Abstract
Objective
To examine the effect of silymarin (SM), a mixture of flavonoids and polyphenols extracted from Silybum marianum, on mesenteric ischemia-reperfusion (I-R) injury in a rat model.
Materials and Methods
Fifty rats were randomly divided into 5 groups (n = 10). Group 1 was sham operated, while groups 2-5 were subjected to mesenteric I-R lasting 1 h. Group 2 received isotonic sodium chloride, group 3 received SM (100 mg/kg/day) for 7 days before I-R, group 4 received SM for 7 days after I-R, and group 5 received SM for 7 days both before and after I-R. The rats were sacrificed by exsanguination in groups 1-3 at the 24th hour and groups 4 and 5 were sacrificed on the 7th day of reperfusion. Blood and intestinal specimens were taken for biochemical and pathological evaluations.
Results
Serum superoxide dismutase (SOD) and heat shock protein 70 levels were significantly higher in group 2 (5.24 ± 1.76 U/l and 261.4 ± 16.8 ng/ml) compared to the sham group (2.08 ± 1.76 U/l and 189.9 ± 28.7 ng/ml) (p < 0.001 and p < 0.0001, respectively). However, SOD activity and the extent and severity of the histopathological lesions were significantly less in groups 3 [3.11 ± 1.18 U/l, 1.0 (range 0.0-2.0)], 4 [2.15 ± 0.87 U/l, 1.0 (range 1.0-3.0)], and 5 [1.80 ± 0.61 U/l, 0.5 (range 0.0-2.0)], treated with SM, than in group 2 [5.24 ± 1.76 U/l, 2.0 (range 2.0-3.0)] (p = 0.002, p < 0.001, and p = 0.0001; p < 0.001, p = 0.007, and p = 0.0001, respectively). Also, TNF-α levels were lower in the SM-supplemented groups compared to group 2. Serum thiobarbituric acid-reactive substance concentrations were low in the pre-/posttreatment groups treated with SM compared to group 2. No statistical difference was observed for protein carbonyls between the groups.
Conclusion
Our findings suggest that SM therapy may attenuate the oxidative and intestinal damage induced by I-R injuries.
Key Words: Silymarin, Ileum, Ischemia-reperfusion injury, Oxidant stress, Histopathology
Introduction
Acute mesenteric vascular occlusion associated with ischemia-reperfusion (I-R), which can damage intestinal tissue if not treated at the earliest clinical course, can be a life-threatening surgical emergency [1]. Approximately 1 out of 1,000 patients admitted to emergency services is associated with acute mesenteric ischemia (AMI) [2]. Despite developments in diagnosis, treatment, and surgical methods in recent years, AMI continues to have a very high mortality rate that varies from 32 to 69% [2,3]. The most common underlying pathophysiological mechanisms of I-R injury are superior mesenteric artery (SMA) embolism, superior artery thrombosis complicated by chronic atherosclerosis and nonocclusive mesenteric ischemia [3], abdominal aortic aneurysm surgery, cardiopulmonary bypass, strangulated hernias, neonatal necrotizing enterocolitis, intestinal transplantation, and hemorrhagic-hypovolemic shocks [4,5].
Blockage of the blood supply results in ischemic injury that causes very quick deterioration of tissue metabolism and damages highly vascularized tissues. Though reestablishment of blood flow to the ischemic tissue is essential to prevent irreversible tissue injury, reperfusion may increase tissue injury in addition to ischemic injury. Also, AMI followed by reperfusion induces severe injury of the intestinal mucosa or cells due to hypoperfusion, accompanied by various pathological, molecular, and biochemical changes that sometimes cause morbidity/mortality of the patient, particularly with late or incorrect interventions [4,5,6]. While ischemic tissue injury is mainly due to oxygen deprivation and energy depletion in relation to cell death, reperfusion also produces a wide array of inflammatory responses that both heighten oxidant stress/local damage and may lead to systemic inflammation or injuries [7,8].
Silymarin (SM), a mixture of flavonoids and polyphenols extracted from Silybum marianum (milk thistle), possesses a variety of pharmacological activities including antioxidant and anti-inflammatory/immunomodulatory, hepatoprotective, neuroprotective, renal-protective (against I-R injury), gastroprotective, antibacterial, antiviral, antithrombotic, and vasodilatory properties [9,10,11,12,13]. While information is not available about its effect on mesenteric injury induced by I-R, SM has been shown to have an anti-ischemic effect on focal ischemia/reperfusion due to its better antioxidant profile [14]. Hence, the aim of this study was to evaluate whether SM can prevent or diminish the mesenteric damage induced by I-R in a rat model using biochemical and pathological findings.
Materials and Methods
This study was approved by the Ethics Committee for Animal Welfare Regulation and was carried out in the Experimental Research Center of Ondokuz Mayıs University (Samsun, Turkey). A total of 50 Sprague-Dawley male rats (250-300 g) were allowed to acclimate to the local environment for 1 week before use. The rats were maintained under standard conditions of controlled temperature and humidity. The animals were fed standard rat chow and water ad libitum and given only water for 12 h before surgery.
Experimental Groups and Design
The animals were fasted overnight and divided into 5 groups (n = 10), and they were anesthetized with an intramuscular injection of 80 mg/kg ketamine HCl (Ketalar®; Pfizer, Istanbul, Turkey) and 3 mg/kg xylazine HCl (Rompun®; Bayer, Istanbul, Turkey). Using sterile techniques, a midline laparotomy was performed. The intestine was dissected and the SMA was isolated in group 1 (sham operated). The SMA was not clamped, and the abdomen was closed in 2 layers with a 2.0 polypropylene suture, followed by a 24-hour reperfusion period. In groups 2-5, after the abdominal midline laparotomy, the SMA was isolated and ischemia was induced by occlusion using a nontraumatic microvascular clamp for 1 h. In group 2, the clamp was carefully removed after 1 h, followed by a 24-hour reperfusion period using isotonic saline; in group 3, SM (Dr. Quick's Milk Thistle Standardized capsule, 100 mg/kg/day, dissolved in saline) solution was given intragastrically via a feeding tube for 7 days prior to I-R; in group 4, the rats received SM for 7 days after I-R while in group 5 the animals were treated with SM for 7 days before and after I-R. All animals received an intramuscular injection of cefuroxime (20 mg/kg/day) after the initial surgical procedures. The body temperature of the rats was maintained close to 37.5°C with a heat lamp throughout the experiment. At the end of the experimental study periods, the rats were anesthetized using ketamine and xylazine; then, blood was drawn from the heart using an injector and collected in microcentrifuge tubes. Serum was separated using a centrifugal device at 3,000 g for biochemical analyses. The abdomen was reopened for collection of the intestinal specimens (6-8 cm long from the terminal ileum). The intestinal samples were washed with cold saline and fixed in 10% neutral formaldehyde solution. All rats were sacrificed by exsanguination.
Biochemical Assays
Circulating heat shock protein (Hsp)-70, superoxide dismutase (SOD) activity, and TNF-α levels were measured in a clinical chemistry laboratory using ELISA kits (EKS-715, Stressgen; E0596r, EIAab Science Co., and ER3TNFA, Thermo Scientific, respectively). Markers of oxidative stress in this study included protein carbonyls (PC; a marker of protein oxidation) and thiobarbituric acid-reactive substance (TBARS; a marker of lipid peroxidation). Serum lipid peroxidation was estimated spectrophotometrically using the TBARS method with slight modifications and expressed as micromoles per liter [15]. Measurements of PC in the serum samples were made according to the method of Evans et al. [16] with slight modifications. Carbonyl values were calculated as follows: carbonyl concentration (nmol/mg of protein) = carbonyl groups (nmol/ml)/protein concentrations (mg/ml). All chemicals used for the assays were of analytical grade and purchased from Sigma-Aldrich Co. LLC (USA).
Histopathological Examinations
The terminal ileum tissues were embedded in paraffin wax, cut into 4- to 5-µm sections, and stained with hematoxylin and eosin (H&E). The histological sections were evaluated with an Olympus BX51 optical microscope (Olympus Optical Co., Osaka, Japan) by a pathologist blinded to the experimental procedures, and photographed. The intestinal lesions were graded according to 5 levels of I-R injury using the scoring system adapted by Quaedackers et al. [17] as follows: grade 0 = normal villus and mucosa; grade 1 = development of a subepithelial space at the villus tip and villous edema and congestion; grade 2 = development of a subepithelial space at the villus tip, hemorrhage and disintegration at the end portion of the villi; grade 3 = loss and disintegration at the end portion of the villi; grade 4 = villi completely separated from the lamina propria; grade 5 = necrosis on the whole wall and disintegration at the lamina propria.
Statistical Analysis
Statistical analyses were performed using SPSS version 15.0 software for Windows (SPSS Inc., Chicago, Ill., USA). Data were tested for the normality assumption analyzed by one-way analysis of variance, and pairwise comparisons were made using the Tukey multiple comparisons test. To evaluate the severity of the histopathological lesions, the Kruskal-Wallis analysis of variance was used to determine the statistical significance of differences in the groups. Then, the Mann-Whitney U test with Bonferroni's correction was used for comparisons between groups. p < 0.05 was considered statistically significant.
Results
Biochemical Results
The serum Hsp-70, TNF-α, SOD, TBARS and PC levels of all groups are given in table 1. The markers of oxidative stress (TBARS and PC) were higher in group 2 after I-R than in sham-operated rats; however, no significant difference (p > 0.05) was observed for either parameter between the SM-supplemented groups. Hsp-70 levels were higher in response to I-R injury in rats treated with or without SM compared to the sham group, and the difference was statistically significant (p < 0.0001). However, its value was significantly lower in rats given SM for 7 days prior to I-R (244.5 ± 26.4 ng/ml) compared to I-R group 2 (261.4 ± 16.8 ng/ml) (p = 0.002). In contrast, the level of Hsp-70 was higher in rats that received SM for 7 days after I-R compared to I-R group 2 (p < 0.001). The TNF-α level in mesenteric I-R injury was lower in rats treated with SM prior to I-R (0.625 ± 0.01 ng/ml) compared to I-R group 2 (0.673 ± 0.09 ng/ml) (p = 0.014). Also, its level was elevated in I-R group 2 compared to the sham group; however, a significant statistical difference was not found compared to the other groups. The SOD activity associated with oxidant stress was significantly higher in mesenteric I-R group 2 (5.24 ± 1.76 U/l) compared to the sham group (2.08 ± 1.76 U/l) (p < 0.001). This elevation was significantly reduced by the SM therapy in rats treated with SM after I-R injury (group 3) compared to I-R group 2 (p = 0.002). A lower level of SOD activity was observed in rats treated with SM before/after I-R (groups 4 and 5) compared to group 2.
Table 1.
Changes in serum biochemical parameters in the sham group, group 2, and groups 3 – 5 treated with SM with regard to mesenteric I-R
| Groups (n = 10) | Parameters |
||||
|---|---|---|---|---|---|
| Hsp-70, ng/ml | TNF-α, ng/ml | SOD, U/l | TBARS, μmol/l | PC, nmol/mg protein | |
| Sham | 189.9 ± 28.7 | 0.649 ± 0.05 | 2.08 ± 1.76 | 0.3406 ± 0.05 | 244.6 ± 68.8 |
| I-R | 261.4 ± 16.8 | 0.673 ± 0.09 | 5.24 ± 1.76 | 0.3485 ± 0.03 | 257.9 ± 66.7 |
| SM+I-R | 244.5 ± 26.4 | 0.625 ± 0.01 | 3.11 ± 1.18 | 0.3380 ± 0.03 | 262.7 ± 29.8 |
| I-R+SM | 280.5 ± 28.4 | 0.665 ± 0.02 | 2.15 ± 0.87 | 0.3380 ± 0.02 | 245.3 ± 46.1 |
| SM before/after I-R | 268.6 ± 27.9 | 0.651 ± 0.01 | 1.80 ± 0.61 | 0.3327 ± 0.02 | 253.6 ± 36.5 |
Histopathological Findings
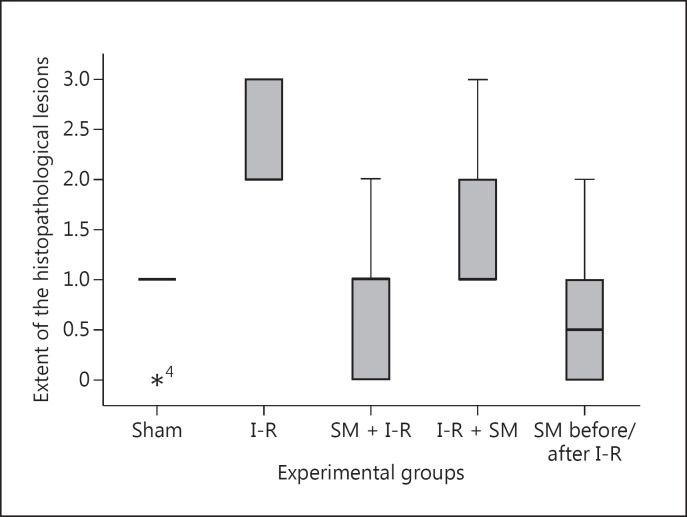
Intestinal specimens from rats after 1 h of ischemia and 24 h of reperfusion exhibited significantly greater damage in the I-R group compared to the sham group as shown in figures 1 and 2 (p < 0.001). However, the extent of the mesenteric histopathological lesions was significantly lower in rats treated with SM in groups 3-5 than in rats in the I-R group (p < 0.001, p = 0.007, and p = 0.0001, respectively) (fig. 1).
Fig. 1.
Effects of SM before (group 3), after (group 4), and before and after treatment (group 5) on changes in the production of histopathological lesions 24 h and 7 days after mesenteric I-R injury in rats. *4 = This observation belongs to sample 4 which is an outlier.
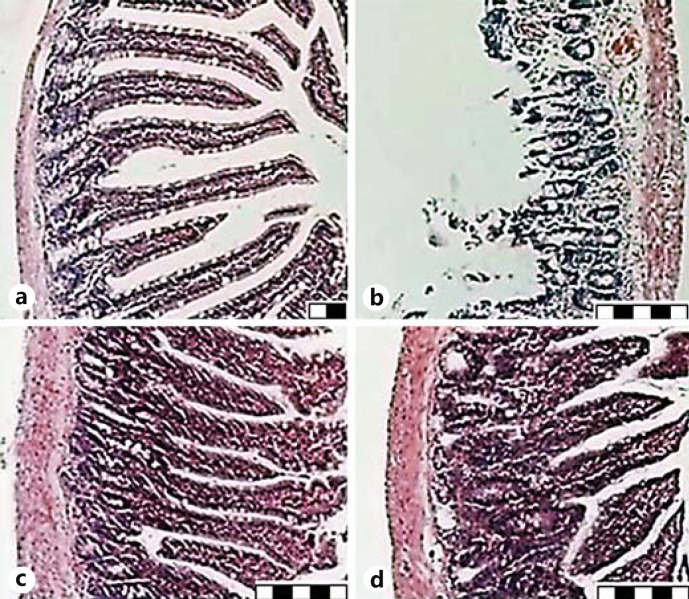
Fig. 2.
Microphotographs present the histopathological findings. Representative photo of the terminal ileum. a Normal intestinal mucosa but presence of edema and vascular congestion within the villous tissue in the sham-operated group. H&E. Scale bar = 20 μm. b Wide disintegration and hemorrhage of the villous tissue at 24 h of reperfusion after 1 h of ischemia in the I-R group. H&E. Scale bar = 50 μm. c Slight edema and vascular congestion in the villous tissue treated with SM prior to I-R. H&E. Scale bar = 50 μm. d Rats that were treated with SM before/after I-R had the lowest degree of edema/inflammatory infiltrates in the villous tissue. H&E. Scale bar = 50 μm.
Discussion
The present study demonstrates that pre-/posttreatment with SM may reduce mesenteric I-R injury in a rat model. The intestinal damage induced by I-R is associated with biochemical findings, e.g. that the serum levels of Hsp-70, TNF-α, SOD, and TBARS decline in connection with pretreatment with SM, and histopathological alterations which are seen to be well ameliorated with the pretreatment and pre-/posttreatment with SM.
Mesenteric I-R injury is a serious clinical problem in patients with AMI, and I-R is a distressful condition for cells or tissues. In cells deprived of oxygen after vascular occlusion, cellular oxidative respiration slows down, with irreversible damage occurring quickly within minutes in sensitive tissues such as brain, myocardium, and intestine. Adverse multiple cellular metabolic and ultrastructural changes result in the course of ischemia. In addition, restoration of blood flow to the ischemic tissue initiates a series of adverse events which may lead to additional cell or tissue damage [5,6,7,8]. Intestinal I-R injuries are characterized by altered microvascular and epithelial permeability and villus damage [18]. Our findings on the histopathological changes in the intestinal wall caused by ileum I-R injury are shown in figures 1 and 2. These findings are almost in line with previous reports [4,18,19]. The extent and severity of the pathological lesions was observed to be higher in the I-R group compared to the sham group. However, pathological lesions were significantly reduced in rats treated with SM before/after I-R injury at the end of 24 h and 1 week. Raza et al. [11] also reported that SM exerts gastroprotective effects against ethanol-induced gastritis. Based on these findings, treatment with SM seems to reduce the pathological lesions on the intestinal wall caused by I-R injury.
In the present study, the serum TNF-α level was higher in group 2 rats compared to sham rats. However, it decreased in group 3 in a statistically significant manner, and its level was lower by an average of 1.2 and 3.3% in groups 4 and 5 (treated with SM) compared to group 2. In this condition, pretreatment with SM for 7 days prior to I-R led to suppression of the release of TNF-α to a smaller degree during reperfusion. Manna et al. [20] also reported that SM suppresses the TNF-induced activation of NF-κB which is an obvious target for anti-inflammatory treatment; SM may be mediated through downregulation of the NF-κB pathway. SM protected rats against cerebral I-R-induced stroke injury through amelioration of the oxidative stresses and inflammation-mediated tissue injury by impeding the activation of the proinflammatory transcription factors (NF-κB and STAT-1) in the upregulation of proinflammatory proteins and cytokines in stroke-damaged sites [21]. Hsp protect cells against stress/apoptosis in response to the different cellular insults. Their expression is upregulated upon exposure to stressful conditions such as I-R, inflammation, oxidant stress, and sepsis [22]. From these proteins, Hsp-70 is rapidly released into the circulation in particularly high stressful courses. In our study, Hsp-70 levels were observed to be high in response to I-R injury in groups 2-5 as compared to the sham group. However, this value was low in rats treated with SM for 7 days prior to I-R compared to group 2. SM in intestinal tissues is likely to exhibit anti-inflammatory and antioxidant effects because TNF-α and Hsp-70 levels were decreased by a 24-hour reperfusion period in rats treated with SM for 7 days prior to I-R. Many published articles have reported that I-R induces inflammation and tissue injury through the endogenous production of toxic oxygen radicals and proinflammatory cytokines like TNF-α, IL-1, and IL-6 [6,8,18,23,24]. The levels of these cytokines were found to be significantly increased by a 4-hour intestinal I-R period [18]. That toxic oxygen free radicals play an important role in the pathogenesis of I-R is supported by a large body of evidence that SOD (a widely specific scavenger of superoxide radicals) prevents gastrointestinal damage induced by I-R [7,8]. In our study, SOD activity was significantly increased in group 2 compared to the sham group; however, its level was gradually decreased in SM-administered groups compared to group 2 as shown in table 1. TBARS that indicate lipid damage induced by oxidant stress were declined in the serum samples treated with SM before/after I-R compared to group 2 as well. The results of TNF-α, Hsp-70, SOD, and TBARS may suggest that SM has a noteworthy antioxidant effect and a moderate anti-inflammatory effect on the mesenteric damage caused by I-R injuries.
Conclusion
Our study found that the histopathological changes on the intestinal wall caused by ileum I-R injury were alleviated by SM therapy. The levels of inflammation and oxidant stress markers were attenuated by the administration of SM before/after mesenteric I-R injury. SM participates in the course of tissue restitution as an antioxidant. The protective effect of SM can be associated with its antioxidant properties; therefore, it most likely acts as an oxygen radical scavenger for protection against the damages induced by I-R.
Acknowledgement
The authors would like to thank Dr. M. Yavuz Gülbahar for providing pathological evaluations.
References
- 1.Lin PH, Kougias P, Bechara C, et al. Arterial disease. In: Brunicardi FC, Andersen DK, Billiar TR, et al., editors. Schwartz's Principles of Surgery. ed 9. New York: McGraw-Hill; 2010. pp. 730–736. [Google Scholar]
- 2.Herbert GS, Steele SR. Acute and chronic mesenteric ischemia. Surg Clin North Am. 2007;87:1115–1134. doi: 10.1016/j.suc.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 3.Park WM, Gloviczki P, Cherry KJ, et al. Contemporary management of acute mesenteric ischemia: factors associated with survival. J Vasc Surg. 2002;35:445–452. doi: 10.1067/mva.2002.120373. [DOI] [PubMed] [Google Scholar]
- 4.Toth S, Jonecova Z, Varga J, et al. Mesenteric ischemia-reperfusion injury: specific impact on different cell populations within the jejunal wall in rats. Acta Histochem. 2012;114:276–284. doi: 10.1016/j.acthis.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Oldenburg WA, Lau LL, Rodenberg TJ, et al. Acute mesenteric ischemia: a clinical review. Arch Intern Med. 2004;164:1054–1062. doi: 10.1001/archinte.164.10.1054. [DOI] [PubMed] [Google Scholar]
- 6.Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bul. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- 7.Dorweiler B, Pruefer D, Andrasi TB, et al. Ischemia-reperfusion injury: pathophysiology and clinical implications. Eur J Trauma Emerg Surg. 2007;6:600–610. doi: 10.1007/s00068-007-7152-z. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki M, Joh T. Oxidative stress and ischemia-reperfusion injury in gastrointestinal tract and antioxidant, protective agents. J Clin Biochem Nutr. 2007;40:1–12. doi: 10.3164/jcbn.40.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mates JM, Segura JA, Alanso FJ, et al. Anticancer antioxidant regulatory functions of phytochemicals. Curr Med Chem. 2011;18:2315–2338. doi: 10.2174/092986711795656036. [DOI] [PubMed] [Google Scholar]
- 10.Turgut F, Bayrak O, Catal F, et al. Antioxidant and protective effects of silymarin on ischemia and reperfusion injury in the kidney tissues of rats. Int Urol Nephrol. 2008;40:453–460. doi: 10.1007/s11255-008-9365-4. [DOI] [PubMed] [Google Scholar]
- 11.Raza SS, Khan MM, Ashafaq M, et al. Silymarin protects neurons from oxidative stress associated damages in focal cerebral ischemia: a behavioral, biochemical and immunohistological study in Wistar rats. J Neurol Sci. 2011;309:45–54. doi: 10.1016/j.jns.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 12.Shin JH, Lee CW, Oh SJ, et al. Protective effect of silymarin against ethanol-induced gastritis in rats: role of sulfhydryls, nitric oxide and gastric sensory afferents. Food Chem Toxicol. 2013;55:353–557. doi: 10.1016/j.fct.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Kiruthiga PV, Karthikeyan K, Archunan G, et al. Silymarin prevents benzo(a)pyrene-induced toxicity in Wistar rats by modulating xenobiotic-metabolizing enzymes. Toxicol Ind Health. 2013 doi: 10.1177/0748233713475524. DOI: 10.1177/0748233713475524. [DOI] [PubMed] [Google Scholar]
- 14.Muley MM, Thakare VN, Patil RR, et al. Silymarin improves the behovioural, biochemical and histoarchitecture alterations in focal ischemic rats: a comparative evaluation with piracetam and protocatachuic acid. Pharmacol Biochem Behav. 2012;102:286–293. doi: 10.1016/j.pbb.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Singh RP, Padmavathi B, Rao R. Modulatory influence of Adhatodavesica leaf extract on the enzmes of xenobiotic metabolism, antioxidant status and lipid peroxidation in mice. Mol Cell Biochem. 2000;213:99–109. doi: 10.1023/a:1007182913931. [DOI] [PubMed] [Google Scholar]
- 16.Evans P, Lyras L, Halliwel B. Measurement of protein carbonyls in human brain tissue. Methods Enzymol. 1999;300:145–156. doi: 10.1016/s0076-6879(99)00122-6. [DOI] [PubMed] [Google Scholar]
- 17.Quaedackers JS, Beuk RJ, Bennet L, et al. An evaluation of methods for grading histologic injury following ischemia/reperfusion of the small bowel. Transplant Proc. 2000;32:1307–1310. doi: 10.1016/s0041-1345(00)01238-0. [DOI] [PubMed] [Google Scholar]
- 18.Spanos CP, Papaconstantinou P, Spanos P, et al. The effect of L-arginine and aprotinin on intestinal ischemia-reperfusion injury. J Gastrointest Surg. 2007;11:247–255. doi: 10.1007/s11605-007-0102-6. [DOI] [PubMed] [Google Scholar]
- 19.Kılıç K, Hancı V, Selek Ş, et al. The effects of dexmedetomidineon mesenteric arterial occlusion-associated gut ischemia and reperfusion-induced gut and kidney injury in rabbits. J Surg Res. 2012;178:223–232. doi: 10.1016/j.jss.2012.03.073. [DOI] [PubMed] [Google Scholar]
- 20.Manna SK, Mukhopadhyay A, Van NT, et al. Silymarin suppresses TNF-induced activation of NF-kappa B, c-Jun N-terminal kinase, and apoptosis. J Immunol. 1999;163:6800–6809. [PubMed] [Google Scholar]
- 21.Hou YC, Liou KT, Chern CM, et al. Preventive effect of silymarin in cerebral ischemia-reperfusion-induced brain injury in rats possibly through impairing NF-κB and STAT-1 activation. Phytomedicine. 2010;17:963–973. doi: 10.1016/j.phymed.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Kalmar B, Greensmith L. Induction of heat shock proteins for protection against oxidative stress. Adv Drug Deliv Rev. 2009;61:310–318. doi: 10.1016/j.addr.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Jung JE, Kim GS, Narasimhan P, et al. Regulation of Mn-superoxide dismutase activity and neuroprotection by STAT3 in mice after cerebral ischemia. J Neurosci. 2009;29:7003–7014. doi: 10.1523/JNEUROSCI.1110-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fridovich I. Oxygen: how do we stand it? Med Princ Pract. 2013;22:131–137. doi: 10.1159/000339212. [DOI] [PMC free article] [PubMed] [Google Scholar]