Abstract
Objective
To evaluate the influence of stroke volume variation (SVV)-based goal-directed therapy (GDT) on splanchnic organ functions and postoperative complications in orthopedic patients.
Subjects and Methods
Eighty patients scheduled for major orthopedic surgery under general anesthesia were randomly allocated to one of two equal groups to receive either intraoperative volume therapy guided by SVV (GDT) or standard fluid management (control). In the SVV group, patients received colloid boluses of 4 ml/kg to maintain an SVV <10s% when in the supine position or an SVV <14s% if prone. In the control group, fluids were given to maintain a mean arterial pressure >65 mm Hg, a heart rate <100 bpm, a central venous pressure of 8–14 mm Hg, and a urine output >0.5 ml/kg/h. Intraoperative organ perfusion, hemodynamic data, hospitalization, postoperative complications, and mortality were recorded.
Results
The heart rate at the end of surgery was significantly lower (p < 0.05), there were fewer hypotensive episodes (p < 0.05), the arterial and gastric intramucosal pH were higher (p < 0.05 for both), the gastric intramucosal PCO2 was lower (p < 0.05), the intraoperative infused colloids and the total infused volume were lower (p < 0.05 for both), and the postoperative time to flatus was shorter (p < 0.05) in the GDT group than in the control group. No differences in the length of hospital stay, complications, or mortality were found between the groups.
Conclusion
SVV-based GDT during major orthopedic surgery reduced the volume of the required intraoperative infused fluids, maintained intraoperative hemodynamic stability, and improved the perioperative gastrointestinal function.
Key Words: Goal-directed fluid therapy, Stroke volume variation, FloTrac/Vigileo system, Orthopedic surgery
Introduction
Fluid balance is a major contributing factor to postoperative morbidity and mortality. Persistent hypovolemia is associated with organ hypoperfusion, systemic inflammatory response syndrome, sepsis, and multiple organ failure. Fluid overload, on the other hand, is associated with edema, ileus, postoperative nausea and vomiting, pulmonary complications, and increased cardiac demands [1]. Traditional methods to monitor the preload are based on measurements of pressure or volume, such as the mean arterial pressure (MAP), the heart rate, or the central venous pressure (CVP). However, these are static parameters and do not accurately reflect fluid responsiveness [2]. The individualized ‘goal-directed therapy’ (GDT) concept [3,4,5] is a more rational strategy for perioperative fluid therapy, which achieves the maximal cardiac stroke volume via targeted administration of i.v. fluids, blood, and/or vasoactive substances. The FloTrac/Vigileo system provides automatic and continuous monitoring of the cardiac output, stroke volume, and stroke volume variation (SVV) based on arterial pulse contour analysis. According to our previous study [6] and data from similar studies [7,8,9], SVV is a sensitive predictor of fluid responsiveness.
Fluid management is a crucial issue for patients undergoing major orthopedic surgery, in which large blood loss, transfusions, fluid shifts, and high incidences of postoperative complications are important concerns. Herein, we report the results of a prospective, randomized, controlled study that compared the intraoperative laboratory parameters of organ functions and postoperative complications between standard care management and SVV-based goal-directed fluid therapy. The primary study endpoint was gastrointestinal function, including the gastric intramucosal partial pressure of carbon dioxide (PgCO2), the gastric intramucosal pH (pHi), and the time to passing the first flatus. We hypothesized that the use of goal-directed volume therapy would result in better gastrointestinal perfusion and fewer postoperative complications in patients undergoing major orthopedic surgery.
Subjects and Methods
Subjects
The Institutional Research Ethics Committee approved this trial and written informed consent was obtained from all patients. Patients scheduled for elective major orthopedic surgery, including total hip arthroplasty, spinal fusion surgery, femoral fracture surgery, and sacral tumor surgery under general anesthesia, with an anticipated blood loss >800 ml, were eligible for inclusion into this study. Patients were excluded if they were under 18 years old or had a BMI >40 or <15, coagulopathy, significant arrhythmia or cardiopulmonary dysfunction, or significant renal or liver diseases.
Eighty patients were randomized preoperatively into either a standard care management group (control, n = 40) or a goal-directed fluid therapy group (GDT, n = 40) using a random number generator in sealed envelopes. The anesthetist (K.P.) responsible for intraoperative management was aware of the group assignment, whereas all other members of the research team, other health care providers, and the patients were not.
Anesthesia and Monitoring
All patients fasted for 6 h before surgery and were premedicated with i.v. midazolam (0.01 mg/kg). A central venous catheter was inserted via the right internal jugular vein and an arterial line was inserted into the radial artery of the nondominant forearm. Standard monitoring included an ECG, MAP, CVP, pulse oximetry, temperature, end-tidal carbon dioxide, and the bispectral index.
In both groups, standard general anesthesia was induced with i.v. fentanyl (3–4 μg/kg), propofol (1.5–2 mg/kg), and vecuronium (0.15 mg/kg). After tracheal intubation, the lungs were ventilated at 8 ml/kg of tidal volume in a volume-controlled mode with 0–3 mm Hg positive end-expiratory pressure. The respiratory rate was set to maintain the end-tidal carbon dioxide at 35–40 mm Hg. The ventilator settings were unchanged during this study. Anesthesia was maintained with sevoflurane 2–3s% in oxygen and fentanyl adjusted to maintain a bispectral index of 45–55. The body temperature was maintained at >36°C by a fluid warmer. A tonometry tube was inserted into the lumen of the stomach via the nasogastric route and was connected to a CO2 monitor (Tonocap).
After induction of anesthesia, patients scheduled for spinal or sacral surgery were placed in the prone position on a prone pad with 4 small pads (2 shoulder and 2 pelvic supports) to allow the chest and abdomen to hang free. All of the patients received an i.v. prophylactic antibiotic and preemptive analgesia of 50 mg flurbiprofen axetil before skin incision. All operations were performed by the same surgical team.
Study Protocol
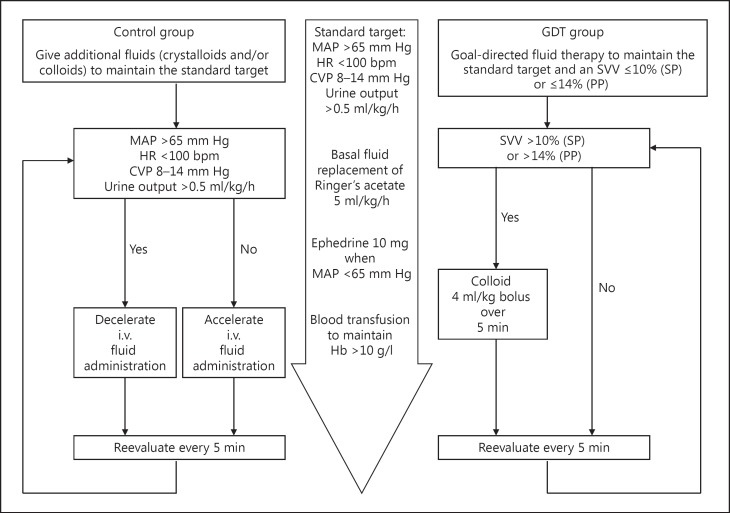
The intraoperative fluid management is shown in figure 1. In both groups, intraoperative basal fluid replacement was achieved by continuous infusion of 5 ml/kg/h crystalloid solution (Ringer's acetate). In the GDT group, an additional bolus of 4 ml/kg colloid solution (Voluven 130/0.4; 6s%) was given when the SVV (measured by the FloTrac/Vigileo 3.0) increased >10s% in the supine position or >14s% in the prone position. Fluid boluses were repeated every 5 min if the criteria were met. In the control group, the anesthesiologist (K.P.) was free to give additional fluids, based on the subject's hemodynamic condition and responses, to maintain an MAP >65 mm Hg, a heart rate <100 bpm, a CVP of 8–14 mm Hg, and a urine output >0.5 ml/kg/h.
Fig. 1.
Intraoperative fluid management. HR = Heart rate; Hb = hemoglobin; SP = supine position; PP = prone position.
In both groups, anemia (hemoglobin level <80 g/l or hematocrit <28s%) and an acute blood loss >20s% of the calculated patient circulatory volume were corrected with transfusions of packed red blood cells and fresh frozen plasma in ratios approaching 2:1. Ephedrine boluses of 10 mg or phenylephrine boluses of 50 µg were given when fluid boluses failed to maintain a systolic arterial pressure >90 mm Hg or an MAP >65 mm Hg. These episodes were recorded as hypotensive events.
Arterial blood samples were taken at the time of skin incision and closure for blood counts, acid-base balance analysis, and other biochemical laboratory tests. At the same time, the PgCO2 was recorded. The mucosal-arterial PCO2 gap was calculated as:
Pg-aCO2 gap = PgCO2 − PaCO2,
where PaCO2 is the arterial carbon dioxide tension.
In addition, the pHi was calculated [10] as:
pHi = arterial pH s+ log10(PaCO2/PgCO2)
Patients were transferred to either the intensive care unit or the postanesthesia care unit and were extubated when they fulfilled the standard clinical criteria (oxygenation, hemodynamics, and protective reflexes). Patient-controlled i.v. analgesia with fentanyl (background infusion of 0.4 µg/kg/h, bolus dose of 0.4 µg/kg, and lockout interval of 10 min) was used during the next 2 postoperative days. Regional anesthesia or analgesia (e.g. epidural catheters or nerve blocks) was not used in this study.
The same surgical team was in charge of the postoperative care, including fluid management (baseline crystalloid infusion of 40–50 ml/kg/day, colloids, and transfusions if required), daily antibiotics for 2–3 days, rescue analgesia (100 mg i.v. flurbiprofen axetil or 10 mg i.m. morphine), and antiemetics (10 mg i.v. metoclopramide or 3 mg i.v. granisetron). The discharge criteria were predefined by the Department of Orthopedics at our institution.
Postoperative complications were defined as follows: (a) cardiac complications: hypotension (MAP <65 mm Hg or SAP <90 mm Hg), arrhythmias (severe arrhythmias resulting in hemodynamic instability), or heart failure; (b) respiratory complications: ventilator support (need for mechanical ventilation in the intensive care unit), acute lung injury, or acute respiratory distress syndrome (PaO2/FiO2 <300 mm Hg); (c) abdominal complications: gastrointestinal hemorrhage (hematemesis and melena), hepatic dysfunction (transaminase >double the upper limit of normal), or hepatic failure (progressive jaundice rise and hepatic encephalopathy); (d) renal complications: renal dysfunction (creatinine >180 μmol/l) or renal failure (creatinine >450 μmol/l); (e) cerebral complications: postoperative cognitive dysfunction or coma; (f) infectious complications: wound infection or wound dehiscence, and (g) others: deep vein thrombosis, nausea, or vomiting. Postoperative complications, fluid management, drainage, length of hospital stay, and mortality were recorded.
Statistical Analysis
The statistical power analysis was based on a review of our hospital database, which showed an average time to passing the first flatus of 15.32 ± 5.16 h. Thirty-six patients per group were required for detection of a 20s% difference in the postoperative first flatus time between the two groups with an α level of significance of 0.05 and a power of 80s% (PASS 11.0.7). To compensate for dropped cases, 40 patients were studied in each group.
The statistical analysis was performed using SPSS 19.0 statistical software (IBM SPSS). Data were checked for normality using the Kolmogorov-Smirnov test. Continuous normally distributed data are presented as means ± SD and were analyzed using paired or unpaired t tests. Nonnormally distributed data are presented as medians (IQR) and were tested using the Mann-Whitney U test and the Wilcoxon rank-sum test for unpaired and paired results, respectively. Categorical data are presented as numbers (s%) and were compared using Fisher's exact test. p < 0.05 was considered statistically significant for all tests.
Results
All 80 patients completed this study. No patient was excluded or dropped out of the study after randomization (fig. 2). There were no significant differences between the groups with regard to demographics and surgical characteristics (table 1).
Fig. 2.
Flowchart of the patients in this study. ICU = Intensive care unit.
Table 1.
Demographic patient data and surgical characteristics
| GDT | Control | p value | |
|---|---|---|---|
| Gender (male/female), n | 17/23 | 18/22 | 0.822 |
| Age, years | 55 ± 13 | 53 ± 10 | 0.505 |
| Weight, kg | 59 ± 10 | 62 ± 11 | 0.216 |
| Height, cm | 162 ± 8 | 164 ± 9 | 0.329 |
| BMI | 22.66 ± 3.22 | 23.25 ± 3.24 | 0.421 |
| Position (supine/prone), n | 25/15 | 22/18 | 0.496 |
| ASA (I/II/III), n | 17/22/1 | 17/21/2 | 0.837 |
| Surgery | |||
| Hip | 21 (52.5) | 20 (50.0) | 0.823 |
| Spine | 16 (40.0) | 18 (45.0) | 0.651 |
| Femur | 2 (5.0) | 1 (2.5) | 1.000 |
| Sacrum | 1 (2.5) | 1 (2.5) | 1.000 |
| Comorbidity | |||
| Hypertension | 11 (27.5) | 15 (37.5) | 0.340 |
| Diabetes mellitus | 2 (5.0) | 7 (17.5) | 0.157 |
| Anemia | 5 (12.5) | 2 (5.0) | 0.432 |
| COPD | 2 (5.0) | 1 (2.5) | 1.000 |
| Multiple trauma | 3 (7.5) | 2 (5.0) | 1.000 |
| Tuberculosis | 2 (5.0) | 0 (0) | 0.494 |
| Coronary artery disease | 0 (0) | 1 (2.5) | 1.000 |
| Heart block | 2 (5.0) | 1 (2.5) | 1.000 |
| Cerebral infarction | 1 (2.5) | 2 (5.0) | 1.000 |
| Duration of surgery, min | 175 ± 100 | 162 ± 80 | 0.520 |
| Intraoperative BIS | 52 ± 2 | 53 ± 2 | 0.298 |
Values are presented as means ± SD or numbers (s%) unless otherwise stated. ASA = American Society of Anesthesiologists physical status classification; COPD = chronic obstructive pulmonary disease; BIS = bispectral index.
In both groups, at the end of surgery, the MAP decreased and the CVP increased compared to the baseline values (table 2). Similarly, the mean hemoglobin and hematocrit decreased significantly in both groups (p < 0.05 for all). The mean heart rate in the GDT group (68 ± 13 bpm) was significantly lower than that in the control group (75 ± 13 bpm, p = 0.028), and there were fewer hypotensive episodes (n = 0, IQR 0–1, compared to n = 1, IQR 0–2, p = 0.021).
Table 2.
Intraoperative hemodynamic data, laboratory parameters, and fluid management
| GDT |
Control |
|||
|---|---|---|---|---|
| baseline | end of surgery | baseline | end of surgery | |
| Hemodynamic data | ||||
| Heart rate, bpm | 68 ± 11 | 68 ± 13a | 73 ± 13 | 75 ± 13 |
| MAP, mm Hg | 88 ± 9 | 79 ± 10b | 91 ± 10 | 81 ± 12b |
| CVP, mm Hg | 8 ± 3 | 10 ± 3b | 8 ± 3 | 10 ± 3b |
| SVV, s% | 9 ± 2 | 7 ± 1b | NA | NA |
| Cardiac output, l/min | 4.41 ± 1.07 | 4.79 ± 1.24 | NA | NA |
| Hypotensive events | 0 (0 – 1)a | 1 (0 – 2) | ||
| Laboratory parameters | ||||
| PgCO2, mm Hg | 29.29 ± 5.57 | 42.90 ± 10.01ab | 30.81 ± 5.63 | 48.96 ± 11.34b |
| PaCO2, mm Hg | 39.10 ± 6.83 | 42.11 ± 9.07b | 40.63 ± 6.11 | 44.26 ± 6.75b |
| Pg–aCO2, mm Hg | −9.80 ± 9.44 | 0.78 ± 14.48b | −9.82 ± 6.76 | 4.52 ± 11.48b |
| pHa | 7.42 ± 0.04 | 7.36 ± 0.06ab | 7.42 ± 0.05 | 7.34 ± 0.05b |
| pHi | 7.55 ± 0.10 | 7.37 ± 0.11ab | 7.54 ± 0.08 | 7.30 ± 0.11b |
| Lactate, mmol/l | 1.54 ± 0.32 | 2.12 ± 0.89b | 1.61 ± 0.57 | 2.35 ± 1.02b |
| Hemoglobin, g/l | 12.03 ± 1.81 | 10.52 ± 1.54b | 11.94 ± 1.69 | 10.28 ± 1.61b |
| Hematocrit | 0.37 ± 0.05 | 0.33 ± 0.04b | 0.37 ± 0.04 | 0.32 ± 0.04b |
| Fluid management | ||||
| Blood loss, ml | 800 (600 – 1,000) | 800 (525 – 1,200) | ||
| Crystalloids infused, ml | 1,000 (712 – 1,000) | 1,000 (500 – 1,000) | ||
| Colloids infused, ml | 500 (312 – 1,000)a | 1,000 (500 – 1,000) | ||
| PRBC infused, ml | 600 (400 – 600) | 600 (400 – 800) | ||
| FFP infused, ml | 0 (0 – 200) | 0 (0 – 200) | ||
| Total volume infused, ml | 1,850 (1,525 – 2,537)a | 2,225 (1,850 – 2,900) | ||
| Urinary output, ml | 300 (200 – 400) | 300 (200 – 475) | ||
| Urinary output, ml/kg/h | 1.98 (1.29 – 2.63) | 2.20 (1.53 – 3.25) | ||
Values are presented as means ± SD or medians (IQR). NA = Not available; PRBC = packed red blood cells; FFP = fresh frozen plasma; pHa = arterial pH; pHi = intramucosal pH; PgCO2 = gastric intramucosal partial pressure of carbon dioxide; PaCO2 = arterial partial pressure of carbon dioxide; Pg–aCO2 = mucosal-arterial PCO2 gap.
p < 0.05 between the two groups.
p < 0.05 in comparison to baseline values.
Also in the GDT group, at the end of surgery the mean SVV (7 ± 1) was significantly lower than the mean preoperative value (9 ± 2, p = 0.000), with a trend toward higher cardiac outputs (4.79 ± 1.24 l/min compared to 4.41 ± 1.07 l/min, p = 0.057). The SVV and cardiac output were not evaluated in the control group.
The arterial and gastric pHi decreased at the end of surgery, while the arterial and gastric intramucosal CO2 tensions, Pg-aCO2, and the lactic acid concentration increased. At the end of surgery, compared to the control group, the mean gastric intramucosal CO2 of the GDT group was significantly lower (48.96 ± 11.34 mm Hg compared to 42.90 ± 10.01 mm Hg, p = 0.013), while the arterial and gastric pHi were higher (7.34 ± 0.05 compared to 7.36 ± 0.06, p = 0.048, and 7.30 ± 0.11 compared to 7.37 ± 0.11, p = 0.007, respectively).
The volume of infused intraoperative colloids was significantly lower in the GDT group than in the control group (500 ml, IQR 312–1,000, compared to 1,000 ml, IQR 500–1,000, p = 0.003). Similarly, the total infused volume in the GDT group was also significantly lower (1,850 ml, IQR 1,525–2,537, compared to 2,225 ml, IQR 1,850–2,900, p = 0.036). The volume of blood loss, infused crystalloids, infused blood products, and urinary output did not differ between the groups.
The time to the first passage of flatus was significantly shorter in the GDT group (10.82 ± 5.83 h compared to 14.97 ± 11.17 h, p = 0.042; table 3). There was no significant difference between the two groups with regard to the postoperative amount of infused fluids, urine output and drainage volume at 0–24 or 24–48 h, or the volume of blood transfusion. There were also no differences in postoperative nausea and vomiting, patient-controlled analgesia requests, the number of patients who developed complications, the length of postoperative hospital stay, or mortality. One patient who underwent resection of a sacral tumor in the GDT group died from septic shock 17 days after surgery.
Table 3.
Postoperative complications and fluid management
| GDT | Control | p value | |
|---|---|---|---|
| Cardiovascular complications | |||
| Hypotension | 3 (7.5) | 2 (5.0) | 0.432 |
| Arrhythmias | 1 (2.5) | 0 (0) | 1.000 |
| Heart failure | 0 (0) | 0 (0) | 1.000 |
| Respiratory complications | |||
| Ventilator support | 2 (5.0) | 2 (5.0) | 1.000 |
| ALI/ARDS | 1 (2.5) | 0 (0) | 1.000 |
| Abdominal complications | |||
| Flatus time, h | 10 ± 5 | 14 ± 11 | 0.042a |
| Gastrointestinal hemorrhage | 0 (0) | 0 (0) | 1.000 |
| Hepatic dysfunction | 5 (12.5) | 6 (15.0) | 0.745 |
| Hepatic failure | 1 (2.5) | 0 (0) | 1.000 |
| Renal complications | |||
| Urine output 0 – 24 h, ml | 1,625 (1,175 – 2,412) | 2,000 (1,150 – 2,700) | 0.263 |
| Urine output 24 – 48 h, ml | 2,500 (1,800 – 3,100) | 2,200 (1,700 – 3,525) | 0.672 |
| Renal dysfunction | 1 (2.5) | 3 (7.5) | 0.615 |
| Renal failure | 1 (2.5) | 0 (0) | 1.000 |
| Central nervous complications | |||
| POCD | 1 (2.5) | 1 (2.5) | 1.000 |
| Coma | 1 (2.5) | 0 (0) | 1.000 |
| Infection-related complications | |||
| Pneumonia | 4 (10.0) | 3 (7.5) | 1.000 |
| Wound infection | 0 (0) | 1 (2.5) | 1.000 |
| Wound dehiscence | 0 (0) | 0 (0) | 1.000 |
| Deep vein thrombosis | 0 (0) | 1 (2.5) | 1.000 |
| Nausea | 5 (12.5) | 8 (20.0) | 0.363 |
| Vomit | 2 (5.0) | 5 (12.5) | 0.432 |
| PCA requests | 0 (0 – 2) | 0 (0 – 2) | 0.719 |
| Fluid management, drainage | |||
| Fluid infused 0 – 24 h, ml | 2,189 ± 659 | 2,109 ± 709 | 0.606 |
| Fluid infused 24 – 48 h, ml | 1,766 ± 965 | 1,806 ± 944 | 0.852 |
| Blood transfusion, ml | 0 (0 – 200) | 0 (0 – 200) | 0.625 |
| Drainage volume 0 – 24 h, ml | 132 (100 – 263) | 107 (38 – 187) | 0.062 |
| Drainage volume 24 – 48 h, ml | 60 (25 – 120) | 47 (16 – 135) | 0.397 |
| Drainage removal time, days | 2 ± 0 | 2 ± 0 | 0.196 |
| Postoperative stay, days | 12 ± 3 | 11 ± 7 | 0.802 |
| Mortality | 1 (2.5) | 0 (0) | 1.000 |
Values are presented as numbers (s%), medians (IQR), or means ± SD. ALI/ARDS = Acute lung injury/acute respiratory distress syndrome; POCD = postoperative cognitive dysfunction; PCA requests = number of analgesic requirements with patient-control analgesia.
p < 0.05 indicates a significant difference.
Discussion
The main findings of the present study are that SVV-based GDT during major orthopedic surgery reduced the required volume of intraoperative infused fluids, maintained intraoperative hemodynamic stability, and improved the perioperative gastrointestinal function.
Perioperative fluid management is challenging in high-risk surgical patients. The aim of volume therapy is not only to prevent hypovolemia but also to reduce the risk of fluid overload. Hypovolemia is recognized as a risk factor for adverse effects, ranging from minor organ dysfunction to multiple organ failure and even death. Conversely, fluid overload may impair pulmonary, cardiac, and gastrointestinal functions, contributing to postoperative complications and a prolonged recovery [11]. Therefore, appropriate hemodynamic monitoring is important for intraoperative fluid management. To our knowledge, this study is the first to directly compare conventional intraoperative management with SVV-based goal-directed fluid therapy with regard to organ functions and postoperative complications in patients undergoing major orthopedic surgery.
Ideally, a simple, affordable, and reliable method to improve intraoperative fluid management is desirable for routine use. Esophageal Doppler has been used to guide fluid management, with good results [12], but its use is partially limited by the need for experienced staff [13]. Also, the reliability of this method in major vascular procedures requiring cross-clamping of the descendent aorta has been questioned. On the other hand, arterial cannulation is routinely used in high-risk patients, and application of the FloTrac/Vigileo system is generally well tolerated by patients, without increased invasion and risk.
As reported in our previous study [6], the SVV obtained by the Vigileo/FloTrac system was a more sensitive predictor of fluid responsiveness than MAP, heart rate, or CVP measurements for intravascular volume assessment. Although the optimal cut-off value for SVV remains uncertain, the 10s% threshold suggested by Manecke [14] for patients in the supine position and the 14s% threshold for patients in the prone position [8] were considered to be the best available estimates for the Vigileo/FloTrac system. In our institution, patients scheduled for spinal or sacral surgery were placed in the prone position on a prone pad with 4 small pads (2 shoulder and 2 pelvic supports) to allow the chest and abdomen to hang free, which is similar to the method described by Biais et al. [8]. Therefore, we used an SVV of 10s% for patients in the supine position and an SVV of 14s% for patients in the prone position as the thresholds for hypovolemia to target volume optimization in our study.
In many studies [15,16,17,18,19], patients who had their fluid requirements managed with a goal-directed protocol received greater amounts of colloids than those who were treated with conventional or restrictive fluid management. Similarly, our results support that the use of intraoperative fluid therapy with accurate targeting of colloid fluid boluses may prevent excessive fluid administration. Greater amounts of infused fluids were less effective and jeopardized gastrointestinal function. In normovolemic healthy volunteers, 16s% of colloids and more than 68s% of the saline solution escaped into the extravascular fluid compartment 1 h after the infusion [20]. Edema of the intestines and other tissues may be responsible for poor tissue oxygenation and postoperative gut dysfunction [21]. Smaller volumes of infused fluids may help protect the gastrointestinal tract from dysfunction, which may help explain the shorter flatus time in the GDT group.
Regarding the lactate level, it is an indirect but sensitive measure of organ perfusion. Increased lactate correlates with an inadequate intravascular volume, tissue hypoxia, and energy failure due to blood flow redistribution [22]. In the present study, lactate-free fluids were used for volume substitution to exclude a potential bias. Our results showed that patients in both groups suffered a significant increase in lactate levels at the end of surgery relative to baseline values, indicating that these major surgical procedures had a great effect on organ perfusion. Tonometry, on the other hand, is a relatively noninvasive technique that measures the PgCO2; from this value, associated parameters such as the pHi and the PCO2 gap (PgCO2 – PaCO2) can be calculated. Low pHi, high PgCO2, and high PCO2 gap values may indicate an inadequate oxygen supply to the bowel, leading to regional acidosis. In addition, intramucosal acidosis has been associated with a poor prognosis and multiple organ failure in critically ill patients, even in the absence of systemic acidosis or hypotension [23,24]. Moreover, it has been shown that the correction of intramucosal acidosis may increase the survival rate of critically ill patients [25]. Recently, PgCO2 and pHi were used as indicators of gastrointestinal function [26,27,28]. In this study, we found that the PgCO2 levels were lower and the pHi levels were higher at the end of surgery in the GDT group. Similarly, in the GDT group, the postoperative flatus time was shorter, indicating better gastrointestinal perfusion compared to the control group.
Our study has some limitations. First, the SVV and CO values were not available for the control group as the FloTrac/Vigileo device was not attached to these patients. Second, the inclusion of different surgical procedures might have influenced our results, because the pathophysiology and complications vary between joint and spinal surgery. Third, the absence of significant differences between the study groups in the incidence of nausea and vomiting, the length of hospital stay, and the incidence of mortality may have been due to our study not being powered enough to detect differences in these complications.
Conclusion
In patients undergoing major orthopedic surgery, SVV-based goal-directed intraoperative fluid therapy reduced the volume of intraoperative infused fluids, maintained intraoperative hemodynamic stability, and improved the perioperative gastrointestinal function relative to conventional treatment.
Acknowledgments
We thank the surgical teams for their technical help and the nurses at the Department of Anesthesiology and Orthopedics for their helpful assistance.
Trial registration: ChiCTR-TRC-12002701.
References
- 1.Bellamy MC. Wet, dry or something else? Br J Anaesth. 2006;97:755–757. doi: 10.1093/bja/ael290. [DOI] [PubMed] [Google Scholar]
- 2.Cavallaro F, Sandroni C, Antonelli M. Functional hemodynamic monitoring and dynamic indices of fluid responsiveness. Minerva Anestesiol. 2008;74:123–135. [PubMed] [Google Scholar]
- 3.Bundgaard-Nielsen M, Holte K, Secher NH, et al. Monitoring of peri-operative fluid administration by individualized goal-directed therapy. Acta Anaesthesiol Scand. 2007;51:331–340. doi: 10.1111/j.1399-6576.2006.01221.x. [DOI] [PubMed] [Google Scholar]
- 4.Funk DJ, Moretti EW, Gan TJ. Minimally invasive cardiac output monitoring in the perioperative setting. Anesth Analg. 2009;108:887–897. doi: 10.1213/ane.0b013e31818ffd99. [DOI] [PubMed] [Google Scholar]
- 5.Phan TD, Ismail H, Heriot AG, et al. Improving perioperative outcomes: fluid optimization with the esophageal Doppler monitor, a meta-analysis and review. J Am Coll Surg. 2008;207:935–941. doi: 10.1016/j.jamcollsurg.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Ji FH, Yang JP. Evaluation of stroke volume variation obtained by the FloTrac/Vigileo system to guide preoperative fluid therapy in patients undergoing brain surgery. J Int Med Res. 2012;40:1175–1181. doi: 10.1177/147323001204000338. [DOI] [PubMed] [Google Scholar]
- 7.Cannesson M, Musard H, Desebbe O, et al. The ability of stroke volume variations obtained with Vigileo/FloTrac system to monitor fluid responsiveness in mechanically ventilated patients. Anesth Analg. 2009;108:513–517. doi: 10.1213/ane.0b013e318192a36b. [DOI] [PubMed] [Google Scholar]
- 8.Biais M, Bernard O, Ha JC, et al. Abilities of pulse pressure variations and stroke volume variations to predict fluid responsiveness in prone position during scoliosis surgery. Br J Anaesth. 2010;104:407–413. doi: 10.1093/bja/aeq031. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Lu B, Sheng X, et al. Accuracy of stroke volume variation in predicting fluid responsiveness: a systematic review and meta-analysis. J Anesth. 2011;25:904–916. doi: 10.1007/s00540-011-1217-1. [DOI] [PubMed] [Google Scholar]
- 10.Huang CC, Tsai YH, Lin MC. Gastric intramucosal PCO2 and pH variability in ventilated critically ill patients. Crit Care Med. 2001;29:88–95. doi: 10.1097/00003246-200101000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Bundgaard-Nielsen M, Secher NH, Kehlet H. ‘Liberal’ vs. ‘restrictive’ perioperative fluid therapy-a critical assessment of the evidence. Acta Anaesthesiol Scand. 2009;53:843–851. doi: 10.1111/j.1399-6576.2009.02029.x. [DOI] [PubMed] [Google Scholar]
- 12.Sinclair S, James S, Singer M. Intraoperative intravascular volume optimisation and length of hospital stay after repair of proximal femoral fracture: randomised controlled trial. BMJ. 1997;315:909–912. doi: 10.1136/bmj.315.7113.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefrant JY, Bruelle P, Aya AG, et al. Training is required to improve the reliability of esophageal Doppler to measure cardiac output in critically ill patients. Intensive Care Med. 1998;24:347–352. doi: 10.1007/s001340050578. [DOI] [PubMed] [Google Scholar]
- 14.Manecke GR. Edwards FloTrac sensor and Vigileo monitor: easy, accurate, reliable cardiac output assessment using the arterial pulse wave. Expert Rev Med Devices. 2005;2:523–527. doi: 10.1586/17434440.2.5.523. [DOI] [PubMed] [Google Scholar]
- 15.Cecconi M, Fasano N, Langiano N, et al. Goal-directed haemodynamic therapy during elective total hip arthroplasty under regional anaesthesia. Crit Care. 2011;15:R132. doi: 10.1186/cc10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer J, Boldt J, Mengistu AM, et al. Goal-directed intraoperative therapy based on autocalibrated arterial pressure waveform analysis reduces hospital stay in high-risk surgical patients: a randomized, controlled trial. Crit Care. 2010;14:R18. doi: 10.1186/cc8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benes J, Chytra I, Altmann P, et al. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care. 2010;14:R118. doi: 10.1186/cc9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Qiao H, He ZY, et al. Intraoperative fluid management in open gastrointestinal surgery: goal-directed versus restrictive. Clinics (Sao Paulo) 2012;67:1149–1155. doi: 10.6061/clinics/2012(10)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheeren TW, Wiesenack C, Gerlach H, et al. Goal-directed intraoperative fluid therapy guided by stroke volume and its variation in high-risk surgical patients: a prospective randomized multicentre study. J Clin Monit Comput. 2013;27:225–233. doi: 10.1007/s10877-013-9461-6. [DOI] [PubMed] [Google Scholar]
- 20.McIlroy DR, Kharasch ED. Acute intravascular volume expansion with rapidly administered crystalloid or colloid in the setting of moderate hypovolemia. Anesth Analg. 2003;96:1572–1577. doi: 10.1213/01.ANE.0000061460.59320.B0. [DOI] [PubMed] [Google Scholar]
- 21.Mythen M, Vercueil A. Fluid balance. Vox Sang. 2004;87(suppl 1):77–81. doi: 10.1111/j.1741-6892.2004.00436.x. [DOI] [PubMed] [Google Scholar]
- 22.Valenza F, Aletti G, Fossali T, et al. Lactate as a marker of energy failure in critically ill patients: hypothesis. Crit Care. 2005;9:588–593. doi: 10.1186/cc3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nordin A, Makisalo H, Mildh L. Gut intramucosal pH as an early indicator of effectiveness of therapy for hemorrhagic shock. Crit Care Med. 1998;26:1110–1117. doi: 10.1097/00003246-199806000-00037. [DOI] [PubMed] [Google Scholar]
- 24.Miller PR, Kincaid EH, Meredith JW. Threshold values of intramucosal pH and mucosal-arterial CO2 gap during shock resuscitation. J Trauma. 1998;45:868–872. doi: 10.1097/00005373-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Gutierrez G, Palizas F, Doglio G, et al. Gastric intramucosal pH as therapeutic index of tissue oxygenation in critically ill patients. Lancet. 1992;339:195–199. doi: 10.1016/0140-6736(92)90002-k. [DOI] [PubMed] [Google Scholar]
- 26.Wang G, Liu S, Liu G. Effects of infusion of different fluids during controlled hypotension on gastric intramucosal pH and postoperative gastroenterological function. J Biomed Res. 2011;25:191–196. doi: 10.1016/S1674-8301(11)60025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arya N, Sharif MA, Lau LL, et al. Retroperitoneal approach to abdominal aortic aneurysm repair preserves splanchnic perfusion as measured by gastric tonometry. Ann Vasc Surg. 2010;24:321–327. doi: 10.1016/j.avsg.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 28.van Haren FM, Pickkers P, Foudraine N, et al. The effects of methylene blue infusion on gastric tonometry and intestinal fatty acid binding protein levels in septic shock patients. J Crit Care. 2010;25:358.e1–358.e7. doi: 10.1016/j.jcrc.2010.02.008. [DOI] [PubMed] [Google Scholar]