Abstract
Context:
Multifocality is often treated as a risk factor for papillary thyroid cancer (PTC), prompting aggressive treatments, but its prognostic value remains unestablished.
Objective:
To investigate the role of tumor multifocality in clinical outcomes of PTC.
Methods:
Multicenter study of the relationship between multifocality and clinical outcomes of PTC in 2638 patients (623 men and 2015 women) with median [interquartile range (IQR)] age of 46 (35 to 58) years and median (IQR) follow-up time of 58 (26 to 107) months at 11 medical centers in six countries. Surveillance, Epidemiology and End Results (SEER) data were used for validation.
Results:
Disease recurrence in multifocal and unifocal PTC was 198 of 1000 (19.8%) and 221 of 1624 (13.6%) (P < 0.001), with a hazard ratio of 1.55 [95% confidence interval (CI), 1.28 to 1.88], which became insignificant at 1.13 (95% CI, 0.93 to 1.37) on multivariate adjustment. Similar results were obtained in PTC variants: conventional PTC, follicular-variant PTC, tall-cell PTC, and papillary thyroid microcarcinoma. There was no association between multifocality and mortality in any of these PTC settings, whereas there was a strong association between classic risk factors and cancer recurrence or mortality, which remained significant after multivariate adjustment. In 1423 patients with intrathyroidal PTC, disease recurrence was 20 of 455 (4.4%) and 41 of 967 (4.2%) (P = 0.892) and mortality was 0 of 455 (0.0%) and 3 of 967 (0.3%) (P = 0.556) in multifocal and unifocal PTC, respectively. The results were reproduced in 89,680 patients with PTC in the SEER database.
Conclusions:
Tumor multifocality has no independent risk prognostic value in clinical outcomes of PTC; its indiscriminate use as an independent risk factor, prompting overtreatments of patients, should be avoided.
Despite common belief, this study shows no independent role of multifocality in clinical outcomes of PTC; its indiscriminate use as a risk factor, prompting overtreatment, should be avoided.
Papillary thyroid cancer (PTC) is a common endocrine malignancy with a rapid rise in incidence in recent years (1–3). This cancer consists of several histological variants, including mainly conventional PTC (CPTC), follicular-variant PTC (FVPTC), and tall-cell PTC (TCPTC), with a prevalence order of CPTC > FVPTC >> TCPTC (4, 5). The standard treatment of PTC is total thyroidectomy or hemithyroidectomy, followed, in appropriately selected cases, by radioiodine ablation and thyrotropin suppression (6–8). These treatments, when used in appropriately defined patients, can reduce disease recurrence and patient mortality but can lead to treatment-associated adverse effects. It is thus important to appropriately define the type and extent of the treatment to maximize the benefit potential while minimizing the adverse risk of the treatment. To this end, accurate risk stratification is critical in helping define appropriate treatments of PTC. This is currently achieved primarily through the evaluation of clinicopathological risk factors, such as advanced patient age, male sex, lymph node metastasis, extrathyroidal extension, and distant metastasis, whose association with poor outcomes of PTC has been well established (8–10).
PTC can occur as a solitary tumor (unifocality) or as multifocal tumors (multifocality). The prevalence of the latter ranged from 32% to 39% in large series of PTC (5, 11, 12). Multifocality is often empirically treated as a high-risk factor for progression of PTC, thereby prompting more aggressive treatments. Yet results from previous studies on the role of tumor multifocality in clinical outcomes of PTC are inconsistent and even contradictory (11–16). Consequently, the prognostic value of multifocality of PTC remains controversial, creating a major confusion and dilemma in the current clinical management of PTC. Thus, overtreatment or undertreatment of PTC may occur, depending on how a clinician understands and uses multifocality as a prognostic risk factor. In the current study, we used a large international multicenter cohort of patients with PTC and the Surveillance, Epidemiology and End Results (SEER) database to definitively address the role of multifocality in the clinical outcomes of PTC and its prognostic value in managing this cancer.
Patients and Methods
With the approval of institutional review boards of the participating institutions and, where required, informed written subject consents, data from 2638 patients with PTC on clinicopathological characteristics, tumor recurrence, and PTC-specific patient death were pooled from 11 medical centers in six countries (listed in Supplemental Table 1 (235.8KB, docx) ). These patients included 623 (23.6%) men and 2015 (76.4%) women, with a median [interquartile range (IQR)] age of 46 (35 to 58) years at the diagnosis of PTC and median (IQR) follow-up time of 58 (26 to 107) months after the initial surgery (Supplemental Table 1 (235.8KB, docx) and Table 1). All the patients were treated with total or near-total thyroidectomy for PTC and were consecutively selected. Histopathological diagnoses of PTC variants were established according to World Health Organization criteria (17). Unifocality of PTC was defined as a solitary cancer focus, and multifocality of PTC was defined as two or more tumor foci. The largest diameter of the largest tumor was used to define the tumor size in each case. Papillary thyroid microcarcinoma (PTMC) was defined as the largest tumor being ≤1.0 cm in diameter. In this sense, multifocal PTMC meant that all the tumor foci were ≤1.0 cm. Disease recurrence was defined as recurrent or persistent PTC identified with standard biochemical, cytological, histological, and radiographical criteria as previously described (9, 10). Unless specifically indicated, recurrence in this study was defined as combined biochemical (serum thyroglobulin) and structural tumor recurrences. Structural tumor recurrence was defined as confirmed physical tumor existence of recurrent or persistent disease, not just positive serum thyroglobulin. A total of 89,680 patients from the SEER database from 2004 to 2013, which started collecting information on multifocality of PTC in 2004, were used here for replication and validation analyses. The study designs and statistical analyses are presented in Supplemental Methods (235.8KB, docx) .
Table 1.
Comparison of the Clinicopathological Characteristics Between Unifocality and Multifocality of PTC
| Characteristics | All PTC | Unifocal PTC | Multifocal PTC | P |
|---|---|---|---|---|
| Total cases, n (%)a | 2638 | 1624 (61.9) | 1000 (38.1) | |
| Age at diagnosis, na | 2638 | 1624 | 1000 | |
| Median (IQR), y | 46 (35–58) | 46 (34–58) | 46 (36–57) | 0.543 |
| Age ≥45 y, n (%)a | 1408/2638 (53.4) | 863/1624 (53.1) | 539/1000 (53.9) | 0.705 |
| Sex (male), n (%)a | 623/2638 (23.6) | 359/1624 (22.1) | 26/11000 (26.1) | 0.019 |
| Tumor size, na | 2601 | 1605 | 996 | |
| Median (IQR), cm | 1.5 (1.0–2.5) | 1.6 (1.0–2.6) | 1.5 (1.0–2.5) | 0.068 |
| Tumor size >1.0 cm, n (%)a | 1820/2601 (70.0) | 1121/1605 (69.8) | 699/996 (70.2) | 0.856 |
| Extrathyroidal extension, n (%)a | 668/2634 (25.4) | 355/1622 (21.9) | 313/1000 (31.3) | <0.001 |
| Lymph node metastasis, n (%)a | 896/2613 (34.3) | 459/1612 (28.5) | 430/987 (43.6) | <0.001 |
| Tumor stage, n (%)a | 2618 | 1613 | 999 | |
| I | 1819 (69.5) | 1152 (71.4) | 664 (66.5) | |
| II | 185 (7.1) | 125 (7.7) | 60 (6.0) | |
| III | 414 (15.8) | 223 (13.8) | 191 (19.1) | |
| IV | 200 (7.6) | 113 (7.0) | 84 (8.4) | 0.001 |
| Tumor stage III or IV, n (%)a | 614/2618 (23.5) | 336/1613 (20.8) | 275/999 (27.5) | <0.001 |
| Distant metastasis, n (%)a | 118/2615 (4.5) | 67/1609 (4.2) | 50/999 (5.0) | 0.313 |
| 131I treatment, n (%)b | 1984/2559 (77.5) | 1149/1553 (74.0) | 82/9992 (83.6) | <0.001 |
| 131I dose, nb | 2540 | 1545 | 982 | |
| Median (IQR), mCi | 100 (30–100) | 100 (0–100) | 100 (50–103) | <0.001 |
| Follow-up time (R), na | 2638 | 1624 | 1000 | |
| Median (IQR), mo | 51 (23–96) | 53 (24–98) | 48 (21–94) | 0.009 |
| Tumor recurrence, n (%)a | 423/2638 (16.0) | 221/1624 (13.6) | 198/1000 (19.8) | <0.001 |
| Structural recurrence, n (%)c | 16/71051 (15.9) | 92/653 (14.1) | 75/398 (18.8) | 0.041 |
| Recurrence of intrathyroidal PTC, n (%)d | 61/1423 (4.3) | 41/967 (4.2) | 20/455 (4.4) | 0.892 |
| Recurrence of PTMC, n (%)e | 59/781 (7.6) | 24/484 (5.0) | 35/297 (11.8) | <0.001 |
| Recurrence of non-PTMC, n (%)f | 352/1820 (19.3) | 190/1121 (16.9) | 162/699 (23.2) | 0.001 |
| Mortality, n (%)a | 58/2638 (2.2) | 37/1624 (2.3) | 20/1000 (2.0) | 0.635 |
| Mortality of intrathyroid PTC, n (%)d | 3/1423 (0.2) | 3/967 (0.3) | 0/455 (0.0) | 0.556 |
| Follow-up time (M), na | 2638 | 1624 | 1000 | |
| Median (IQR), mo | 58 (26–107) | 60 (28–108) | 56 (24–107) | 0.113 |
| BRAF mutation, n (%)a | 1094/2618 (41.8) | 654/1615 (40.5) | 438/989 (44.3) | 0.057 |
In cases where the total patient number in the “All PTC” category is larger than the sum of the numbers of patients in corresponding “Unifocal PTC” and “Multifocal PTC” categories, there were one or more patients with the information available on the parameter under examination but missing information on tumor focus status.
Abbreviations: M, mortality; R, recurrence.
These data on characteristics were from 2638 patients from medical centers 1 to 11, with complete information on patient age and sex but missing information on multifocality, tumor size, extrathyroidal extension, lymph node metastasis, tumor stage, distant metastasis, tumor recurrence, follow-up time for R, M, follow-up time for M, and BRAF mutation status in 14, 37, 4, 25, 20, 23, 0, 0, 0, 0, and 20 cases, respectively.
For 131I treatment and radioiodine dosage, the data were from medical centers 1 to 5 and 7 to 11 with a total of 2562 cases, missing 3 and 22 cases, respectively.
Structural recurrence data were from medical center 1, with a total of 1051 cases.
Data on recurrence and mortality of patients with intrathyroidal PTC defined as PTC without extrathyroidal extension, lymph node metastasis, and distant metastasis were pooled from medical centers 1 to 11, with a total of 1423 cases.
Data on recurrence in PTMC defined as PTC ≤1.0 cm were pooled from medical centers 1 to 11, with a total of 781 cases.
Data on recurrence in non-PTMC were pooled from medical centers 1 to 11, with a total of 1820 cases.
Results
Association between multifocality and high-risk clinicopathological characteristics of PTC in univariate analyses
Multifocality was seen in 1000 of 2624 (38.1%) PTC cases, 731 of 1888 (38.7%) CPTC cases, 183 of 524 (34.9%) FVPTC cases, and 42 of 100 (42.0%) TCPTC cases, with no difference between them (P = 0.203).
On the overall analysis of the 2638 cases of PTC (Table 1), when compared with unifocality of PTC, multifocality was associated with extrathyroidal extension, lymph node metastasis, and, correspondingly, advanced tumor stage III or IV. Multifocality was also associated with more frequent use and higher dosage of radioiodine-131 (131I) in the treatment of PTC. There was no difference between multifocality and unifocality in patient age, tumor size, and BRAF mutation status (P > 0.05 in all). The recurrence of PTC was moderately higher in multifocality compared with unifocality, being 198 of 1000 (19.8%) in the former and 221 of 1624 (13.6%) in the latter (P < 0.001). This pattern was also seen when we examined PTMC and non-PTMC (e.g., tumors >1.0 cm) separately, with the recurrence in multifocal and unifocal PTMC and non-PTMC being 35 of 297 (11.8%) and 24 of 484 (5.0%) (P < 0.001) and 162 of 699 (23.2%) and 190 of 1121 (16.9%) (P = 0.001), respectively. The data from the Johns Hopkins patients also had information on structural recurrence of PTC, which was specifically analyzed and found to be moderately higher in multifocality compared with unifocality, being 75 of 398 (18.8%) in the former and 92 of 653 (14.1%) in the latter (P = 0.041).
On the analysis of the 1893 patients with CPTC (Supplemental Table 2 (235.8KB, docx) ), multifocality was similarly associated with extrathyroidal extension, lymph node metastasis, and, correspondingly, advanced tumor stage III or IV. Multifocality was also associated with more frequent use and higher dosage of 131I in the treatment of CPTC. No association was seen between multifocality and other characteristics. Recurrence of CPTC was moderately higher in multifocality compared with unifocality, being 155 of 731 (21.2%) in the former and 163 of 1157 (14.1%) in the latter (P < 0.001). This pattern was similarly seen in PTMC and non-PTMC in the CPTC cohort, with the recurrence in multifocal and unifocal PTMC and non-PTMC being 30 of 237 (12.7%) and 20 of 395 (5.1%) (P = 0.001) and 124 of 493 (25.2%) and 137 of 748 (18.3%) (P = 0.004), respectively. Structural recurrence of CPTC was not significantly different between multifocality and unifocality, being 59 of 305 (19.3%) in the former and 70 of 488 (14.3%) in the latter (P = 0.063).
In FVPTC, no high-risk clinicopathological characteristic was associated with multifocality except for lymph node metastasis (Supplemental Table 3 (235.8KB, docx) ). The recurrence was marginally associated with multifocality compared with unifocality, being 25 of 183 (13.7%) in the former and 28 of 341 (8.2%) in the latter (P = 0.049). In TCPTC, no association of any high-risk clinicopathological characteristic with multifocality was observed, including disease recurrence (Supplemental Table 4 (235.8KB, docx) ). There was no association between multifocality and distant metastasis in any of the PTC settings investigated in this study (Table 1, Supplemental Tables 2 to 4 (235.8KB, docx) ).
Lack of an independent role of multifocality in clinical outcomes of PTC
On univariate analyses, moderately significant hazard ratios (HRs) of multifocality for recurrences were observed in several settings of PTC (Supplemental Table 5 (235.8KB, docx) ). However, on multivariate adjustment for classic clinicopathological risk factors of patient age, male sex, tumor size, extrathyroidal extension, and lymph node metastasis, the significance of HRs of multifocality for recurrence was lost in all the PTC settings (Table 2). In contrast, the HRs of patient age, male sex, tumor size, extrathyroidal extension, and lymph node metastasis for PTC recurrence remained significant in most of these settings after multivariate adjustment, consistent with their well-established role in poor clinical outcomes of PTC (6–8). Similar results were obtained when the three PTC variants, CPTC, FVPTC, and TCPTC, were individually analyzed (Supplemental Table 6 (235.8KB, docx) ).
Table 2.
HRs of the Clinicopathological Characteristics for Tumor Recurrence of PTC in Multivariate Cox Proportional Hazards Analysis
| Characteristics |
All Variants of PTC |
|
|---|---|---|
| HR (95% CI) | P | |
| Recurrence in all PTC | ||
| Age at diagnosis | 1.02 (1.01–1.03) | <0.001 |
| Sex (male) | 1.33 (1.08–1.64) | 0.007 |
| Tumor size | 1.13 (1.08–1.17) | <0.001 |
| Extrathyroidal extension | 1.93 (1.56–2.39) | <0.001 |
| Lymph node metastasis | 5.40 (4.24–6.87) | <0.001 |
| Multifocality | 1.13 (0.93–1.37) | 0.233 |
| Recurrence in PTMC | ||
| Age at diagnosis | 1.02 (1.00–1.04) | 0.069 |
| Sex (male) | 1.63 (0.93–2.85) | 0.089 |
| Tumor size | 2.93 (0.96–8.99) | 0.060 |
| Extrathyroidal extension | 1.24 (0.67–2.28) | 0.495 |
| Lymph node metastasis | 11.33 (5.97–21.51) | <0.001 |
| Multifocality | 1.43 (0.83–2.47) | 0.194 |
| Recurrence in non-PTMC | ||
| Age at diagnosis | 1.02 (1.01–1.03) | <0.001 |
| Sex (male) | 1.31 (1.05–1.65) | 0.017 |
| Tumor size | 1.10 (1.05–1.16) | <0.001 |
| Extrathyroidal extension | 1.97 (1.57–2.48) | <0.001 |
| Lymph node metastasis | 4.55 (3.51–5.89) | <0.001 |
| Multifocality | 1.10 (0.89–1.36) | 0.400 |
| Recurrence in intrathyroidal PTC | ||
| Age at diagnosis | 1.01 (0.99–1.03) | 0.474 |
| Sex (male) | 1.75 (1.01–3.05) | 0.048 |
| Tumor size | 1.21 (1.05–1.41) | 0.011 |
| Multifocality | 1.14 (0.66–1.95) | 0.643 |
| Structural recurrence | ||
| Age at diagnosis | 1.01 (1.00–1.02) | 0.013 |
| Sex (male) | 1.20 (0.88–1.65) | 0.256 |
| Tumor size | 1.22 (1.12–1.33) | <0.001 |
| Extrathyroidal extension | 2.37 (1.68–3.34) | <0.001 |
| Lymph node metastasis | 5.87 (3.97–8.68) | <0.001 |
| Multifocality | 1.10 (0.81–1.50) | 0.547 |
For each clinicopathological risk factor, multivariate adjustment was performed to adjust the effects of the remaining factors indicated in the same section group.
Abbreviation: CI, confidence interval.
These results strongly suggest that multifocality has no independent role in PTC recurrence and that its association with recurrence observed on univariate analyses reflects the effects of coexisting classic high-risk factors, such as lymph node metastasis and extrathyroidal extension. To definitively test this supposition, we identified and examined a special cohort of 1423 patients with intrathyroidal PTC, defined as tumors lacking extrathyroidal extension, lymph node metastasis, and distant metastasis. Among these patients, 455 of 1422 (32.0%) cases had multifocality. The overall rate of intrathyroidal multifocality among all PTC was 455 of 2624 (17.3%). Even on univariate analysis, no association between multifocality and disease recurrence was observed in patients with intrathyroidal PTC. Specifically, recurrence in multifocal and unifocal PTC in these patients was 20 of 455 (4.4%) and 41 of 967 (4.2%) (P = 0.892) (Table 1), with an HR of 1.08 [95% confidence interval (CI), 0.63 to 1.84] (Supplemental Table 5 (235.8KB, docx) ), which remained insignificant at 1.14 (95% CI, 0.66 to 1.95) after multivariate adjustment (Table 2). Lack of association between multifocality and recurrence of intrathyroidal PTC was similarly observed in CPTC (Supplemental Table 2 (235.8KB, docx) ), FVPTC (Supplemental Table 3 (235.8KB, docx) ), and TCPTC (Supplemental Table 4 (235.8KB, docx) ), with insignificant HRs (Supplemental Tables 5 and 6 (235.8KB, docx) ). In contrast, multifocality of intrathyroidal PTC was significantly associated with increased use and dosage of 131I (Supplemental Table 7 (235.8KB, docx) ). Specifically, patients with multifocality or unifocality received 131I treatment in 328 of 451 (72.7%) and 604 of 928 (65.1%) patients (P = 0.004) with dosages of 80 (IQR, 50 to 100) mCi and 55 (IQR, 0 to 100) mCi (P < 0.001), respectively. Similar results were observed in intrathyroidal CPTC, FVPTC, and TCPTC. However, such 131I treatments did not affect disease recurrence or patient mortality in intrathyroidal PTC, because the differences in recurrence and mortality between multifocality and unifocality remained insignificant on multivariate adjustments for the factors of 131I treatment and 131I dosage, whether on the analysis of all PTC or intrathyroidal PTC (Supplemental Table 8 (235.8KB, docx) ).
There was no association between multifocality and PTC-related patient mortality in any of the PTC settings examined in this study, whether on univariate or multivariate analyses (Table 1, Table 3, Supplemental Tables 2 to 5 (235.8KB, docx) , Supplemental Table 8 (235.8KB, docx) ). For example, on the univariate analysis of the entire cohort of patients, mortality in multifocal and unifocal PTC was 20 of 1000 (2.0%) and 37 of 1624 (2.3%) (P = 0.635) (Table 1), corresponding to an HR of 0.90 (95% CI, 0.52 to 1.55) (Supplemental Table 5 (235.8KB, docx) ), which remained insignificant at 0.88 (95% CI, 0.49 to 1.56) on multivariate adjustment (Table 3). A similar lack of association between multifocality and mortality was seen on analyses of CPTC (Supplemental Table 2 (235.8KB, docx) ), FVPTC (Supplemental Table 3 (235.8KB, docx) ), and TCPTC (Supplemental Table 4 (235.8KB, docx) ), with insignificant HRs (Table 3 and Supplemental Table 5 (235.8KB, docx) ). In the group of patients with intrathyroidal PTC of all variants, the mortality was extremely low in multifocal PTC and unifocal PTC, being 0 of 455 (0.0%) in the former and 3 of 967 (0.3%) in the latter (P = 0.556) (Table 1). Similar results were observed in the individual PTC variants (Supplemental Tables 2 to 4 (235.8KB, docx) ). In all these PTC settings, HRs of multifocality for mortality were insignificant (Table 3 and Supplemental Table 5 (235.8KB, docx) ). In contrast, the classic clinicopathological risk factors were associated with patient mortality, which remained significant after multivariate adjustments (Table 3). These results were replicated and validated in the analysis of 89,680 patients with PTC in the SEER database (Supplemental Table 9 (235.8KB, docx) ). Specifically, whereas the classic clinicopathological risk factors were independently associated with PTC-related mortality, multifocality did not adversely affect mortality. There was also no difference in mortality between multifocality and unifocality of intrathyroidal PTC in the SEER data.
Table 3.
HRs of the Clinicopathological Characteristics for PTC-Associated Patient Mortality in Multivariate Cox Proportional Hazards Analysis
| Characteristics |
All Variants |
CPTC |
FVPTC |
TCPTC |
||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Mortality in all PTC | ||||||||
| Age at diagnosis | 1.09 (1.07–1.11) | <0.001 | 1.10 (1.07–1.13) | <0.001 | 1.11 (1.00–1.24) | 0.060 | 1.01 (0.95–1.06) | 0.830 |
| Sex (male) | 1.53 (0.89–2.64) | 0.128 | 2.30 (1.19–4.45) | 0.014 | 0.21 (0.02–2.58) | 0.221 | 4.91 (0.67–36.15) | 0.119 |
| Tumor size | 1.23 (1.14–1.40) | <0.001 | 1.14 (0.96–1.36) | 0.137 | 1.08 (0.55–2.13) | 0.830 | 2.10 (1.38–3.20) | <0.001 |
| Extrathyroidal extension | 2.73 (1.43–5.22) | 0.002 | 3.44 (1.58–7.51) | 0.002 | 2.67 (0.23–31.03) | 0.433 | 0.23 (0.03–1.97) | 0.181 |
| Lymph node metastasis | 6.06 (2.96–12.38) | <0.001 | 5.22 (2.30–11.83) | <0.001 | —a | 0.932 | 13.91 (1.00–194.30) | 0.050 |
| Multifocality | 0.88 (0.49–1.56) | 0.653 | 0.69 (0.34–1.43) | 0.317 | 0.74 (0.10–5.76) | 0.772 | 0.46 (0.08–2.61) | 0.381 |
| Mortality in intrathyroidal PTC | ||||||||
| Age at diagnosis | 1.07 (0.98–1.17) | 0.151 | 4.80 (0.18–125.26) | 0.346 | —b | —b | 1.28 (0.02–111.60) | 0.915 |
| Sex (male) | 5.05 (0.42–60.89) | 0.202 | —a | 0.904 | —b | —b | —a | 0.918 |
| Tumor size | 1.40 (0.86–2.28) | 0.172 | 5.33 (0.06–512.24) | 0.473 | —b | —b | —a | 0.843 |
| Multifocality | —a | 0.976 | —a | 0.756 | —b | —b | —a | 0.940 |
For each clinicopathological risk factor, multivariate adjustment was performed to adjust the effects of the remaining factors indicated in the same section group.
Because of the low death number, HR could not be calculated.
HR and P value could not be calculated because of the zero death in unifocal and multifocal FVPTC in the intrathyroidal cohort.
Lack of independent effects of multifocality on Kaplan-Meier survival curves of patients with PTC
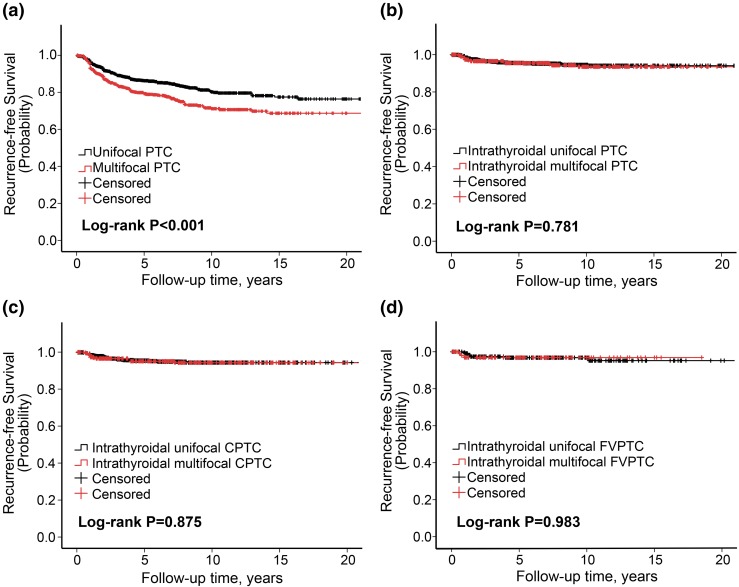
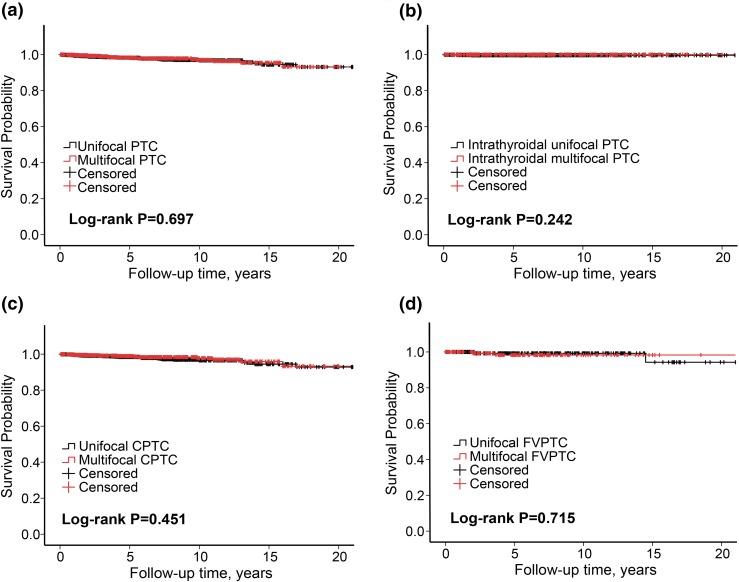
Compared with unifocality, multifocality was associated with a moderate decline in recurrence-free survival curves of patients in the analysis of the entire cohort of patients [Fig. 1(a)] or those with CPTC [Supplemental Fig. 1(A (235.8KB, docx) )] or FVPTC [Supplemental Fig. 1(B (235.8KB, docx) )]. In striking contrast, there was no difference in recurrence-free survival curves in patients between multifocality and unifocality when intrathyroidal PTC was analyzed, either in intrathyroidal PTC of all variants [Fig. 1(b)], intrathyroidal CPTC [Fig. 1(c)], or intrathyroidal FVPTC [Fig. 1(d)]. There was no difference in PTC-specific survival curves of patients between multifocality and unifocality, either in general PTC of all variants [Fig. 2(a)], intrathyroidal PTC of all variants [Fig. 2(b)], general CPTC [Fig. 2(c)], or general FVPTC [Fig. 2(d)]. Thus, these results clearly demonstrated that multifocality had no effects on the survival curves of patients with PTC.
Figure 1.
Kaplan-Meier survival curve analysis of the effect of multifocality on disease recurrence–free probability in patients with various types of PTC. Shown are comparisons of recurrence-free survival probabilities between multifocality and unifocality in patients with (a) all PTC of the entire cohort, (b) intrathyroidal PTC of all variants, (c) intrathyroidal CPTC, and (d) intrathyroidal FVPTC. Log-rank test was performed to compare multifocality and unifocality in each group pair, with corresponding P values shown in each panel. The follow-up time is truncated at 20 years.
Figure 2.
Kaplan-Meier survival curve analysis of the effect of multifocality on disease-specific survival probability of patients with various types of PTC. Shown are comparisons of disease-specific survival probabilities between multifocality and unifocality in patients with (a) all PTC of the entire cohort, (b) intrathyroidal PTC of all variants, (c) CPTC, and (d) FVPTC. Log-rank test was performed to compare multifocality and unifocality in each group pair, with corresponding P values shown in each panel. The follow-up time is truncated at 20 years.
Discussion
Appropriate clinicopathological risk stratification is important in the clinical management of PTC to optimize the balance between treatment-associated benefits and risk of adverse complications. This is achieved primarily by evaluating clinicopathological risk factors. In this context, tumor multifocality is often empirically treated by clinicians in practice as a risk factor for poor prognosis of PTC, prompting more aggressive treatments. However, there is no established evidence to support this practice. In fact, previous studies have been controversial on the prognostic value of multifocality in PTC (11–16, 18–21), leaving clinicians with a dilemma on how to use multifocality as a risk factor to assist in managing patients with PTC. A general limitation of the previous studies is that they used mostly single-institution cohorts; many were small and used only univariate analyses. Moreover, few studies investigated the effect of multifocality on PTC-related mortality, the most concerning clinical outcome of PTC. The recent clinical guidelines on the management of thyroid cancer from the American Thyroid Association recommended no more aggressive treatments, such as radioiodine ablation, for multifocality only in PTMC, but they were unable to make specific recommendations on multifocality in PTC >1.0 cm (8). Thus, a major practical challenge in the current management of PTC is how to understand and use tumor multifocality as a risk factor in the treatment of patients, particularly patients with multifocality of tumors >1.0 cm.
In this study of a large multicenter cohort of patients, complemented with the SEER database, we extensively investigated the prognostic value of multifocality in clinical outcomes of PTC. As found in previous studies (11, 12, 18, 19, 21), our univariate analyses revealed an association between multifocality and lymph node metastasis and extrathyroidal extension as well as PTC recurrence. However, on multivariate adjustment for classic clinicopathological risk factors, the association between multifocality and disease recurrence was lost in all PTC settings. In contrast, the association between the established clinicopathological risk factors and recurrence of PTC remained significant even after multivariate adjustments. When only intrathyroidal PTC was analyzed, there was no association between multifocality and recurrence, whether on univariate analysis or multivariate analysis. This was true whether the entire cohort of 1423 cases of intrathyroidal PTC of all variants or individual variants of intrathyroidal CPTC, FVPTC, or TCPTC were examined. These results clearly demonstrate no independent role of multifocality in PTC recurrence.
We also found no association between multifocality and distant metastasis in PTC, even in univariate analyses. This finding is worth noting because distant metastasis is the most aggressive tumor risk factor for PTC-related mortality. In fact, whether on univariate or multivariate analyses, there was no association between multifocality and patient mortality in any of the PTC settings investigated. As in unifocal intrathyroidal PTC, the mortality was also extremely low in multifocal intrathyroidal PTC, being 0.3% in the former and 0.0% in the latter. Importantly, all these results were replicated and validated in the large SEER database.
The findings of this study may have important clinical implications at two levels: the preoperative level and the postoperative level. For the former, knowledge of PTC multifocality from preoperative diagnostic efforts may favor a more aggressive surgical approach in appropriately selected patients, given the association of multifocality with lymph node metastasis and extrathyroidal extension. For the latter, multifocality itself should not affect defining postoperative treatments, such as the need for radioiodine ablation and thyrotropin suppression, which should be dictated instead by the status of classic clinicopathological risk factors. Similarly, intrathyroidal multifocality localized in one thyroid lobe may not justify total thyroidectomy for the treatment of PTC, as it otherwise could in current clinical practice. This conservative approach to the treatment of multifocal intrathyroidal PTC may have a general application, as supported by the current study, including tumors >1.0 cm, not just PTMC, as suggested by the recent American Thyroid Association guidelines (8).
More aggressive treatments of multifocality over unifocality, such as more frequent and higher-dose radioiodine treatments, were commonly reported in previous studies (11, 12) and observed in the current study. It is likely that in many cases such multifocality-associated aggressive treatments were empirically prompted by the multifocality status itself. This speculation is directly supported by the finding in the current study that even in patients with intrathyroidal PTC without classic high-risk factors, those with tumor multifocality were treated with 131I more often and with higher doses than patients with unifocality. This could theoretically be a cofounding factor for the comparability of clinical outcomes of patients between multifocal and unifocal intrathyroidal PTC in the current study. In reality, however, this was not the case because the increased use of 131I did not actually affect the clinical outcomes of PTC in the current study, as demonstrated by the fact that on multivariate adjustment for 131I treatments there was still no difference in disease recurrence and patient mortality between multifocal and unifocal PTC. Consistent with the finding of the extremely low patient mortality associated with intrathyroidal PTC in the current study, intrathyroidal PTC is generally of low-risk stage I or II disease, for which it has been well established that high-dose 131I has no additional therapeutic benefit compared with low-dose 131I, as exemplified by the demonstration that 100 mCi and 30 mCi 131I had comparable effects on clinical outcomes of such PTC (22, 23). It is likely that multifocality may also empirically prompt more aggressive thyrotropin suppression, which could be theoretically also a cofounding factor for the comparability of clinical outcomes of patients between multifocal and unifocal intrathyroidal PTC in the current study. This, in reality, was not the case because large studies have generally shown no therapeutic benefit of aggressive thyrotropin suppression and radioiodine treatments for PTC of stage I or II disease (24–27). Thus, more aggressive treatments of PTC empirically prompted by multifocality did not improve clinical outcomes and played no role in the comparability of clinical outcomes between multifocality and unifocality observed in the current study. More aggressive treatments for multifocal intrathyroidal PTC are unnecessary overtreatments and may increase the risk of treatment-associated adverse complications.
Although its multicenter nature is a strength of this study, it is also potentially associated with the problem of inherent data heterogeneity between centers. This problem is minimized by several factors, however: The participating centers are world-renowned thyroid tumor centers that have been closely following contemporary standard guidelines to treat patients with thyroid cancer; only patients with total or nearly total thyroidectomy were included in the study; the main parameter studied, multifocality, is simply defined by the number of cancer foci and should not cause variations between centers; all participating centers used the same predesigned protocol to collect data according to the same criteria for this study; and this was a large cohort, and in fact the largest multicenter one to provide a large power to study multifocality. These and other strengths should ably minimize the heterogeneity issue and help reach solid conclusions. The fact that the findings from the analyses of these multicenter data were completely replicated in the analysis of the SEER data provides additional support for their reliability.
Conclusions
This large, comprehensive multicenter study, complemented by the SEER data analyses, provides definitive evidence reconciling the controversies on the role and prognostic value of tumor multifocality in clinical outcomes of PTC. Although multifocality is associated with lymph node metastasis and extrathyroidal invasion and can therefore be potentially useful in facilitating preoperative surgical decision making for PTC, it has no independent impact on the ultimate clinical outcomes of disease recurrence and patient mortality. Postoperatively discovered multifocality of PTC itself has limited role in guiding the management of these patients; it is the established classic clinicopathological risk factors, if present, that can help facilitate decision making for the postoperative treatment of PTC. In particular, postoperatively confirmed intrathyroidal multifocal PTC bears no increased risk and should not prompt more aggressive treatments for patients. Given the common occurrence of tumor multifocality, seen in 32.0% of intrathyroidal PTC or 38.1% of all PTC patients, this study will probably have a broad impact on the current clinical management of PTC.
Acknowledgments
Acknowledgments
This study was supported by U.S. National Institutes of Health (NIH) Grants R01CA113507 and R01CA189224 to M.X. Additional supports at the individual participating centers include the following: Polish National Center of Research and Development MILESTONE Project: Molecular Diagnostics and Imaging in Individualized Therapy for Breast, Thyroid, and Prostate Cancer, grant STRATEGMED2/267398/4/NCBR/2015 (Poland, A.C., B.J.); grants from Menzies Health Institute, Queensland and Queensland Smart State fellowship (Australia; A.K.L.); grants SAF2013-44709-R (MINECO and FEDER), RD12/0036/0030, PI14/01980 (ISCIII), and GCB14142311CRES (AECC Foundation) (Spain; P.S. and G.R.-E.); grant IG 9338 from the Fondazione Cassa di Risparmio di Perugia and Associazione Italiana per la Ricerca sul Cancro (Italy) and the Beadle Family Foundation (San Antonio, TX; E.P.); grants AZV 16-32665A and MH CZ-DRO (Institute of Endocrinology-EU, 00023761) (Czech Republic; B.B., V.S.); grants from the New South Wales Cancer Institute (C.J.O.) and Cancer Council of New South Wales (Australia; R.C.-B.); NIH/National Institute on Aging grant 5R03AG042334-02 (L.Y.); grants from the Ministero della Istruzione Universitaria e Ricerca Scientifica, the Associazione Italiana per la Ricerca sul Cancro, the Istituto Toscano Tumori, and the Ministero della Salute (Italy; D.V., R.E.); and the Health Department of Shandong Province, 2013 WS0266 and the Innovative Platform Project of Qingdao, 12-1-2-15-jch (China; S.Z.).
The content of this article is solely the responsibility of the authors and does not necessarily reflect the official views of the U.S. NIH or the funding entities of the individual centers participating in this study. The funding organizations had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Author contributions: conception and design, M.X. and S.Z.; acquisition, analysis, and interpretation of data, all authors; drafting of the manuscript, F.W. and M.X.; critical revision of the manuscript for important intellectual content, all authors; statistical analysis, F.W., X.S., and M.X.; obtaining funding, M.X.; administrative, technical, or material support, all authors; and study supervision, M.X.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CI
- confidence interval
- CPTC
- conventional papillary thyroid cancer
- FVPTC
- follicular-variant papillary thyroid cancer
- HR
- hazard ratio
- 131I
- radioiodine-131
- IQR
- interquartile range
- PTC
- papillary thyroid cancer
- PTMC
- papillary thyroid microcarcinoma
- SEER
- Surveillance, Epidemiology and End Results
- TCPTC
- tall-cell papillary thyroid cancer.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Mao Y, Xing M. Recent incidences and differential trends of thyroid cancer in the USA. Endocr Relat Cancer. 2016;23(4):313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, eds. SEER Cancer Statistics Review, 1975–2013, National Cancer Institute. April 2016. Available at: http://seer.cancer.gov/csr/1975_2013/. Accessed 21 May 2016.
- 4.Lam AK, Lo CY, Lam KS. Papillary carcinoma of thyroid: a 30-yr clinicopathological review of the histological variants. Endocr Pathol. 2005;16(4):323–330. [DOI] [PubMed] [Google Scholar]
- 5.Shi X, Liu R, Basolo F, Giannini R, Shen X, Teng D, Guan H, Shan Z, Teng W, Musholt TJ, Al-Kuraya K, Fugazzola L, Colombo C, Kebebew E, Jarzab B, Czarniecka A, Bendlova B, Sykorova V, Sobrinho-Simões M, Soares P, Shong YK, Kim TY, Cheng S, Asa SL, Viola D, Elisei R, Yip L, Mian C, Vianello F, Wang Y, Zhao S, Oler G, Cerutti JM, Puxeddu E, Qu S, Wei Q, Xu H, O’Neill CJ, Sywak MS, Clifton-Bligh R, Lam AK, Riesco-Eizaguirre G, Santisteban P, Yu H, Tallini G, Holt EH, Vasko V, Xing M. Differential clinicopathological risk and prognosis of major papillary thyroid cancer variants. J Clin Endocrinol Metab. 2016;101(1):264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JWA, Wiersinga W; European Thyroid Cancer Taskforce . European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol. 2006;154(6):787–803. [DOI] [PubMed] [Google Scholar]
- 7.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer . Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer [published correction appears in Thyroid. 2010;20(8):942]. Thyroid. 2009;19(11):1167–1214. [DOI] [PubMed] [Google Scholar]
- 8.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xing M, Alzahrani AS, Carson KA, Viola D, Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, Robenshtok E, Fagin JA, Puxeddu E, Fugazzola L, Czarniecka A, Jarzab B, O’Neill CJ, Sywak MS, Lam AK, Riesco-Eizaguirre G, Santisteban P, Nakayama H, Tufano RP, Pai SI, Zeiger MA, Westra WH, Clark DP, Clifton-Bligh R, Sidransky D, Ladenson PW, Sykorova V. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309(14):1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xing M, Alzahrani AS, Carson KA, Shong YK, Kim TY, Viola D, Elisei R, Bendlová B, Yip L, Mian C, Vianello F, Tuttle RM, Robenshtok E, Fagin JA, Puxeddu E, Fugazzola L, Czarniecka A, Jarzab B, O’Neill CJ, Sywak MS, Lam AK, Riesco-Eizaguirre G, Santisteban P, Nakayama H, Clifton-Bligh R, Tallini G, Holt EH, Sýkorová V. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015;33(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HJ, Sohn SY, Jang HW, Kim SW, Chung JH. Multifocality, but not bilaterality, is a predictor of disease recurrence/persistence of papillary thyroid carcinoma. World J Surg. 2013;37(2):376–384. [DOI] [PubMed] [Google Scholar]
- 12.Kim KJ, Kim SM, Lee YS, Chung WY, Chang HS, Park CS. Prognostic significance of tumor multifocality in papillary thyroid carcinoma and its relationship with primary tumor size: a retrospective study of 2,309 consecutive patients. Ann Surg Oncol. 2015;22(1):125–131. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Jiao D, Liu B, Sun S. Analysis of risk factors contributing to recurrence of papillary thyroid carcinoma in Chinese patients who underwent total thyroidectomy. Med Sci Monit. 2016;22:1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leboulleux S, Rubino C, Baudin E, Caillou B, Hartl DM, Bidart JM, Travagli JP, Schlumberger M. Prognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid capsule at initial diagnosis. J Clin Endocrinol Metab. 2005;90(10):5723–5729. [DOI] [PubMed] [Google Scholar]
- 15.Neuhold N, Schultheis A, Hermann M, Krotla G, Koperek O, Birner P. Incidental papillary microcarcinoma of the thyroid: further evidence of a very low malignant potential: a retrospective clinicopathological study with up to 30 years of follow-up. Ann Surg Oncol. 2011;18(12):3430–3436. [DOI] [PubMed] [Google Scholar]
- 16.Grogan RH, Kaplan SP, Cao H, Weiss RE, Degroot LJ, Simon CA, Embia OMA, Angelos P, Kaplan EL, Schechter RB. A study of recurrence and death from papillary thyroid cancer with 27 years of median follow-up. Surgery. 2013;154(6):1436–1446, discussion 1446–1447. [DOI] [PubMed] [Google Scholar]
- 17.DeLellis RA, Lioyd RV, Heitz PU, Eng C, eds. WTO Classification of Tumours: Pathology and Genetics of Tumours of Endocrine Organs. Lyon, France: IARC Press; 2004. [Google Scholar]
- 18.Vasileiadis I, Karakostas E, Charitoudis G, Stavrianaki A, Kapetanakis S, Kouraklis G, Karatzas T. Papillary thyroid microcarcinoma: clinicopathological characteristics and implications for treatment in 276 patients. Eur J Clin Invest. 2012;42(6):657–664. [DOI] [PubMed] [Google Scholar]
- 19.Qu N, Zhang L, Wu WL, Ji QH, Lu ZW, Zhu YX, Lin DZ. Bilaterality weighs more than unilateral multifocality in predicting prognosis in papillary thyroid cancer. Tumour Biol. 2016;37(7):8783–8789. [DOI] [PubMed] [Google Scholar]
- 20.Zhu J, Wang X, Zhang X, Li P, Hou H. Clinicopathological features of recurrent papillary thyroid cancer. Diagn Pathol. 2015;10:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pyo JS, Sohn JH, Kang G. Detection of tumor multifocality is important for prediction of tumor recurrence in papillary thyroid microcarcinoma: a retrospective study and meta-analysis. J Pathol Transl Med. 2016;50(4):278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, Nicol A, Clark PM, Farnell K, McCready R, Smellie J, Franklyn JA, John R, Nutting CM, Newbold K, Lemon C, Gerrard G, Abdel-Hamid A, Hardman J, Macias E, Roques T, Whitaker S, Vijayan R, Alvarez P, Beare S, Forsyth S, Kadalayil L, Hackshaw A. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. 2012;366(18):1674–1685. [DOI] [PubMed] [Google Scholar]
- 23.Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, Bardet S, Leenhardt L, Bastie D, Schvartz C, Vera P, Morel O, Benisvy D, Bournaud C, Bonichon F, Dejax C, Toubert M-E, Leboulleux S, Ricard M, Benhamou E; Tumeurs de la Thyroïde Refractaires Network for the Essai Stimulation Ablation Equivalence Trial . Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med. 2012;366(18):1663–1673. [DOI] [PubMed] [Google Scholar]
- 24.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer. 1998;83(12):2638–2648. [DOI] [PubMed] [Google Scholar]
- 25.Hay ID, Thompson GB, Grant CS, Bergstralh EJ, Dvorak CE, Gorman CA, Maurer MS, McIver B, Mullan BP, Oberg AL, Powell CC, van Heerden JA, Goellner JR. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg. 2002;26(8):879–885. [DOI] [PubMed] [Google Scholar]
- 26.Durante C, Attard M, Torlontano M, Ronga G, Monzani F, Costante G, Ferdeghini M, Tumino S, Meringolo D, Bruno R, De Toma G, Crocetti U, Montesano T, Dardano A, Lamartina L, Maniglia A, Giacomelli L, Filetti S; Papillary Thyroid Cancer Study Group . Identification and optimal postsurgical follow-up of patients with very low-risk papillary thyroid microcarcinomas. J Clin Endocrinol Metab. 2010;95(11):4882–4888. [DOI] [PubMed] [Google Scholar]
- 27.Carhill AA, Litofsky DR, Ross DS, Jonklaas J, Cooper DS, Brierley JD, Ladenson PW, Ain KB, Fein HG, Haugen BR, Magner J, Skarulis MC, Steward DL, Xing M, Maxon HR, Sherman SI. Long-term outcomes following therapy in differentiated thyroid carcinoma: NTCTCS registry analysis 1987–2012. J Clin Endocrinol Metab. 2015;100(9):3270–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]