Abstract
Context:
Adrenal incidentalomas (AIs) are found commonly on axial imaging. Around 30% exhibit autonomous cortisol secretion (ACS) associated with increased cardiovascular events and death.
Objective:
We hypothesized that AI/ACS patients have an abnormal cortisol rhythm that could be reversed by use of carefully timed short-acting cortisol synthesis blockade, with improvement in cardiovascular disease markers.
Design, Setting, and Participants:
In a phase 1/2a, prospective study (Eudract no. 2012-002586-35), we recruited six patients with AI/ACS and two control groups of six sex-, age-, and body mass index–matched individuals: (1) patients with AI and no ACS (AI/NoACS) and (2) healthy volunteers with no AI [healthy controls (HC)]. Twenty-four-hour circadian cortisol analysis was performed to determine any differences between groups and timing of intervention for cortisol lowering using the 11β-hydroxylase inhibitor metyrapone. Circadian profiles of serum interleukin-6 (IL-6) were assessed.
Results:
Serum cortisol levels in group AI/ACS were significantly higher than both group AI/NoACS and group HC from 6 pm to 10 pm [area under the curve (AUC) difference: 0.81 nmol/L/h; P = 0.01] and from 10 pm to 2 am (AUC difference: 0.86 nmol/L/h; P < 0.001). In light of these findings, patients with ACS received metyrapone 500 mg at 6 pm and 250 mg at 10 pm, and cortisol rhythms were reassessed. Postintervention evening serum cortisol was lowered, similar to controls [6 pm to 10 pm (AUC difference: –0.06 nmol/L/h; P = 0.85); 10 pm to 2 am (AUC difference: 0.10 nmol/L/h; P = 0.76)]. Salivary cortisone showed analogous changes. IL-6 levels were elevated before treatment [10 pm to 2 pm (AUC difference: 0.42 pg/mL/h; P = 0.01)] and normalized post treatment.
Conclusions:
In AI/ACS, the evening and nocturnal cortisol exposure is increased. Use of timed evening doses of metyrapone resets the cortisol rhythm to normal. This unique treatment paradigm is associated with a reduction in the cardiovascular risk marker IL-6.
In patients with AI and ACS, evening doses of metyrapone reset the abnormal cortisol rhythm to normal and reduce the cardiovascular risk marker IL-6.
Patients with adrenal incidentalomas (AIs) and low-grade excess cortisol secretion have higher mortality due to cardiovascular events and infections (1, 2). The prevalence of AI is <1% of those aged 20 years but increases to around 10% of those aged 70 years (3). Between 30% and 50% of AI exhibit low-grade cortisol secretion variously termed “subclinical Cushing,” “subclinical hypercortisolism,” or, more recently, “autonomous cortisol secretion (ACS)” (4, 5), because patients lack the classical clinical features of Cushing syndrome (6, 7). Nevertheless, patients with AI/ACS have more cardiovascular events, osteoporosis and fractures, and incident diabetes (1, 2, 8–11). Together these data strongly support that AI/ACS is detrimental to health and that this is a common problem.
A blunted cortisol circadian rhythm, with higher evening and night levels, is a sensitive indicator of overt Cushing syndrome (6, 12). In AI, however, the cortisol rhythm has never been systematically studied in fine detail by measuring hourly cortisol levels. Normally, cortisol concentrations reach a nadir around midnight, and rise at around 2 am to 4 am, peaking 30 to 60 minutes after waking and then gradually decline toward the quiescent phase in the early evening. In overt Cushing, inappropriately high cortisol levels result in multiple complications, including impaired glucose tolerance and diabetes, visceral obesity, psychiatric illness, metabolic bone and cardiovascular disease, infections, and increased mortality (6, 13). Interleukin-6 (IL-6) is high in patients with Cushing syndrome (14, 15), and this cytokine is associated with endothelial dysfunction and implicated in the pathogenesis of atherosclerosis (16), a recognized complication of AI/ACS (17).
We hypothesized, therefore, that patients with AI/ACS, as identified using the 1-mg dexamethasone suppression test (ONDST), have higher evening cortisol concentrations due to autonomous secretion from the adrenal, with this being associated with a state of low-grade inflammation, as indicated by IL-6 levels. We have assessed the baseline cortisol and IL-6 rhythms in these patients and then tested whether administration of a short-acting 11β-hydroxylase inhibitor, metyrapone, at specific time points could “reset” the cortisol rhythm in patients and what impact this would have acutely on IL-6 levels.
Methods
Study design and patients
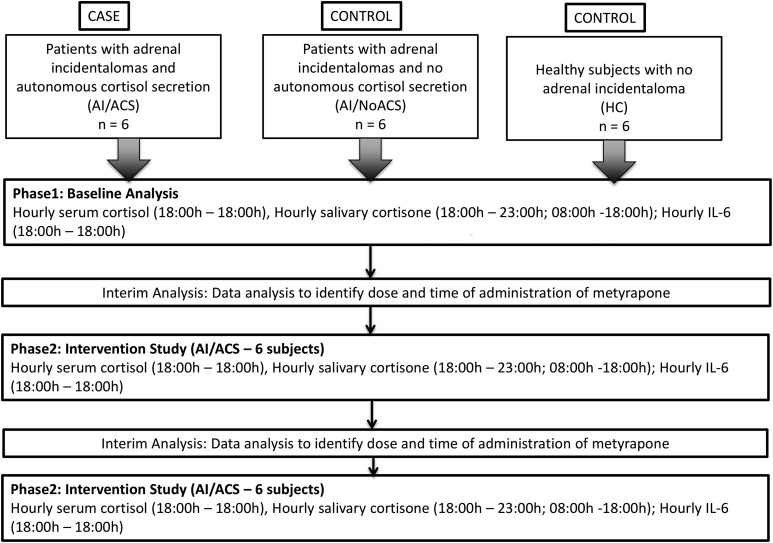
This was a phase 1/2a, prospective, open-label, controlled, single-center study carried out in the National Institute for Health Research Clinical Research Facility, Sheffield Teaching Hospitals National Health Service Foundation Trust, United Kingdom, in patients diagnosed with AI (Fig. 1). Six patients (group AI/ACS) with unilateral or bilateral AI showing benign characteristics on a computed tomography scan (precontrast <10 Hounsfield units) or magnetic resonance imaging together with an ONDST serum cortisol >80 nmol/L or 60 to 80 nmol/L with an adrenocorticotropic hormone (ACTH) <2.2 pmol/L (10 pg/mL) and no features of clinical Cushing syndrome were recruited. Baseline characteristics were similar to the target population of interest (Supplemental Table 1) (8). Two control groups of six sex-, body mass index (BMI)–, and age-matched subjects were recruited who had an ONDST serum cortisol <50 nmol/L: (1) AI and no ACS (group AI/NoACS) or (2) normal adrenal glands on abdominal magnetic resonance imaging [group healthy controls (HCs)]. Inclusion criteria were 45- to 80-year-old males and postmenopausal females and stable antihypertensive and diabetic medications for 4 weeks prior to screening. Exclusion criteria were patients with clinical features associated with overt Cushing syndrome, history of malignancy, alcohol dependence or drug abuse, primary adrenocortical insufficiency, severe uncontrolled diabetes [fasting plasma glucose >15.0 mmol/L or glycated hemoglobin >9% (75 mmol/mol)], severe uncontrolled hypertension (>190/120 mm Hg), severe liver disease, renal impairment (serum creatinine ≥120 μmol/L), clinically significantly impaired cardiovascular function, uncontrolled severe active infection, night-shift workers, patients with depression or psychosis, treatment with glucocorticoids (oral, spray, or cream) in the last 3 months, concomitant treatment with any other drug known to affect the hypothalamo-pituitary-adrenal axis, cortisol-binding globulin, or the CYP450 3A4 cytochrome system, and adrenocortical tumors >4 cm (Supplemental Fig. 1 (652.5KB, tif) ).
Figure 1.
Adaptive study design. Figure shows study design highlighting two study phases: baseline analysis (phase 1) and intervention study (phase 2). Interim analysis between phases was organized for research team to analyze data and assess what dose and at what time metyrapone should be administered. At interim analysis 1, all baseline data were analyzed, and based on the results, the decision was taken to administer 500 mg of metyrapone at 6 pm. At interim analysis 2, all data from phase 2 were analyzed, and based on the results, the decision was taken to administer 500 mg of metyrapone at 6 pm and 250 mg at 10 pm.
The study protocol was approved by the East Leeds National Research Ethics Service committee and the Medicines and Health Regulatory Authority, United Kingdom. Written informed consent was obtained from all participants. The study was registered with the European Clinical Trials Database (Eudract no. 2012-002586-35) and is reported according to the Transparent Reporting of Evaluations with Nonrandomized Designs.
Baseline study
For study visits, participants arrived at the clinical research facility around 2 hours before study start (approximately 4 pm) for a 24-hour sampling period. An intravenous cannula was inserted with a three-way tap for sampling via long extension line to avoid disturbing sleep and kept patent by slow intravenous 0.9% normal saline. Dead space volume was discarded at each sampling time point prior to sample collection. Standard meals were provided at 6 pm, 7:30 am, and 12:30 pm. The study was carried out under stable environmental conditions, and lights were turned off at 11 pm. Hourly blood samples (day 1 6 pm to day 2 6 pm) and hourly salivary cortisol/cortisone during waking hours between 6 pm and 11 pm and between 8 am and 6 pm were collected. Patients were not allowed to eat, drink, or wash their teeth for 30 minutes before salivary tests. Full blood count, liver function tests, urea, creatinine and electrolytes, fasting blood glucose and lipids, and 8 am ACTH were also measured.
Interventional study
By a priori design (Fig. 1), an interim analysis was performed on all baseline data to assess if there were differences in the cortisol rhythms between groups. These data were used to determine the timing of administration of metyrapone. Based on these analyses, group AI/ACS was administered 500 mg of metyrapone at 6 pm after a standard meal. Hourly serum cortisol and salivary cortisol/cortisone levels (measured during waking hours only) were measured. These data were then analyzed, and on the basis of these results, a subsequent intervention was performed with metyrapone 500 mg at 6 pm after a standard meal and 250 mg at 10 pm with a snack in patients (group AI/ACS). Similar investigations to those carried out during the baseline visit were performed during these interventions.
Assays
Serum and salivary cortisol and salivary cortisone were measured by liquid chromatography and tandem mass spectrometry, as previously described (18). Serum IL-6 was measured by a bead-based immunoassay, and the limit of detection was 1.6 pg/mL (19). The normal upper limit of IL-6 is 4 pg/mL (20). The intra-assay coefficient of variation (CV) was 3% at 35.6 pg/mL, and the interassay CV was 6% at 38.8 pg/mL. Plasma ACTH was measured in a Siemens Immulite 2000 chemiluminescent assay (Siemens, Frimley, United Kingdom): analytical range, 1.1 to 275 pmol/L; interassay CVs, 6.1% at 7.5 pmol/L and 4.3% at 100 pmol/L. Dehydroepiandrosterone was measured by LC-MS/MS.
Statistical analysis
Data are summarized per group and time points by descriptive statistics using number of patients (N), mean, standard deviation (SD) for continuous variables, and absolute counts and relative frequencies (n and %) for categorical data. Pharmacokinetic analyses were performed using WinNonlin Professional version 5.3 software (Certara USA, Princeton, NJ) and Matlab version 8.2 (Mathworks, Natick, MA). Concentration time profiles were designed for serum cortisol, salivary cortisone, and serum IL-6 at baseline and postintervention (metyrapone). As salivary cortisone has been shown to be a superior surrogate marker for serum cortisol when compared with salivary cortisol, our analysis is based on salivary cortisone measurements (18). The primary endpoint for the analysis was the geometric 4-hourly areas under the curve (AUCs) over 24 hours starting from 6 pm. Wilcoxon signed rank test was used for paired sample analysis. Where samples were unpaired, the differences and P values are computed using bootstrapped (N = 50,000) Welch’s t test. AUC was computed from log10 transformed data. Missing values were linearly interpolated. The full 24-hour profile was also assessed for salivary cortisone. Missing values (nighttime and sporadic) are imputed by applying the inverse of our published linear mixed-effects model to serum cortisol values (18). Similar AUCs were measured for IL-6.
For 24-hour AUCs, independent sample t test was used for normally distributed data and Mann-Whitney U test for nonnormally distributed data. Other secondary endpoints were the peak (Cmax), time of peak (Tmax) from midnight, trough (Cmin), and relative amplitude [absolute amplitude (50% of the difference between the level attained at Cmax and the level attained at Cmin) expressed as a percentage of the 24-hour mean cortisol]. Analysis of variance (normally distributed) with least significance difference as post hoc test or Kruskal-Wallis tests (not normally distributed) were used to assess for differences in pharmacokinetic parameters between groups.
Results
A total of 20 participants were included in the study: six healthy subjects, six patients with AI/NoACS, and eight patients with AI/ACS (Table 1). Two AI/ACS patients were withdrawn from the study after the first intervention, one for an unrelated serious adverse event (prostate adenocarcinoma, diagnosed during the study) and the other because of work-related time commitment reasons. Two additional AI/ACS patients were included for the subsequent intervention with metyrapone 500 mg at 6 pm and 250 mg at 10 pm. Baseline data for group AI/AS taken into account in the final analysis are from the six patients who concluded the study.
Table 1.
Patient Characteristics
| Age (y) | Weight (kg) | BMI (kg/m2) | Sex | Tumor size (cm) | Dex cortisol (nmol/L) | ACTH (pmol/L) | DHEA (nmol/L) | HT | DM | Statins | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | 60 | 103.2 | 43 | F | 3.5 | 95 | 2.0 | 2.4 | Y | Y | N |
| A2 | 67 | 57 | 24 | M | 2.7 | 59 | 1.6 | 3.3 | N | N | N |
| A3 | 61 | 73.3 | 29.4 | F | 2.6 | 93 | 1.1 | 1.6 | N | N | N |
| A4 | 70 | 66.3 | 25 | F | 1.5 | 101 | 3.1 | 7.0 | N | N | N |
| A5 | 73 | 104 | 32.5 | M | 3.5 | 101 | 6.3 | <1.0 | Y | N | Y |
| A6 | 59 | 95.3 | 31.5 | M | 2.3 | 127 | 1.3 | 2.4 | Y | N | Y |
| B7 | 62 | 80.3 | 33.4 | F | 2.8 | 29 | 2.9 | 8.0 | N | N | Y |
| B8 | 62 | 62.9 | 25.2 | F | 0.7 | 29 | 1.1 | 12.0 | N | N | N |
| B9 | 63 | 104.9 | 29.9 | M | 1.3 | 22 | 2.6 | 5.0 | Y | N | Y |
| B10 | 65 | 93.9 | 31.4 | M | 2.0 | 31 | 4.0 | 2.6 | N | Y | N |
| B11 | 65 | 85.6 | 36.6 | F | 2.0 | 24 | 4.6 | 3.3 | Y | N | Y |
| B12 | 71 | 57.2 | 22.9 | F | 1.8 | 28 | 5.1 | 5.1 | N | N | Y |
| C13 | 64 | 109 | 43.7 | F | 22 | 8.4 | 7.4 | N | N | N | |
| C14 | 72 | 61.9 | 24.8 | F | 29 | 1.8 | 18.8 | N | N | N | |
| C15 | 64 | 113.2 | 35.7 | M | 31 | 4.4 | 7.1 | N | N | N | |
| C16 | 60 | 73.1 | 24.7 | M | 22 | 5.3 | 35.1 | Y | N | N | |
| C17 | 59 | 58.6 | 21.5 | F | 30 | 2.6 | 10.9 | N | N | N | |
| C18 | 68 | 72.5 | 22.6 | M | 30 | 3.1 | 7.4 | Y | N | N |
Abbreviations: Dex, dexamethasone-suppressed serum cortisol; DHEA, dehydroepiandrosterone; DM, diabetes mellitus; F, female; HT, hypertension; M, male; N, no; Y, yes.
There were no differences in sex, mean (SD) age [65 (5.8) vs 65 (3.4) vs 65 (4.9) years; P = 0.9], or BMI [31 (6.9) vs 30 (5.1) vs 29 (8.9) kg/m2; P = 0.6] between groups (Supplemental Table 1). In the AI/ACS group, ONDST serum cortisol varied between 59 and 127 nmol/L; the highest ONDST serum cortisol in the other groups was 31 nmol/L (Supplemental Table 1).
Serum cortisol rhythm at baseline
Analysis of the baseline 24-hour serum cortisol rhythm showed that there were no differences in AUCs between AI/NoACS and HC throughout the entire 24-hour period (P = 0.33). In contrast, levels in AI/ACS were significantly higher than in these groups only between 6 pm and 10 pm (AUC difference: 0.81 nmol/L/h; P = 0.01) and between 10 pm and 2 am (AUC difference: 0.86 nmol/L/h; P < 0.001) [Fig. 2(a)].
Figure 2.
Serum cortisol rhythms. (a) Baseline: Concentration time profiles (geometric mean ± standard error of the mean) of cortisol rhythm in groups AI/ACS, AI/NoACS, and HC. Higher nighttime cortisol exposure between 6 pm and 2 am is evident in group AI/ACS. To convert nmol/L to ug/dL, divide by 27.59. (b) Reset rhythm after metyrapone: Concentration time profiles (geometric mean ± standard error of the mean) show that by administering metyrapone 500 mg at 6 pm and 250 mg at 10 pm, one is able to restore the cortisol rhythm to approximate normal physiological concentrations comparable to groups AI/NoACS and HC. After intervention, all 24-hour AUCs of all three concentration time profiles in the three groups of subjects were similar (P = 0.29). Log-transformed AUC between 6 pm and 10 pm (P = 0.85) and between 10 pm and 2 am (P = 0.76) normalized to physiological levels. To convert nmol/L to ug/dL, divide by 27.59.
The mean (SD) relative amplitude in the AI/ACS group at 85% (21) was significantly lower than either group AI/NoACS, 144% (28), or group HC, 120% (19) (P = 0.002), whereas Cmin was significantly higher in group AI/ACS 94 (45) nmol/L compared with group AI/NoACS 53 (16) nmol/L and group HC 44 (19) nmol/L (P = 0.03), but Cmax (P = 0.18) and Tmax (P = 0.84) were similar in all groups. These data demonstrate blunting of the physiological cortisol rhythm in patients with AI/ACS due to higher minimum evening values.
Serum cortisol rhythm postintervention
In view of the identified serum cortisol differences, the sampling study was repeated in patients with AI/ACS with administration of metyrapone 500 mg at 6 pm to assess if this could “reset” the rhythm to normal. There was a significant reduction in log-converted AUC6 pm–10 pm (P = 0.03) but not in AUC10 pm–2 am (P = 0.17), resulting in serum cortisol levels similar to controls in the 4-hour period after 6 pm (AUC difference: –0.40 nmol/L/h; P = 0.93), but with values then rising toward baseline after 10 pm. In view of this, sampling was repeated in AI/ACS patients with metyrapone 500 mg at 6 pm and 250 mg at 10 pm, resulting in no differences between group AI/ACS and controls in log-transformed AUC between 6 pm and 10 pm (AUC difference: –0.06 nmol/L/h; P = 0.85) and between 10 pm and 2 am (AUC difference: 0.10 nmol/L/h; P = 0.76) in serum cortisol levels throughout the 24-hour period (P = 0.29) and in mean (SD) relative amplitude [117% (39%); P = 0.27], Cmin [56 (26) nmol/L; P = 0.62], Cmax [442 (108) nmol/L; P = 0.21], and Tmax [9 (2.5) hours; P = 0.80]. These data indicate that cortisol exposure in group AI/ACS had been normalized by “resetting” the cortisol rhythm [Fig. 2(b)].
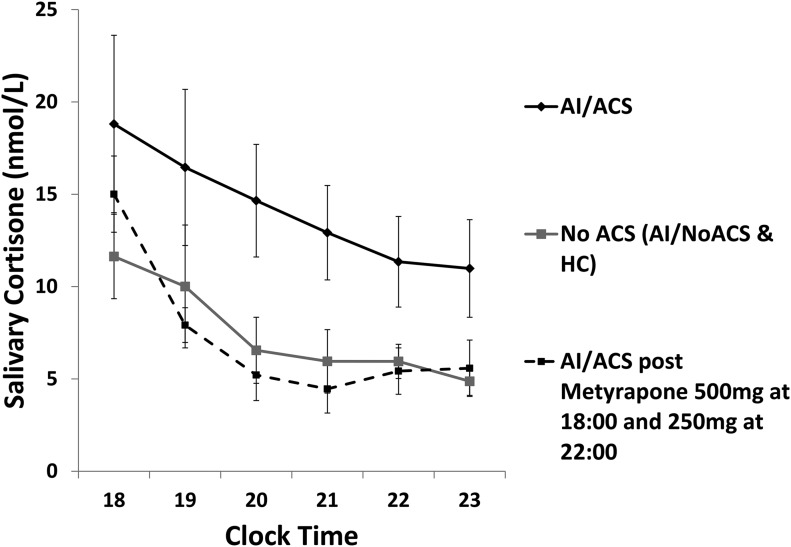
Salivary cortisone and cortisol at baseline
Salivary cortisone accurately reflects total and free serum cortisol levels (18, 21). Log-transformed AUC8 pm–11 pm salivary cortisone was significantly higher (AUC difference: 0.83 nmol/L/h; P < 0.001) in AI/ACS compared with control groups (Fig. 3), whereas AUC8 pm–11 pm salivary cortisone in each control group was similar (AUC difference: 0.4 nmol/L/h; P = 0.4). The correlation between serum cortisol and salivary cortisone was strong (Pearson’s r = 0.94). Results for salivary cortisol were less robust both in the correlation between serum cortisol and salivary cortisol (Pearson’s r = 0.78) and when comparing AI/ACS with control groups (AUC8 pm–11 pm difference: 0.67 nmol/L/h; P < 0.03) (Supplement Fig. 2 (148.1KB, tif) ). Salivary cortisol levels were similar between control groups (AUC8 pm–11 pm difference: 0.28 nmol/L/h; P = 0.35) (Supplement Fig. 2 (148.1KB, tif) ). Because nighttime values for salivary assessments were missing, the fixed-effects components (slope and intercept) of previously described mixed-effects models (18) relating (log) serum cortisol to (log) salivary cortisol or cortisone were used to infer salivary values from measured serum levels through back calculation (inversion). From this, 4-hourly AUCs could be estimated across the full 24 hours. There was no significant difference in 4-hourly AUC salivary cortisone and salivary cortisol between control groups throughout the entire 24-hour period. Calculated salivary cortisone AUC was, however, significantly higher in AI/ACS compared with both control groups between 8 pm and 12 am (AUC difference: 1.1 nmol/L/h; P < 0.001) and between 10 pm and 2 am (AUC difference: 0.96 nmol/L/h; P < 0.001).
Figure 3.
Concentration time profile for salivary cortisone in the evening (geometric mean ± standard error of the mean). Patients with AI/ACS have significantly higher salivary cortisone levels than subjects with no ACS (P < 0.001). Levels are restored to normality after administration of metyrapone 500 mg at 6 pm and 250 mg at 10 pm. The measurement of salivary cortisone could hence be considered an alternative means to calculate changes in serum cortisol rhythm.
Salivary cortisone rhythm postintervention
Post administration of metyrapone 500 mg at 6 pm and 250 mg at 10 pm, AUC8 pm–11 pm salivary cortisone levels in patients with AI/ACS decreased to levels similar to the control groups (AUC difference: –0.12 nmol/L/h; P = 0.71) (Fig. 3). Similarly, between 8 pm and 12 am (AUC difference: –0.1 nmol/L/h; P = 0.82) and between 10 pm and 2 am (AUC difference: 0.10 nmol/L/h; P = 0.79), AUC salivary cortisone normalized, such that salivary cortisone rhythm was reset to normal physiological levels.
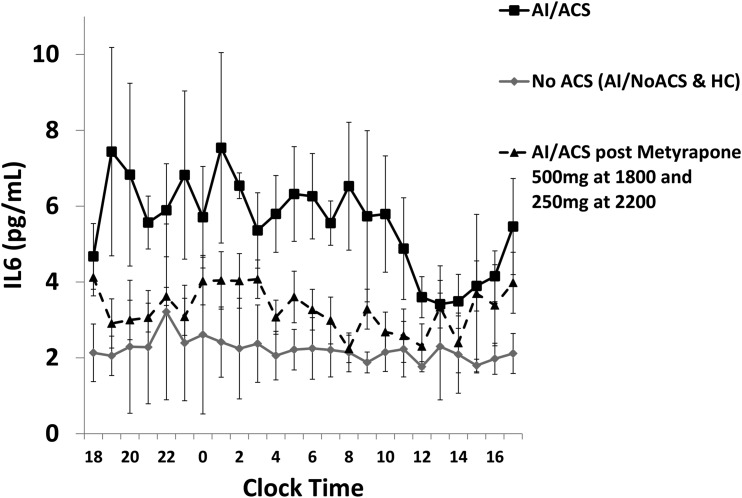
Il-6 levels before and after intervention
At baseline, serum IL-6 levels were higher in patients with AI/ACS compared with both patients with AI/NoACS and subjects in group HC at all log-converted, 4-hourly AUCs between 10 pm and 2 pm (AUC difference: 0.42 pg/mL/h; P = 0.01). There was no difference in serum IL-6 at any time point between patients with AI/NoACS and HC (P = 0.77). After administration of metyrapone 500 mg at 6 pm and 250 mg at 10 pm, serum IL-6 levels in patients with AI/ACS normalized to levels similar to the other two control groups over all time points over 24 hours (P = 0.08) (Fig. 4).
Figure 4.
Concentration time profiles of serum IL-6 levels before and post administration of metyrapone in patients with AI/ACS (geometric mean ± standard error of the mean). Figure indicates higher IL-6 levels in patients with AI/ACS when compared with patients with no ACS (P = 0.01). Concentrations decrease to normal levels, similar to patients with no ACS after the administration of metyrapone (P = 0.08).
Safety profile
For the AI/ACS group that had intervention, six adverse events were reported by four subjects. These included four mild headaches (one possibly related), one episode of hypertension, and an episode of mild dizziness with a serum cortisol level of 22 nmol/L at 11 pm (levels in normal individuals at this time were 5 to 68 nmol/L) (18).
Discussion
We have shown that patients with AI/ACS have an abnormal cortisol rhythm with excess evening/nocturnal cortisol exposure and higher IL-6 levels. To our knowledge, this is the first time where patients with AI have been investigated by detailed analysis of the serum 24-hour cortisol rhythm. Conversely, an ONDST serum cortisol of 31 nmol/L or less in patients with AI is associated with as normal a physiological cortisol rhythm as HCs. Groups were carefully matched for age, sex, and BMI, minimizing the potential impact that these parameters might have on our observations. Administration of metyrapone specifically in the evening allowed the cortisol rhythm to be “reset,” with an immediate improvement in IL-6 levels, a unique mechanism of action in AI/ACS.
Circadian misalignment, as seen in shift workers, is associated with higher cortisol levels in the evening and higher 24-hour IL-6 levels (22, 23). Moreover, disturbances in the quiescent phase of cortisol secretion in the evening have been associated with impaired glucose tolerance (24) and predict future new-onset type 2 diabetes (25). In overt Cushing syndrome, circulating IL-6 levels are high, with associated endothelial dysfunction and increased cardiovascular risk (14, 15). Increased circulating IL-6 may cause direct endothelial damage by disturbance of immune and inflammatory processes or through mechanisms mediated by insulin resistance (26) and may also impair endothelium-dependent vasodilatation independent of insulin sensitivity (16). Furthermore, higher tertile serum IL-6 is an independent predictor of sudden death in asymptomatic European men (27). In our patients with AI/ACS, serum IL-6 levels were high at baseline but were reduced immediately after “resetting” of the cortisol rhythm, strongly suggesting that this intervention was causal for the improvement.
Our data show that nocturnal cortisol exposure can be lowered, while leaving cortisol levels unaltered throughout the rest of the day. This approach is based on the advantages of using a rapidly acting 11β-hydroxylase inhibitor with a short duration of action (28) so that the reduction in cortisol could be fine-tuned. This represents an entirely unique paradigm of intervention for these patients for whom debate exists over the best strategy of care (5). Currently, guidelines recommend either observation with treatment to comorbidities potentially related to cortisol or adrenal surgery. The problem with the latter is that patients may be referred for surgery inappropriately due to misdiagnosis, as there is a high risk for false positivity of diagnostic tests. After metyrapone administration, cortisol levels were decreased to the range seen for normal individuals, no adrenal insufficiency event was reported, and, importantly, waking cortisol values were unaffected. Such treatment may allow identification of whether comorbidities observed in an individual patient improve and so may be a means to stratify patients to adrenal surgery or treat medically in the longer term (personalized precision medicine). A randomized, controlled, prospective study in a larger sample of AI/ACS patients is needed to assess these notions and to explore the impact of metyrapone treatment on clinical outcomes.
Although an ONDST cut-off of serum cortisol of 50 nmol/L is recommended by both the Endocrine Society and European Society of Endocrinology to identify hypercortisolemia in AI (5, 12), other data suggest that a lower level of serum cortisol may be associated with true normality. Patients with apparently “nonfunctioning” AI, with an ONDST serum cortisol level of >30 nmol/L, have increased atherosclerosis risk in the absence of conventional cardiovascular risk factors (17). Moreover, the prevalence of type 2 diabetes in a population with no AI is around 14%, similar to patients with AI and an ONDST serum cortisol around 30 nmol/L, but is significantly higher in patients with AI and ONDST serum cortisol levels above this value (11). In our study, our two control groups showed ONDST serum cortisol of 31 nmol/L or less, supporting this cut-point as being associated with a normal cortisol rhythm. The cut-offs used in this study, 60 to 80 nmol/L with ACTH <2.2 pmol/L or >80 nmol/L, are based on previous data showing increased risk of cardiovascular events and adverse metabolic outcomes with higher postdexamethasone cortisol levels (8). Furthermore, higher cut-offs than 50 nmol/L increase specificity, especially in the presence of a suppressed plasma ACTH.
Midnight salivary cortisol is an established means to screen for Cushing syndrome (12, 29). Despite this, studies in patients with AI have shown that late-night salivary cortisol levels have poor sensitivity for the diagnosis of subclinical hypercortisolism (30, 31). Late-night salivary cortisone may be a better marker for subclinical hypercortisolism in patients with AI, although this needs testing in large cohorts. Additionally, our data show that salivary cortisone may have utility for monitoring of medical intervention, with the advantage that it may be used in the community setting without the need for hospital attendance.
The study has limitations: It is small, patients were not dosed over repeated days and were only studied over 24 hours, and therefore no clinical end points were assessed. IL-6 is known to have interindividual and intraindividual variability, and postinterventional changes could be related to this variability (32). Conversely, the study has been carried out in a stable environment, and cortisol and cytokine rhythms were assessed in fine detail. It is, to our knowledge, the first time that the cortisol rhythm has been systematically studied in this patient group, and control groups were carefully matched.
In summary, we have identified that patients with AI/ACS have an abnormal cortisol rhythm with elevated nocturnal cortisol exposure. This can be “reset” to normal by using a short-acting 11β-hydroxylase inhibitor, metyrapone, in the evening, resulting in subsequent decrease in IL-6 levels. This has the potential for an entirely unique approach to intervention in these commonly encountered patients.
Acknowledgments
We thank the Clinical Research Facility and the research staff at the Northern General Hospital National Health Service Foundation Trust in Sheffield where the study was carried out, the participants of the study, Peter Trainer at Christie Hospital in Manchester who supported the recruitment of patients, and Richard Ross at the University of Sheffield for helpful discussions.
Acknowledgments
This work was supported by HRA PHARMA and National Institute for Health Research Fellowship Grant DH_BFR-2011-005 (to M.D.).
Author contributions: M.D. did the literature search and contributed to data collection, data analysis, data interpretation, and writing of the report. R.F.H. contributed to statistical analysis and data interpretation. R.C. contributed to study design, data analysis, and writing of the report. C.G. was the research coordinator and contributed to writing the report. J.-L.A. contributed to study design, data analysis, and writing of the report. J.N.-P. contributed to study design, was responsible for study oversight, and contributed to data analysis, data interpretation, and writing of the report. All authors contributed to the research project and approved its submission.
Clinical trial registry: Eudract no. 2012-002586-35 (registered 8 June 2012).
Disclosure Summary: R.C. and J.-L.A. were HRA PHARMA employees at the time of study conduct and analysis. C.G. is an HRA PHARMA employee. J.N.-P. has received research funding and honoraria from HRA Pharma. The remaining authors have nothing to disclose.
Footnotes
- ACS
- autonomous cortisol secretion
- ACTH
- adrenocorticotropic hormone
- AI
- adrenal incidentaloma
- AI/NoACS
- adrenal incidentaloma and no autonomous cortisol secretion
- AUC
- area under the curve
- BMI
- body mass index
- Cmax
- peak
- Cmin
- trough
- CV
- coefficient of variation
- HC
- healthy control
- IL-6
- interleukin-6
- ONDST
- 1-mg dexamethasone suppression test
- SD
- standard deviation
- Tmax
- time of peak.
References
- 1.Di Dalmazi G, Vicennati V, Garelli S, Casadio E, Rinaldi E, Giampalma E, Mosconi C, Golfieri R, Paccapelo A, Pagotto U, Pasquali R. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol. 2014;2(5):396–405. [DOI] [PubMed] [Google Scholar]
- 2.Debono M, Bradburn M, Bull M, Harrison B, Ross RJ, Newell-Price J. Cortisol as a marker for increased mortality in patients with incidental adrenocortical adenomas. J Clin Endocrinol Metab. 2014;99(12):4462–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young WF., Jr Clinical practice. The incidentally discovered adrenal mass. N Engl J Med. 2007;356(6):601–610. [DOI] [PubMed] [Google Scholar]
- 4.Chiodini I. Clinical review: diagnosis and treatment of subclinical hypercortisolism. J Clin Endocrinol Metab. 2011;96(5):1223–1236. [DOI] [PubMed] [Google Scholar]
- 5.Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, Tabarin A, Terzolo M, Tsagarakis S, Dekkers OM. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175(2):G1–G34. [DOI] [PubMed] [Google Scholar]
- 6.Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing’s syndrome. Lancet. 2006;367(9522):1605–1617. [DOI] [PubMed] [Google Scholar]
- 7.Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing’s syndrome. Lancet. 2015;386(9996):913–927. [DOI] [PubMed] [Google Scholar]
- 8.Di Dalmazi G, Vicennati V, Rinaldi E, Morselli-Labate AM, Giampalma E, Mosconi C, Pagotto U, Pasquali R. Progressively increased patterns of subclinical cortisol hypersecretion in adrenal incidentalomas differently predict major metabolic and cardiovascular outcomes: a large cross-sectional study. Eur J Endocrinol. 2012;166(4):669–677. [DOI] [PubMed] [Google Scholar]
- 9.Chiodini I, Mascia ML, Muscarella S, Battista C, Minisola S, Arosio M, Santini SA, Guglielmi G, Carnevale V, Scillitani A. Subclinical hypercortisolism among outpatients referred for osteoporosis. Ann Intern Med. 2007;147(8):541–548. [DOI] [PubMed] [Google Scholar]
- 10.Morelli V, Reimondo G, Giordano R, Della Casa S, Policola C, Palmieri S, Salcuni AS, Dolci A, Mendola M, Arosio M, Ambrosi B, Scillitani A, Ghigo E, Beck-Peccoz P, Terzolo M, Chiodini I. Long-term follow-up in adrenal incidentalomas: an Italian multicenter study. J Clin Endocrinol Metab. 2014;99(3):827–834. [DOI] [PubMed] [Google Scholar]
- 11.Lopez D, Luque-Fernandez MA, Steele A, Adler GK, Turchin A, Vaidya A. “Nonfunctional” adrenal tumors and the risk for incident diabetes and cardiovascular outcomes: a cohort study. Ann Intern Med. 2016;165(8):533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93(5):1526–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dekkers OM, Horváth-Puhó E, Jørgensen JO, Cannegieter SC, Ehrenstein V, Vandenbroucke JP, Pereira AM, Sørensen HT. Multisystem morbidity and mortality in Cushing’s syndrome: a cohort study. J Clin Endocrinol Metab. 2013;98(6):2277–2284. [DOI] [PubMed] [Google Scholar]
- 14.Barahona MJ, Sucunza N, Resmini E, Fernández-Real JM, Ricart W, Moreno-Navarrete JM, Puig T, Farrerons J, Webb SM. Persistent body fat mass and inflammatory marker increases after long-term cure of Cushing’s syndrome. J Clin Endocrinol Metab. 2009;94(9):3365–3371. [DOI] [PubMed] [Google Scholar]
- 15.Valassi E, Biller BM, Klibanski A, Misra M. Adipokines and cardiovascular risk in Cushing’s syndrome. Neuroendocrinology. 2012;95(3):187–206. [DOI] [PubMed] [Google Scholar]
- 16.Esteve E, Castro A, López-Bermejo A, Vendrell J, Ricart W, Fernández-Real JM. Serum interleukin-6 correlates with endothelial dysfunction in healthy men independently of insulin sensitivity. Diabetes Care. 2007;30(4):939–945. [DOI] [PubMed] [Google Scholar]
- 17.Androulakis II, Kaltsas GA, Kollias GE, Markou AC, Gouli AK, Thomas DA, Alexandraki KI, Papamichael CM, Hadjidakis DJ, Piaditis GP. Patients with apparently nonfunctioning adrenal incidentalomas may be at increased cardiovascular risk due to excessive cortisol secretion. J Clin Endocrinol Metab. 2014;99(8):2754–2762. [DOI] [PubMed] [Google Scholar]
- 18.Debono M, Harrison RF, Whitaker MJ, Eckland D, Arlt W, Keevil BG, Ross RJ. Salivary cortisone reflects cortisol exposure under physiological conditions and after hydrocortisone. J Clin Endocrinol Metab. 2016;101(4):1469–1477. [DOI] [PubMed] [Google Scholar]
- 19.Dabitao D, Margolick JB, Lopez J, Bream JH. Multiplex measurement of proinflammatory cytokines in human serum: comparison of the Meso Scale Discovery electrochemiluminescence assay and the Cytometric Bead Array. J Immunol Methods. 2011;372(1-2):71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimoto N, Kanakura Y, Aozasa K, Johkoh T, Nakamura M, Nakano S, Nakano N, Ikeda Y, Sasaki T, Nishioka K, Hara M, Taguchi H, Kimura Y, Kato Y, Asaoku H, Kumagai S, Kodama F, Nakahara H, Hagihara K, Yoshizaki K, Kishimoto T. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106(8):2627–2632. [DOI] [PubMed] [Google Scholar]
- 21.Perogamvros I, Keevil BG, Ray DW, Trainer PJ. Salivary cortisone is a potential biomarker for serum free cortisol. J Clin Endocrinol Metab. 2010;95(11):4951–4958. [DOI] [PubMed] [Google Scholar]
- 22.Morris CJ, Purvis TE, Mistretta J, Scheer FA. Effects of the internal circadian system and circadian misalignment on glucose tolerance in chronic shift workers. J Clin Endocrinol Metab. 2016;101(3):1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci USA. 2016;113(10):E1402–E1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plat L, Leproult R, L’Hermite-Baleriaux M, Fery F, Mockel J, Polonsky KS, Van Cauter E. Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab. 1999;84(9):3082–3092. [DOI] [PubMed] [Google Scholar]
- 25.Hackett RA, Kivimäki M, Kumari M, Steptoe A. Diurnal cortisol patterns, future diabetes, and impaired glucose metabolism in the Whitehall II cohort study. J Clin Endocrinol Metab. 2016;101(2):619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, Xiao X, Shan ZL, Zhang Y, Yao P, Liu LG. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;36(1):166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Empana JP, Jouven X, Canouï-Poitrine F, Luc G, Tafflet M, Haas B, Arveiler D, Ferrieres J, Ruidavets JB, Montaye M, Yarnell J, Morange P, Kee F, Evans A, Amouyel P, Ducimetiere P. C-reactive protein, interleukin 6, fibrinogen and risk of sudden death in European middle-aged men: the PRIME study. Arterioscler Thromb Vasc Biol. 2010;30(10):2047–2052. [DOI] [PubMed] [Google Scholar]
- 28.Verhelst JA, Trainer PJ, Howlett TA, Perry L, Rees LH, Grossman AB, Wass JA, Besser GM. Short and long-term responses to metyrapone in the medical management of 91 patients with Cushing’s syndrome. Clin Endocrinol (Oxf). 1991;35(2):169–178. [DOI] [PubMed] [Google Scholar]
- 29.Carroll T, Raff H, Findling JW. Late-night salivary cortisol for the diagnosis of Cushing syndrome: a meta-analysis. Endocr Pract. 2009;15(4):335–342. [DOI] [PubMed] [Google Scholar]
- 30.Masserini B, Morelli V, Bergamaschi S, Ermetici F, Eller-Vainicher C, Barbieri AM, Maffini MA, Scillitani A, Ambrosi B, Beck-Peccoz P, Chiodini I. The limited role of midnight salivary cortisol levels in the diagnosis of subclinical hypercortisolism in patients with adrenal incidentaloma. Eur J Endocrinol. 2009;160(1):87–92. [DOI] [PubMed] [Google Scholar]
- 31.Nunes ML, Vattaut S, Corcuff JB, Rault A, Loiseau H, Gatta B, Valli N, Letenneur L, Tabarin A. Late-night salivary cortisol for diagnosis of overt and subclinical Cushing’s syndrome in hospitalized and ambulatory patients. J Clin Endocrinol Metab. 2009;94(2):456–462. [DOI] [PubMed] [Google Scholar]
- 32.Agorastos A, Hauger RL, Barkauskas DA, Moeller-Bertram T, Clopton PL, Haji U, Lohr JB, Geracioti TD Jr, Patel PM, Chrousos GP, Baker DG. Circadian rhythmicity, variability and correlation of interleukin-6 levels in plasma and cerebrospinal fluid of healthy men. Psychoneuroendocrinology. 2014;44:71–82. [DOI] [PubMed] [Google Scholar]