Abstract
Cystic fibrosis (CF) is a multisystem disease causing severe chronic sinopulmonary disease and loss of pancreatic exocrine function, which affects approximately 70,000 individuals worldwide. New therapeutic developments over the last few decades have resulted in a significant increase in survival, with the median predicted survival now reaching the late thirties and more and more CF patients living well into adulthood. However, with this advent of new therapies and the associated increase in survival, new challenges in CF care have also emerged. Two of these challenges, i.e. chronic methicillin-resistant Staphylococcus aureus lung infection and patient adherence to very complicated and time-consuming therapeutic regimens, are reviewed in detail here. In addition, the ultimate challenge of treating the underlying cause of CF by correcting the dysfunction of the CF transmembrane conductance regulator chloride channel is reviewed, as agents to correct channel function will likely significantly alter CF clinical outcomes and treatment approaches in the next decade.
Key Words: Cystic fibrosis, Methicillin-resistant Staphylococcus aureus, Adherence, Cystic fibrosis transmembrane conductance regulator
Introduction
Cystic fibrosis (CF) is a multisystem disease that leads to chronic sinopulmonary disease, pancreatic exocrine impairment, elevated sweat chloride, and male infertility. These phenotypic abnormalities are caused by dysfunction of the CF transmembrane conductance regulator (CFTR) protein, a chloride channel present at the apical membrane of the epithelia of most luminal surfaces of the body. CFTR maintains the homeostasis of the pulmonary airway surface liquid layer through its actions as a chloride channel and its influence on the epithelial sodium channel. Nearly 2,000 mutations in the CFTR gene that can lead to dysfunction of the CFTR protein and result in a CF phenotype have been identified. CFTR dysfunction particularly affects airway epithelial and glandular cells and results in a decrease in the depth of the airway surface liquid layer in the lungs, an increase in the viscosity of airway secretions, and a decreased ability to clear bacterial infections.
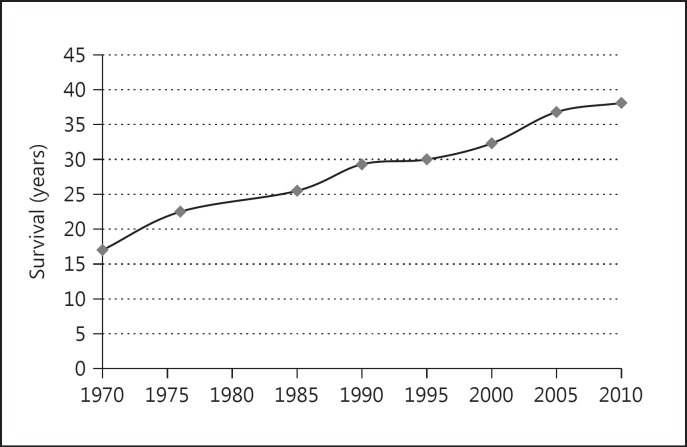
CF is a common inherited disorder of Caucasians, affecting 1 in 2,500 births, and it is the most common lethal autosomal recessive genetic disorder in this population [1]. Over the last few decades, advancements in disease management have resulted in improved patient survival, with the median survival now exceeding 38 years of age [2] (fig. 1). This represents a dramatic improvement in survival compared to previous decades, as the median predicted survival was approximately 25.5 years in 1985 and 17 years in 1970 [1]. Approximately 50s% of all individuals with CF are now 18 years of age or older [2].
Fig. 1.
Cystic fibrosis median survival (1970–2010). Source: Cystic Fibrosis Foundation Patient Registry data [2].
This improvement in outcomes is a result of the development of multiple new medications that slow the progression of lung disease, including mucolytics designed to address the increased viscosity of airway secretions and intravenous and inhaled antibiotics allowing better management of chronic infections. In addition, the development of standardized treatment guidelines and organized care teams has aided in the improvement of clinical outcomes [3].
Even in this setting of improving care, however, new challenges have continued to emerge in the treatment of CF. Three particularly important areas of challenge that have been identified as obstacles for continued improvement of outcomes include: (1) the increasing prevalence of difficult-to-treat bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA), (2) the growing burden of numerous new therapies leading to patient nonadherence to treatment regimens, and (3) the need to treat the underlying CFTR dysfunction to prevent progressive lung disease. This review will highlight recent findings and ongoing research in each of these three important emerging areas.
Emerging CF Challenge No. 1: Increasing Prevalence of MRSA
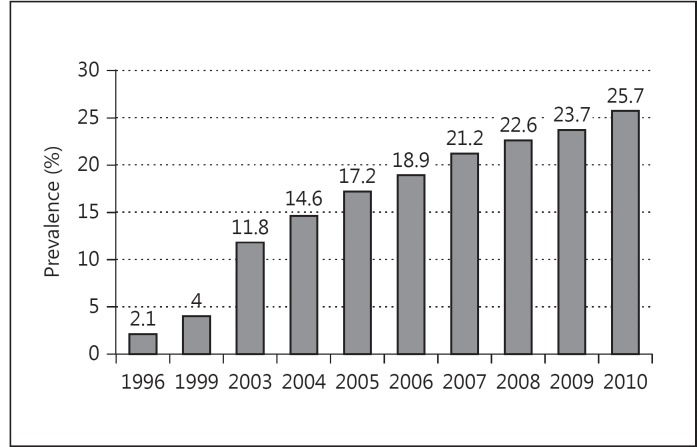
Despite improvements in outcomes, the majority of CF patients still die from pulmonary complications, and treatment of bacterial lung infection remains one of the primary goals of CF care [4,5]. MRSA has emerged as a particularly vexing component of this challenge. While known pathogens such as Pseudomonas aeruginosa and Burkholderia cepacia continue to affect CF disease progression, over the last decade MRSA has demonstrated a notable increase in prevalence, including among individuals with CF, increasing from 4s% in 1999 to 25.7s% in 2010 [2,6] (fig. 2). At some US CF care centers, nearly 50s% of the patient population has been demonstrated to have MRSA pulmonary infection [2].
Fig. 2.
Prevalence of MRSA in CF.
Along with concerns about increasing prevalence, emerging research has demonstrated that MRSA pulmonary infection has a significant clinical impact on individuals with CF. Two large observational studies utilizing the US Cystic Fibrosis Foundation National Patient Registry (CFFPR) database demonstrated that persistent infection with MRSA is associated with worse outcomes. The first analysis demonstrated an association between persistent MRSA infection and a more rapid rate of decline in lung function, as measured by the FEV1 s% predicted [7]. This association was noted in individuals with CF aged 8–21 years even after controlling for other factors known to contribute to lung function decline. Patients with persistent MRSA were found to have a 43s% more rapid rate of decline of FEV1 s% predicted compared with MRSA-negative patients. Moreover, when examining FEV1 in individual patients over time there was a 25s% more rapid rate of decline in lung function after acquisition of persistent MRSA compared to the time prior to infection.
Recent research has also demonstrated that chronic MRSA infection in CF has an effect on survival. In a separate analysis of the CFFPR, CF patients with persistent MRSA-positive respiratory cultures were shown to have a median survival time 6.2 years shorter than that of patients who remained MRSA negative during the study period [8]. The hazard ratio of mortality was calculated using Cox regression models, with adjustment for known contributors to worse survival. The adjusted hazard ratio of mortality for those 5,759 patients with MRSA was 1.27 (95s% CI 1.11–1.45, p < 0.001). A time-lagged analysis also demonstrated a significant increase in the adjusted hazard ratio of mortality associated with MRSA, suggesting that MRSA is an independent risk factor for death and not just a marker of disease severity or end of life in individuals with CF.
The role of CF MRSA microbiologic epidemiology has been investigated and unique molecular characteristics of MRSA infection in CF have been identified that can contribute to worse outcomes. Specifically, virulent SCCmec I clonal strains able to grow as biofilms, small-colony variant MRSA, and presence of the Panton-Valentine leukocidin cytotoxin have all been demonstrated to influence the clinical course of MRSA infection in CF. Glikman et al. [9] demonstrated that hospital-acquired MRSA (identified by SCCmec II) was predominant in a pediatric population of chronically MRSA-infected CF patients, and community-acquired MRSA (SCCmec IV) was predominant in CF patients newly infected with MRSA [9]. In a separate analysis of MRSA isolates, Molina et al. [10] identified a particular MRSA clone, i.e. ST228-SCCmec I, which appeared to be associated with persistent infection in the CF patients at their care center. This clone demonstrated an increased ability to grow as a biofilm and the authors hypothesized that this characteristic could contribute to persistent respiratory infection. Additionally, small-colony variant MRSA has been recognized for its ability to contribute to persistent infection in a number of different clinical settings [11]. This may be attributable to the ability of small-colony variant MRSA to grow intracellularly, as well as increased antibiotic resistance, when compared with normal-colony variant MRSA [12,13]. Finally, the emergence of Panton-Valentine leukocidin-positive MRSA in a CF population has been described, demonstrating the ability of this cytotoxin to cause more serious respiratory infections compared to Panton-Valentine leukocidin-negative MRSA [13].
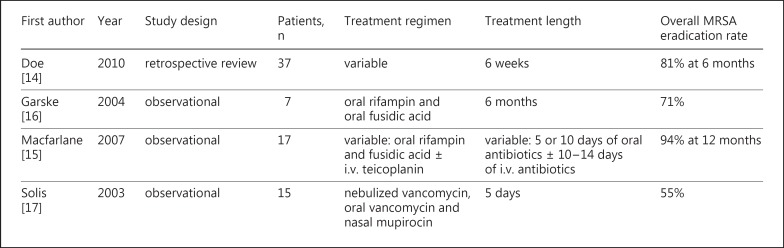
Given the growing evidence of the effect of MRSA on clinical outcomes, a number of small clinical studies have investigated the optimal therapy for MRSA in CF (fig. 3). These studies, however, have been limited by small study populations, a lack of control groups, their single-center retrospective design, variable follow-up, and failure to differentiate incident versus persistent infection [14,15,16,17]. Doe et al. [14] conducted a retrospective review of 37 adult patients at their CF center in Manchester, UK (average age 25.6 years, mean FEV1 2.2 liters, and 75s% with P. aeruginosa). The study examined multiple eradication regimens in which most patients were treated with a combination of 2 oral antibiotics (rifampin, trimethoprim, or fusidic acid) for a minimum of 6 weeks. Eighteen patients were also treated with a course of nebulized vancomycin (200 mg 4 times a day for 5 days). They reported an overall MRSA eradication rate of 81s% at 6 months (although no distinction was made between patients with incident vs. persistent MRSA). Garske et al. [16] conducted a small study focusing on the treatment of persistent MRSA in adults with CF. Seven patients with known persistent MRSA (average FEV1 36s% predicted, all with chronic P. aeruginosa, 6/7 previously treated with i.v. antibiotics for MRSA) were treated with oral rifampin and fusidic acid for 6 months. Five of the 7 patients (71s%) were culture negative for MRSA after completing this treatment regimen. Macfarlane et al. [15] investigated the treatment of incident MRSA in 17 pediatric patients cared for at their CF center in Belfast, Northern Ireland. Patients were treated with a 5-day course of oral rifampin and fusidic acid. This course of treatment was repeated if patients remained culture positive for MRSA, and a 10- to 14-day course of i.v. teicoplanin was initiated in patients who failed to clear MRSA after 10 days of oral antibiotics. Utilization of this protocol resulted in a 94s% eradication rate at 12 months. Solis et al. [17] also investigated the treatment of incident MRSA in pediatric CF patients. Fifteen patients were treated with nebulized vancomycin (200 mg 4 times a day for 5 days), oral vancomycin, and nasal mupirocin, and a 55s% eradication rate was achieved with the use of this protocol.
Fig. 3.
Previous studies of MRSA treatment in CF.
Given the recognition of the clinical significance of MRSA pulmonary infection in CF and the limited data available to guide current therapeutic approaches, two larger clinical trials have been initiated that are designed to assess the efficacy of treatment for both newly incident and persistent MRSA infection. The STAR-CF Too trial (‘STaph. Aureus Resistance in CF, Treat Or Observe?’) is a multicenter trial (University of North Carolina, Seattle Children's Hospital, and the CF Foundation Therapeutics Development Network), funded by the Cystic Fibrosis Foundation, in which 80 patients with incident MRSA infection will be randomized 1:1 to an observation arm or an eradication arm. The eradication treatment will consist of two oral antibiotics, topical mupirocin, chlorhexidine gargle, and environmental decontamination. Subjects will be followed for 6 months. The trial is designed to assess if an early eradication protocol is effective for the eradication of a new MRSA infection. Additionally, the trial aims to better characterize MRSA infection in CF and evaluate spontaneous clearance versus persistent MRSA infection in those not initially treated. The primary outcome measure is the proportion of subjects in each arm with MRSA-negative respiratory cultures at day 28. Secondary outcome measures include the use of antibiotics and the frequency of pulmonary exacerbations over the 6-month study period.
The PMEP Trial (Persistent Methicillin-resistant S. aureus Eradication Protocol) is a two-center trial (Johns Hopkins University and Case Western Reserve University), funded by the US CF Foundation, that is currently enrolling 40 CF patients with known, persistent MRSA pulmonary infection. The trial is designed to assess if a 28-day inhaled and oral antibiotic protocol can effectively eradicate persistent MRSA infection. The study is a double-blind, comparator-controlled, parallel-group study, with 1:1 randomization assignment to either vancomycin for inhalation (250 mg twice daily) or taste-matched placebo. Additionally, both groups will receive oral rifampin, a second oral antibiotic (trimethoprim- sulfa or doxycycline), mupirocin intranasal cream, and chlorhexidine body washes. The primary outcome is the percentage of patients who are MRSA free by respiratory culture 1 month after the 28-day treatment protocol in the intervention arm versus the control arm. Secondary outcomes include: the percentage of patients who are MRSA free 3 months after completion of the treatment protocol, the change in FEV1 from baseline on days 28 and 118, the change in MRSA colony-forming units, and the time to the first pulmonary exacerbation.
MRSA pulmonary infection presents a formidable clinical challenge that is increasingly encountered in the care of individuals with CF. Research to date has successfully identified the increasing prevalence of MRSA in CF and established the significant clinical impact MRSA can have on outcomes. Ongoing trials will provide further insight into the role of the molecular epidemiology of MRSA in outcomes and direct future therapeutic approaches toward effective management of MRSA infection in CF.
Emerging CF Challenge No. 2: Maintaining Patient Adherence to Complicated CF Medical Regimens
At the same time that the rise in prevalence of MRSA lung infection is presenting CF caregivers with a difficult challenge, individuals with CF are also facing an increasingly difficult challenge: adhering to their complicated CF medical regimen. Between use of inhaled mucolytics, inhaled antibiotics, airway clearance, nutritional enzymes, supplements, and equipment maintenance, most individuals with CF spend hours a day on their medications. Recent research has highlighted both the challenge of adherence to these complicated CF medical regimens and the impact that the degree of adherence can have on clinical outcomes.
Using the Epidemiologic Study of Cystic Fibrosis (ESCF) database, Konstan et al. [18] quantified changes in prescribing rates between 1995 and 2005. The use of most therapies increased significantly, including airway clearance (69.9–89.6s%), inhaled bronchodilators (72.0–84.0s%), dornase alfa (44.8–67.2s%), inhaled corticosteroids (16.0–49.3s%), inhaled antibiotics (6.5–43.1s%), oral nutritional supplements (18.3–24.5s%), and insulin/oral hypoglycemic agents (4.9–10.2s%). In addition, several new medications, such as oral macrolide antibiotics, inhaled hypertonic saline, and leukotriene inhibitors, became routine. It is no surprise then that individuals with CF now have regimens that are complex and burdensome and tend to only become more so over time.
Adherence to these complicated CF medication regimens is only approximately 50s%, with estimates ranging from 31 to 79s% depending on the specific regimen and the person's age [19,20,21,22]. There is, however, often substantial variation even within individuals in terms of daily adherence. For example, studies have demonstrated that children with CF adhere more to their nebulized medications on evenings versus mornings, weekdays versus weekends, and when school is in session versus while on vacation [23,24].
Several recent studies highlight the importance of adhering to treatments. Two studies have demonstrated the importance of inhaled tobramycin adherence by finding that poorer adherence was associated with an increased risk of hospitalization and increased health care costs [25,26]. A different study found no association between dornase alfa nonadherence and the frequency of inpatient respiratory exacerbations but did demonstrate an association with longer length of stays in the hospital [27]. While the previous studies relied on health care claims data, a fourth study of 95 people reviewed clinical records and determined that a lower overall pulmonary medication adherence (average adherence to dornase alfa, hypertonic saline, azithromycin, and inhaled tobramycin) was associated with a more frequent occurrence of pulmonary exacerbations requiring i.v. antibiotics, and lower baseline lung function [19].
Together these data highlight that the level of adherence to medications does affect pulmonary health outcomes and the frequency of hospitalization. When it comes to costs, however, savings from reducing health care utilization are often offset by higher spending on prescription medications. Future research is needed on larger samples over longer periods of time to better quantify the degree to which different levels of overall adherence and varying patterns in daily adherence affect health outcomes and health care costs and whether the strength of these associations varies by subgroups such as age, gender, or disease severity.
To accomplish this, it will be helpful in the future to incorporate electronic monitors, such as the I-neb™ Adaptive Aerosol Device, into daily clinical practice because they capture the date, the time, and often the duration of medication use in an unobtrusive manner that does not place an additional burden on individuals with CF [28]. Other approaches to measuring adherence, such as patient report and clinician estimates, are notoriously inaccurate [20], and pharmacy refill data requires a longer period of time to establish an accurate estimate and therefore is insensitive to fluctuations in patterns of adherence [29]. Understanding patterns of nonadherence will aid in identifying adherence barriers and facilitators of adherence and determining the efficacy of interventions. Unfortunately, electronic monitors can be costly, require additional staffing to manage, and are not yet widely approved for use as a drug delivery device.
Many studies have evaluated potential adherence barriers and facilitators of adherence among adolescents and young adults with CF [30,31,32,33,34]. Commonly identified barriers include the overall treatment burden, competing social and work demands, forgetfulness, lack of a perceived necessity for the treatment, and being uncomfortable doing treatments in front of others. Factors identified as having a positive influence on adherence include attending a CF clinic, getting pulmonary function test results, support from significant others, medicine reminders, the presence of a perceived health benefit from the therapy, and having treatment lead to increased body satisfaction (e.g. wanting to gain weight when under the 50th BMI percentile). Interestingly, some people with CF report being intentionally nonadherent to rebel against having a routine, permit spontaneity, or reward themselves for high adherence at other times. Mental health problems, such as depression, can also affect adherence [35]. Barriers range from easy fixes that require a one-time intervention to more complicated psychosocial challenges that will require ongoing support and possible referral to behavioral health counseling. What is clear from these results is that people with CF face a wide variety of challenges and, more often than not, several barriers concurrently, suggesting that an individualized approach to reducing barriers is critical (fig. 4).
Fig. 4.
Factors influencing adherence to treatment regimens in CF [29,30,31,32,33,34].
Unfortunately, there are few conclusive published studies of adherence interventions in CF, although there are several trials underway. Systematic reviews of adherence to date have concluded that education-only interventions are less effective than multicomponent interventions in improving adherence and illness self-management [36,37]. Multicomponent interventions almost always include education but additionally provide one or more of the following: behavioral modification, parent training, problem solving, motivational enhancement, behavioral and health feedback, and social support. A recent Cochrane review of self-management education interventions for CF by Savage et al. [38] concluded that the interventions increased patients' knowledge about what they ‘should be doing’ immediately following intervention delivery. Unfortunately, this knowledge alone did not effectively translate into sustained behavior changes or improved health outcomes. Thus, much like in other illnesses, interventions to support adherence in CF will need to include more than just education but also multicomponent strategies for behavioral modification.
In just the past few years, the field of CF adherence research has progressed dramatically. There is greater understanding about the importance of adherence, how to measure it, and which interventions might be effective. With several adherence trials in progress, our knowledge and our ability to help people with CF balance the need to follow their complex regimen will expand in the coming years.
Emerging CF Challenge No. 3: Correcting the Underlying CFTR Protein Dysfunction
The ultimate way to address many of the ongoing treatment challenges in CF will be to treat the basic defect underlying the CF phenotype. Recent developments have made addressing the basic CF defect realistic for the first time.
The CFTR protein acts to maintain the airway surface liquid layer through its function as a chloride channel and its regulation of the epithelial sodium channel [17]. This liquid layer lines the surface of the airways and allows cilia to protect the lungs through continuous mucociliary clearance. When mutations in the CFTR gene cause a lack of CFTR production or dysfunction of the CFTR protein, the result is increased sodium resorption from the airways and formation of a contracted viscous surface liquid layer [39,40]. This abnormal liquid layer forms the basis of CF lung disease and results in a cycle of recurrent mucous plugging, infection, and inflammation which causes progressive lung damage [41].
In the past, pulmonary therapies for CF have always targeted the viscous mucus or chronic airway infection characteristic of CF lung disease. While this resulted in improved outcomes and better survival, it never addressed the underlying basis of the CF lung disease and did not halt the progression of lung disease. Since the discovery of the CF gene in 1989, there has been a desire to address the underlying chloride channel defect characteristic of CF [42,43,44]. Recent results of a landmark phase III study in the 4s% of CF individuals carrying a mutation (G551D-CFTR) that gives rise to a G551D substitution in the CFTR protein have demonstrated that correction of the underlying channel defect of CFTR is both possible and results in a significant clinical benefit for individuals with CF [45]. These results usher in a new era in CF therapeutics in which treatments will improve patient outcomes by correcting the underlying chloride channel defect in CF. The emerging challenge now will be to correct the many different types of underlying chloride channel defects seen in CF.
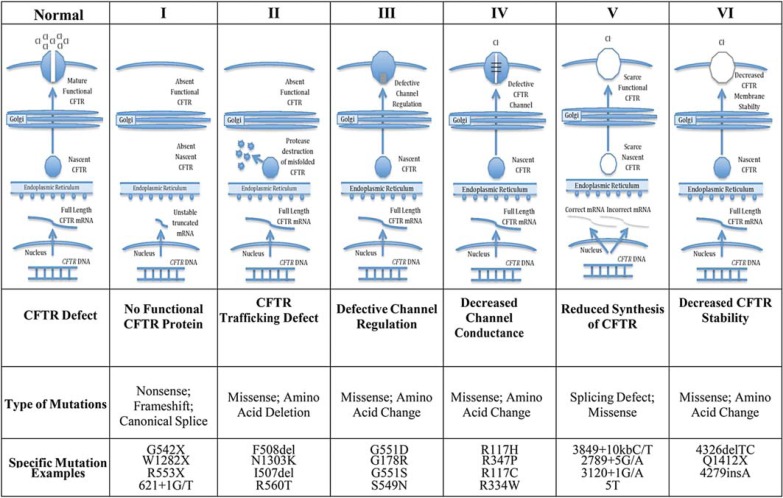
CF follows a classic recessive inheritance pattern, so an individual must have a disease-causing CFTR mutation on each chromosome to develop a CF phenotype. Nearly 2,000 different CFTR mutations have been identified which when paired can result in a CF phenotype. These CFTR mutations can be grouped into different classes based on their effect on CFTR protein production, trafficking, function, and stability (fig. 5) [46]. Class I mutations result in no functional CFTR protein being made and include nonsense mutations causing premature stop codons that result in the production of truncated unstable RNA (G542X, W1282X, and R553X) [47]. Class II mutations, the most common type, cause protein misfolding that prevents CFTR from trafficking correctly to the cell surface and results in minimal functional CFTR reaching the cell membrane. F508del-CFTR, a 3-bp deletion that causes a single amino acid deletion from CFTR, is the best example of a class II trafficking mutation caused by misfolding. F508del-CFTR is the most common mutation in CF, with close to 90s% of individuals with CF worldwide having the F508del-CFTR mutation on at least one CFTR gene and approximately 50s% being homozygous for two F508del-CFTR mutations [47]. CFTR protein affected by mutations in classes III and IV reaches the cell surface but does not function normally as a chloride channel. Class III mutations result in the CFTR chloride channel opening time being significantly reduced. G551D-CFTR is the most common class III mutation [47]. Mutations in class IV also result in CFTR protein being present at the surface, but the chloride transport is reduced even when the channel is open [47]. Class V mutations cause a decrease in the overall chloride transport because there is a reduced amount of normal CFTR at the surface, usually due to intron mutations that reduce the efficiency of the production of CFTR by affecting splicing [47]. Class VI mutations are rare and decrease the stability of mature CFTR once at the cell membrane.
Fig. 5.
Classes of CFTR protein mutations.
By understanding the classes of CFTR dysfunction based on mutation classes, it is easier to understand the emerging challenge of correcting the basic CFTR defect. These classes make clear that some CFTR mutations will be more challenging to correct than others – particularly class I and II mutations in which little or no CFTR is present at the cell surface. However, at the present time all 6 classes have emerging therapies and/or ongoing clinical trials with the potential to address the underlying CFTR defect.
As mentioned previously, recent results of a study in individuals with the class III mutation G551D-CFTR, where the CFTR channel is present at the cell surface but is not open, demonstrated convincingly that correction of the underlying channel defect of CFTR results in a significant clinical benefit for individuals with CF. The small-molecule compound ivacaftor, previously called VX-770, increases the opening time of CFTR and increases the chloride flux through the CFTR channel. In a phase III clinical trial of ivacaftor in 144 individuals with CF carrying at least one G551D-CFTR mutation, twice daily oral therapy led to a mean absolute improvement of 10.5s% in FEV1 s% predicted (17s% relative improvement) within 2 weeks of starting the therapy. This improvement was maintained throughout the 48-week treatment period [45]. Treated participants also experienced a 55s% reduction in CF pulmonary exacerbations, a mean weight gain of 2.7 kg, and significant improvement in the measured quality of life (p < 0.001) [45]. A follow-up trial in 52 CF children aged 6–11 years with at least one G551D-CFTR mutation demonstrated nearly identical effects on lung function, weight, and sweat chloride, with the mean improvement in absolute FEV1 s% predicted being 10.0s% at week 48 (relative 15.1s%) [48].
The results for ivacaftor in G551D patients were groundbreaking because they demonstrated that correcting CFTR chloride transport improves clinical outcomes. While only 4s% of individuals with CF carry a G551D-CFTR mutation, it is known from in vitro studies that ivacaftor also increases chloride transport in many other mutations in which CFTR is present at the apical membrane, including other mutations in class III and mutations from classes IV and V [49]. This provides an opportunity for ivacaftor to impact a larger number of patients – estimated to be 15s% of patients or more. Phase III clinical trials of ivacaftor in other class III mutations and in the class IV mutation R117H-CFTR are now underway.
Class II mutations are by far the most important CFTR mutation class, however, because of how common they are. In most countries, close to 90s% of individuals with CF have at least one of the class II mutations F508del-CFTR. While ivacaftor increases chloride conductance for F508del-CFTR once the protein reaches the cell surface, protein misfolding prevents most of the protein from reaching the surface [50]. Lumacaftor (VX-809) is a new small molecular compound that has demonstrated its ability, in cell culture and in early phase II studies, to increase the amount of F508del-CFTR trafficking to the cell surface [51]. By using lumacaftor or another new CFTR corrector called VX-661 to aid in moving F508del-CFTR to the cell surface, and ivacaftor to increase the opening time and chloride conductance once it is there, it appears possible that function for class II mutations may be corrected. This combination was tested in a phase II study of individuals carrying the F508del-CFTR mutation and initial results suggested a beneficial effect on lung function in F508del-CFTR homozygotes [52]. A phase III study of the combination is ongoing in 2013–2014.
Class I mutations are the most difficult CFTR defect to address because they result in an absence of stable CFTR protein. Correcting CFTR function in patients with class I mutations will require either replacing the defective CFTR gene or altering the manner in which the protein is made. Both of these strategies are currently being pursued. The UK Gene Therapy Consortium is currently conducting a phase II trial in CF utilizing a nonviral lipid vector for DNA delivery that seeks to provide a corrected copy of CFTR DNA. The protocol includes monthly dosing by inhalation for a year, with results anticipated sometime in 2014 [53]. A second strategy for class I mutations is promoting full transcription by allowing read-through of the premature stop codons present in the majority of patients. PTC Therapeutics has attempted to do this with a small molecular compound, i.e. ataluren (previously PTC124), which allows read-through of premature stop codons, particularly ‘UGA’, the premature stop codon present in G542X-CFTR [54,55]. While initial studies using measurements of nasal chloride transport suggested ataluren to be effective, a recently concluded phase III trial of ataluren in CF patients with stop mutations failed to meet its primary end point of improvement in FEV1 at 48 weeks [56,57,58]. The development of next-generation agents allowing read-through of premature stop codons is ongoing and will focus on both increased efficacy and specificity given the potential for read-through of other stop codons.
Recent clinical trials have demonstrated that correcting CFTR-mediated chloride transport results in significant improvement in clinical outcomes in CF. Taking this approach which is now available only for a small subset of patients and developing it for all classes of CF is perhaps the most important emerging challenge in CF, and it offers the potential to dramatically alter the outlook for individuals with CF worldwide.
Conclusion
Continuing to improve survival in CF will require a multipronged attack against the many emerging challenges. Three particularly important emerging CF challenges, i.e. resistant bacterial infections, adherence to complicated medical regimens, and correction of the underlying CFTR defect, will require a multidisciplinary approach involving clinicians, care teams, microbiologists, and basic scientists, but addressing these challenges offers the opportunity to significantly alter outcomes in CF in the next decade.
References
- 1.Boyle MP. Adult cystic fibrosis. JAMA. 2007;298:1787–1793. doi: 10.1001/jama.298.15.1787. [DOI] [PubMed] [Google Scholar]
- 2.Cystic Fibrosis Foundation . Patient Registry, 2011 Annual Data Report. Bethesda: Cystic Fibrosis Foundation; 2012. [Google Scholar]
- 3.Mogayzel PJ, Naureckas ET, Robinson KA, et al. Cystic fibrosis pulmonary guidelines. Am J Respir Crit Care Med. 2013;187:680–689. doi: 10.1164/rccm.201207-1160oe. [DOI] [PubMed] [Google Scholar]
- 4.Ratjen F, Doring G. Cystic fibrosis. Lancet. 2003;361:681–689. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 5.Flume P, O'Sullivan B, Robinson K, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2007;176:957–969. doi: 10.1164/rccm.200705-664OC. [DOI] [PubMed] [Google Scholar]
- 6.Udo EE. Community-acquired methicillin-resistant Staphylococcus aureus: the new face of an old foe? Med Princ Pract. 2013 doi: 10.1159/000354201. DOI 10.11591000354201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dasenbrook EC, Merlo CA, Diener-West M, et al. Persistent methicillin-resistant Staphylococcus aureus and rate of FEV1 decline in cystic fibrosis. Am J Respir Crit Care Med. 2008;178:814–821. doi: 10.1164/rccm.200802-327OC. [DOI] [PubMed] [Google Scholar]
- 8.Dasenbrook EC, Checkley W, Merlo CA, et al. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA. 2010;303:2386–2392. doi: 10.1001/jama.2010.791. [DOI] [PubMed] [Google Scholar]
- 9.Glikman D, Siegel J, David M, et al. Complex molecular epidemiology of methicillin-resistant Staphylococcus aureus isolates from children with cystic fibrosis in the era of epidemic community-associated methicillin-resistant S. aureus. Chest. 2008;133:1381–1387. doi: 10.1378/chest.07-2437. [DOI] [PubMed] [Google Scholar]
- 10.Molina A, Del Campo R, Maiz L, et al. High prevalence in cystic fibrosis patients of multiresistant hospital-acquired methicillin-resistant Staphylococcus aureus ST228-SCCmecI capable of biofilm formation. J Antimicrob Chemother. 2008;62:961–967. doi: 10.1093/jac/dkn302. [DOI] [PubMed] [Google Scholar]
- 11.Besier S, Smaczny C, von Mallinckrodt C, et al. Prevalence and clinical significance of Staphylococcus aureus small-colony variants in cystic fibrosis lung disease. J Clin Microbiol. 2007;45:168–172. doi: 10.1128/JCM.01510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahl B, Herrmann M, Everding AS, et al. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J Infect Dis. 1998;177:1023–1029. doi: 10.1086/515238. [DOI] [PubMed] [Google Scholar]
- 13.Elizur A, Orscheln RC, Ferkol TW, et al. Panton-Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus lung infection in patients with cystic fibrosis. Chest. 2007;131:1718–1725. doi: 10.1378/chest.06-2756. [DOI] [PubMed] [Google Scholar]
- 14.Doe SJ, McSorley A, Isalska B, et al. Patient segregation and aggressive antibiotic eradication therapy can control methicillin-resistant Staphylococcus aureus at large cystic fibrosis centres. J Cyst Fibros. 2010;9:104–109. doi: 10.1016/j.jcf.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Macfarlane M, Leavy A, McCaughan J, et al. Successful decolonization of methicillin-resistant Staphylococcus aureus in paediatric patients with cystic fibrosis (CF) using a three-step protocol. J Hosp Infect. 2007;65:231–236. doi: 10.1016/j.jhin.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Garske LA, Kidd TJ, Gan R, et al. Rifampicin and sodium fusidate reduces the frequency of methicillin-resistant Staphylococcus aureus (MRSA) isolation in adults with cystic fibrosis and chronic MRSA infection. J Hosp Infect. 2004;56:208–214. doi: 10.1016/j.jhin.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Solis A, Brown D, Hughes J, et al. Methicillin-resistant Staphylococcus aureus in children with cystic fibrosis: an eradication protocol. Pediatr Pulmonol. 2003;36:189–195. doi: 10.1002/ppul.10231. [DOI] [PubMed] [Google Scholar]
- 18.Konstan MW, VanDevanter DR, Rasouliyan L, et al. Trends in the use of routine therapies in cystic fibrosis: 1995–2005. Pediatr Pulmonol. 2010;45:1167–1172. doi: 10.1002/ppul.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eakin MN, Bilderback A, Boyle MP, et al. Longitudinal association between medication adherence and lung health in people with cystic fibrosis. J Cyst Fibros. 2011;10:258–264. doi: 10.1016/j.jcf.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daniels T, Goodacre L, Sutton C, et al. Accurate assessment of adherence: self-report and clinician report vs. electronic monitoring of nebulizers. Chest. 2011;140:425–432. doi: 10.1378/chest.09-3074. [DOI] [PubMed] [Google Scholar]
- 21.Latchford G, Duff A, Quinn J, et al. Adherence to nebulised antibiotics in cystic fibrosis. Patient Educ Couns. 2009;75:141–144. doi: 10.1016/j.pec.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Faulkner C, Taper LJ, Scott M. Adherence to pancreatic enzyme supplementation in adolescents with cystic fibrosis. Can J Diet Pract Res. 2012;73:196–199. doi: 10.3148/73.4.2012.196. [DOI] [PubMed] [Google Scholar]
- 23.Ball R, Southern KW, McCormack P, et al. Adherence to nebulised therapies in adolescents with cystic fibrosis is best on week-days during school term-time. J Cyst Fibros. 2013;12:440–444. doi: 10.1016/j.jcf.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 24.McNamara PS, McCormack P, McDonald AJ, et al. Open adherence monitoring using routine data download from an adaptive aerosol delivery nebuliser in children with cystic fibrosis. J Cyst Fibros. 2009;8:258–263. doi: 10.1016/j.jcf.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Briesacher BA, Quittner AL, Saiman L, et al. Adherence with tobramycin inhaled solution and health care utilization. BMC Pulm Med. 2011;11:5. doi: 10.1186/1471-2466-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wertz DA, Chang CL, Stephenson JJ, et al. Economic impact of tobramycin in patients with cystic fibrosis in a managed care population. J Med Econ. 2011;14:759–768. doi: 10.3111/13696998.2011.621004. [DOI] [PubMed] [Google Scholar]
- 27.Nasr SZ, Chou W, Villa KF, et al. Adherence to dornase alfa treatment among commercially insured patients with cystic fibrosis. J Med Econ. 2013;16:801–808. doi: 10.3111/13696998.2013.787427. [DOI] [PubMed] [Google Scholar]
- 28.Geller DE, Kesser KC. The I-neb Adaptive Aerosol Delivery System enhances delivery of alpha1-antitrypsin with controlled inhalation. J Aerosol Med Pulm Drug Deliv. 2010;23((suppl 1)):S55–S59. doi: 10.1089/jamp.2009.0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hess LM, Raebel MA, Conner DA, et al. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40:1280–1288. doi: 10.1345/aph.1H018. [DOI] [PubMed] [Google Scholar]
- 30.George M, Rand-Giovannetti D, Eakin MN, et al. Perceptions of barriers and facilitators: self-management decisions by older adolescents and adults with CF. J Cyst Fibros. 2010;9:425–432. doi: 10.1016/j.jcf.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bregnballe V, Schiotz PO, Boisen KA, et al. Barriers to adherence in adolescents and young adults with cystic fibrosis: a questionnaire study in young patients and their parents. Patient Prefer Adherence. 2011;5:507–515. doi: 10.2147/PPA.S25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bucks RS, Hawkins K, Skinner TC, et al. Adherence to treatment in adolescents with cystic fibrosis: the role of illness perceptions and treatment beliefs. J Pediatr Psychol. 2009;34:893–902. doi: 10.1093/jpepsy/jsn135. [DOI] [PubMed] [Google Scholar]
- 33.Dziuban EJ, Saab-Abazeed L, Chaudhry SR, et al. Identifying barriers to treatment adherence and related attitudinal patterns in adolescents with cystic fibrosis. Pediatr Pulmonol. 2010;45:450–458. doi: 10.1002/ppul.21195. [DOI] [PubMed] [Google Scholar]
- 34.Simon SL, Duncan CL, Horky SC, et al. Body satisfaction, nutritional adherence, and quality of life in youth with cystic fibrosis. Pediatr Pulmonol. 2011;46:1085–1092. doi: 10.1002/ppul.21477. [DOI] [PubMed] [Google Scholar]
- 35.Smith BA, Modi AC, Quittner AL, et al. Depressive symptoms in children with cystic fibrosis and parents and its effects on adherence to airway clearance. Pediatr Pulmonol. 2010;45:756–763. doi: 10.1002/ppul.21238. [DOI] [PubMed] [Google Scholar]
- 36.Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008:CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 37.Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol. 2008;33:590–611. doi: 10.1093/jpepsy/jsm128. [DOI] [PubMed] [Google Scholar]
- 38.Savage E, Beirne PV, Ni Chroinin M, et al. Self-management education for cystic fibrosis. Cochrane Database Syst Rev. 2011:CD007641. doi: 10.1002/14651858.CD007641.pub2. [DOI] [PubMed] [Google Scholar]
- 39.Boucher RC. Cystic fibrosis: a disease of vulnerability to airway surface dehydration. Trends Mol Med. 2007;13:231–240. doi: 10.1016/j.molmed.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Gentzsch M, Dang H, Dang Y, et al. The cystic fibrosis transmembrane conductance regulator impedes proteolytic stimulation of the epithelial Nas+ channel. J Biol Chem. 2010;285:32227–32232. doi: 10.1074/jbc.M110.155259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med. 2007;261:5–16. doi: 10.1111/j.1365-2796.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- 42.Kerem B, Rommens J, Buchanan J, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 43.Amaral M, Kunzelmann K. Molecular targeting of CFTR as a therapeutic approach to cystic fibrosis. Trends Pharmacol Sci. 2007;28:334–341. doi: 10.1016/j.tips.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 44.Clunes M, Boucher R. Front-runners for pharmacotherapeutic correction of the airway ion transport defect in cystic fibrosis. Curr Opin Pharmacol. 2008;8:292–299. doi: 10.1016/j.coph.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramsey B, Davies J, McElvaney N, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sloane P, Rowe S. Cystic fibrosis transmembrane conductance regulator protein repair as a therapeutic strategy in cystic fibrosis. Curr Opin Pulm Med. 2010;16:591–597. doi: 10.1097/MCP.0b013e32833f1d00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.CFTR2 Clinical and Functional Translation of CFTR US CF Foundation, Johns Hopkins University et al. http://www.cftr2.org/browse.php. 2011–2012. http://www.cftr2.org
- 48.Davies JC, Li H, Yen K, et al. Ivacaftor in subjects 6 to 11 years of age with cystic fibrosis and the G551D-CFTR mutation (abstract) J Cyst Fibros. 2012;11:S13. [Google Scholar]
- 49.Yu H, Burton B, Huang C, et al. Ivacaftor potentiation of multiple CFTR channels with gating mutations. J Cyst Fibros. 2012;11:237–245. doi: 10.1016/j.jcf.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Flume P, Liou T, Borowitz D, et al. Ivacaftor in subjects with cystic fibrosis who are homozygous for the F508del-CFTR mutation. Chest. 2012;142:718–724. doi: 10.1378/chest.11-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Goor F, Straley K, Cao D, et al. Rescue of DeltaF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol Lung Cell Mol Physiol. 2009;106:18825–18830. doi: 10.1152/ajplung.00169.2005. [DOI] [PubMed] [Google Scholar]
- 52.Boyle MP, Bell S, Konstan M, et al. The investigational CFTR corrector, VX-809 (lumacaftor) co-administered with the oral potentiator ivacaftor improved CFTR and lung function in F508del homozygous patients: phase II study results. Pediatr Pulmonol. 2012;47:315. [Google Scholar]
- 53.Corbyn Z. Promising new era dawns for cystic fibrosis treatment. Lancet. 2012;379:1475–1476. doi: 10.1016/s0140-6736(12)60617-5. [DOI] [PubMed] [Google Scholar]
- 54.Du M, Liu X, Welch E, et al. PTC124 is an orally bioavailable compound that promotes suppression of the human CFTR-G542X nonsense allele in a CF mouse model. Proc Natl Acad Sci USA. 2008;105:2064–2069. doi: 10.1073/pnas.0711795105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Welch E, Barton E, Zhuo J, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 56.Kerem E, Hirawat S, Armoni S, et al. Effectiveness of PTC124 treatment of cystic fibrosis caused by nonsense mutations: a prospective phase II trial. Lancet. 2008;372:719–727. doi: 10.1016/S0140-6736(08)61168-X. [DOI] [PubMed] [Google Scholar]
- 57.Wilschanski M, Miller L, Shoseyov D, et al. Chronic ataluren (PTC124) treatment of nonsense mutation cystic fibrosis. Eur Respir J. 2011;38:59–69. doi: 10.1183/09031936.00120910. [DOI] [PubMed] [Google Scholar]
- 58.Konstan M, Accurso F, De Boeck K, et al. Targeting class 1 mutations: update on ataluren as a promising treatment for nonsense mutation in CF. Pediatr Pulmonol. 2012;47:108–109. [Google Scholar]