testing. The patient was not anemic and her renal function was intact. Serum albumin was 3.2 g/dL. A urine analysis showed large protein and fifty red blood cells per high power field. Urine sediment exam was without dysmorphic cells or casts. Given that total serum cholesterol was normal, a full lipid panel was not obtained. However, the serum specimen was not lipemic, therefore significant elevation of serum triglycerides were unlikely.
Table 1. Patient's Laboratory Values.
| Upon Presentation | Reference Ranges | ||
|---|---|---|---|
| Patient 1 | Patient 2 | ||
| Aspartate Aminotransferase | 30 | 23 | 10-37 U/L |
| Alanine Aminotransferase | 27 | 22 | 5-37 U/L |
| Albumin | 3.2 | 3.9 | 4-5.2 g/dL |
| Total protein | 5.3 | 6.0 | 6.3-8.1 g/dL |
| Blood Urea Nitrogen | 34 | 19 | 6-20 mg/dL |
| Creatinine | 0.9 | 0.8 | 0.6-1.3 mg/dL |
| Cholesterol | 200 | 194 | <200 mg/dL |
| Hemoglobin | 13.9 | 12.8 | 11.5-16 g/dL |
| White Blood Cell Count | 3.3 | 3.8 | 4-11 1,000/μL |
| Hematocrit | 42 | 38.7 | 34-46% |
| Mean Corpuscular Volume | 97 | 38 | 82-98 fL |
| Anion Gap | 4 | 2 | 8-16 mEq/L |
| Sodium | 133 | 142 | 136-144 mEq/L |
| Potassium | 4.6 | 4.2 | 3.5-5.1 mEq/L |
| Chloride | 102 | 105 | 98-109 mEq/L |
| Complement 3 | 122 | 88 | 81-157 mg/dL |
| Complement 4 | 31 | 15 | 13-39 mg/dL |
Her chyluria screen (Table 2) was positive for chylomicrons and ->400 mg/dL of triglycerides. A twenty four hour urine collection revealed 8.8 grams of protein with normal electrophoresis. Imaging for a lymphatic leak by lymphoscintigraphy was unrevealing. Serologic studies including IgG western blot for cysticercus and IgG ELISA for filariasis were negative. The rapid plasma reagin (RPR) was negative. Multiple midnight blood smears were negative for filarial parasites. Urine culture was without mycobacteria or schistosoma; stool studies failed to demonstrate ova or parasites.
Table 2. Results of Chlyuria Screen.
| Patient 1 | Patient 2 | ||
|---|---|---|---|
| Chyluria Screen | Upon Presentation | Upon Remission | Upon Presentation |
| Cholesterol | 10 mg/dL | <5 mg/dL | 10mg/dL |
| Triglycerides | >400 mg/dL | <3 mg/dL | >400mg/dl |
| Chylomicrons | Present | Absent | Present |
Case Two
74 year old asian female with remote history of mitral valve replacement due to endocarditis and congenital solitary left kidney presented to renal service with 2 weeks history of sudden onset milky urine. Patient denied any flank pain, fever or dysuria but reported several episodes of gross hematuria in the same time period. Patient's vital signs were normal; the weight was 52.8 kg and the height 160 cm. The physical exam was unremarkable. Table 1 shows the patient's initial laboratory testing. Her chyluria screen (Table 2) was positive. A urine analysis showed large protein and fifty red blood cells per high power field. Urine sediment exam was without dysmorphic cells or casts. Urinary protein to creatinine ratio was 7.5. Midnight blood smears were negative for filarial parasites. Urine ova and parasites, IgG western blot for cysticercus and mycobacterial cultures were negative. Both CT urogram and lymphoscintigraphy were unremarkable. Patient was offered suppsoritve care and reported resolution of milky urine few weeks after last follow up.
Discussion
Chyle is cholesterol rich fluid formed in the small intestine during the digestive process. Thereafter, it is absorbed by specialized intestinal lymph nodes, known as lacteals. Chyle in the lacteals then transits through the extensive lymphatic vessels interwoven among the organ systems and eventually reaches the thoracic duct. It then enters the systemic circulation as the thoracic duct feeds into the left subclavian vein [3]. Among the organs the lymphatic system courses through are the renal parenchyma, the ureters, and the bladder. As such, obstruction or stenosis at the level of the larger caliber and more distal lymphatics generates elevated intra-lymphatic pressures that lead to the development of lymphatic varices within the retroperitoneum, kidney, and genitourinary system. These varices can rupture leading to a lymphatico-urinary fistula and chyluria [4, 5].
Early recognition of chyluria is imperative to avoid unnecessary workup of nephrotic syndrome of glomerular origin. Typically patients with chyluria present with malnutrition, weight loss, hypocholesterolemia and are not edematous. In contradistinction, weight gain, pitting edema and hypercholesterolemia are the norm in patients with a glomerular nephrotic syndrome [6].
The most common cause of chyluria is parasitic infection (Table 3) with granulomatous diseases or structural etiologies less frequently encountered [7-10]. Among the various parasitic causes-cysticercosis, echinococcus, malaria, filariasis, ascariasis filariasis, an organism endemic to Southeast Asia, and portions of Africa and South America [11] is the most common parasite. However, to date, there are no appropriate studies to identify whether or not gender, ethnicity predominates in endemic areas. Similar to other infectious causes, filariasis causes lymphatic obstruction at the level of the thoracic duct generating elevated intra-lymphatic pressures leading to a retrograde flow of lymph and variceal formation. Non-parasitic infectious causes of chyluria include granulomatous disease such as tuberculosis, fungal infection, and leprosy. Finally, structural etiologies such as trauma, surgery, or lymphatic anomalies have been described [12, 13] and should be excluded.
Table 3. Causes of Chyluria.
| Parasitic Causes | Non-parasitic Causes |
|---|---|
| Lymphatic Filariasis | Traumatic lesions (surgery) |
| Cysticercosis | Tumors |
| Echincoccosis | Lymphangioma |
| Malaria | Pregnancy |
| Ascariasis | Aortic Aneurysm |
| Granulomatous Infections |
Once the presence of triglycerides and chylomicrons has been detected in the urine, radiographic evaluation is indicated to locate the fistula source. Visualization can be achieved via lymphoscintigraphy, an albumin tagged radioisotope, or less commonly by retrograde pyelography or lymphangiography [14]. Thereafter, if an infectious cause is suspected, serologic testing for cysticercosis, malaria, echinococcus, tuberculosis and ascariasis should be pursued. In contrast, owing to the nocturnal periodicity of filariasis, detection of the microorganism is best realized by evaluation of a midnight blood smear.
Apart from eradication of the parasite, the treatment of chyluria is largely conservative with to 50% of parasitic cases remitting spontaneously [15]. Expectant management consists of a low fat, high fiber diet and substitution of dietary long chain triglycerides with medium chain fats as the latter are rapidly absorbed from the gut thereby bypassing the lymphatic circulation. In cases of more severe chyluria (malnutrition, immune dysfunction) invasive approaches such as silver nitrate sclerotherapy of the renal pelvis can be employed; however, recurrence is not uncommon.
Conclusion
In our cases, radiologic visualization via lymphoscintigraphy was unrevealing as was an extensive infectious work up. The patients were managed conservatively and underwent spontaneous remission marked by the disappearance of chyluria within several weeks to months of initial diagnosis. Our report emphasizes that in certain cases of idiopathic chyluria watchful waiting maybe indicated with eventual resolution of the symptoms and avoidance of invasive procedures.
Acknowledgments
This work is supported in part by MSK NIH National Cancer Institute Cancer Center Support Grant/Core Grant (P30 CA008748).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lang EK, Redetzki JE, Brown RL. Lymphangiographic demonstration of lymphatico-caliceal fistulas causing chyluria (filariasis) J Urol. 1972;108:321–24. doi: 10.1016/s0022-5347(17)60727-4. [DOI] [PubMed] [Google Scholar]
- 2.Swanson GW. Lymphangiography. Radiology. 1963;81:473–78. doi: 10.1148/81.3.473. [DOI] [PubMed] [Google Scholar]
- 3.Singh I, Dargan P, Sharma N. Chyluria-a clinical and diagnostic stepladder algorithm with review of literature. Indian Journal Urol. 2004;20:79–85. [Google Scholar]
- 4.Akisada M, Tani S. Filarial chyluria in Japan. Lymphography, etiology and treatment in 30 cases Radiology. 1968;90:311–17. [Google Scholar]
- 5.Graziani G, Cucchiari D, Verdesca S, Balzarini L, Montanelli A, Ponticelli C. Chyluria associated with nephrotic-range proteinuria: pathophysiology, clinical picture and therapeutic options. Nephron Clinical Practice. 2011;119:c248–53. doi: 10.1159/000329154. [DOI] [PubMed] [Google Scholar]
- 6.Hemal AK, Gupta NP. Retroperitoneoscopic lymphatic management of intractable chyluria J Urol. 2002;167:2473–76. [PubMed] [Google Scholar]
- 7.Verjans V, Peluso J, Oyen R, Maes B. Magnetic resonance imaging of nontropical chyluria due to distal thoracic duct obstruction. Nephrol Dial Transplant. 2004;19:3200–01. doi: 10.1093/ndt/gfh356. [DOI] [PubMed] [Google Scholar]
- 8.Kekre NS, Arun N, Date A. Retroperitoneal cystic lymphangioma causing intractable chyluria. Br J Urol. 1998;81:327–28. doi: 10.1046/j.1464-410x.1998.00337.x. [DOI] [PubMed] [Google Scholar]
- 9.Wilson RS, White RJ. Lymph node tuberculosis presenting as chyluria. Thorax. 1976;31:617–20. doi: 10.1136/thx.31.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung DE, Koo JW, Kim SW, Cheong HI. A case of a child with non-parasitic chyluria. Korean J Pediatr. 2006;49:326–28. [Google Scholar]
- 11.Koo CG, Van Langenberg A. Chyluria. A clinical study J R Coll Surg Edinb. 1969;14:31–41. [PubMed] [Google Scholar]
- 12.Garrido P, Arcas R, Bobadilla JF, Albertos J, González Santos JM, Vallejo JL, Bastida E. Thoracic aneurysm as a cause of chyluria: resolution by surgical treatment. Ann Thorac Surg. 1995;60:687–89. doi: 10.1016/0003-4975(95)00333-G. [DOI] [PubMed] [Google Scholar]
- 13.Chen HS, Yen TS, Lu YS, Yang JC, Ko YL. Transient ‘milky urine’ after cardiac catheterization: another unreported cause of non-parasitic chyluria. Nephron. 1996;72:367–68. doi: 10.1159/000188892. [DOI] [PubMed] [Google Scholar]
- 14.Kim RJ, Joudi FN. Chyluria after partial nephrectomy: case report and review of the literature. Sci World J. 2009;18:1–4. doi: 10.1100/tsw.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohyama C, Saita H, Miyasato N. Spontaneous remission of chyluria. J Urol. 1979;121:316–7. doi: 10.1016/s0022-5347(17)56767-1. [DOI] [PubMed] [Google Scholar]


