Abstract
Objective
To report a case of spontaneous tumor lysis syndrome (STLS) of a solid tumor in a patient who had undiagnosed metastatic hepatocellular carcinoma.
Clinical Presentation and Intervention
A 70-year-old man with a medical history of alcohol abuse, withdrawal seizure and hypertension presented to the emergency department after being found unresponsive by his landlord. The patient had a bulky mass in the liver, classic laboratory abnormalities, oliguric renal failure and elevated alpha fetoprotein. He had never been treated with cytotoxic therapy. He was treated aggressively with fluid resuscitation and sodium bicarbonate, but he continued to be oliguric and the deterioration of his renal function also continued. Due to a minimal response to treatment and a poor prognosis, he was discharged to hospice for palliative care.
Conclusion
This case showed that STLS should be in the differential diagnosis of a patient who has malignant disease and has developed classic laboratory abnormalities and renal failure even without previous cytotoxic therapy.
Key Words: Tumor lysis syndrome, Hepatocellular carcinoma, Neoplastic diseases
Introduction
Tumor lysis syndrome (TLS) is an oncological emergency and a potentially life-threatening complication of antineoplastic treatment that most commonly occurs in patients with rapidly proliferating, treatment-responsive hematological malignancies [1,2]. However, spontaneous tumor lysis syndrome (STLS) of solid tumors, which occurs without any therapeutic interventions, is considered to be a rare disease requiring prompt recognition and management.
We present a case concerning a 70-year-old male who developed STLS in the setting of previously undiagnosed metastatic hepatocellular carcinoma (HCC). To our knowledge, this is the third case ever reported of STLS occurring in the context of HCC without antineoplastic treatment.
Case Report
A 70-year-old male with a medical history of alcohol abuse, withdrawal seizure and hypertension presented to the emergency department after being found unresponsive by his landlord. It was not clear how long the patient had been unconscious, although the landlord noted the patient was covered with bugs at the time of discovery. The history provided by the patient himself was very limited due to an altered mental status. In the emergency department, his vitals were significant for a temperature of 94.5°F, a heart rate of 94 beats per minute, a respiratory rate of 20 breaths per minute, blood pressure of 103/69 mm Hg and oxygen saturation 95% on room air. The physical examination was significant for cachexia, confusion and hepatomegaly upon abdominal exam.
Laboratory findings were notable for the following values: sodium 152 mmol/l, potassium 5.0 mmol/l, chloride 114 mmol/l, bicarbonate 14 mmol/l, anion gap 24 mmol/l, blood urea nitrogen 111 mg/dl, creatinine 3.4 mg/dl, glucose 118 mg/dl, corrected calcium 11.0 mg/dl, lactic acid 3.7 U/l, magnesium 3.2 mg/dl, aspartate aminotransferase 279 U/l, alanine aminotransferase 17 U/l, alkaline phosphatase 258 U/l, lactate dehydrogenase (LDH) 473 U/l, creatine phosphokinase 69 U/l, albumin 3.0 g/dl, thyroid-stimulating hormone 6.60 IU/ml and free thyroxine 0.88 ng/dl. Serologies for HIV and hepatitis A, B and C were all negative.
A computed tomography (CT) scan of the head showed chronic small-vessel ischemic changes and volume loss. Chest X-ray showed bilateral hazy perihilar opacities. Abdominal ultrasound revealed markedly abnormal hepatic echotexture, gallbladder sludge, a small amount of ascites and a 3.6 × 3 × 3 cm mass in the portahepatis region.
The patient was admitted to the intensive care unit and was started on intravenous fluids and antibiotics and was closely monitored. Once he had been stabilized, a chest CT was performed which showed extensive hilaradenopathy, large bilateral pleural effusions and a nodule in the left upper lobe. Abdominal CT without contrast revealed marked hepatomegaly, multiple areas of diminished density in the liver with a conglomerate lesion 14 × 14 cm and multiple, enlarged lymph nodes in the portahepatis and the mediastinum (fig. 1, 2).
Fig. 1.
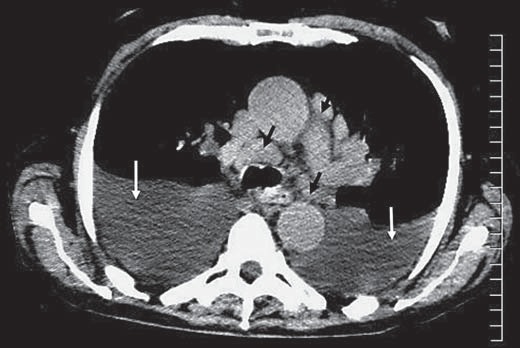
Axial noncontrast CT image through the mid-thorax demonstrates mediastinal lymphadenopathy (black arrows) and medium-size bilateral pleural effusions (white arrows).
Fig. 2.
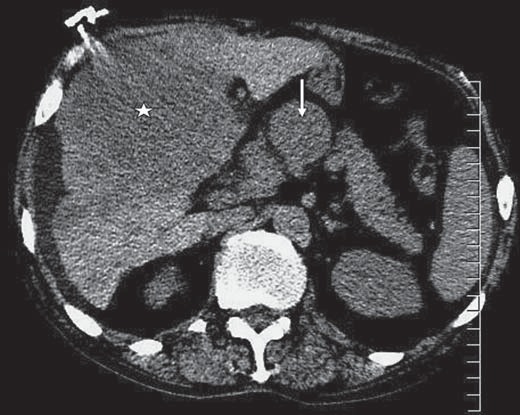
Axial noncontrast CT image through the upper abdomen shows a large heterogeneous mass of relatively low attenuation within a large portion of the liver (white star) and portahepatis lymphadenopathy (white arrow).
The test for alpha fetoprotein came back significantly high (>60,500 ng/ml). Based on this and the CT findings, the patient was presumed to have high-grade, metastatic HCC. Subsequent laboratory results revealed increasing levels of potassium (maximum 6.0 mmol/l), phosphorus (maximum 6.9 mg/dl), uric acid (maximum 22.9 mg/dl) and LDH (maximum 703 U/l). Corrected calcium decreased slightly from 11.0 mg/dl upon admission to 9.6 mg/dl after fluid resuscitation. An oncologist and a nephrologist were consulted and a consensus was reached that the patient had developed STLS from metastatic HCC. A biopsy of the liver mass was not obtained because his condition was critical. Despite aggressive fluid resuscitation, he continued to be oliguric and his renal function continued to deteriorate with the creatinine level increasing from 3.4 mg/dl upon admission to 4.6 mg/dl over 6 days. He initially received sodium bicarbonate for TLS and metabolic acidosis, but this was discontinued due to the minimal response. The prognosis was thought to be poor and he was discharged to hospice for palliative care.
Discussion
Our case showed STLS caused by HCC. The patient had oliguric renal failure and characteristic laboratory abnormalities that included hyperkalemia and hyperuricemia in the setting of a large hepatic mass and a very high AFP. To our knowledge, only 2 case reports of STLS caused by HCC have previously been reported in the literature [3,4].
STLS typically develops within 12–72 h of treatment with cytotoxic chemotherapy and, rarely, also spontaneously, when large numbers of neoplastic cells are simultaneously lysed and release intracellular ions and metabolic byproducts into the systemic circulation. The cardinal signs of TLS are hyperuricemia causing uric acid nephropathy, hyperphosphatemia with secondary hypocalcemia and hyperkalemia. Clinical complications such as acute kidney injury, cardiac arrhythmias and seizures can develop and may ultimately lead to multiorgan failure and death [1].
The incidence of TLS and STLS by solid tumors is unknown because collection of the data is limited to case reports and small case series. Several case reports have associated the development of TLS in patients with HCC after transarterial chemoembolization and sorafenib administration [5,6].
Several authors have identified risk factors, in particular for TLS, that are caused by solid tumors: large tumor burden, renal insufficiency, elevated LDH, hyperuricemia, metastatic disease (especially of the liver), bone marrow involvement, extrinsic compression of urinary tract by tumors and pretreatment azotemia [7,8]. In our case, the risk factors included liver involvement, bulky disease, elevated LDH (>2 times the upper limit of normal) and azotemia.
The current management guideline suggests diuretics as an adjunct option to intravenous fluid therapy, since the combination of these could improve the excretion of uric acid and phosphate [9]. Furthermore, allopurinol, a xanthine analog, is commonly used for the prevention and treatment of TLS. Rasburicase, a recombinanturate oxidase, has proved to be more effective than allopurinol for the prevention of TLS in a randomized control trial and is also considered effective for TLS treatment [5,10]. Urine alkalinization with the use of sodium bicarbonate is controversial: it increases uric acid solubility but inhibits calcium-phosphate solubility, which can then be precipitated throughout the body, including the kidneys. The current guideline therefore recommends this measure only for patients with metabolic acidosis and not those receiving rasburicase [9]. Dialysis, or hemofiltration, is also a therapeutic option for TLS that is complicated by severe renal failure but, given his poor prognosis, our case was not a suitable candidate for such treatment.
Conclusion
This case showed that STLS should be in the differential diagnosis for a patient who has malignant disease and developed classic laboratory abnormalities and renal failure, even without previous cytotoxic therapy.
References
- 1.Howard SC, Jones DP, Pui C-H. The tumor lysis syndrome. N Engl J Med. 2011;36419:1844–1854. doi: 10.1056/NEJMra0904569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gemici C. Tumour lysis syndrome in solid tumours. Clin Oncol. 2006;18:773–780. doi: 10.1016/j.clon.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Kekre N, Djordjevic B, Touchie C. Spontaneous tumour lysis syndrome. CMAJ. 2012;184:913–916. doi: 10.1503/cmaj.111251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaisban E, Braester A, Mosenzon O, et al. Spontaneous tumor lysis syndrome in solid tumors: really a rare condition? Am J Med Sci. 2003;3251:38–40. doi: 10.1097/00000441-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Chao C-T, Chiang C-K. Rasburicase for huge hepatocellular carcinoma with tumor lysis syndrome: case report. Med Princ Pract. 2012;215:498–500. doi: 10.1159/000339083. [DOI] [PubMed] [Google Scholar]
- 6.Huang W-S, Yang C-H. Sorafenib induced tumor lysis syndrome in an advanced hepatocellular carcinoma patient. World J Gastroenterol. 2009;1535:4464–4466. doi: 10.3748/wjg.15.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shenoy C. Acute spontaneous tumor lysis syndrome in a patient with squamous cell carcinoma of the lung. QJM. 2009;102:71–73. doi: 10.1093/qjmed/hcn129. [DOI] [PubMed] [Google Scholar]
- 8.Baeksgaard L, Sørensen JB. Acute tumor lysis syndrome in solid tumors – a case report and review of the literature. Cancer Chemother Pharmacol. 2003;51:187–192. doi: 10.1007/s00280-002-0556-x. [DOI] [PubMed] [Google Scholar]
- 9.Coiffier B, Altman A, Pui C-H, et al. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. 2008;26:2767–2778. doi: 10.1200/JCO.2007.15.0177. [DOI] [PubMed] [Google Scholar]
- 10.Goldman SC, Holcenberg JS, Finklestein JZ, et al. A randomized comparison between rasburicase and allopurinol in children with lymphoma or leukemia at high risk for tumor lysis. Blood. 2001;9710:2998–3003. doi: 10.1182/blood.v97.10.2998. [DOI] [PubMed] [Google Scholar]


