Abstract
Context:
Preeclampsia is a leading cause of fetal and maternal morbidity and mortality during pregnancy. Although the etiology of preeclampsia is unknown, preeclampsia offspring have increased risks of developing cardiovascular disorders in adulthood, implicating that preeclampsia programs fetal vasculature in utero.
Objective:
We hypothesize that preeclampsia alters expression profiles of endothelial microRNAs (miRNAs) in fetal endothelial cells and disturbs the vascular endothelial growth factor A (VEGFA)- and fibroblast growth factor 2 (FGF2)-induced endothelial function.
Design and Setting:
Unpassaged (P0) human umbilical vein endothelial cells (HUVECs) were isolated immediately after cesarean-section delivery from normotensive (NT) and preeclamptic (PE) pregnancies. Differentially expressed miRNAs between P0-HUVECs from NT and PE pregnancies were identified using a miRNA polymerase chain reaction (PCR) array and confirmed using reverse transcription quantitative PCR. To determine the function of these differentially expressed miRNAs, miRNAs of interest were knocked down in NT-HUVECs following by cell functional assays.
Results:
Sixteen miRNAs, including miR-29a/c-3p, were downregulated in P0-HUVECs from the PE group compared with the NT group. Bioinformatics analysis predicted the PI3K–v-akt murine thymoma viral oncogene homolog 1 (AKT) signaling pathway was dysregulated in P0-HUVECs from the PE group, which was associated with the miR-29a/c-3p downregulation. We further demonstrated that miR-29a/c-3p knockdown inhibited the VEGFA- and FGF2-induced endothelial migration as well as FGF2-induced AKT1 phosphorylation in HUVECs. However, miR-29a/c-3p knockdown did not alter the extracellular signal-regulated kinase 1/2 phosphorylation, cell proliferation, and endothelial monolayer integrity in response to VEGFA and FGF2 in HUVECs.
Conclusions:
Preeclampsia-downregulated miR-29a/c-3p may impair fetal endothelial function by disturbing the FGF2-activated PI3K-AKT signaling pathway, hence inhibiting endothelial cell migration.
Preeclampsia downregulates 16 miRNAs, including miR-29a/c-3p, in fetal endothelial cells, which may impair fetal endothelial cell migration by disturbing the FGF2-stimulated PI3K-AKT1 pathway.
Preeclampsia is a leading cause of fetal and maternal morbidity and mortality during human pregnancy, which accounts for ∼50,000 to 75,000 maternal deaths worldwide per year (1). Preeclampsia occurs in 3% to 8% of all pregnancies and is associated with hypertension and proteinuria during pregnancy (2). Although the etiology of preeclampsia is unknown, it is considered to manifest as a maternal endothelial disorder (3, 4). However, emerging evidence indicates that preeclampsia also has adverse impacts on fetal endothelial function, such as defective cell permeability (5), Ca2+ responses and nitric oxide production (4, 6, 7), all of which are associated with peptide growth factors-induced endothelial function (8, 9). More importantly, offspring from preeclampsia exhibit increased risk of developing cardiovascular disorders later in life (10, 11), implying that preeclampsia may also program fetal endothelial function in utero for life-long deleterious hemodynamic consequences.
MicroRNAs (miRNAs) are a class of small noncoding RNAs that mainly function as posttranscriptional regulators of gene expression by sequence-selective targeting of messenger RNAs, leading to degradation of messenger RNA or repression of translation (12). Although miRNAs are involved in the regulation of human placental development (13, 14) and numerous aspects of endothelial function including angiogenesis (15), the function of miRNAs in human fetal endothelial cells is poorly defined. Several recent studies have suggested that miRNAs play important roles in regulating placental development and function (13, 16–18). In addition, the dysregulation of miRNAs has been linked to many pregnancy disorders including preeclampsia (19). To date, most of our knowledge about preeclampsia-associated miRNAs is based on studies utilizing whole placental tissue and maternal blood (14, 16–18). Thus, the exact role of miRNAs in endothelial dysfunction, a hallmark of the pathogenesis of preeclampsia, remains unclear.
Endothelial function is tightly regulated by peptide growth factors, such as vascular endothelial growth factor A (VEGFA) and fibroblast growth factor 2 (FGF2), which are key regulators of placental angiogenesis and vasodilation (20, 21). PE significantly alters the levels of VEGFA (22) and FGF2 (23) in human placenta. Both VEGFA and FGF2 regulate cellular responses primarily by activation of a cascade of protein kinases, including mitogen-activated protein kinase 3/1 [also known as extracellular signal-regulated kinase (ERK)1/2] and v-akt murine thymoma viral oncogene homolog 1 (AKT1) (20, 21). In addition, we have previously reported that VEGFA- and FGF2- induce activation of ERK1/2 and AKT1 in human umbilical vein endothelial cells (HUVECs), which is a classical cell model for studying human endothelial cells, particularly for fetal endothelial cells (24, 25).
In the current study, we hypothesize that preeclampsia dysregulates expression profile of endothelial function-associated miRNAs in fetal endothelial cells, which disturbs the VEGFA- and FGF2-induced endothelial function using HUVECs as a model. Specifically, we compared the expression profile of 1008 miRNAs between unpassaged (P0) HUVECs freshly isolated from normotensive (NT) and preeclamptic (PE) pregnancies using a human miRNome polymerase chain reaction (PCR) array (miScript miRNome miRNA PCR Array; Qiagen, Valencia, CA) and further confirmed using quantitative reverse transcription PCR (RT-qPCR). Bioinformatics analysis was performed on the confirmed differentially expressed (DE)-miRNAs and their target genes to predict the pathways and biological processes that might be dysregulated in PE vs NT P0-HUVECs. Selected DE-miRNAs of interest were knocked down in cultured NT-HUVECs to further explore their roles in regulating the ERK1/2 and AKT1 pathways, as well as cell migration, cell proliferation, and cell monolayer integrity in response to VEGFA and FGF2.
Material and Methods
Isolation and characterization of HUVECs
Tissue collection procedures were approved by the Institutional Review Boards of Meriter Hospital and the University of Wisconsin–Madison Health Sciences. The obstetric clinicians at the Meriter Hospital Birth Center where umbilical cords were collected made the clinical diagnosis of NT and PE. PE was defined according to the standard criteria (26).
The HUVECs were isolated from umbilical veins immediately after cesarean-section delivery from NT and PE (Table 1) and purified after 16 hours of culture (Supplemental Methods) as described (24, 27). The majority of cells [referred as passage 0 (P0) HUVECs] were immediately frozen in liquid nitrogen until total RNA and miRNA isolation. Approximately 10% of the cells were used for the acetylated low-density lipoprotein labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate uptake assay (Supplemental Methods) to validate the purity of each cell preparation (27). Only cell preparations exhibiting acetylated low-density lipoprotein labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate uptake ≥96% were used.
Table 1.
Patient Demographics
| N | Systolic BP, mm Hg | Diastolic BP, mm Hg | Maternal Age, y | Gestational Age, wk | Fetal Weight, g | |
|---|---|---|---|---|---|---|
| Human miRNome PCR array | ||||||
| NT | 3 | 114 ± 7.5 | 69 ± 16.3 | 33.0 ± 5.6 | 39.0 ± 0.0 | 3817.2 ± 504.6 |
| PE | 3 | 156 ± 16.94 | 95 ± 8.7 | 30.3 ± 2.9 | 38.1 ± 1.9 | 3279.6 ± 251.9 |
| P value | 0.018 | 0.069 | 0.502 | 0.456 | 0.174 | |
| MiRNA RT-qPCR validation | ||||||
| NT PE P value | 6 | 113 ± 3.3 | 67 ± 7.0 | 34 ± 2.5 | 39 ± 0.0 | 3884 ± 216.7 |
| 6 | 157 ± 7.0 | 94 ± 3.8 | 30 ± 1.3 | 36 ± 2.2 | 2772 ± 518.3 | |
| 0.002 | 0.011 | 0.098 | 0.135 | 0.059 |
Abbreviation: BP, blood pressure.
RNA isolation and quality control
Total RNA samples with enriched small RNA (<200 nucleotides) portion including miRNAs were isolated from HUVECs using the miRNeasy Mini Kit (Qiagen). The concentration and quality of each RNA sample were assessed using NanoDrop™ ND-2000 spectrophotometer (NanoDrop Technologies, Wilmington, DE) and Agilent 2100-bioanalyzer (Agilent Technologies, Santa Clara, CA). Only RNA samples with high RNA integrity number (>8.5) were used in this study.
Screening and verification of differentially expressed miRNAs Between NT and PE P0 HUVECs
Screening of DE-miRNAs between P0 HUVECs isolated from NT and PE (Table 1; n = 3 individual cell preparations/group) was performed using the human miScript miRNome PCR Array (Qiagen), which contained a total of 1008 mature miRNAs (Supplemental Methods). All DE-miRNAs identified by the miRNome PCR Array were validated by RT-qPCR (Supplemental Methods and Supplemental Table 1 (212KB, pdf) ) using a separate set of NT and PE P0-HUVECs (Table 1; n = 6).
Bioinformatics analysis
Further miRNA-targeted gene/pathway enrichment analyses were performed on the confirmed DE-miRNAs using the DIANA Tools (DIANA-TarBasev7.0, -mirPathv.3, and -microT-CDS software) (28) and Kyoto Encyclopedia of Genes and Genomes pathway database. The enriched pathways were determined by the Fisher’s exact test with false discovery rate (FDR) P value correction for multiple testing (28).
Knockdown of miR-29a/c-3p in NT-HUVECs
MiRNome PCR Array and RT-qPCR analyses showed that miR-29a-3p and miR-29c-3p were significantly downregulated in PE vs NT P0-HUVECs. In addition, bioinformatics analysis suggested that miR-29a-3p and miR-29c-3p regulate many endothelial function-associated pathways. Hence, in this study we focused on dissecting roles of miR-29a-3p and miR-29c-3p in regulating fetal endothelial function using HUVECs as a model.
Due to the limited cell number of P0-HUVECs from each preparation (∼3 × 105 – 1 × 106/preparation), we used passages 3 to 4 NT-HUVECs preparations pooled from five individuals as described (27) for all miRNA knockdown and following cell functional assays in this study.
Knockdown of miRNAs (Supplemental Methods) was performed using miScript miRNA Inhibitors [Qiagen, referred as miRNA-(i)], which are chemically synthesized and modified single-strand RNAs that specifically bind and inhibit each target miRNA (29). Cells transfected with only the transfection reagent and miScript inhibitor negative control (NC) were used as the vehicle and NCs, respectively (29). RT-qPCR (Supplemental Methods) was used to verify efficiency of miRNA knockdown (n = 3 per group).
Western blot analysis
HUVECs were transfected with vehicle, NC, and miRNA-(i) for 24 hours. After 16 hours of serum starvation in endothelial culture medium (ECM)-basal (ECM-b, serum-free, ScienCell Research Laboratories, Carlsbad, CA; control), HUVECs were treated with ECM (ECM-b supplemented with 5% fetal bovine serum and 1% endothelial cell growth supplement), VEGFA (10 ng/mL, category number 80006-RNAB, Sino Biological, China), and FGF2 (10 ng/mL, category number 10014-HNAE, Sino Biological) for 10 minutes and immediately processed to protein isolation followed by western blot analysis (n = 4, Supplemental Methods and Supplemental Table 2 (212KB, pdf) ) (22, 24).
Cell functional assays
Cell functional assays were performed on HUVECs that were transfected with vehicle, NC, and miRNA-(i) (Supplemental Methods). Cell migration was assessed using the scratch healing assay (n = 4) (30). Cell proliferation was assessed by the methylthiazolyl tetrazolium assay (n = 6) using the methylthiazolyl tetrazolium assay kit (Cayman Chemical, Ann Arbor, MI) as described (31). Cell monolayer integrity was determined using the ECIS Zθ+ 96-well array station (Applied BioPhysics, Troy, NY) using 96W10E+ plates (n = 4) (32).
Statistical analyses
SigmaPlot software (Jandel Co., San Rafael, CA) was used for statistical analyses. Data are represented as the median ± standard deviation (SD). Data analyses were performed using the Mann-Whitney rank sum test and Kruskal-Wallis test when appropriate. Differences were considered to be statistically significant when P < 0.05. Benjamini and Hochberg FDR (33, 34) adjustment was used for multiple comparison correction when appropriate.
Results
Differentially expressed miRNAs Between NT and PE P0-HUVECs and bioinformatics analysis
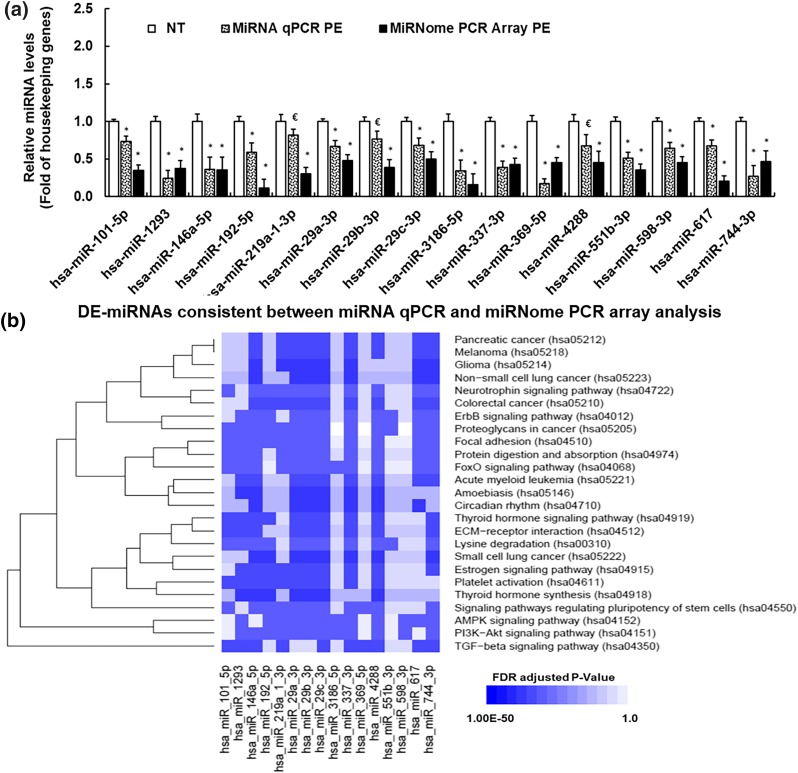
Among the 1008 miRNAs examined in human miRNome PCR Array analysis, 930 were detected in both NT and PE P0-HUVECs (Supplemental Table 3 (212KB, pdf) ). Compared with NT, 23 DE-miRNAs (P < 0.05, fold change >|2|) (34) were identified in PE P0-HUVECs (Supplemental Table 3 (212KB, pdf) ). All of these DE-miRNAs were further verified using miRNA RT-qPCR with a separate set of NT and PE P0-HUVECs samples. Among the 23 DE-miRNAs, 16 displayed the same trend of differential expression between miRNome PCR Array and miRNA RT-qPCR analyses [Fig. 1(a)], all of which were downregulated in PE vs NT P0-HUVECs. In addition, two DE-miRNAs (miR-2116-5p and miR-374c-5p) were not detectable in most samples in miRNA RT-qPCR analysis, and five exhibited inconsistent trends between miRNome PCR Array and miRNA RT-qPCR analyses. Among the 16 confirmed DE-miRNAs, 3 were with borderline P values (0.05 < P < 0.1) in miRNA RT-qPCR analysis [Fig. 1(a)]. Specifically, all three members of miR-29 family (29a/b/c) were expressed in NT and PE P0-HUVECs [Fig. 1(a)]. In both miRNome PCR Array and miRNA RT-qPCR analyses, miR-29a-3p and miR-29c-3p (referred as miR-29a/c-3p) were downregulated (P < 0.05) in PE vs NT P0-HUVECs [Fig. 1(a)]. The level of miR-29b-3p was decreased in PE vs NT P0-HUVECs with a borderline P value in miRNA RT-qPCR analysis, although this decrease reached significant (P < 0.05) in miRNome PCR Array analysis [Fig. 1(a)].
Figure 1.
(a) RT-qPCR validation of preeclampsia-induced DE-miRNAs in PE vs NT P0-HUVECs. *Differ (P < 0.05, Mann–Whitney Rank Sum Test) from NT; €differ from NT with a borderline P value (0.05 < P < 0.1). (b) Kyoto Encyclopedia of Genes and Genomes (KEGG) signaling pathways associated with preeclampsia-induced DE-miRNAs and their targeted genes. MiRNA targeted gene/pathway enrichment analyses were performed using the DIANA Tools and KEGG pathway database. The 25 significantly enriched (FDR adjusted P < 0.05) pathways were determined by the Fisher’s exact test with FDR P value correction for multiple testing. x-axis: number of DE-miRNA associated with each pathway; y-axis: names of the enriched KEGG signaling pathways. *Pathways that are associated with miR-29a/c-3p.
Bioinformatics analyses of these 16 confirmed DE-miRNAs and their targeted genes identified 25 enriched pathways [FDR adjusted P < 0.05; Fig. 1(b), Supplemental Table 4 (212KB, pdf) ] indicating dysregulations of these pathways in PE HUVECs. Specifically, the 16 DE-miRNAs were enriched with miRNAs that regulate key endothelial function-associated signaling pathways including Estrogen signaling pathway (10 DE-miRNAs), transforming growth factor (TGF)-β signaling pathway (10 DE-miRNAs), Focal adhesion (12 DE-miRNAs), and PI3K-AKT signaling pathway (12 DE-miRNAs). Among all PE-downregulated miRNAs, miR-29a/c-3p were predicted to be associated with all 25 enriched pathways. In addition, VEGFA was predicted to be a regulation target of both miR-29a/c-3p. Further Gene Ontology analysis revealed that many members of the FGF receptor pathway were regulation targets of miR-29a/c-3p, whereas FGF2 is not a direct regulation target of miR-29a/c-3p (Supplemental Table 5 (212KB, pdf) ). As dysregulation of miR-29a/c-3p is associated with severe cardiovascular diseases such as cardiac fibrosis (35) and heart failure (36) in adults, we focused on exploring the effect of miR-29a/c-3p on endothelial function in this study.
Knockdown of miR-29a/c-3p
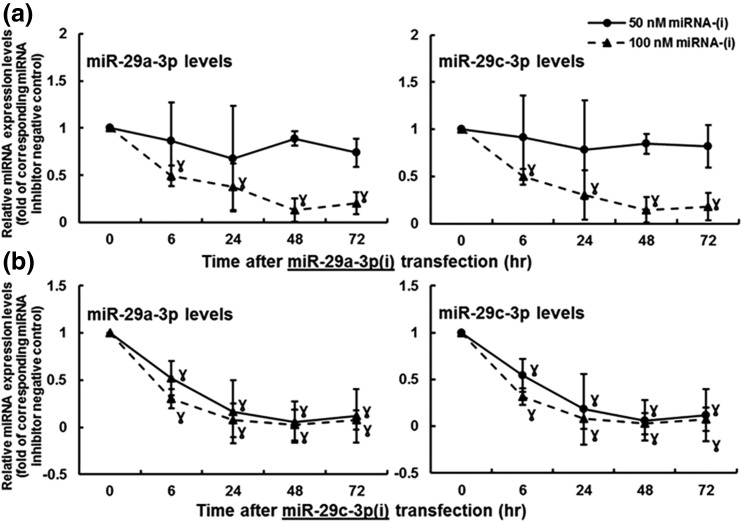
To evaluate the efficiency of miR-29a/c-3p knockdown, RT-qPCR analysis was performed in passages 3 to 4 NT-HUVECs (Fig. 2). The 50-nM miR-29a-3p inhibitor [miR-29a-3p(i)] decreased (P < 0.05) miR-29a-3p levels by 12% and 23% at 48 and 72 hours, respectively, whereas it did not change miR-29c-3p levels in HUVECs up to 72 hours [Fig. 2(a)]. The 100-nM miR-29a-3p(i) effectively decreased (P < 0.05) both miR-29a/c-3p levels by about 60% at 24 hours, and this inhibitory effect maintained up to 72 hours [Fig. 2(a)]. The 50-nM miR-29c-3p inhibitor [miR-29c-3p(i)] also decreased (P < 0.05) both miR-29a/c-3p levels by about 60% at 24 hours, and this inhibitory effect maintained up to 72 hours in HUVECs [Fig. 2(b)]. The 100-nM miR-29c-3p(i) decreased (P < 0.05) miR-29a/c-3p levels by about 80% at 24 hours; the inhibition was further enhanced to about 90% at 48 hours and maintained up to 72 hours in HUVECs [Fig. 2(b)]. Because miR-29c-3p(i) at 100 nM exhibited the most significant and stable inhibition on both miR-29a/c-3p levels, this dose was used for miR-29a/c-3p knockdown in cell functional assays described next.
Figure 2.
Efficiency of (a) miR-29a-3p and (b) miR-29c-3p knockdown in NT-HUVECs. Subconfluent HUVECs were transfected with miR-29a-3p(i), miR-29c-3p(i), or miRNA-(i) NC. The miRNA levels were determined using miRNA RT-qPCR. Data normalized to housekeeping genes are expressed as median ± SD fold of NC at each corresponding time point. ƔDiffer (P < 0.05, Kruskal–Wallis test) from 0 hours, n = 3.
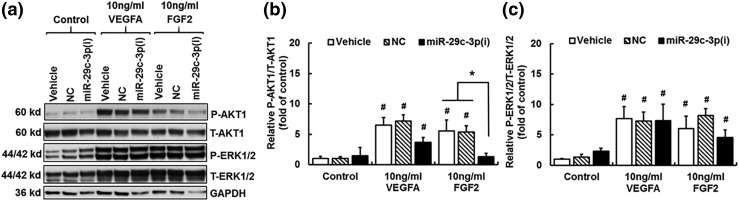
Knockdown of miR-29a/c-3p reduces the FGF2-induced AKT1 phosphorylation
We examined the effects of miR-29a/c-3p knockdown on activation of the PI3K-AKT1 and ERK1/2 pathways, both of which are critical in regulating endothelial function in HUVECs (8, 20, 21, 24, 37). Compared with control, 10 minutes of VEGFA (10 ng/mL) and FGF2 (10 ng/mL) treatments increased (P < 0.05) the phosphorylated (P)-AKT1/total (T)-AKT1 ratio in cells treated with vehicle and NC [Fig. 3(a) and 3(b)]. The VEGFA-induced P-AKT1/T-AKT1 ratio was slightly decreased in cells with miR-29c-3p(i) treatment, but this decrease was not statistically significant. In contrast, the FGF2-induced increases in P-AKT1/T-AKT1 ratio were completely blocked by the miR-29c-3p(i) treatment.
Figure 3.
Effects of miR-29a/c-3p knockdown on the FGF2- and VEGFA-induced phosphorylation (P) of AKT1 and ERK1/2. After a 24-hour treatment with vehicle, NC, and miR-29c-3p(i), followed by a 16-hour serum starvation, HUVECs were treated with ECM-b (control), VEGFA (10 ng/mL) or FGF2 (10 ng/mL) for 10 minutes. Protein samples were subjected to western blotting. (a) Representative blots of P-AKT1, T-AKT1, P-ERK1/2, T-ERK1/2, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Effects of miR-29a/c-3p knockdown on FGF2- and VEGFA-induced relative AKT1 (b) and ERK1/2 (c) phosphorylation (P-AKT1/T-AKT1 and P-ERK1/2/T-ERK1/2, respectively). Data are expressed as median ± SD fold of corresponding control within the same transfection treatment (n = 4). Kruskal–Wallis test was performed. #Differ (P < 0.05) from control group with vehicle treatment; *differ (P < 0.05) among transfection treatment groups.
Compared with control, 10 minutes of VEGFA and FGF2 treatments significantly increased (P < 0.05) the P-ERK1/2/T-ERK1/2 ratio in cells treated with vehicle, NC, and miR-29c-3p(i) [Fig. 3(a) and 3(c)]. In comparison with the vehicle and NC groups, miR-29c-3p(i) did not changed the relative ERK1/2 phosphorylation in HUVECs.
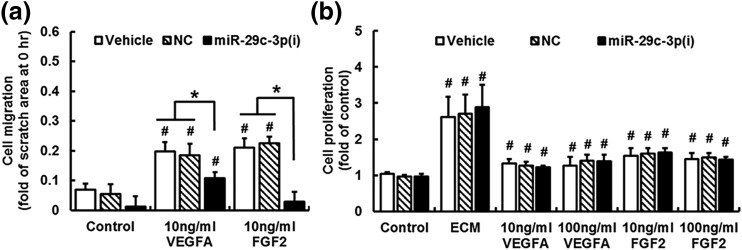
Knockdown of miR-29a/c-3p inhibits the VEGFA- and FGF2-induced cell migration
Results of the scratch healing assay are shown in Fig. 4(a). Compared with vehicle and NC, knockdown of miR-29a/c-3p alone did not significantly alter the cell migration after 20 hours of ECM-b treatment (control). Compared with control, both VEGFA and FGF2 increased (P < 0.05) cell migration by approximately twofold. Knockdown of miR-29a/c-3p substantially inhibited (P < 0.05) the VEGFA-induced cell migration by 44%, while it completely abrogated (P < 0.05) the FGF2-induced cell migration.
Figure 4.
Knockdown of miR-29a/c-3p inhibits VEGFA- and FGF2-stimulated cell migration (a) but not cell proliferation (b) in NT-HUVECs. (a) After treating with vehicle, NC, or miR-29c-3p(i) for 24 hours, confluent cells were scratched followed by treating cells with ECM-b (control), VEGFA, and FGF2 for 20 hours. The scratch areas were measured at 0 and 20 hours. Data normalized to total scratch area at 0 hour are expressed as median ± SD fold of 0 hour (n = 4). Kruskal–Wallis test was performed. #Differ (P < 0.05) from the corresponding control; *differ (P < 0.05) among treatment groups. (b) After treating with Vehicle, NC, and miR-29c-3p(i) for 24 hours and serum-starved in ECM-b for 8 hours, subconfluent cells were treated with ECM-b (control), ECM, VEGFA (10 and 100 ng/mL), or FGF2 (10 and 100 ng/mL). After 42 hours of treatment, cells were subjected to the methylthiazolyl tetrazolium assay. Data are expressed as median ± SD fold of control (n = 6). Kruskal–Wallis test was performed. #Differ (P < 0.05) from control.
Knockdown of miR-29a/c-3p does not affect the VEGFA- and FGF2-induced cell proliferation
After 42 hours of treatment, ECM induced (P < 0.05) the cell proliferation by threefold; VEGFA at 10 ng/mL and 100 ng/mL stimulated (P < 0.05) cell proliferation by 1.3- to ∼1.4-fold; and FGF2 at 10 ng/mL and 100 ng/mL induced (P < 0.05) the cell proliferation by 1.4- to ∼1.6-fold. However, miR-29c-3p(i) did not significantly alter ECM-, VEGFA-, and FGF2-stimulated cell proliferation compared with vehicle and NC [Fig. 4(b)].
Knockdown of miR-29a/c-3p does not affect the FGF2-strengthened and VEGFA-weakened monolayer integrity in HUVECs
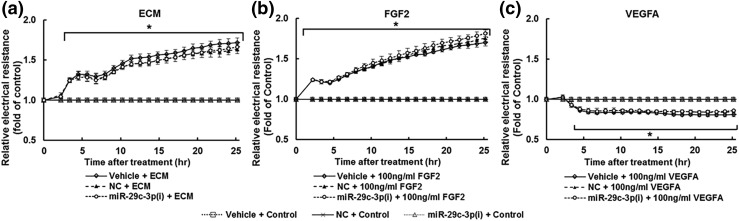
Both ECM [Fig. 5(a)] and FGF2 [Fig. 5(b)] progressively increased (P < 0.05) the electrical resistance of the monolayer of cells, indicating strengthened cell monolayer integrity in a time-dependent manner. Specifically, ECM and FGF2 significantly increased (P < 0.05) the electrical resistance after 2 hours of treatment, and this stimulatory effect was continuously elevated, reaching 164% and 169% of time 0, respectively after 25 hours of treatment. In contrast, VEGFA [100 ng/mL; Fig. 5(c)] decreased (P < 0.05) the electrical resistance by 16% after 6 hours, and this effect was maintained up to 25 hours. No significant difference was observed among cells transfected with vehicle, NC, and miR-29c-3p(i) within all treatment groups (ECM, FGF2, and VEGFA).
Figure 5.
Knockdown of miR-29a/c-3p does not affect the (a) ECM- and (b) FGF2-strengthened monolayer integrity as well as (c) VEGFA-weakened monolayer integrity in NT-HUVECs. After treating with vehicle, NC, or miR-29c-3p(i), cells were cultured until reaching confluent (∼24 hours). Cells were treated with ECM-b (control), ECM, VEGFA (100 ng/mL), or FGF2 (100 ng/mL). The electrical resistance at 4000 Hz was constantly recorded up to 25 hours. Data are expressed as median ± SD fold of control at corresponding time (n = 4). Kruskal–Wallis test was performed. *Differ (P < 0.05) from control within each corresponding transfection treatment.
Discussion
Preeclampsia is characterized by impaired maternal endothelial function including endothelial proliferations and vasodilator production (4–7). Although little is known about preeclampsia-associated fetal endothelial dysfunction and its underlying mechanisms, PE-offspring exhibit increased risk of developing cardiovascular disorders (such as stroke) later in life (10, 11). In addition, preeclampsia adversely affects Ca2+ responses and nitric oxide production in fetal endothelial cells (4, 6, 7). In this study, we identified a set of preeclampsia-associated DE-miRNAs in highly purified, P0 fetal endothelial cells, which might closely mimic the status of fetal endothelial cells in vivo. We also demonstrated (1) the 16 DE-miRNAs including miR-29a/c-3p were all downregulated in PE vs NT P0-HUVECs, which predicted to be associated with dysregulation of many key signaling pathways; (2) knockdown of miR-29a/c-3p inhibited the FGF2-induced relative AKT1 phosphorylation in HUVECs; and (3) knockdown of miR-29a/c-3p greatly inhibited the VEGFA- and FGF2-induced endothelial cell migration in HUVECs. These data indicate that preeclampsia dysregulates an array of miRNAs that may play important roles in fetal endothelial function. Additionally, our current data suggest that downregulation of miR-29a/c-3p impairs the VEGFA- and FGF2-stimulated fetal endothelial cell migration, the latter of which is associated with decreased activation of the AKT1 signaling pathway.
To date, little is known about PE-associated DE-miRNAs in fetal endothelial cells and effects of these miRNAs on fetal endothelial function. Previous genome-wide miRNA profiling studies have identified several sets of DE-miRNAs in PE placentas (14, 16–18). However, these sets of DE-miRNAs are highly variable, possibly due to the heterogeneity of methodologies used in tissue collection and analysis among different studies. It is difficult to assess the quantitative changes and roles of miRNAs in any single cell type in the placenta when DE-miRNAs are identified from whole placentas. In contrast, we identified a set of 16 PE-associated DE-miRNAs in a single population of HUVECs. Among these miRNAs, miR-101-5p (38), miR-1293 (39), miR-146a-5p (14), miR-192-5p (14), miR-29a-3p (19, 40), miR-29b-3p (41), and miR-744-3p (40) have been previously linked to preeclampsia in different tissues including cord blood (38), maternal plasma (19), and placentas (14, 40). To our knowledge, this is the first reported study that relates these miRNAs with preeclampsia in P0-HUVECs. More importantly, this is the first report, to our knowledge, that connects differential expression of miR-219a-1-3p, miR-29c-3p, miR-3186-5p, miR-337-3p, miR-369-5p, miR-4288, miR-551b-3p, miR-598-3p, and miR-617 with preeclampsia. These results indicate the existence of fetal endothelial cells-specific dysregulation of miRNAs in preeclampsia.
It is noted that all 16 DE-miRNAs identified in this study were downregulated in PE vs NT P0-HUVECs. The mechanism underlying this downregulation is unknown. However, it is possible that multiple factors, including dysregulation of growth factors and cytokines as well as hypoxia, could be involved, as they are associated with development of preeclampsia (4, 6, 7). Moreover, given that miRNAs mainly function as negative posttranscriptional regulators of their target genes (42), downregulation of these miRNAs might increase expression of their direct target genes and potentially lead to fetal endothelial dysfunction in preeclampsia (4, 6, 7).
Bioinformatics analyses of the 16 PE-downregulated miRNAs predicted that many endothelial function-associated signaling pathways are dysregulated in PE P0-HUVECs compared with NT P0-HUVECs, including estrogen signaling pathway (43), TGF-β signaling pathway (44), Focal adhesion (45), and PI3K-Akt signaling pathway (20, 46) [Fig. 1(b)]. These pathways are important in regulating pregnancy-associated cardiovascular adaptations in normal vascular development and various aspects of endothelial function, such as proliferation, migration, and endothelial monolayer integrity. It is possible that these DE-miRNAs contribute to the preeclampsia-associated endothelial dysfunction via dysregulating these endothelial function-associated pathways. Specifically, miR-29a/c-3p was associated with all of the pathways that were predicted to be dysregulated in PE vs NT P0-HUVECs [Fig. 1(b)]. This prediction strongly supports the notion that miR-29a/c-3p might be key miRNAs involved in dysregulation of fetal endothelial function in preeclampsia.
Altered expression of the miR-29 family is associated with cardiac fibrosis (35) and heart failure (36) in adults. The miR-29 family is expressed in human endothelium and regulates many aspects of angiogenesis (47–49). For instance, miR-29a overexpression enhances serum-induced cell proliferation and cell cycle process while miR-29a suppression inhibits cell proliferation (47) and completely blocks TGF-β1-stimulated angiogenesis via the AKT signaling pathway (48) in HUVECs. In addition, overexpression of miR-29a also promotes angiogenesis in vivo (48). As VEGFA (50) and many members of the FGF receptor pathway are regulation targets of miR-29a/c-3p (Supplemental Table 5 (212KB, pdf) ), miR-29a/c-3p downregulation is likely to disturb the VEGFA- and FGF2-induced fetal endothelial function in preeclampsia. To elucidate the effect of miR-29a/c-3p downregulation on different aspects of fetal endothelial function, we performed cell functional assays on preestablished NT-HUVECs (24, 27) with miR-29a/c-3p knockdown. Our present findings extend the role of miR-29a/c-3p in the regulation of VEGFA- and FGF2-induced cellular responses in HUVECs.
Consistent with our previous reports (24, 27), our current data show that VEGFA and FGF2 significantly induced the AKT1 and ERK1/2 protein phosphorylation in HUVECs. AKT and VEGF are direct regulation targets of miR-29c, and overexpression of miR-29c inhibits the total VEGF and phosphorylation of AKT under serum-free condition (49). Since miRNAs function as negative posttranscriptional regulators, it is unsurprising that knockdown of miR-29a/c-3p does not change the relative phosphorylation of AKT1 in HUVECs under serum-free condition. Importantly, miR-29a/c-3p knockdown specifically inhibited the FGF2-induced relative phosphorylation of AKT1 protein (P-AKT1/T-AKT1 ratio; indicative for AKT1 activation). This observation is in agreement with our current bioinformatics analyses showing that the PI3K/AKT signaling pathway is a regulation target of miR-29a/c-3p [Fig. 1(b)]. These data suggest that miR-29a/c-3p may regulate the FGF2-induced endothelial function through the AKT1 pathway. Our data also revealed that miR-29a/c-3p knockdown did not significantly change phosphorylation of ERK1/2 (P-ERK1/2 /T-ERK1/2 ratio). In addition, the ERK1/2 pathway was not predicted as an enriched pathway among preeclampsia-induced DE-miRNAs in our bioinformatics analysis. It is reasonable to conclude that miR-29a/c-3p knockdown does not affect the ERK1/2 pathway in HUVECs. Thus, miR-29a/c-3p may play important roles in the FGF2-induced activation of AKT1, but not the ERK1/2 pathway in preeclampsia HUVECs. Although miR-29a/c-3p knockdown partially inhibited the VEGFA-induced cell migration, our data suggest that this inhibition is independent of AKT1 and ERK1/2 pathways in HUVECs.
The fact that miR-29a/c-3p knockdown inhibited FGF2- and VEGFA-induced cell migration in HUVECs indicates that miR-29a/c are proangiogenic, which is consistent with previous findings (47, 48). These data are also in line with a previous report that miR-29c overexpression promotes the cell migration in HUVECs under serum-free condition (49). Notably, these results indicate that although miR-29a/c-3p are important in the VEGFA-induced cell migration, they are required for FGF2-induced cell migration in HUVECs. In addition, though overexpression of miR-29a (47) and miR29c (49) both promote cell proliferation in HUVECs under serum-free condition, knockdown of miR-29a/c-3p did not change basal cell proliferation in HUVECs in the current study. It is noteworthy that miR-29a/c-3p knockdown only significantly affected the VEGFA- and FGF2-induced cell migration, but not cell proliferation and monolayer integrity in HUVECs, suggesting that miR-29a/c-3p differentially regulate different aspects of fetal angiogenesis.
A limitation of our study is that the sample sizes in this study are relatively small. In addition, all miRNA knockdown studies were performed in passages 3 to 4 NT-HUVECs. Because NT-HUVECs, but not PE-HUVECs, should maintain many of their original phenotypes in culture as reported (5, 51).
Conclusions
Preeclampsia downregulates expression of 16 miRNAs including miR-29a/c-3p in fetal endothelial cells. In addition, preeclampsia-induced downregulation of miR-29a/c-3p may impair fetal endothelial cell migration by disturbing the FGF2-stimulated PI3K-AKT1 pathway. Though more studies are necessary, these observations suggest that preeclampsia-induced downregulation of miRNAs in fetal endothelial cells might contribute to early fetal programming that may be associated with developmental origins of cardiovascular diseases of preeclampsia offspring (10, 11), as impaired angiogenesis is a hallmark of various cardiovascular diseases.
Perspectives
Although the concept of fetal origins of adult diseases is widely accepted, reliable molecular biomarkers for predicting late-life vascular disorders are not well-defined. Dysregulation of miRNAs has been linked with preeclampsia; however, the mechanisms underlying such miRNAs dysregulation and miRNAs’ roles in maternal and fetal endothelial function in preeclampsia are still not well understood. If clinical importance of these preeclampsia-associated DE-miRNAs in fetal endothelial cells are fully confirmed, they may serve as novel risk predictors for the development of cardiovascular diseases in preeclampsia offspring later in life.
Acknowledgments
We thank Lori Uttech-Hanson from the Office of Grant Writing and Collaborative Project Development, University of Wisconsin–Madison for English editing and Dr. Emmanuel Sampene from Department of Biostatistics and Medical Informatics, University of Wisconsin–Madison for statistical analysis consulting. We also thank Rosalina Villalon-Landeros, Vladimir L.Vargas, Mayra B. Pastore, and Bryan C. Ampey for their help in preparing this manuscript.
Current affiliation: R.R. Magness’s current affiliation is the Department of Obstetrics and Gynecology, Perinatal Research Center, University of South Florida, Tampa, FL 33620.
Acknowledgments
This study is supported by National Institutes of Health Grants P01HD38843 (J.Z. and R.R.M.) and R01HL117341 (R.R.M.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AKT1
- v-akt murine thymoma viral oncogene homolog 1
- DE
- differentially expressed
- ECM
- endothelial culture medium
- ECM-b
- endothelial culture medium-basal
- FDR
- false discovery rate
- FGF2
- fibroblast growth factor 2
- HUVEC
- human umbilical vein endothelial cell
- miRNA
- microRNA
- NC
- negative control
- NT
- normotensive
- P-AKT1
- phosphorylated AKT1
- PCR
- polymerase chain reaction
- PE
- preeclamptic
- RT-qPCR
- quantitative reverse transcription polymerase chain reaction
- T-AKT1
- total AKT1
- TGF
- transforming growth factor
- P0
- unpassaged
- VEGFA
- vascular endothelial growth factor A.
References
- 1.Askie LM, Duley L, Henderson-Smart DJ, Stewart LA; PARIS Collaborative Group . Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791–1798. [DOI] [PubMed] [Google Scholar]
- 2.Anderson UD, Olsson MG, Kristensen KH, Åkerström B, Hansson SR. Review: biochemical markers to predict preeclampsia. Placenta. 2012;33(Suppl):S42–S47. [DOI] [PubMed] [Google Scholar]
- 3.Ducat A, Doridot L, Calicchio R, Méhats C, Vilotte JL, Castille J, Barbaux S, Couderc B, Jacques S, Letourneur F, Buffat C, Le Grand F, Laissue P, Miralles F, Vaiman D. Endothelial cell dysfunction and cardiac hypertrophy in the STOX1 model of preeclampsia. Sci Rep. 2016;6:19196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123(24):2856–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Gu Y, Granger DN, Roberts JM, Alexander JS. Endothelial junctional protein redistribution and increased monolayer permeability in human umbilical vein endothelial cells isolated during preeclampsia. Am J Obstet Gynecol. 2002;186(2):214–220. [DOI] [PubMed] [Google Scholar]
- 6.Boeldt DS, Hankes AC, Alvarez RE, Khurshid N, Balistreri M, Grummer MA, Yi F, Bird IM. Pregnancy programming and preeclampsia: identifying a human endothelial model to study pregnancy-adapted endothelial function and endothelial adaptive failure in preeclamptic subjects. Adv Exp Med Biol. 2014;814:27–47. [DOI] [PubMed] [Google Scholar]
- 7.Bird IM, Boeldt DS, Krupp J, Grummer MA, Yi FX, Magness RR. Pregnancy, programming and preeclampsia: gap junctions at the nexus of pregnancy-induced adaptation of endothelial function and endothelial adaptive failure in PE. Curr Vasc Pharmacol. 2013;11(5):712–729. [DOI] [PubMed] [Google Scholar]
- 8.Wang K, Zheng J. Signaling regulation of fetoplacental angiogenesis. J Endocrinol. 2012;212(3):243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munaron L, Fiorio Pla A. Endothelial calcium machinery and angiogenesis: understanding physiology to interfere with pathology. Curr Med Chem. 2009;16(35):4691–4703. [DOI] [PubMed] [Google Scholar]
- 10.Koleganova N, Benz K, Piecha G, Ritz E, Amann K. Renal, cardiovascular and metabolic effects of fetal programming. Nephrol Dial Transplant. 2012;27(8):3003–3007. [DOI] [PubMed] [Google Scholar]
- 11.Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke. 2009;40(4):1176–1180. [DOI] [PubMed] [Google Scholar]
- 12.Piva R, Spandidos DA, Gambari R. From microRNA functions to microRNA therapeutics: novel targets and novel drugs in breast cancer research and treatment (review). Int J Oncol. 2013;43(4):985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie L, Mouillet JF, Chu T, Parks WT, Sadovsky E, Knöfler M, Sadovsky Y. C19MC microRNAs regulate the migration of human trophoblasts. Endocrinology. 2014;155(12):4975–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Feng L, Zhang H, Hachy S, Satohisa S, Laurent LC, Parast M, Zheng J, Chen DB. Preeclampsia up-regulates angiogenesis-associated microRNA (i.e., miR-17, -20a, and -20b) that target ephrin-B2 and EPHB4 in human placenta. J Clin Endocrinol Metab. 2012;97(6):E1051–E1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu F, Yang Z, Li G. Role of specific microRNAs for endothelial function and angiogenesis. Biochem Biophys Res Commun. 2009;386(4):549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enquobahrie DA, Abetew DF, Sorensen TK, Willoughby D, Chidambaram K, Williams MA. Placental microRNA expression in pregnancies complicated by preeclampsia. Am J Obstet Gynecol. 2011;204(2):178.e12–178.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu XM, Han T, Sargent IL, Yin GW, Yao YQ. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. Am J Obstet Gynecol. 2009;200(6):661.e1–661.e7. [DOI] [PubMed] [Google Scholar]
- 18.Choi SY, Yun J, Lee OJ, Han HS, Yeo MK, Lee MA, Suh KS. MicroRNA expression profiles in placenta with severe preeclampsia using a PNA-based microarray. Placenta. 2013;34(9):799–804. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Ge Q, Guo L, Lu Z. Maternal plasma miRNAs expression in preeclamptic pregnancies. BioMed Res Int. 2013;2013:970265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. [DOI] [PubMed] [Google Scholar]
- 21.Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10(2):116–129. [DOI] [PubMed] [Google Scholar]
- 22.Chung JY, Song Y, Wang Y, Magness RR, Zheng J. Differential expression of vascular endothelial growth factor (VEGF), endocrine gland derived-VEGF, and VEGF receptors in human placentas from normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2004;89(5):2484–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozkan S, Vural B, Filiz S, Coştur P, Dalçik H. Placental expression of insulin-like growth factor-I, fibroblast growth factor-basic, and neural cell adhesion molecule in preeclampsia. J Matern Fetal Neonatal Med. 2008;21(11):831–838. [DOI] [PubMed] [Google Scholar]
- 24.Jiang YZ, Li Y, Wang K, Dai CF, Huang SA, Chen DB, Zheng J. Distinct roles of HIF1A in endothelial adaptations to physiological and ambient oxygen. Mol Cell Endocrinol. 2014;391(1-2):60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91(10):3527–3561. [PubMed] [Google Scholar]
- 26.National Institutes of Health; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program. Working Group Report on High Blood Pressure in Pregnancy. 2000. NIH publication no. 00-3029. Available at: https://www.nhlbi.nih.gov/files/docs/guidelines/hbp_preg_archive.pdf
- 27.Jiang YZ, Wang K, Li Y, Dai CF, Wang P, Kendziorski C, Chen DB, Zheng J. Transcriptional and functional adaptations of human endothelial cells to physiological chronic low oxygen. Biol Reprod. 2013;88(5):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vlachos IS, Zagganas K, Paraskevopoulou MD, Georgakilas G, Karagkouni D, Vergoulis T, Dalamagas T, Hatzigeorgiou AG. DIANA-miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res. 2015;43(W1):W460–W466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ukai T, Sato M, Akutsu H, Umezawa A, Mochida J. MicroRNA-199a-3p, microRNA-193b, and microRNA-320c are correlated to aging and regulate human cartilage metabolism. J Orthop Res. 2012;30(12):1915–1922. [DOI] [PubMed] [Google Scholar]
- 30.Dai CF, Jiang YZ, Li Y, Wang K, Liu PS, Patankar MS, Zheng J. Expression and roles of Slit/Robo in human ovarian cancer. Histochem Cell Biol. 2011;135(5):475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Wang K, Zou QY, Magness RR, Zheng J. 2,3,7,8-Tetrachlorodibenzo-p-dioxin differentially suppresses angiogenic responses in human placental vein and artery endothelial cells. Toxicology. 2015;336:70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong F, Zhou X, Li C, Yan S, Deng X, Cao Z, Li L, Tang B, Allen TD, Liu J. Dihydroartemisinin targets VEGFR2 via the NF-κB pathway in endothelial cells to inhibit angiogenesis. Cancer Biol Ther. 2014;15(11):1479–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57(1):289–300. [Google Scholar]
- 34.Zhou C, Dobrinsky J, Tsoi S, Foxcroft GR, Dixon WT, Stothard P, Verstegen J, Dyck MK. Characterization of the altered gene expression profile in early porcine embryos generated from parthenogenesis and somatic cell chromatin transfer. PLoS One. 2014;9(3):e91728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thum T, Lorenzen JM. Cardiac fibrosis revisited by microRNA therapeutics. Circulation. 2012;126(7):800–802. [DOI] [PubMed] [Google Scholar]
- 36.Thum T, Galuppo P, Wolf C, Fiedler J, Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J, Haverich A, Gross C, Engelhardt S, Ertl G, Bauersachs J. MicroRNAs in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation. 2007;116(3):258–267. [DOI] [PubMed] [Google Scholar]
- 37.Podar K, Anderson KC. The pathophysiologic role of VEGF in hematologic malignancies: therapeutic implications. Blood. 2005;105(4):1383–1395. [DOI] [PubMed] [Google Scholar]
- 38.Ching T, Ha J, Song MA, Tiirikainen M, Molnar J, Berry MJ, Towner D, Garmire LX. Genome-scale hypomethylation in the cord blood DNAs associated with early onset preeclampsia. Clin Epigenetics. 2015;7(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castelli EC, Moreau P, Oya e Chiromatzo A, Mendes-Junior CT, Veiga-Castelli LC, Yaghi L, Giuliatti S, Carosella ED, Donadi EA. In silico analysis of microRNAS targeting the HLA-G 3′ untranslated region alleles and haplotypes. Hum Immunol. 2009;70(12):1020–1025. [DOI] [PubMed] [Google Scholar]
- 40.Zhang C, Li Q, Ren N, Li C, Wang X, Xie M, Gao Z, Pan Z, Zhao C, Ren C, Yang W. Placental miR-106a∼363 cluster is dysregulated in preeclamptic placenta. Placenta. 2015;36(2):250–252. [DOI] [PubMed] [Google Scholar]
- 41.Li P, Guo W, Du L, Zhao J, Wang Y, Liu L, Hu Y, Hou Y. microRNA-29b contributes to pre-eclampsia through its effects on apoptosis, invasion and angiogenesis of trophoblast cells. Clin Sci (Lond). 2013;124(1):27–40. [DOI] [PubMed] [Google Scholar]
- 42.Kinoshita T, Nohata N, Hanazawa T, Kikkawa N, Yamamoto N, Yoshino H, Itesako T, Enokida H, Nakagawa M, Okamoto Y, Seki N. Tumour-suppressive microRNA-29s inhibit cancer cell migration and invasion by targeting laminin-integrin signalling in head and neck squamous cell carcinoma. Br J Cancer. 2013;109(10):2636–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jobe SO, Tyler CT, Magness RR. Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertension. 2013;61(2):480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Huang XR, Wei LH, Chung AC, Yu CM, Lan HY. miR-29b as a therapeutic agent for angiotensin II-induced cardiac fibrosis by targeting TGF-β/Smad3 signaling. Mol Ther. 2014;22(5):974–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quadri SK, Bhattacharjee M, Parthasarathi K, Tanita T, Bhattacharya J. Endothelial barrier strengthening by activation of focal adhesion kinase. J Biol Chem. 2003;278(15):13342–13349. [DOI] [PubMed] [Google Scholar]
- 46.Wang K, Jiang YZ, Chen DB, Zheng J. Hypoxia enhances FGF2- and VEGF-stimulated human placental artery endothelial cell proliferation: roles of MEK1/2/ERK1/2 and PI3K/AKT1 pathways. Placenta. 2009;30(12):1045–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Z, Wu L, Zhu X, Xu J, Jin R, Li G, Wu F. MiR-29a modulates the angiogenic properties of human endothelial cells. Biochem Biophys Res Commun. 2013;434(1):143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Wang Y, Wang Y, Ma Y, Lan Y, Yang X. Transforming growth factor β-regulated microRNA-29a promotes angiogenesis through targeting the phosphatase and tensin homolog in endothelium. J Biol Chem. 2013;288(15):10418–10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu Y, Deng F, Song J, Lin J, Li X, Tang Y, Zhou J, Tang T, Zheng L. Evaluation of miR-29c inhibits endotheliocyte migration and angiogenesis of human endothelial cells by suppressing the insulin like growth factor 1. Am J Transl Res. 2015;7(3):489–501. [PMC free article] [PubMed] [Google Scholar]
- 50.Kishore S, Jaskiewicz L, Burger L, Hausser J, Khorshid M, Zavolan M. A quantitative analysis of CLIP methods for identifying binding sites of RNA-binding proteins. Nat Methods. 2011;8(7):559–564. [DOI] [PubMed] [Google Scholar]
- 51.Krupp J, Boeldt DS, Yi FX, Grummer MA, Bankowski Anaya HA, Shah DM, Bird IM. The loss of sustained Ca(2+) signaling underlies suppressed endothelial nitric oxide production in preeclamptic pregnancies: implications for new therapy. Am J Physiol Heart Circ Physiol. 2013;305(7):H969–H979. [DOI] [PMC free article] [PubMed] [Google Scholar]