Abstract
Objective
To investigate the status of the oxidant/antioxidant balance in patients with multiple myeloma compared to healthy controls.
Materials and Methods
This study was conducted on 40 multiple myeloma patients and 40 healthy controls of matched age and sex. Serum total thiol, oxidative stress index (OSI), total oxidant status (TOS), and total antioxidant status (TAS) were measured using colourimetric methods; paraoxonase-1 and arylesterase enzyme activities were also quantified.
Results
Serum paraoxonase-1 and arylesterase activities and total thiol levels were significantly lower (p = 0.0001, p = 0.036 and p < 0.0001, respectively), whereas TOS and OSI levels were significantly higher (p < 0.0001 for both parameters) in multiple myeloma patients compared to controls. However, no significant differences in TAS were identified when the two groups were compared.
Conclusions
Our findings indicate an impaired oxidative/antioxidative balance in multiple myeloma. We recommend further studies with larger groups to investigate the possible relationship between oxidative stress and the aetiopathogenesis of multiple myeloma.
Key Words: Antioxidant, Arylesterase, Free radical, Multiple myeloma, Paraoxonase
Introduction
Multiple myeloma is a cancer of the plasma cells. It is the second most frequent malignancy of the blood in the USA after non-Hodgkin's lymphoma, accounting for 1% of neoplastic diseases and 13% of haematological malignancies [1]. Clonal enlargement of the tumour cells results in excessive generation of monoclonal immunoglobulin, which is used for the diagnosis of the disease. Collection of monoclonal immunoglobulin light chains in the kidneys can lead to renal failure. Another feature of multiple myeloma is the accumulation of malignant cells in the bone marrow, where they lead to osteolytic bone destruction and impaired haematopoiesis [1,2].
Human serum paraoxonase (PON1) and arylesterase (ARE) are lipophilic antioxidant enzymes. Serum PON1 binds to high-density lipoprotein (HDL) and contributes to the elimination of organophosphorus compounds and free radicals. PON1 is one of the endogenous free-radical scavenger systems in the human organism [3]. Serum PON1 and ARE have been shown to function as a single enzyme [4]. Human serum PON1 indicates neither age-related changes in activity nor gender differences [5]. Reduced PON1 enzyme activities have been shown in several groups of patients with hypercholesterolaemia, diabetes mellitus, and cardiovascular disease, in which the patients are under increased oxidative stress [6,7].
The serum levels of different oxidant species can be determined separately in laboratories. However, these measurements are time consuming and expensive to perform, and require complicated equipment [8,9,10]. Recently, lipid peroxidation concentrations were observed by determining the total oxidant status (TOS) [8]. Total antioxidant status (TAS) correlates with the activity of antioxidants in a medium, such as serum or plasma [9]. Therefore, measurements of TAS and TOS can give information on an individual's overall serum oxidative stress index (OSI) [10].
Thiols are endogenous compounds that include the sulphydryl group (SH) attached to a carbon atom. Both extracellular and intracellular redox states of thiols play a critical role in the determination of protein function and structure, regulation of the enzymatic activity of transcription factors and antioxidant protection [11]. The major thiol antioxidant is the tripeptide glutathione; the reduced form is glutathione (GSH) and the oxidized form is glutathione disulphide (GSSG). An oxidative environment leads to the rapid modification of protein sulphydryls. Oxidation yields sulphenic acids, and one-electron oxidation yields thiyl radicals. These partially oxidized products react with GSH and form S-glutathiolated protein, which is reduced further by the glutathione cycle mainly through glutathione reductase, to restore protein sulphydryls.
The aim of this study was to investigate the activity of serum PON1 and ARE in patients with multiple myeloma in comparison to healthy controls, and to investigate possible changes with regard to oxidative stress.
Materials and Methods
Forty patients (18 women and 22 men) with multiple myeloma admitted to the Antalya Education and Research Hospital were included in this study. All of the patients were newly diagnosed, depending on their clinical and laboratory findings, and were in various stages of the disease. The diagnosis of multiple myeloma was confirmed by serum protein electrophoresis and immunofixation. Forty healthy gender- and age-matched control subjects (19 women and 21 men) were also enrolled for comparison. None of the participants in this study was using hypolipidaemic agents or antioxidant drugs. Other exclusion criteria were patients and controls with other systemic diseases such as hepatic failure, diabetes mellitus, coronary artery disease and active infection.
A 5-ml blood sample in a tube with clot activator was obtained from patients and controls after overnight fasting. The sample was centrifuged at 3,000 rpm for 10 min and the serum was separated. Lipid parameters and other routine parameters were measured anew. The remaining portion of the serum was stored at −80°C and used to analyse PON1, ARE, TOS, TAS and total thiol concentrations.
Measurement of PON1 and ARE Activities in Serum
PON1 and ARE enzyme activities were measured using commercially available kits (Relassay®; Turkey). The fully automated PON1 activity measurement method consisted of two different sequential reagents; the first reagent was an appropriate Tris buffer and it also contained a calcium ion, which was a cofactor of the PON1 enzyme. The linear increase in the absorbance of p-nitrophenol, produced from paraoxon, was followed in the kinetic measurement mode. The non-enzymatic hydrolysis of paraoxon was subtracted from the total rate of hydrolysis. The molar absorptivity of p-nitrophenol was 18,290 M-1·cm-1, and 1 unit of paraoxonase activity is equal to 1 mol of paraoxon hydrolysed per litre per minute at 37°C [12]. Phenylacetate was used as a substrate to measure the ARE activity. PON1, present in the sample, hydrolysed phenylacetate to phenol and acetic acid. The produced phenol was colourimetrically measured via oxidative coupling with 4-aminoantipyrine and potassium ferricyanide. Non-enzymatic hydrolysis of phenyl acetate was subtracted from the total rate of hydrolysis. The molar absorptivity of the coloured complex was 4,000 M-1·cm-1, and 1 unit of ARE activity is equal to 1 mmol of phenylacetate hydrolysed per litre per minute at 37°C [13].
Measurement of the TOS of the Serum
The TOS of the serum was measured using an automated colourimetric measurement method developed by Erel [8]. In this method, oxidants present in the sample oxidize the ferrous ion-chelator complex to a ferric ion, which produces a coloured complex with a chromogen in an acidic medium. The colour intensity, which was measured spectrophotometrically, was related to the total amount of oxidant molecules present in the sample. The results are expressed in terms of micromolar hydrogen peroxide equivalents per litre (μmol H2O2 Eq/l).
Measurement of the TAS of the Serum
The TAS of the serum was measured using an automated colourimetric measurement method also developed by Erel [9]. In this method, antioxidants in the sample reduce dark blue-green coloured 2,2’-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) radicals to the colourless reduced ABTS form. The change of absorbance at 660 nm is related to the total antioxidant level of the sample. This method determined the antioxidative effect of the sample against the potent free-radical reactions initiated by the produced hydroxyl radical. The results are expressed as micromolar trolox equivalents per litre.
Oxidative Stress Index
The percentage ratio of the TOS level to the TAS level was accepted as the OSI [10]. The resulting micromolar unit of TAS was changed to millimoles per litre, and the OSI value was calculated according to the following formula: OSI = TOS (μmol H2O2 Eq/l)/TAS (μmol trolox Eq/l).
Measurement of Thiol Activities
The total serum total thiol concentration or SHs were measured via the methods originally described by Ellman [14] and modified by Hu [15]. The concentration of sulphydryl groups was calculated using reduced glutathione as the free sulphydryl group standard, and the results are expressed as millimoles. The CV for the measurement of the serum SH level was 3.6%.
Other Parameters
The levels of triglycerides (TG), total cholesterol, HDL cholesterol, low-density lipoprotein (LDL) cholesterol, calcium (Ca), protein, albumin, creatinine, blood urea nitrogen (BUN) and uric acid were determined using commercially available assay kits (Abbott) with an autoanalyser (Architect® c16000; Abbott Diagnostics). The levels of haemoglobin were determined using a fully automated haematology analyser (Sysmex® xt−2000i; Roche Diagnostics). Serum protein electrophoresis and immunofixation electrophoresis were detected using a Helena Biosciences Europe electrophoresis instrument.
Statistical Analysis
Statistical analyses were carried out using statistical software (version 11.5.1.0; MedCalc, Mariakerke, Belgium). In normally distributed groups, the results are presented as means and SD; otherwise they are presented as medians and interquartile ranges (IQR). The significance of the differences between groups was determined using Student's unpaired t test for normal distributions, and the Mann-Whitney U test for abnormal distributions. Categorical variables were evaluated using Fisher's exact test. Pearson's correlation coefficient and Spearman's correlation coefficient were used to test the strength of any associations between different variables. p < 0.05 was considered statistically significant.
Results
The demographic data and laboratory findings for the multiple myeloma patients and control subjects are summarized in table 1. Serum total protein, BUN, creatinine, and uric acid levels were significantly higher in multiple myeloma patients (p < 0.0001, p < 0.0001, p < 0.0001 and p = 0.0033, respectively) compared to controls. The levels of haemoglobin and albumin were significantly lower in multiple myeloma patients (p < 0.0001 for both parameters) compared to controls.
Table 1.
Biochemical parameters of the patients and controls
| Parameter | Patients (n = 40) | Controls (n = 40) | p |
|---|---|---|---|
| Age, years | 67.5 ± 8.4 | 66.4 ± 6.8 | 0.47 |
| Males, n (%) | 22 (55) | 21 (52.5) | 0.99 |
| Smokers, n (%) | 12 (30) | 16 (40) | 0.48 |
| Total protein, g/dl | 9.5 ± 2.4 | 7.54 ± 0.5 | <0.0001 |
| Albumin, g/dl | 3.4 ± 0.8 | 4.4 ± 0.2 | <0.0001 |
| Haemoglobin, g/dl | 9.1 ± 2.3 | 13.7 ± 1.23 | <0.0001 |
| Calcium, mg/dl | 9.5 (9.2 – 9.7) | 9.2 (9.1 – 9.4) | 0.15 |
| BUN, mg/dl | 20 (16 – 25) | 14 (12 – 16) | <0.0001 |
| Creatinine, mg/dl | 1 (0.8 – 1.2) | 0.7 (0.6 – 0.7) | <0.0001 |
| Uric acid, mg/dl | 7.3 ± 3.5 | 5 ± 1.3 | 0.0003 |
| Total cholesterol, mg/dl | 154 ± 58.6 | 167 ± 26.3 | 0.18 |
| TG, mg/dl | 103 ± 97 | 145 ± 99 | 0.61 |
| LDL cholesterol, mg/dl | 93 ± 43 | 92 ± 25 | 0.36 |
| HDL cholesterol, mg/dl | 35 ± 13 | 38.6 ± 5.2 | 0.11 |
Values are means ± SD or medians (IQR) unless otherwise stated.
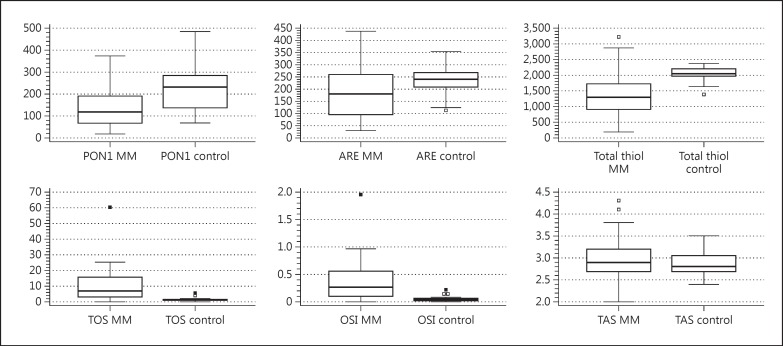
Serum PON1, ARE activity, and total thiol levels were significantly lower (p = 0.0001, p = 0.036 and p < 0.0001, respectively), while TOS and OSI levels were significantly higher (p < 0.0001 for both parameters) in multiple myeloma patients than in controls. However, no significant differences in TAS levels were identified (p = 0.24) (table 2; fig. 1).
Table 2.
Oxidative and antioxidative parameters for multiple myeloma patients and controls
| Parameter | Patients (n = 40) | Controls (n = 40) | p |
|---|---|---|---|
| PON1, U/l | 117 (84 – 165) | 233 (175 – 271) | <0.0001 |
| ARE, kU/l | 182 (121 – 246) | 241 (212 – 260) | 0.036 |
| Total thiol, mm/l | 1,309 (980 – 1,635) | 2,062 (1,992 – 2,172) | <0.0001 |
| TAS, nmol trolox/l | 2.9 (2.7 – 3.1) | 2.8 (2.7 – 3) | 0.24 |
| TOS, μmol H2O2 Eq/l | 6.9 (4 – 13) | 1.4 (1 – 1.6) | <0.0001 |
| OSI | 0.26 (0.1 – 0.4) | 0.05 (0.04 – 0.06) | <0.0001 |
Values are presented as medians (IQR).
Fig. 1.
PON1 and ARE activities and total thiol and TAS in multiple myeloma (MM) patients and controls.
No statistically significant correlation was identified between haemoglobin, creatinine, calcium, and albumin levels and PON1, ARE, total thiol, TAS, TOS, and OSI levels.
Discussion
Data from this study demonstrated significantly lower levels of PON1 and ARE activities and total thiol for multiple myeloma patients in comparison to healthy subjects, while OSI and TOS levels were significantly increased. However, a parallel change in TAS levels was not observed. These results indicate a clear shift in oxidative/antioxidative balance towards oxidation in patients with multiple myeloma.
All cells in the human body sustain a condition of homeostasis between the oxidant and antioxidant species [16]. Oxidant/antioxidant balance is very important for normal metabolism, signal transduction and regulation of cellular functions. When an increase in oxidants and a decrease in the antioxidant defence system occur, the oxidative/antioxidative balance eventually shifts toward the oxidative status. Proteins, lipids and DNA are significant targets for oxidative attack, and modification of these molecules can increase the risk of somatic mutations and neoplastic transformation. In fact, the development of cancers and their progression have been linked to DNA mutations and damage, genome instability, and cell proliferation caused by oxidative stress [16,17]. This study not only sheds light on such an impaired oxidative balance in multiple myeloma but it also presents potential laboratory markers to measure it.
The human body has a number of endogenous free-radical scavengers systems. HDL-associated PON1 and ARE are the enzymes involved in such systems. These enzymes contribute to the detoxification of organophosphorus compounds and carcinogenic lipid-soluble radicals from lipid peroxidation [3,4,5]. Studies have revealed that PON1 expression is alleviated in human lung cancer [18] and pancreatic [19] and gastric cancers [20]. Accordingly, in this study, PON1 and ARE activities were significantly lower in the multiple myeloma patients compared to the healthy subjects. A probable explanation could be an increase in intracellular oxidants that led to a parallel decrease in antioxidants and finally disrupted the structure of enzymes such as PON1 and ARE. However, cachexia and malnutrition in cancer patients are important problems caused by a variety of mechanisms. In the later stages of the disease, malnutrition and inflammation suppress protein synthesis [21]. PON1 and ARE activities may decrease, due to suppressed protein synthesis, cachexia and malnutrition, as the host response to the tumour.
Sharma et al. [22] examined the levels of oxidative stress markers in 50 patients with multiple myeloma and 50 healthy controls. These markers were SOD, GPX and catalase enzymatic activities in whole blood as well as the circulating levels of MDA and vitamins E and C. They determined that the average levels of SOD, GPX, catalase and vitamins E and C were lower in the patients with multiple myeloma, while MDA levels were significantly higher. Gangemi et al. [23] evaluated the serum levels of advanced oxidation protein products, advanced glycation end products, and protein nitrosylation as markers of oxidative stress. They included in their study monoclonal gammopathy of uncertain significance patients in addition to multiple myeloma and control patients. They reported higher levels of these markers in the multiple myeloma group compared to both the monoclonal gammopathy of uncertain significance group and the control group and detected no differences between stages of the disease [23]. These findings indicate the role of oxidative stress at the very critical point of passage through uncertain significance to true malignant neoplasia. Our findings were similar to theirs, but the markers of our study have more practical applications because there are ready-to-use kits, suitable for autoanalysers. There was no significant difference in TAS levels between the groups with multiple myeloma and controls; this is probably due to the state of the disease that failed to show a proportional response to the increase in oxidative status. Higher uric acid (an effective antioxidant) levels in multiple myeloma patients were of concern as contributors to TAS. Measurement of the TOS is extremely valuable as it is the sum of all oxidants, including those oxidants not yet recognized or not easily measured. On the other hand, it is an unrefined, vague result, making it crucial to examine some subclasses of oxidants.
Thiols are organic compounds that include a sulphydryl group. They play a significant role in the defence against free-radical species. The antioxidant features of thiols include: the quenching of free-radical species, the renewal of exogenous and endogenous antioxidants such as vitamins C and E and glutathione, the chelation of redox metals including Fe2+ and Cu2+, and the repair of oxidized proteins [16]. In our study, a striking feature was the significantly lower levels of total thiols in multiple myeloma patients in comparison to healthy subjects. An increase in intracellular oxidants can lead to a parallel decrease in thiols and disrupt the structure of many enzymes [16]. There is no reason to think that PON1 and ARE are exceptions.
Haemoglobin, creatinine, calcium and albumin levels are important prognostic factors for the Durie-Salmon and international staging system [24,25]. The correlations between haemoglobin, creatinine, calcium, and albumin levels and PON1, ARE, total thiol, TAS, TOS, and OSI levels were not significant. Based on these findings, we can assume that the change in oxidant/antioxidant balance did not correlate with disease activity. Such a correlation would have been helpful in developing and planning novel treatments for multiple myeloma. Therefore, further studies are needed to investigate the possible relationship between oxidative stress and the prognosis of multiple myeloma.
A major limitation of this study was the small number of patients. Further studies with larger groups are necessary to investigate the possible relationship between oxidative stress and the aetiopathogenesis of multiple myeloma.
Conclusion
The presence of impaired oxidative balance was identified in patients with multiple myeloma due to oxidative stress. Unfortunately, it was not possible to determine whether oxidative stress led to the disease or was caused by it.
References
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 2.Reab MS, Podar K, Breitkreuz, et al. Multiple myeloma. Lancet. 2009;374:324–339. doi: 10.1016/S0140-6736(09)60221-X. [DOI] [PubMed] [Google Scholar]
- 3.Aviram M, Rosenblat M, Bisgaier CL, et al. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions: a possible peroxidative role for paraoxonase. J Clin Invest. 1998;101:1581–1590. doi: 10.1172/JCI1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gan KN, Smolen A, Eckerson HW, et al. Purification of human serum paraoxonase/arylesterase: evidence for one esterase catalyzing both activities. Drug Metab Dispos. 1991;19:100–106. [PubMed] [Google Scholar]
- 5.Geldmacher-von Mallinckrodt M, Diepgen TL, Duhme C, et al. A study of the polymorphism and ethnic distribution differences of human serum paraoxonase. Am J Phys Anthropol. 1983;62:235–241. doi: 10.1002/ajpa.1330620302. [DOI] [PubMed] [Google Scholar]
- 6.Ayub A, Mackness MI, Arrol S, et al. Serum paraoxonase after myocardial infarction. Arterioscler Thromb Vasc Biol. 1999;19:330–335. doi: 10.1161/01.atv.19.2.330. [DOI] [PubMed] [Google Scholar]
- 7.Mackness MI, Harty D, Bhatnagar D, et al. Serum paraoxonase activity in familial hypercholesterolaemia and insulin-dependent diabetes mellitus. Atherosclerosis. 1991;86:193–199. doi: 10.1016/0021-9150(91)90215-o. [DOI] [PubMed] [Google Scholar]
- 8.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Harma M, Erel O. Increased oxidative stress in patients with hydatidiform mole. Swiss Med Wkly. 2003;133:563–566. doi: 10.4414/smw.2003.10397. [DOI] [PubMed] [Google Scholar]
- 11.Wlodek L. Beneficial and harmful effects of thiols. Pol J Pharmacol. 2002;54:215–223. [PubMed] [Google Scholar]
- 12.Eckerson HW, Wyte MC, LaDu BN. The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet. 1983;35:1126–1138. [PMC free article] [PubMed] [Google Scholar]
- 13.Haagen L, Brock A. A new automated method for phenotyping arylesterase (E.C.3.1.1.2.) based upon inhibition of enzymatic hydrolysis of 4-nitrophenyl acetate. Eur J Clin Chem Clin Biochem. 1992;30:391–395. doi: 10.1515/cclm.1992.30.7.391. [DOI] [PubMed] [Google Scholar]
- 14.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 15.Hu ML. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994;233:380–385. doi: 10.1016/s0076-6879(94)33044-1. [DOI] [PubMed] [Google Scholar]
- 16.Valko M, Rhodes CJ, Moncola J, et al. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Gupte A, Russell JM. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev. 2009;35:32–46. doi: 10.1016/j.ctrv.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Elkiran ET, Mar N, Aygen B, et al. Serum paraoxonase and arylesterase activities in patients with lung cancer in a Turkish population. BMC Cancer. 2007;15:48. doi: 10.1186/1471-2407-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akcay MN, Polat MF, Yilmaz I, et al. Serum paraoxonase levels in pancreatic cancer. Hepatogastroenterology. 2003;50:ccxxv–ccxxvii. [PubMed] [Google Scholar]
- 20.Akcay MN, Yilmaz I, Polat MF, et al. Serum paraoxonase levels in gastric cancer. Hepatogastroenterology. 2003;50:cclxxiii–cclxxv. [PubMed] [Google Scholar]
- 21.von Meyenfeldt M. Cancer-associated malnutrition: an introduction. Eur J Oncol Nurs. 2005;9(suppl 2):35–38. doi: 10.1016/j.ejon.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Sharma A, Tripathi M, Satyam A, et al. Study of antioxidant levels in patients with multiple myeloma. Leuk Lymphoma. 2009;50:809–815. doi: 10.1080/10428190902802323. [DOI] [PubMed] [Google Scholar]
- 23.Gangemi S, Allegra A, Alonci A, et al. Increase of novel biomarkers for oxidative stress in patients with plasma cell disorders and in multiple myeloma patients with bone lesions. Inflamm Res. 2012;61:1063–1067. doi: 10.1007/s00011-012-0498-7. [DOI] [PubMed] [Google Scholar]
- 24.Durie BG, Salmon SE. A clinical staging system for multiple myeloma: correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36:842–854. doi: 10.1002/1097-0142(197509)36:3<842::aid-cncr2820360303>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 25.Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]