Abstract
Objective
To investigate the effect of thoracic paravertebral block (PVB) on pain control and morphine consumption in percutaneous nephrolithotomy operations.
Subjects and Methods
This randomized controlled clinical study was performed on 60 American Society of Anesthesiologists (ASA) I-II patients between the ages of 18 and 60 years who underwent percutaneous nephrolithotomy with approval of the ethical committee and written consent of the patients. Patients were randomly allocated into two groups: group P had 4 ml of 0.5% levobupivacaine injected at each of the T10, T11, and T12 paravertebral spaces and a standard PVB, and group C received 4 ml of 0.9% NaCl solution. All patients were given standard general anesthesia. The follow-up of saturation, heart rate, peripheral oxygen, and blood pressure values was recorded before induction, intraoperatively, and postoperatively. At postoperative 1, 2, 6, 12, and 24 h, the visual analog scale (VAS), Ramsey sedation score, respiratory rate, and 24-hour total morphine consumption were recorded. In addition, side effects and satisfaction of patients were recorded.
Results
VAS scores and total morphine consumption were lower in group P than in group C: 2.3 vs. 4.3 and 22.3 vs. 43.2 mg, respectively (p < 0.05). The level of satisfaction was higher in group P than group C. Differences between groups in other parameters were not significant.
Conclusions
Thoracic PVB with levobupivacaine provided a good postoperative analgesia and increased patient satisfaction for those who underwent percutaneous nephrolithotomy.
Key Words: Pain management, Postoperative pain, Paravertebral block, Levobupivacaine, Morphine consumption
Introduction
Percutaneous nephrolithotomy is a very common less invasive method for the management of patients with renal calculi. However, the procedure still causes postoperative pain [1], mostly due to dilatation of the renal capsule, the parenchymal tract, and peritubal distressing of the nephrostomy tube [1,2].
Postoperative pain is one of the important factors affecting morbidity concerning the cardiovascular, pulmonary, and emotional systems in surgical patients [1,3]. Pain during the postoperative phase of anesthesia and surgery is still an important problem that requires continued efforts for improvement because adequate analgesia during the postoperative phase not only decreases complications, but facilitates faster recovery [4]. Side effects such as respiratory depression due to opioids are another problem for patients with postoperative pain [5]. Appropriate and adequate treatment of postoperative pain may decrease the incidence of complications, requirements for hospitalization, recovery times, and health costs [4].
Paravertebral block (PVB) is a successful regional method that has been used for the pain management of several procedures such as thoracotomy, breast surgery, abdominal herniorrhaphy, and lithotripsy [6,7,8,9], and provides nonopioid analgesia to the somatic nerve roots in a dermatome distribution with no side effects [9,10]. Although PVB has been used for many procedures, there is limited data for its use for the management of pain in percutaneous nephrolithotomy operations.
In this study, the effect of thoracic PVB with levobupivacaine on pain management and morphine consumption was evaluated in patients having percutaneous nephrolithotomy, and the advantages and disadvantages of PVB were examined.
Subjects and Methods
The study was conducted between October 2010 and November 2011, after approval of the ethics committee of our institution and written informed consent was obtained from each patient. Sixty percutaneous nephrolithotomy patients between the ages of 18 and 60 years with a risk classification of ASA I-II were enrolled in the study, which was a prospective, randomized, controlled clinical trial.
Exclusion criteria were a history of allergy to local anesthesia, hepatic or cardiac dysfunction, unregulated diabetes mellitus, hypertension, coagulation disorders, sepsis, pathological obesity (BMI >35), local infection at the application site, and not volunteering to the study or uncooperative to the use of patient-controlled analgesia (PCA).
Patients were visited 1 day prior to the surgery and briefed on PCA pumps and visual analog scale (VAS) scores for assessing the level of their pain. Mean arterial pressure (MAP), heart rate (HR), respiration rate (RR), and peripheral oxygen saturation (SpO2) values of patients were monitored in the operating room before the procedure. After peripheral venous catheterization, a 10-ml·kg−1 Ringer lactate infusion was given. Intravenous fluids were administered and subsequently maintained by 0.9% NaCl at the discretion of the anesthesiologist (S.G.). Patients were randomly assigned to one of two groups: group P (PVB) and group C (control) by using the closed envelope technique during the interview with patients, who were blinded to their groups. The two study groups were similar in the terms of age, gender, ASA, height, and weight. Patients were not informed as to which group they had been assigned.
Preoperative basal MAP, HR, RR, SpO2, and doses of the drugs used were recorded by anesthetists (C.D. and K.K.) who were blinded to the groups. Anesthesia was induced by 5–7 mg·kg−1 thiopental sodium (Pental Sodyum; IE Ulugay, Istanbul, Turkey) and 0.6 mg·kg−1 rocuronium (Esmeron; Organon, Holland). Patients were ventilated by a 50% O2 and 50% N2O mixture and 4–6% desflurane (Suprane; Baxter-Eczacibasi, Turkey). In group P patients, at the end of the surgical procedure, the patients were turned to the lateral position with the side to be operated and blocked upwards. Using aseptic precautions and a C-armed fluoroscope, a 22-gauge 88-mm Quincke needle was inserted perpendicular to the skin 2.5 cm lateral from the cephalic edge of each of the spinous processes of T10, T11, and T12 using the loss of resistance technique. The needle was withdrawn and redirected in the cephalic direction to walk off the transverse process after the transverse process was contacted. The aim was to insert the needle to a depth of 1 cm past the transverse process. Four millilers of 5% levobupivacaine were injected at each of three levels in group P. Group C patients were given 4 ml of normal saline by the same procedure with a 22-gauge Quincke needle for each level. Block solutions were prepared by an anesthesiologist other than the anesthesiologist who performed the blocks and this anesthesiologist was not involved in any other aspect of the study. All block procedures were performed by the same anesthesiologist who was blinded to the block solutions. After the block procedure, patients were awakened and transferred to the postoperative care unit (PACU). Patients with an Aldrete score of 9 and over were transferred to the inpatient service [11]. Patients with an Aldrete score of 8 and below were monitored in the PACU until the Aldrete score reached 9, and then they were transferred to the inpatient service too.
The block evaluations were made by pinprick test by an anesthesiologist (A.C.I.) who was blinded to the groups. Analgesia evaluated by pinprick test including the T10, T11, and T12 segments was defined as a successful PVB. If the blockade did not include all 3 segments, it was defined as a failed PVB and excluded from analysis. MAP, HR, RR, SpO2, and Ramsey sedation scores [12] were recorded at postoperative 1, 2, 6, 12, and 24 h. The VAS pain scores were evaluated using a 0- to 10-cm visual scale: (no pain: 0; to very sharp pain: 10) at postoperative 1, 2, 6, 12, and 24 h by directly asking patients about their maximum sensation of pain. All the patients in the postoperative period received a PCA device upon arrival at the recovery room and a loading dose of intravenous morphine, 0.1 mg·kg−1, by PCA for initiation. The PCA device was set for a 1-mg bolus dose with a 10-min lock-out interval and a 4-hour limit of 10 mg. Time to first PCA use and morphine consumption over 24 h were recorded. Duration of PACU stay was also recorded. If any patient had a VAS score above 5 out of 10, diclofenac sodium analgesic treatment was given intramuscularly in the postoperative period. No other analgesic was used. If patients had nausea, 10 mg metoclopramide hydrochloride (Primperan 10 mg amp, Biofarma Ilac, Turkey) was given intravenously. Patient satisfaction data was evaluated using a 5-point scale (1: very bad; 2: bad; 3: good; 4: very good; 5: perfect).
Three patients from group P were excluded due to inadequate block and 2 patients from group C were excluded due to wrong use of the PCA device. A total of 55 patients were included in the study. Data were analyzed using SPSS (ver. 14.0). Age, height, weight, duration of the operation, VAS scores, and morphine consumption were analyzed by using a t test (all were distributed normally as tested by K.S.), while the χ2 test was used to compare binary variables of nausea, vomiting, itching, additional analgesic requirement, and satisfaction. A p value <0.05 was considered significant. Data are given as means ± SD. The amount of morphine consumption at 24 h was the primary endpoint for statistical analysis. A power analysis based on a pilot study in which the amount of postoperative morphine consumption was 35 ± 12 mg in the control group and 24 ± 9 mg in the PVB group showed that two groups of 30 patients each would be required to demonstrate a 25% difference in postoperative morphine consumption with α = 0.05 and β = 0.20.
Results
There were no significant differences between the groups in terms of surgical characteristics (table 1). There were also no significant differences concerning MAP, HR, RR, and SpO2 values between groups (p > 0.05; tables 2, 3, 4, 5). Blocked segments were the same in all patients as including the T10-T12 segments. No epidural spread of local anesthetics was observed.
Table 1.
Demographic and surgical data
| Group P (n = 27) | Group C (n = 28) | p value | |
|---|---|---|---|
| Age, years | 48.8 ± 9.9 | 50.6 ± 9.6 | 0.450 |
| Gender (female/male) | 12/15 | 14/14 | 0.168 |
| Height, cm | 164.5 ± 13.9 | 167.1 ± 14.2 | 0.266 |
| Weight, kg | 77.7 ± 10.9 | 80.7 ± 12.5 | 0.350 |
| ASA (I/II) | 11/16 | 10/18 | 0.147 |
| Operation side (right/left) | 12/15 | 13/15 | 0.621 |
| Duration of operation, min | 52.3 ± 15.2 | 53.5 ± 15.9 | 0.900 |
Values are given as means 8 SD or n.
Table 2.
MAP data (in mm Hg)
| Group P (n = 27) | Group C (n = 28) | p value | |
|---|---|---|---|
| Basal | 98.25 ± 13.18 | 99.20 ± 11.62 | 0.685 |
| Postoperative 1 h | 97.00 ± 9.17 | 95.25 ± 5.18 | 0.671 |
| Postoperative 2 h | 89.10 ± 6.20 | 89.30 ± 7.98 | 0.891 |
| Postoperative 6 h | 84.50 ± 8.68 | 88.55 ± 5.61 | 0.071 |
| Postoperative 12 h | 83.05 ± 8.17 | 86.20 ± 7.48 | 0.235 |
| Postoperative 24 h | 83.90 ± 8.49 | 86.20 ± 6.08 | 0.269 |
Data are means ± SD.
Table 3.
HR data (in beats per minute)
| Group P (n = 27) | Group C (n = 28) | p value | |
|---|---|---|---|
| Basal | 78.20 ± 12.43 | 75.60 ± 8.68 | 0.607 |
| Postoperative 1 h | 83.75 ± 6.99 | 82.30 ± 8.63 | 0.498 |
| Postoperative 2 h | 79.00 ± 8.27 | 74.70 ± 18.57 | 0.616 |
| Postoperative 6 h | 76.80 ± 6.46 | 71.90 ± 18.12 | 0.378 |
| Postoperative 12 h | 75.95 ± 6.96 | 75.65 ± 8.39 | 0.889 |
| Postoperative 24 h | 75.55 ± 6.73 | 75.10 ± 6.71 | 0.674 |
Data are means ± SD.
Table 4.
RR data (in breathing per minute)
| Group P (n = 27) | Group C (n = 28) | p value | |
|---|---|---|---|
| Basal | 20.80 ± 1.19 | 20.60 ± 0.94 | 0.667 |
| Postoperative 1 h | 22.40 ± 0.88 | 23.00 ± 1.02 | 0.052 |
| Postoperative 2 h | 21.40 ± 0.94 | 21.70 ± 0.73 | 0.262 |
| Postoperative 6 h | 20.20 ± 0.61 | 20.30 ± 0.73 | 0.637 |
| Postoperative 12 h | 20.40 ± 0.82 | 20.40 ± 0.82 | 1.00 |
| Postoperative 24 h | 20.65 ± 0.93 | 20.30 ± 0.73 | 0.167 |
Data are means ± SD.
Table 5.
SpO2 data (in s%)
| Group P (n = 27) | Group C (n = 28) | p value | |
|---|---|---|---|
| Basal | 95.96 ± 2.34 | 95.96 ± 2.28 | 0.530 |
| Postoperative 1 h | 99.30 ± 0.79 | 99.36 ± 0.49 | 0.624 |
| Postoperative 2 h | 99.46 ± 0.68 | 99.53 ± 0.57 | 0.490 |
| Postoperative 6 h | 99.23 ± 0.93 | 99.43 ± 0.62 | 0.463 |
| Postoperative 12 h | 99.10 ± 0.88 | 99.36 ± 0.71 | 0.271 |
| Postoperative 24 h | 99.10 ± 0.98 | 99.50 ± 0.57 | 0.061 |
Data are means 8 SD.
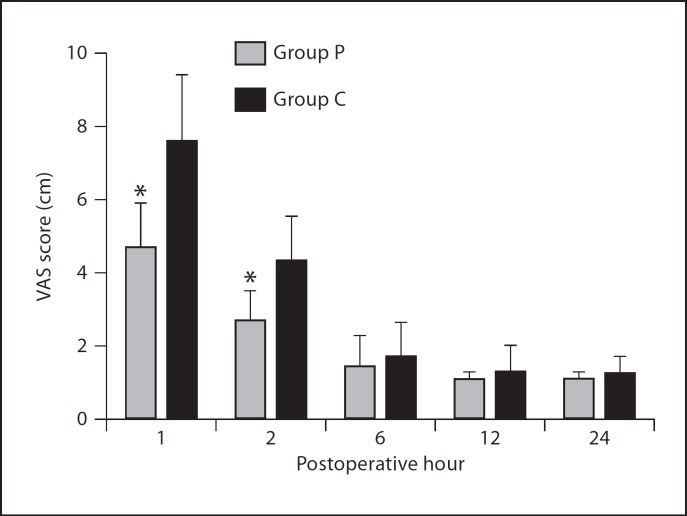
Postoperative VAS values at the first and second hours were significantly lower in group P than group C (p < 0.05; fig. 1). Time to first PCA use value was less in group C than in group P (p < 0.05), and 24-hour morphine consumption was found to be significantly lower in group P (22.3 ± 6.1 mg) than the control group (43.2 ± 9.5 mg) at both time points (p < 0.05; table 6). The need for additional analgesic (diclofenac sodium) 24 h postoperatively was significantly lower in group P than group C (p < 0.05; table 6). The duration of PACU stay of both groups was similar.
Fig. 1.
VAS pain scores. *p < 0.05 compared to group C.
Table 6.
Postoperative data
| Group P (n = 27) | Group C (n = 28) | p value | |
|---|---|---|---|
| Time to first PCA use1, min | 94.2 ± 24.1 | 48.3 ± 17.4 | 0.001 |
| Morphine consumption | |||
| 0–24 h2, mg | 22.3 ± 6.1 | 43.2 ± 9.5 | 0.001 |
| Nausea (s+/−) | 2/25 | 12/16 | 0.015 |
| Vomiting (s+/−) | 0/27 | 2/26 | 0.900 |
| Itching (s+/−) | 5/22 | 7/21 | 0.910 |
| Additional analgesic (s+/−) | 1/26 | 9/19 | 0.001 |
| Overall satisfaction score at 24 h3 | 4.2 ± 0.6 | 2.4 ± 0.5 | 0.001 |
Calculated from transferring time to PACU.
Recorded from PCA device.
1: very bad; 2: bad; 3: good; 4: very good; 5: perfect.
Patient satisfaction scores of group P were higher than that of group C (p < 0.05; table 6). The Ramsey sedation scores of the two groups were similar, 2 (cooperated, oriented and quiet), at all postoperative hours.
There were no side effects such as respiratory depression, delirium, urinary retention, hypotension, and bradycardia. No significant differences were seen in itching and vomiting between groups; nausea was significantly lower in group P (p < 0.05). Additionally, there were no complications related to PVB.
Discussion
In this study, the problem of postoperative pain was overcome by adequate analgesia, which a single injection of PVB provided, as evidenced by high satisfaction scores and low morphine consumption after percutaneous nephrolithotomy operations. It has been reported that preoperative or intraoperative application of PVB before awakening from anesthesia decreased acute postoperative pain as well as chronic pain after surgery in a variety of patient populations [13,14,15,16,17]. Consequently, PVB is being used for postoperative analgesia management and provides effective analgesia in many surgical procedures such as outpatient lithotripsy [9], renal surgery [18], abdominal laparotomy [19], thoracotomies [13,14], breast surgeries [15,16,17], and open prostatectomies [20].
Other techniques have been reported to provide postoperative analgesia for percutaneous nephrolithotomy, such as spinal and epidural block, local analgesic infiltration, and systemic analgesic therapy modalities such as nonsteroidal analgesic drugs and opioids [21,22,23,24]. Singh et al. [21], Karacalar et al. [22], Chen et al. [23], and Aravantinos et al. [24] all found other techniques that are effective for postoperative pain management of percutaneous nephrolithotomies. However, we think that PVB would provide better analgesia than these other techniques, while reducing additional analgesic doses. PVB is also an easy-to-use technique that has only one injection intraoperatively. Other techniques such as spinal epidural anesthesia provides analgesia, but also has some unwanted effects that would not occur with PVB, such as prolonged motor blockade, bowel movement impairment, and nausea and vomiting. These advantages of PVB make it a better analgesic technique.
Although there is no study comparing PVB and spinal-epidural block in patients undergoing percutaneous nephrolithotomy, there are several studies which have compared two techniques as thoracic epidural block and continuous PVB in thoracic surgery and lower abdominal surgery. Richardson et al. [25] reported that PVB was superior to the epidural block while Kotze et al. [26] reported that PVB in postthoracotomy provided stronger analgesia and fewer systemic opioids. However, Messina et al. [27] reported that epidural block was more effective than PVB. Further, Lönnqvist et al. [28] reported that continuous thoracic PVB provided effective postoperative analgesia equal to or even better than classical lumbar epidural block. Because no comparative studies had been done between these other techniques and PVB, such studies will be necessary for postoperative pain relief of percutaneous nephrolithotomies.
Conclusion
Thoracic PVB using levobupivacaine was an effective regional technique with low morphine consumption, high patient satisfaction, and no side effects for postoperative pain management of patients undergoing percutaneous nephrolithotomy. Hence, a low thoracic PVB technique should be considered as a safe alternative treatment to conventional methods for postoperative pain management of percutaneous nephrolithotomy operations.
References
- 1.Parikh GP, Shah VR, Modi MP, Chauhan NC. The analgesic efficacy of peritubal infiltration of 0.25s% bupivacaine in percutaneous nephrolithotomy – a prospective randomized study. J Anaesthesiol Clin Pharmacol. 2011;27:481–484. doi: 10.4103/0970-9185.86591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalela D, Goel A, Singh P, Shankhwar SN. Renal capsular block: a novel method for performing percutaneous nephrolithotomy under local anesthesia. J Endourol. 2004;18:544–546. doi: 10.1089/end.2004.18.544. [DOI] [PubMed] [Google Scholar]
- 3.Tuzuner Oncul AM, Yazicioglu D, Alanoglu Z, Demiralp S, Ozturk A, Ucok C. Postoperative analgesia in impacted third molar surgery: the role of preoperative diclofenac sodium, paracetamol and lornoxicam. Med Princ Pract. 2011;20:470–476. doi: 10.1159/000327658. [DOI] [PubMed] [Google Scholar]
- 4.McHugh GA. The management of pain following day-case surgery. Anaesthesiology. 2002;57:270–275. doi: 10.1046/j.1365-2044.2002.2366_2.x. [DOI] [PubMed] [Google Scholar]
- 5.Khademi S, Ghaffarpasand F, Heiran HR, Yavari MJ, Motazedian S, Dehghankhalili M. Intravenous and peritonsillar infiltration of ketamine for postoperative pain after adenotonsillectomy: a randomized placebo-controlled clinical trial. Med Princ Pract. 2011;20:433–437. doi: 10.1159/000327657. [DOI] [PubMed] [Google Scholar]
- 6.Klein SM, Bergh A, Steele SM, Georgiade GS, Greengrass RA. Thoracic paravertebral block for breast surgery. Anesth Analg. 2000;90:1402–1405. doi: 10.1097/00000539-200006000-00026. [DOI] [PubMed] [Google Scholar]
- 7.Greengrass R, Buckenmaier CC., 3rd Paravertebral anesthesia/analgesia for ambulatory surgery. Best Pract Res Clin Anaesthesiol. 2002;16:271–283. doi: 10.1053/bean.2002.0238. [DOI] [PubMed] [Google Scholar]
- 8.Klein SM, Pietrobon R, Nielsen KC, Steele SM, Warner DS, Moylan JA, Eubanks WS, Greengrass RA. Paravertebral somatic nerve block compared with peripheral nerve blocks for outpatient inguinal herniorrhaphy. Reg Anesth Pain Med. 2002;27:476–480. doi: 10.1053/rapm.2002.35147. [DOI] [PubMed] [Google Scholar]
- 9.Jamieson BD, Mariano ER. Thoracic and lumbar paravertebral blocks for outpatient lithotripsy. J Clin Anesth. 2007;19:149–151. doi: 10.1016/j.jclinane.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Rudkin GE, Gardiner SE, Cooter RD. Bilateral thoracic paravertebral block for abdominoplasty. J Clin Anesth. 2008;20:54–56. doi: 10.1016/j.jclinane.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth. 1995;7:89–91. doi: 10.1016/0952-8180(94)00001-k. [DOI] [PubMed] [Google Scholar]
- 12.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–659. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navlet MG, Garutti I, Olmedilla L, Pérez- Peña JM, San Joaquin MT, Martinez-Ragues G, Gomez-Caro L. Paravertebral ropivacaine, 0.3s%, and bupivacaine, 0.25s%, provide similar pain relief after thoracotomy. J Cardiothorac Vasc Anesth. 2006;20:644–647. doi: 10.1053/j.jvca.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 14.Vogt A, Stieger DS, Theurillat C, Curatolo M. Single-injection thoracic paravertebral block for postoperative pain treatment after thoracoscopic surgery. Br J Anaesth. 2005;95:816–821. doi: 10.1093/bja/aei250. [DOI] [PubMed] [Google Scholar]
- 15.Ibarra MM, S-Carralero GC, Vicente GU, Cuartero del Pozo A, López Rincón R, Fajardo del Castillo MJ. Chronic postoperative pain after general anesthesia with or without a single-dose preincisional paravertebral nerve block in radical breast cancer surgery. Rev Esp Anestesiol Reanim. 2011;58:290–294. doi: 10.1016/s0034-9356(11)70064-0. [DOI] [PubMed] [Google Scholar]
- 16.Boughey JC, Goravanchi F, Parris RN, Kee SS, Frenzel JC, Hunt KK, Ames FC, Kuerer HM, Lucci A. Improved postoperative pain control using thoracic paravertebral block for breast operations. Breast J. 2009;15:483–488. doi: 10.1111/j.1524-4741.2009.00763.x. [DOI] [PubMed] [Google Scholar]
- 17.Dabbagh A, Elyasi H. The role of paravertebral block in decreasing postoperative pain in elective breast surgeries. Med Sci Monit. 2007;13:464–467. [PubMed] [Google Scholar]
- 18.Berta E, Spanhel J, Smakal O, Smolka V, Gabrhelik T, Lönnqvist PA. Single injection paravertebral block for renal surgery in children. Paediatr Anaesth. 2008;18:593–597. doi: 10.1111/j.1460-9592.2008.02592.x. [DOI] [PubMed] [Google Scholar]
- 19.Visoiu M, Yang C. Ultrasound-guided bilateral paravertebral continuous nerve blocks for a mildly coagulopathic patient undergoing exploratory laparotomy for bowel resection. Paediatr Anaesth. 2011;21:459–462. doi: 10.1111/j.1460-9592.2010.03511.x. [DOI] [PubMed] [Google Scholar]
- 20.Chelly JE, Ploskanych T, Dai F, Nelson JB. Multimodal analgesic approach incorporating paravertebral blocks for open radical retropubic prostatectomy: a randomized double-blind placebo-controlled study. Can J Anaesth. 2011;58:371–378. doi: 10.1007/s12630-010-9442-x. [DOI] [PubMed] [Google Scholar]
- 21.Singh V, Sinha RJ, Sankhwar SN, Malik A. A prospective randomized study comparing percutaneous nephrolithotomy under combined spinal-epidural anesthesia with percutaneous nephrolithotomy under general anesthesia. Urol Int. 2011;87:293–298. doi: 10.1159/000329796. [DOI] [PubMed] [Google Scholar]
- 22.Karacalar S, Bilen CY, Sarihasan B, Sarikaya S. Spinal-epidural anesthesia versus general anesthesia in the management of percutaneous nephrolithotripsy. J Endourol. 2009;23:1591–1597. doi: 10.1089/end.2009.0224. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Zhou Z, Sun W, Zhao T, Wang H. Minimally invasive percutaneous nephrolithotomy under peritubal local infiltration anesthesia. World J Urol. 2011;29:773–777. doi: 10.1007/s00345-011-0730-z. [DOI] [PubMed] [Google Scholar]
- 24.Aravantinos E, Kalogeras N, Stamatiou G, Theodorou E, Moutzouris G, Karatzas A, Melekos M. Percutaneous nephrolithotomy under a multimodal analgesia regime. J Endourol. 2009;23:853–856. doi: 10.1089/end.2008.0448. [DOI] [PubMed] [Google Scholar]
- 25.Richardson J, Sabanathan S, Jones J, Shah RD, Cheema S, Mearns AJ. A prospective, randomized comparison of preoperative and continuous balanced epidural or paravertebral bupivacaine on post-thoracotomy pain, pulmonary function and stress responses. Br J Anaesth. 1999;83:387–392. doi: 10.1093/bja/83.3.387. [DOI] [PubMed] [Google Scholar]
- 26.Kotze A, Scally A, Howell S. Efficacy and safety of different techniques of paravertebral block for analgesia after thoracotomy: a systematic review and metaregression. Br J Anesth. 2009;103:626–636. doi: 10.1093/bja/aep272. [DOI] [PubMed] [Google Scholar]
- 27.Messina M, Boroli F, Landoni G, Bignami E, Dedola E, N'zepa Batonga J, Magrin A, Zangrillo S. A comparison of epidural vs. paravertebral blockade thoracic surgery. Minerva Anestesiol. 2009;75:616–621. [PubMed] [Google Scholar]
- 28.Lönnqvist PA. Paravertebral vs epidural block in children. Effects on postoperative morphine requirement after renal surgery. Acta Anaesthesiol Scand. 1994;38:346–349. doi: 10.1111/j.1399-6576.1994.tb03905.x. [DOI] [PubMed] [Google Scholar]