Abstract
Context:
Fetuses exposed to the high thyroid hormone (TH) levels of mothers with resistance to thyroid hormone beta (RTH-β), due to mutations in the THRB gene, have low birth weight and suppressed TSH.
Objective:
Determine if such exposure to high TH levels in embryonic life has a long-term effect into adulthood.
Design:
Observations in humans with a parallel design on animals to obtain a preliminary information regarding mechanism.
Setting:
University research centers.
Patients or other participants:
Humans and mice with no RTH-β exposed during intrauterine life to high TH levels from mothers who were euthyroid due to RTH-β. Controls were humans and mice of the same genotype but born to fathers with RTH-β and mothers without RTH-β and thus, with normal serum TH levels.
Interventions:
TSH responses to stimulation with thyrotropin-releasing hormone (TRH) during adult life in humans and male mice before and after treatment with triiodothyronine (T3). We also measured gene expression in anterior pituitaries, hypothalami, and cerebral cortices of mice.
Results:
Adult humans and mice without RTH-β, exposed to high maternal TH in utero, showed persistent central resistance to TH, as evidenced by reduced responses of serum TSH to TRH when treated with T3. In mice, anterior pituitary TSH-β and deiodinase 3 (D3) mRNAs, but not hypothalamic and cerebral cortex D3, were increased.
Conclusions:
Adult humans and mice without RTH-β exposed in utero to high maternal TH levels have persistent central resistance to TH. This is likely mediated by the increased expression of D3 in the anterior pituitary, enhancing local T3 degradation.
We studied adult humans and mice exposed to high TH levels during fetal life and found that they have resistance to TH likely due to increasing D3-mediated TH inactivation in the anterior pituitary.
The consequences of maternal hyperthyroidism on progeny are not fully understood, mostly because of the inability to separate the effects maternal thyrotoxicosis from the direct effects of thyroid hormone (TH) on the fetus (1, 2). In resistance to thyroid hormone beta (RTH-β), a syndrome of reduced end-organ responsiveness to TH due to mutations of the TH receptor beta (THRB) gene (3), the resulting TH excess in serum is not associated with metabolic consequences or is limited to some organ systems (4). In this sense, the study of women with RTH-β carrying wild-type (WT, without THRB gene mutations) fetuses provides a unique opportunity to evaluate the direct effect of maternal TH excess on the fetus. In previous studies in an extended Azorean family harboring the THRB gene mutation R243Q, we have shown that affected mothers miscarry a greater proportion of WT embryos and that infants born to such mothers have suppressed neonatal thyrotropin (TSH) and low birth weight (5). Taken together, these findings indicate that WT fetuses exposed to high maternal TH levels during pregnancy develop hyperthyroid features. In neonates born with fetal hyperthyroidism, the resulting central hypothyroidism is transient (6, 7). In contrast, normal mice born to dams with high TH levels due to lack of TH receptor beta (Thrb) gene develop partial central resistance to TH despite the presence of a normal TH receptor beta (8). These data from adult animals suggest that exposure of human embryos to high TH levels may not only have transient effect on the newborn but possibly also permanent effects on the progeny.
In the current study, we examined WT adults without THRB gene mutations from the same Azorean family born to mothers with THRB gene mutations. The control group consisted also of WT adults but born to WT euthyroid mothers and conceived by fathers with THRB gene mutations. To gain information regarding the mechanism whereby intrauterine exposure to high TH levels affects the adult progeny, we used genetically manipulated mice with disruption of both alleles of Thrb gene, which mimics the phenotype of humans harboring a heterozygous THRB gene mutation. They were mated to carry normal progeny in dams with RTH-β as well as in normal dams mated to male mice with RTH-β (9, 10). Only humans and mice born to mothers with RTH-β having high TH levels developed reduced central sensitivity to TH that was persistent during adulthood.
Materials and Methods
Humans and in vivo study design
The human study groups consisted of WT (without THRB gene mutations) adults belonging to the same extended Azorean family born to mothers with RTH-β due the mutation R243Q in one allele of the THRB gene. Control subjects were WT adults born to WT mothers but to fathers with RTH-β due the same THRB gene mutation. Each group had three female subjects and one male subject. The overall age range was 22 to 54 years.
Heterozygous individuals with missense mutations in the hormone-binding domain of the THRB gene, such as that harbored in this family, produce the phenotype of RTH-β due to interference of the mutant receptor with the normal one. This phenomenon is known as the “dominant negative effect” (11). In contrast, deletion of a WT THRB gene in one allele has no phenotype because a single WT THRB gene is sufficient for normal function, and therefore the absence of a mutant allele does not exert a dominant negative effect. However, deletion of both THRB alleles produces the RTH-β phenotype (12). Individuals with RTH-β have high TH levels with a nonsuppressed TSH. None of the subjects studied had autoimmune thyroid disease as documented clinically and by the absence of thyroid autoantibodies.
Each individual was given intravenously 200 µg thyrotropin-releasing hormone (TRH) before and again 3 months later after 3 days treatment with 25 µg of triiodothyronine (liothyronine, L-T3) taken orally twice daily. Serum was collected at −15, 0, 15, 30, 45, 60, 90, 120, and 180 minutes relative to the TRH administration for measurement of TSH and prolactin (PRL). The following additional substances were measured in the samples obtained at −15 minutes: total T4, T3, and reverse T3 (rT3), free T4 index (FT4I), thyroglobulin, sex hormone–binding globulin, ferritin, cholesterol, and creatinine kinase. A graphic depiction of the protocol is provided in Fig. 1(c) and 1(d).
Figure 1.
Serum TSH changes in response to the administration of TRH in humans. (a) WT progeny of fathers with RTH-β. (b) WT progeny of mothers with RTH-β. (c) Diagram depicting the experimental protocol with the time of TRH administration, times of blood sampling, and the substances measured. (d) The schedule of TRH testing and L-T3 administration. The same four individuals in each group, as indicated by the same color, were tested before (open symbols) and after (closed symbols) treatment with L-T3. Results from male subjects are in blue. CK, creatinine kinase; i.v., intravenously; SHBG, sex hormone–binding globulin; TG, thyroglobulin.
The tests described previously were obtained at baseline from a larger group of adult subjects belonging to the same extended family. The number of individuals in each group is provided in the tables. The study was approved by the Institutional Review Boards of Hospital Divino Espírito Santo and The University of Chicago.
Mice and in vivo study design
The TH receptor beta knockout mouse (Thrb−/−) was produced as described (13), and mice were maintained in the C57BL/6 background. As in humans lacking one allele of the THRB gene (12), heterozygous Thrb+/− mice were indistinguishable in all respects from the WT Thrb+/+ mice (Table 1). Thrb−/− mice were chosen for the comparative studies because the magnitudes of serum T4, FT4I, and T3 concentrations were comparable to those of humans heterozygous for the THRB R243Q when expressed as percent difference from the respective WT humans and Thrb+/− mice (Table 1).
Table 1.
Thyroid Function Tests in Humans and Mice Without and With RTH-β
| Determinations |
Humansa |
Mice |
||||||
|---|---|---|---|---|---|---|---|---|
| WT Relatives | RTH-β R243Q | % Differenceb | WT Thrb+/+ | Hetero Thrb+/− | % Difference | RTH-β Thrb−/− | % Difference | |
| Number of subjects | 33 | 40 | 12 | 12 | 12 | |||
| T4, µg/dL | 8.4 ± 0.2 | 15.4 ± 0.5 | 183 | 3.8 ± 0.1 | 4.1 ± 0.2 | 108 | 7.8 ± 0.3 | 205 |
| T3, ng/dL | 139 ± 8 | 252 ± 15 | 181 | 84 ± 3 | 90 ± 4 | 107 | 152 ± 12 | 181 |
| rT3, ng/dL | 19 ± 1 | 42 ± 3 | 224 | 32 ± 2 | 43 ± 3 | 130 | 146 ± 10 | 456 |
| FT4I | 8.8 ± 0.2 | 18.5 ± 0.6 | 210 | 4.5 ± 0.3 | 4.9 ± 0.3 | 109 | 11.7 ± 1.4 | 260 |
| TSH, mU/L | 2.5 ± 0.2 | 7.3 ± 4.6 | 292 | 25 ± 3 | 24 ± 3 | 96 | 136 ± 18 | 544 |
To convert to SI units: T4 µg/dl × 0.0129 = µmol/L; T3 and rT3 ng/dl × 0.0154 = nmol/L.
All humans belong to the same extended Azorean family.
% difference is that compared with WT relatives for humans and normal (Thrb+/+) for mice. None of the differences between Thrb+/+ and Thrb+/− mice was significant. All differences between WT and R243Q humans and Thrb+/− (wthout RTH-β) and Thrb−/− (with RTH-β) mice were significant, with P values of at least <0.001.
Mating strategies were as described in detail (8). Briefly, female mice with RTH-β (Thrb−/−) were mated to male mice without RTH-β (Thrb+/−) to produce a 50% progeny without RTH-β (Thrb+/−). As controls, female mice without RTH-β (Thrb+/−) were mated to male mice with RTH-β (Thrb−/−) to produce 50% progeny without RTH-β (Thrb+/−). The difference between these two mating strategies is that the progeny without RTH-β born to dams with RTH-β were exposed to high levels of TH during gestation, whereas in the reciprocal crossing the progeny were not exposed to hyperiodothyroninemic intrauterine environment. Mouse DNA extraction and genotyping were performed as previously described (10).
Thrb+/− adult male progeny (60 to 80 days old) were used in the study because half of female mice without RTH-β have serum TSH values below the limit of the assay sensitivity (14). Mice were given daily intraperitoneal injections of a low dose (0.2 µg) of L-T3/100 g body weight daily for four consecutive days. About 18 hours after the last injection, blood was obtained before and 15 minutes after intraperitoneal injection of 0.280 µg TRH. Serum was separated and frozen before determination of TSH concentration in both samples. Baseline measurements of total T4, T3, and rT3 were obtained in all animals.
Groups of untreated and L-T3–treated mice were dissected, and the anterior pituitary glands, hypothalami, and cerebral cortices were collected. The number of animals used in each group is provided in the tables or legends to figures. Animal experiments were performed at The University of Chicago and at the University of Duisburg-Essen according to protocols approved by the respective Institutional Animal Care and Use Committees.
Hormones and RNA measurements
In human serum, total T4, and T3, TSH, and PRL were measured by chemiluminescence immunometric assays using the Elecsys Automated System (Roche Molecular Biochemicals GmbH and Hitachi, Ltd., Indianapolis, IN). Total rT3 was measured by radioimmunoassay (Adaltis Italia S.p.A, Bologna, Italy), and thyroglobulin was measured by an in-house radioimmunoassay. FT4I, an estimate of the serum-free T4, is the product of serum T4 concentration and the normalized resin T4 uptake ratio.
In mouse serum, total T4, T3, and rT3 as well as TSH were measured by radioimmunoassay using methods described in detail in the supplement to a publication by Ferrara et al. (15). Before tissue harvesting, mice were perfused under anesthesia with heparinized phosphate-buffered saline through a needle placed in the left ventricle. The anterior pituitary, hypothalamus, and cerebral cortex were dissected, immediately frozen, and kept at −80°C until homogenized. RNA was extracted and used for measurement of specific mRNAs by quantitative real-time polymerase chain reaction as described in detail (15). Amplification of the housekeeping gene ribonucleic acid polymerase 2 was used as internal control. Primer sequences are provided in Supplemental Table 1 (86.3KB, pdf) .
Statistical analyses
All results are expressed as mean ± standard error of the mean. Statistical analysis of multiple groups was by two-way analysis of variance with Fisher protected least significant difference test. Student t test was used when there were only two groups to compare. P < 0.05 was considered to be significant.
Results
Humans
Baseline thyroid function tests were not significantly different between subjects born to mothers with RTH-β compared with those born to fathers with RTH-β but WT mothers (Table 2). Thyroid tests obtained from a larger group of subjects born to mothers with RTH-β showed a significant increase in rT3 and a reduction in T3, which did not reach significance, compared with those born to fathers with RTH-β (Table 3). However, the rT3/T3 ratio was significantly higher (P < 0.03). After L-T3 treatment, both groups had equally elevated serum T3 and suppressed TSH (Table 2). Before L-T3 treatment, mean peak serum TSH responses in individuals born to mothers with RTH-β, compared with those born to fathers with RTH-β, were 11.8 ± 1.7 and 13.2 ± 1.7 mU/L, respectively (P = 0.57). After L-T3 treatment, the corresponding TSH values were 6.8 ± 1.0 vs 1.6 ± 0.5 (P < 0.003) [Fig. 1(a) and 1(b); Table 2]. Differences were significant when expressed as the delta response relative to the basal values [Fig. 2(a)] and as the percentage of the basal value or the area under the curve (data not shown). There was no overlap between the post–L-T3 treatment responses in individual subjects of both groups [Fig. 1(a)]. This demonstrates, despite the small number of subjects tested, a significantly reduced sensitivity to T3 in adult subjects exposed during intrauterine life to elevated maternal TH levels. There were no differences in basal and stimulated serum PRL levels between the two groups before and after L-T3 treatment (Table 2). Furthermore, no significant differences between the two groups were found for markers of peripheral tissue action of TH, including serum sex hormone–binding globulin, ferritin, cholesterol, and creatinine kinase (data not shown).
Table 2.
Baseline Thyroid Function Tests in Humans Before and After Treatment With L-T3
| Test | Born to RTH-β Fathers | Born to RTH-β Mothers | P Value |
|---|---|---|---|
| Number of subjects | 4 | 4 | |
| Before treatment | |||
| T4, µg/dL | 9.7 ± 0.7 | 8.6 ± 1.3 | NS |
| T3, ng/dL | 131 ± 2 | 110 ± 7 | NS |
| rT3, ng/dL | 25.1 ± 2.7 | 24.7 ± 3.2 | NS |
| FT4I | 9.4 ± 0.4 | 9.7 ± 1.4 | NS |
| TSH, mU/L | 1.42 ± 0.16 | 1.62 ± 0.18 | NS |
| Peak stimulated TSH, mU/L | 13.2 ± 1.7 | 10.4 ± 0.8 | NS |
| PRL, ng/mL | 15.5 ± 1.4 | 11.4 ± 3.8 | NS |
| Peak stimulated PRL, ng/mL | 98 ± 25 | 61 ± 39 | NS |
| After treatment | |||
| T4, µg/dL | 7.3 ± 0.55 | 6.6 ± 0.61 | NS |
| T3, ng/dL | 252 ± 33 | 248 ± 24 | NS |
| rT3, ng/dL | 24.7 ± 1.7 | 20.0 ± 2.3 | NS |
| FT4I | 7.6 ± 0.12 | 7.3 ± 0.39 | NS |
| TSH, mU/L | 0.14 ± 0.04 | 0.26 ± 0.10 | NS |
| Peak stimulated TSH, mU/L | 1.58 ± 0.52 | 6.83 ± 0.98 | <0.003 |
| PRL, ng/mL | 20.1 ± 11.5 | 10.6 ± 3.5 | NS |
| Peak stimulated PRL, ng/mL | 72.5 ± 9.4 | 62.8 ± 15.4 | NS |
Peak stimulated TSH and PRL values are after administration of TRH.
Abbreviation: NS, not significant.
Table 3.
Thyroid Function Tests in WT Progeny Born to RTH-β Fathers (Controls) and RTH-β Mothers
| Groups |
Humans |
Mice |
||||
|---|---|---|---|---|---|---|
| Born to RTH-β Fathers | Born to RTH-β Mothers | P Value | Born to RTH-β Sires | Born to RTH-β Dams | P Value | |
| Number of subjects | 9 | 15 | 8 | 7 | ||
| Age | 35 ± 6 y | 46 ± 5 y | NS | 64 ± 1 d | 62 ± 1 d | NS |
| T4, µg/dL | 8.6 ± 0.3 | 8.3 ± 0.3 | NS | 5.2 ± 0.1 | 4.8 ± 0.2 | NS |
| T3, ng/dL | 150 ± 8 | 130 ± 9 | NS | 63 ± 6 | 54 ± 5 | NS |
| rT3, ng/dL | 15.7 ± 0.9 | 19.2 ± 1.1 | <0.05 | 23.5 ± 1.3 | 25.8 ± 2.7 | NS |
| rT3/T3 ratio | 0.11 ± 0.01 | 0.16 ± 0.01 | <0.03 | 0.36 ± 0.02 | 0.48 ± 0.02 | <0.01 |
| FT4I | 9.1 ± 0.3 | 8.6 ± 0.3 | NS | |||
| TSH, mU/L | 2.9 ± 0.8 | 1.7 ± 0.2 | NS | 18 ± 2 | 41 ± 10 | <0.01 |
Abbreviation: NS, not significant.
Figure 2.
Mean peak responses of serum TSH to TRH after the administration of L-T3 (a) in humans and (b) in mice without RTH-β. Open bars show data from adult controls born to fathers with RTH-β, and black bars show data from adults born to mothers with RTH-β and thus exposed to the high maternal levels of TH. (c) The schedule of TRH testing in mice. There are four human subjects and six male mice in each of the respective groups.
Mice
Mice without RTH-β (Thrb+/−) born to dams with RTH-β (Thrb−/−), but not those born to fathers with RTH-β, showed significantly reduced suppression of their serum TSH response to TRH after treatment with L-T3 [Fig. 2(b)], replicating the human phenotype of reduced sensitivity to TH. Whereas differences in baseline serum T3 and rT3 concentration between the two groups did not reach statistical significance, the rT3/T3 ratio did (Table 3). In addition, and contrary to humans, the mice without RTH-β born to dams with RTH-β had higher baseline serum TSH.
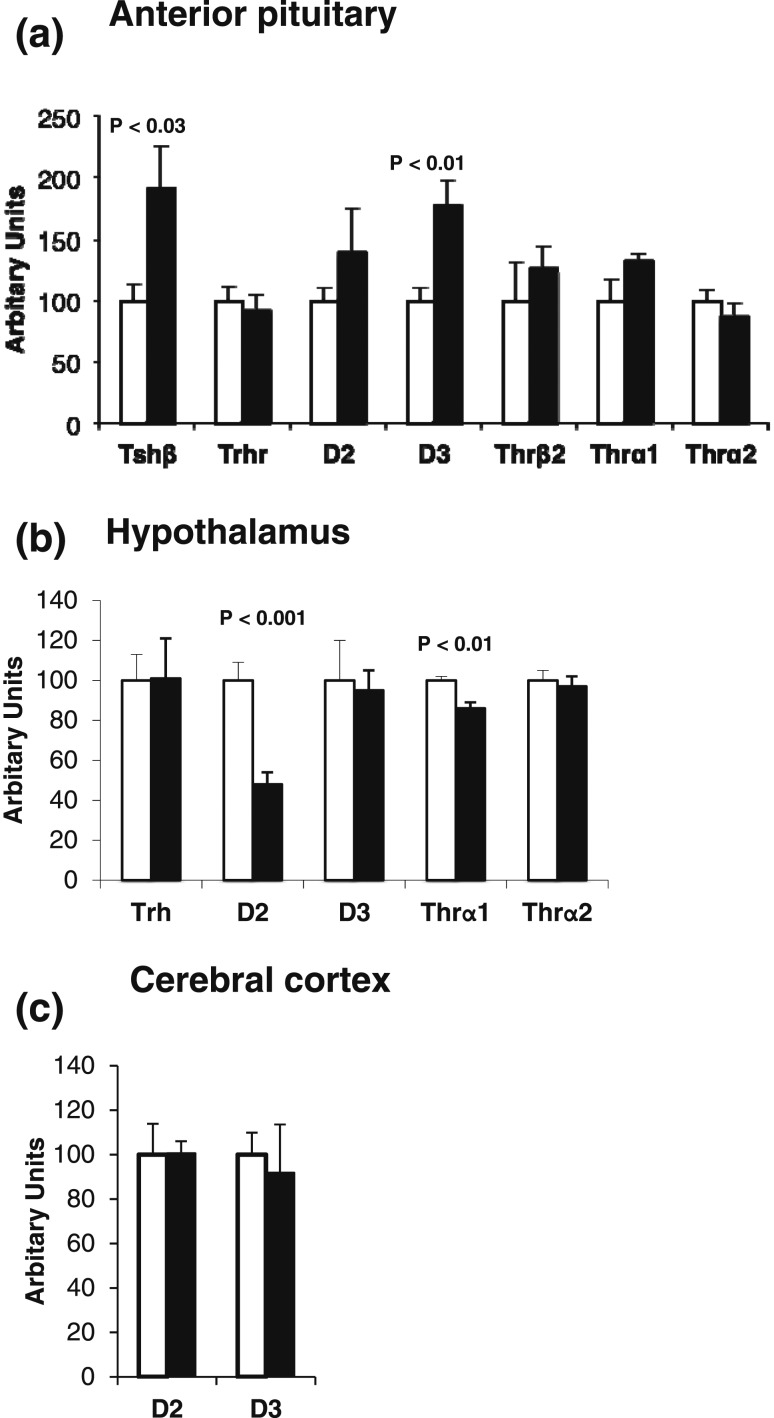
To study the underlying mechanism, we examined the expression of genes involved in the hypothalamo-pituitary-thyroid axis. Results showed that TSH-β and D3 were increased in the pituitaries of mice born to dams with RTH-β [Fig. 3(a)]. This was observed in mice treated and in those not treated with L-T3 (Supplemental Fig. 1 (86.3KB, pdf) ). The expression of other candidate genes was examined in anterior pituitaries and hypothalami. Other than the observed increases in anterior pituitary TSH-β and D3, expression of deiodinase 2 (D2) and TH receptor α1 (Thr-α1) was significantly reduced in hypothalami of mice born to dams with RTH-β [Fig. 3(b)]. No differences in D2 and D3 expression were found in the cerebral cortex [Fig. 3(c)].
Figure 3.
(a) Gene expression in the anterior pituitaries of male mice born to mothers with RTH-β and thus exposed to high maternal TH levels during intrauterine life (black bars), as compared with those born to fathers with RTH-β as controls (open bars), because they were exposed to normal maternal levels of TH. (b) Gene expression in hypothalami of the same two groups of mice. (c) Gene expression in cerebral cortices of the same two groups of mice. Note the increase of D3 in anterior pituitary of progeny of mothers with RTH-β is not shared by the hypothalamus and cerebral cortex. There are six mice in each of the respective groups.
Discussion
Our results show that exposure of human embryos to high maternal TH concentrations not only affects the newborn (5) but also reduces thyrotroph sensitivity to TH during adult life. Whether other tissues or only those involved in the central feedback regulation are affected remains to be determined. The lack of significant differences in peripheral tissue markers between the two groups of individuals at baseline and after L-T3 treatment does not rule out more general effects because these markers require larger changes of TH to respond (4). However, it appears certain that the effect was mediated by the high levels of TH derived from the mother acting on the fetus. Indeed, by virtue of the RTH-β, the mothers were not thyrotoxic and maintained high serum TH levels without abnormal thyroid-stimulating substances, whereas their WT newborns were thyrotoxic given their low birth weight and suppressed TSH (5).
We used mice with RTH-β as models to probe into the mechanism because these mice maintained increased serum TH levels of similar magnitude as the humans with THRB R243Q under study (Table 1). As in humans, the effect of TH excess is confined to the progeny and limited to their intrauterine life. We were able to demonstrate that adult mice without RTH-β born to dams with RTH-β had also reduced suppression of their TSH response to TRH after L-T3 treatment. This did not occur in mice without RTH-β born to fathers with RTH-β whose mothers without RTH-β had normal serum TH levels. Thus, they provided a good model to study the mechanism of this central resistance due to the availability of anterior pituitary and brain tissues.
As expected, TSH-β mRNA was increased in anterior pituitaries of mice born to dams with RTH-β, in agreement with their increased serum TSH in response to TRH. However, expression of the TRH receptor in the anterior pituitary and all three TH receptors was not altered [Fig. 3(a)]. Although, based on the altered response to TRH, we expected a defect at the level of the anterior pituitary rather than the hypothalamus, the latter tissue was also examined. Expression of TRH and TH receptor α2 was not significantly different in both groups of mice.
The other alteration in the anterior pituitary, besides the expected increase in TSH-β expression, was an increase in D3 mRNA. This enzyme, which inactivates both T3 and T4, reduces the concentration of biologically active TH. Increased D3 expression and consequently increased T3 degradation explain the reduced sensitivity of the anterior pituitary to administered L-T3 in mice. Direct measurement of D3 enzymatic activity will require pooling of anterior pituitary tissue. However, the quantitative polymerase chain reaction, using 32 ng template RNA, was sufficiently robust (28 to 29 cycles), and, contrary to D2, there is a good correlation between D3 enzymatic activity and mRNA concentration in other tissues that have been examined (16, 17). As shown in D3-deficient mice, this enzyme protects the fetus from the maternal TH excess (18). D3 is imprinted in both mice and humans, and the imprinting is tissue- and age specific (19, 20). Thus, D3 is a good candidate for the apparent epigenetic effect of intrauterine exposure to high TH concentrations. It is unknown whether the increase in D3 involves all anterior pituitary cells or only the thyrotrophs. If the effect is cell specific, it must be quite robust because the thyrotrophs represent a minority of the anterior pituitary cell population.
A decrease in D2 activity, the enzyme that activates TH, was observed in the hypothalami of mice born to dams with RTH-β. This would decrease the generation of T3, further contributing to the hyposensitivity to TH. In fact, posttranscriptional regulation of D2 in the hypothalamus is less dependent on ubiquitination, and the observed decrease in D2 mRNA concentration may correlate with its enzyme activity (21). However, reduction of hypothalamic D2 could not have a role in the reduced sensitivity to the administered L-T3. The small amount of anterior pituitary tissue and the low concentration of this hormone in the hypothalamus precluded direct measurement of T3 tissue content. The observed slight (14%) decrease in the expression of the Thr-α1 in the hypothalamus is not expected to affect the TSH level as reported in Thr-α1 receptor–deficient mice and humans (22, 23).
Several differences between results in humans and mice were observed. In progeny born to mothers with high TH, serum rT3 was significantly increased in humans but not in mice, and although the mean decrease in serum T3 was not significant in both species, the rT3/T3 ratio was. Furthermore, mice born to mothers with RTH-β showed a significant increase in basal serum TSH, whereas the corresponding humans did not (Table 3). In general, mice have a more robust response than humans to changes of serum TH of similar magnitude. This is also manifested by the higher proportional elevation of serum TSH in mice with RTH-β than in humans despite a similar relative difference in TH levels (Table 1). Because metabolism of TH in the anterior pituitary cannot account for changes in serum TH concentrations, the observed differences in rT3/T3 ratios suggest that exposure to high TH during intrauterine life may alter the metabolism of TH in other tissues. However, subtle differences in endogenous concentrations of TH were excluded due to the observed reduced sensitivity to TH by the administration of L-T3, which greatly increased, and, to the same degree, due to the serum T3 concentration in the two groups of humans (Table 2).
In summary, our data show that intrauterine exposure to increased TH concentrations has permanent consequences on the progeny. Increased inactivation of TH in the anterior pituitary may be protective in utero, but this effect persists into adulthood. This is likely due to an effect mediated through an increased expression of D3. Whether this apparent epigenetic effect is transmitted to the following generation is under investigation.
Acknowledgments
Acknowledgments
This work was supported by National Institutes of Health Grant R37DK15070 (to S.R.) and by Seymour J. Abrams and Rabbi Morris Esformes Thyroid Funds. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- D2
- deiodinase 2
- D3
- deiodinase 3
- FT4I
- free T4 index
- L-T3
- liothyronine
- PRL
- prolactin
- rT3
- reverse triiodothyronine
- RTH-β
- resistance to thyroid hormone β
- T3
- triiodothyronine
- T4
- thyroxine
- TH
- thyroid hormone
- Thr-α1
- thyroid hormone receptor α1
- TRH
- thyrotropin-releasing hormone
- TSH
- thyrotropin
- WT
- wild-type.
References
- 1.Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18(3):404–433. [DOI] [PubMed] [Google Scholar]
- 2.Abramson J, Stagnaro-Green A. Thyroid antibodies and fetal loss: an evolving story. Thyroid. 2001;11(1):57–63. [DOI] [PubMed] [Google Scholar]
- 3.Dumitrescu AM, Refetoff S. The syndromes of reduced sensitivity to thyroid hormone. Biochim Biophys Acta 2013;1830:3987–4003. [DOI] [PMC free article] [PubMed]
- 4.Refetoff S, Weiss RE, Usala SJ. The syndromes of resistance to thyroid hormone. Endocr Rev. 1993;14(3):348–399. [DOI] [PubMed] [Google Scholar]
- 5.Anselmo J, Cao D, Karrison T, Weiss RE, Refetoff S. Fetal loss associated with excess thyroid hormone exposure. JAMA. 2004;292(6):691–695. [DOI] [PubMed] [Google Scholar]
- 6.Matsuura N, Konishi J, Fujieda K, Kasagi K, Iida Y, Hagisawa M, Fujimoto S, Fukushi M, Takasugi N. TSH-receptor antibodies in mothers with Graves’ disease and outcome in their offspring. Lancet. 1988;1(8575-6):14–17. [DOI] [PubMed] [Google Scholar]
- 7.Higuchi R, Miyawaki M, Kumagai T, Okutani T, Shima Y, Yoshiyama M, Ban H, Yoshikawa N. Central hypothyroidism in infants who were born to mothers with thyrotoxicosis before 32 weeks’ gestation: 3 cases. Pediatrics. 2005;115(5):e623–e625. [DOI] [PubMed] [Google Scholar]
- 8.Alonso M, Goodwin C, Liao X, Page D, Refetoff S, Weiss RE. Effects of maternal levels of thyroid hormone (TH) on the hypothalamus-pituitary-thyroid set point: studies in TH receptor beta knockout mice. Endocrinology. 2007;148(11):5305–5312. [DOI] [PubMed] [Google Scholar]
- 9.Forrest D, Hanebuth E, Smeyne RJ, Everds N, Stewart CL, Wehner JM, Curran T. Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor β: evidence for tissue-specific modulation of receptor function. EMBO J. 1996;15(12):3006–3015. [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss RE, Forrest D, Pohlenz J, Cua K, Curran T, Refetoff S. Thyrotropin regulation by thyroid hormone in thyroid hormone receptor β-deficient mice. Endocrinology. 1997;138(9):3624–3629. [DOI] [PubMed] [Google Scholar]
- 11.Nagaya T, Jameson JL. Thyroid hormone receptor dimerization is required for dominant negative inhibition by mutations that cause thyroid hormone resistance. J Biol Chem. 1993;268(21):15766–15771. [PubMed] [Google Scholar]
- 12.Takeda K, Sakurai A, DeGroot LJ, Refetoff S. Recessive inheritance of thyroid hormone resistance caused by complete deletion of the protein-coding region of the thyroid hormone receptor- β gene. J Clin Endocrinol Metab. 1992;74(1):49–55. [DOI] [PubMed] [Google Scholar]
- 13.Gauthier K, Chassande O, Plateroti M, Roux J-P, Legrand C, Pain B, Rousset B, Weiss R, Trouillas J, Samarut J. Different functions for the thyroid hormone receptors TRalpha and TRbeta in the control of thyroid hormone production and post-natal development. EMBO J. 1999;18(3):623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pohlenz J, Maqueem A, Cua K, Weiss RE, Van Sande J, Refetoff S. Improved radioimmunoassay for measurement of mouse thyrotropin in serum: strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid. 1999;9(12):1265–1271. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara AM, Liao XH, Gil-Ibáñez P, Marcinkowski T, Bernal J, Weiss RE, Dumitrescu AM, Refetoff S. Changes in thyroid status during perinatal development of MCT8-deficient male mice. Endocrinology. 2013;154(7):2533–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez A, St Germain DL. Dexamethasone inhibits growth factor-induced type 3 deiodinase activity and mRNA expression in a cultured cell line derived from rat neonatal brown fat vascular-stromal cells. Endocrinology. 2002;143(7):2652–2658. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez A, Morte B, Belinchón MM, Ceballos A, Bernal J. Critical role of types 2 and 3 deiodinases in the negative regulation of gene expression by T3in the mouse cerebral cortex. Endocrinology. 2012;153(6):2919–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez A, Martinez ME, Liao XH, Van Sande J, Refetoff S, Galton VA, St Germain DL. Type 3 deiodinase deficiency results in functional abnormalities at multiple levels of the thyroid axis. Endocrinology. 2007;148(12):5680–5687. [DOI] [PubMed] [Google Scholar]
- 19.Martinez ME, Charalambous M, Saferali A, Fiering S, Naumova AK, St Germain D, Ferguson-Smith AC, Hernandez A. Genomic imprinting variations in the mouse type 3 deiodinase gene between tissues and brain regions. Mol Endocrinol. 2014;28(11):1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez ME, Cox DF, Youth BP, Hernandez A. Genomic imprinting of DIO3, a candidate gene for the syndrome associated with human uniparental disomy of chromosome 14. Eur J Hum Genet. 2016;24(11):1617–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bianco AC, Kim BW. Deiodinases: implications of the local control of thyroid hormone action. J Clin Invest. 2006;116(10):2571–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bochukova E, Schoenmakers N, Agostini M, Schoenmakers E, Rajanayagam O, Keogh JM, Henning E, Reinemund J, Gevers E, Sarri M, Downes K, Offiah A, Albanese A, Halsall D, Schwabe JWR, Bain M, Lindley K, Muntoni F, Vargha-Khadem F, Dattani M, Farooqi IS, Gurnell M, Chatterjee K. A mutation in the thyroid hormone receptor alpha gene. N Engl J Med. 2012;366(3):243–249. [DOI] [PubMed] [Google Scholar]
- 23.Forrest D, Vennström B. Functions of thyroid hormone receptors in mice. Thyroid. 2000;10(1):41–52. [DOI] [PubMed] [Google Scholar]