ABSTRACT
Dickkopf-1(DKK-1), the downstream target of β-catenin/T-cell factor, participates in a negative feedback loop in the Wnt signaling and reported as an important biomarker in many tumors. In this study, we analyzed the expression of DKK-1 in pancreatic ductal adenocarcinoma (PDAC) patients at both mRNA and protein levels. We used real-time PCR to detect the expression of DKK-1 in 32 PDAC and paired adjacent non-tumor tissues, results suggested that the expression of DKK-1 was increased in PDAC tissues. We found the similar results in the analysis of 3 independent microarray data sets. Immunohistochemical staining of 311 pairs of PDAC tissues suggested that DKK-1 expression was significantly associated with T classification (P = 0.039) and lymph node metastasis (P = 0.035). Furthermore, Kaplan-Meier analysis for DKK-1 expression demonstrated that patients with higher DKK-1 level had shorter overall survival (OS) and relapse-free survival (RFS) time in Ren Ji cohort and online PDAC database at both mRNA and protein levels. Univariable and multivariable Cox regression analysis confirmed that DKK-1 as well as lymph node metastasis and histology were independent predictors of OS in patients with PDAC. This study demonstrated that DKK-1 may be a predictor for prognosis in PDAC patients.
KEYWORDS: DKK-1, Pancreatic cancer, Pancreatic ductal adenocarcinoma, Tissue microarray, Prognosis, Biomarker, Wnt signaling
Introduction
Pancreatic cancer (PC) is one of the most malignant tumors, leading the fourth cause of cancer related death in the USA.1 Among all the PCs, pancreatic ductal adenocarcinoma (PDAC) accounts the most, for nearly 90%.2 In the past decades, little advances in early detection and improvements in the treatment have been achieved. When diagnosed, most of the PDACs are advanced and only 15–20% of these patients have the chance to receive operation.3 The 5-year overall survival rate is less than 5% and only 10–20% of cases who have radical resection can survive 5 y.4 Therefore, new insights into the biology and genetics of PDAC are required and it is essential to identify novel biologic markers for better diagnosis and prediction of prognosis and thus develop new targeted therapy.
In the past years, accumulating studies have revealed the role of the Wnt signaling pathway in cell proliferation, differentiation, migration and tumorigenesis.5,6 Dickkopf-1 (DKK-1), as a crucial protein in head formation in vertebrate development7 and a member of Wnt signaling pathway,8 has been found to be overexpressed in a variety of tumors such as hepatoblastoma, Wilms' tumor, hepatocellular carcinoma (HCC), non-small cell lung cancer, esophageal carcinoma and so on, including PDAC.9-13 Meanwhile, it was also demonstrated that DKK-1 participates in the tumorigenesis and can serve as a novel prognostic predictor for HCC, lung cancer and esophageal carcinoma patients. In 2012, Shen et al14 assessed serum DKK-1 in 831 participants, and discovered that the serum DKK-1 of the patients with HCC was significantly higher than that of chronic liver disease patients and normal people, and it is helpful for diagnosis of early HCC especially small HCC, even better than α fetoprotein (AFP). Recently, serum DKK-1 level was reported to be elevated in pancreatic cancer patients, and might be a diagnostic/prognostic biomarker for pancreatic cancer.15 However, a comprehensive investigation remains to elucidate the clinical significance and prognostic value of the expression of DKK-1 in PDAC tissues specifically.
In this retrospective study, we further validated the expression pattern of DKK-1 at both mRNA and protein level in a relative large scale, and investigated the relationship of DKK-1 expression with corresponding clinicopathological parameters and prognosis. Importantly, the results suggested that high expression of DKK-1 is a reliable indicator for the poor prognosis of PDAC patients.
Results
DKK-1 mRNA and protein are significantly upregulated in PDAC
To further verify the expression pattern of DKK-1 in PDAC, we first analyzed 3 independent microarray data sets in PDAC research from GEO database. The results showed that DKK-1 mRNA expression was significantly upregulated in PDAC tissues compared with normal pancreatic tissues using GSE15471 (P < 0.0001, Fig. 1A), GSE28735 (P < 0.0001, Fig. 1B) and GSE71729 (P < 0.0001, Fig. 1C). In present study, similar result was also observed in 32 paired PDAC and non-cancerous tissues (Ren Ji cohort 1, fresh tissues) as demonstrated by qRT-PCR (P < 0.0001, Fig. 1D). To further address the protein changes of DKK-1 in PDAC, Western blot was conducted in 8 PDAC cell lines and 1 nonmalignant HPDE6-C7 cells and immunohistochemical analysis was performed in tissue microarray (TMA) containing 311 pairs of PDAC samples (Ren Ji cohort 2, paraffin-embedded tissues). The results also confirmed that DKK-1 protein displayed higher expression in both PDAC cells (Fig. 2A) and tissues (65.9% vs. 39.5%, P < 0.0001, Fig. 2B–C) than their own counterparts, and the immunoreactivity of DKK-1 was mostly distributed in both cytoplasm and cell membrane of PDAC cells (Fig. 2B). Collectively, these results further validated that both the mRNA and protein level of DKK-1 were significantly upregulated in PDAC, which was consistent with previous studies.
Figure 1.
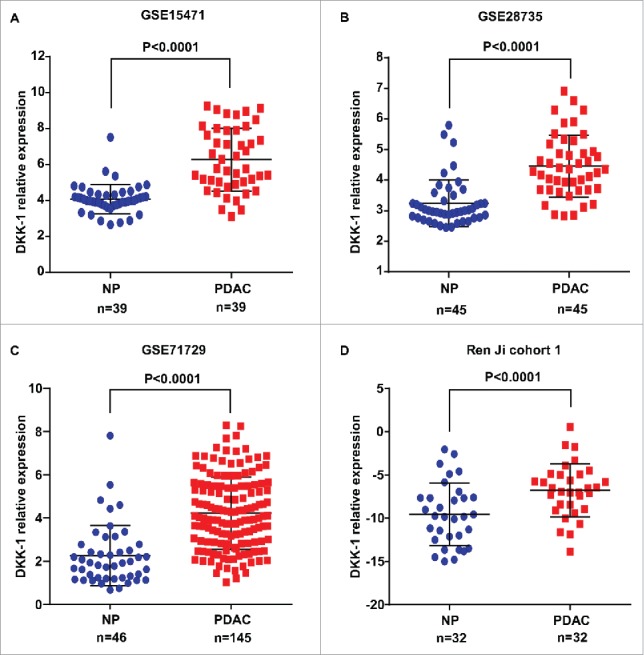
DKK-1 expression in PDAC tissue at mRNA level. (A-C). Expression of DKK-1 was significantly increased in PDAC tumor tissues compared with the adjacent non-tumor tissues (ANT) in GSE 15471, GSE 28735 and GSE 71729. (D) The result of qRT-PCR in Ren Ji cohort 1 showed that the expression of DKK-1 was elevated in PDAC tissue compared with normal pancreas (NP).
Figure 2.
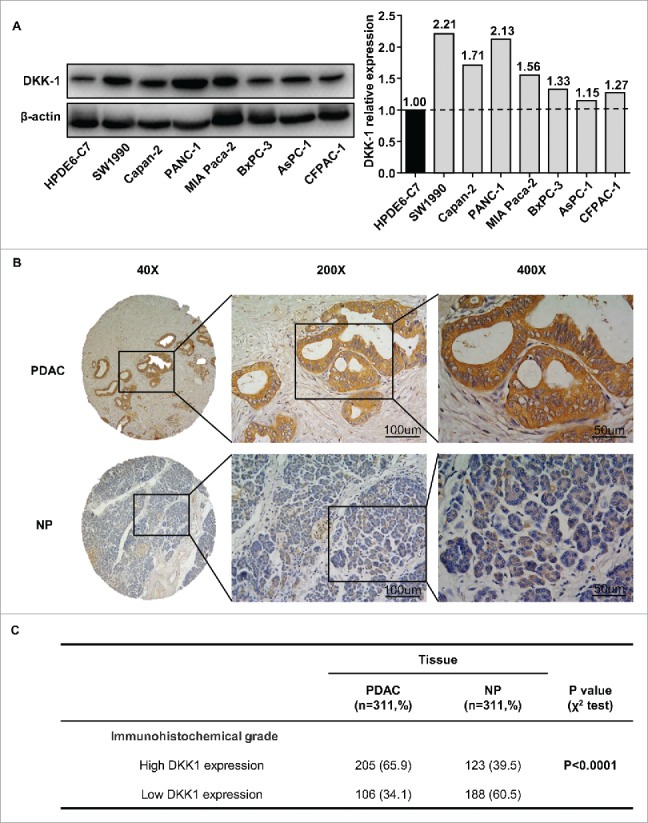
DKK-1 expression in PDAC tissue at protein level. (A) Western blot of 8 PDAC cell lines and 1 nonmalignant HPDE6-C7 cells and the quantification of western blot demonstrated that expression of DKK-1 was increased in PDAC cell lines. (B) Typical imagine of DKK-1 expression in PDAC tissue microarray by IHC. (C) In Ren Ji cohort 2, the rate of high DKK-1 expression was significantly higher in PDAC group compared with NP group.
Correlation between DKK-1 expression and clinicopathological parameters in patients with PDAC
To explore the clinical significance of DKK-1 expression in PDAC, we analyzed the relationship between DKK-1 expression and corresponding patients' clinicopathological characteristics in Ren Ji cohort 2. As shown in Table 1, DKK-1 expression was significantly associated with adverse clinicopathological features of PDAC, including T classification (P = 0.039) and lymph node metastasis (P = 0.035). No correlations between DKK-1 expression and age, gender, tumor location, TNM stage, tumor size, T classification, distant metastasis, vascular invasion, perineural invasion and histological differentiation were found.
Table 1.
Correlations between DKK1 expression and clinicopathologic features in Ren Ji cohort 2 patients with pancreatic ductal adenocarcinoma (PDAC).
| Expression of DKK1 |
||||
|---|---|---|---|---|
| Clinicopathological feature | Total 311 | Low (n = 106, 34.1%) | High (n = 205, 65.9%) | P value (χ2 test) |
| Age(years) | ||||
| <65 | 145 | 55 (37.9) | 90 (62.1) | 0.181 |
| ≥ 65 | 166 | 51 (30.7) | 115 (69.3) | |
| Gender | ||||
| Male | 176 | 54 (30.7) | 122 (69.3) | 0.148 |
| Female | 135 | 52 (38.5) | 83 (61.5) | |
| Tumor location | ||||
| Head | 206 | 69 (33.5) | 137 (66.5) | 0.759 |
| Body/tail | 105 | 37 (35.2) | 68 (64.8) | |
| TNM stage(AJCC) | ||||
| Stage I + II | 243 | 88 (36.2) | 155 (63.8) | 0.134 |
| Stage III + IV | 68 | 18 (26.5) | 50 (73.5) | |
| Tumor size | ||||
| ≤ 3cm | 112 | 40 (35.7) | 72 (64.3) | 0.649 |
| >3cm | 199 | 66 (33.2) | 133 (66.8) | |
| T classificattion | ||||
| T1+T2 | 39 | 19 (48.7) | 20 (51.3) | 0.039 |
| T3+T4 | 272 | 87 (32.0) | 185 (68.0) | |
| Lymph node metastasis | ||||
| Absent | 195 | 75 (38.5) | 120 (61.5) | 0.035 |
| Present | 116 | 31 (26.7) | 85 (73.3) | |
| Distant metastasis | ||||
| Absent | 281 | 100 (35.6) | 181 (64.4) | 0.087 |
| Present | 30 | 6 (20.0) | 24 (80.0) | |
| Vascular invasion | ||||
| Absent | 280 | 98 (35.0) | 182 (65.0) | 0.306 |
| Present | 31 | 8 (25.8) | 23 (74.2) | |
| Perineural invasion | ||||
| Absent | 165 | 56 (33.9) | 109 (66.1) | 0.955 |
| Present | 146 | 50 (34.2) | 96 (65.8) | |
| Histological differentiation | ||||
| Well/moderate | 194 | 66 (34.0) | 128 (66.0) | 0.976 |
| Poor | 117 | 40 (34.2) | 77 (65.8) | |
Note. Values in parentheses indicate percentage values. The bold number represents the P-values with significant differences.
Up-regulated DKK-1 predicts poor prognosis of PDAC patients
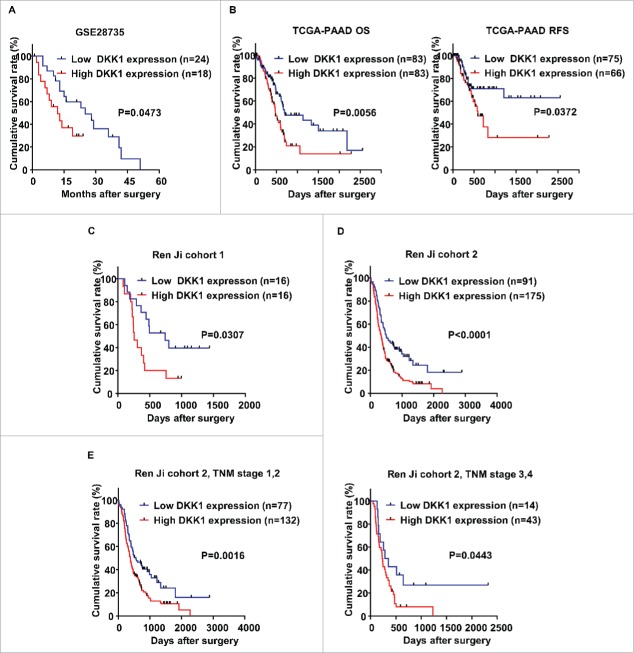
To further evaluate the prognostic value of DKK-1, the correlation between DKK-1 expression and corresponding clinical follow-up information were analyzed by Kaplan-Meier analysis and log-rank test. We first determined the prognostic value of DKK-1 at mRNA level using GSE28735, TCGA-PAAD and Ren Ji cohort 1. As shown in Fig. 3A–C, patients with higher DKK-1 level had significantly shorter overall survival (OS) or relapse-free survival (RFS) time than those with a lower DKK-1 level (P = 0.0473, GSE28735; P = 0.0056, OS, P = 0.0372, RFS, TCGA-PAAD; P = 0.0307, Ren Ji cohort 1). At protein level, as demonstrated in Ren Ji cohort 2, patients with higher DKK-1 expression had dramatically decreased survival time than those with lower DKK-1 expression (P<0.0001, Fig. 3D). In addition, we determined the correlation between DKK-1 expression and OS in PDAC patients in early or advanced TNM stage. Kaplan-Meier analysis showed that OS was shorter in PDAC patients with higher DKK-1 expression regardless the state of TNM stage (Fig. 3E).
Figure 3.
DKK-1 expression is associated with survival in PDAC patients. A. Kaplan-Meier survival curves demonstrated that patients with high expression of DKK-1 lived significantly shorter than those with low expression of DKK-1 in GSE28735. B. Data from TCGA-PAAD database showed that patients with high expression of DKK-1 had shorter OS and RFS than the patients in low DKK-1 group. C-D. In Ren Ji cohort 1 and 2, we defined the DKK-1 expression based on the results of qRT-PCR and IHC analysis, the Kaplan-Meier survival analysis suggested that high expression of DKK-1 is positive correlated with shorter OS in patients with PDAC. E. In Ren Ji cohort 2, we found that patients with high expression of DKK-1 lived shorter independent of TNM stage.
Furthermore, univariable and multivariable Cox regression analysis were performed to identify the risk factors correlated with patients' prognosis in Ren Ji cohort 2. Univariable analyses displayed that DKK-1 expression, TNM stage, tumor size, T classification, lymph node metastasis, distant metastasis, vascular invasion and histology were significant prognostic factors for OS prediction (Table 2). Meanwhile, multivariable analysis suggested that DKK-1 expression, lymph node metastasis and histology were independent predictors of OS in patients with PDAC after pancreatectomy (Table 2). Taken together, these data demonstrated that high DKK-1 expression status may be a predictor for prognosis in PDAC patients.
Table 2.
Univariable and multivariable analyses of prognostic parameters for survival in Ren Ji cohort 2 patients with pancreatic ductal adenocarcinoma (PDAC).
| Univariable analysis |
Multivariable analysis |
|||||
|---|---|---|---|---|---|---|
| Prognostic parameter | HR | 95% CI | P value | HR | 95% CI | P value |
| Expression of DKK1 (high vs. low) | 1.828 | 1.354–2.467 | 0.000 | 1.623 | 1.189–2.215 | 0.002 |
| Age (≥ 65 vs. <65) | 1.314 | 1.000–1.727 | 0.050 | − | − | − |
| Gender (male vs. female) | 0.861 | 0.655–1.132 | 0.283 | − | − | − |
| TNM stage (III , IV vs.I , II) | 1.827 | 1.326–2.518 | 0.000 | 1.480 | 0.961–2.279 | 0.075 |
| Tumor Size (> 3 cm vs. ≤ 3 cm) | 1.753 | 1.313–2.341 | 0.000 | 1.353 | 0.995–1.839 | 0.054 |
| T classification (T3, 4 vs. T1, 2) | 2.014 | 1.252–3.240 | 0.004 | 1.429 | 0.868–2.352 | 0.160 |
| Lymph node metastasis (present vs. absent) | 1.686 | 1.279–2.223 | 0.000 | 1.408 | 1.054–1.881 | 0.021 |
| Distant metastasis (present vs. absent) | 1.858 | 1.201–2.874 | 0.005 | 0.983 | 0.554–1.747 | 0.954 |
| Vascular invasion (present vs. absent) | 1.830 | 1.195–2.802 | 0.005 | 1.504 | 0.959–2.359 | 0.075 |
| Perineural invasion (present vs. absent) | 1.147 | 0.876–1.502 | 0.319 | − | − | − |
| Tumor location (head vs. body/tail) | 1.039 | 0.782–1.380 | 0.791 | − | − | − |
| Histology (well/moderate vs. poor) | 1.682 | 1.274–2.222 | 0.000 | 1.490 | 1.115–1.990 | 0.007 |
Note. HR: Hazard ratio; CI: Confidence interval. The bold number represents the P-values with significant differences.
Discussion
Despite the development of modern surgical techniques, peri-surgical management and adjuvant chemoradiotherapies in the past decades, PDAC remains to be the disease bearing the worst prognosis among malignant tumors. Once confirmed, more than 50% of the PDAC patients were diagnosed with distant metastasis while the others progress rapidly after surgery.1 Therefore, it is urgent to discover novel biomarkers for the choice of the most appropriate individualized treatment.
We did a genome-wide expression profile analysis to identify genes which might be potential novel diagnostic marker and therapeutic target. Finally, we chose dkk-1, a gene encoding secretory protein DKK-1. DKK-1, identified in 1998,7 is a 35-kDa secreted protein containing a signal peptide sequence and 2 cysteine-rich domains.16 It is a member of a secreted proteins family and plays an important role in head formation,7 which expressed in placental and embryonic tissues and many cancer tissues,16,17 but rarely in normal human adult tissues. Meanwhile, its protein expression can be easily detected in serum and tissue by ELISA and Immunohistochemistry. Furthermore, it has been reported to be novel target for immunotherapy in myeloma.18
Wnt signaling pathway is a kind of highly conservative signal transduction pathway in the process of biologic evolution, it plays an important role in physiologic and pathological process, including in the embryonic development and the occurrence and development of tumor and it has been identified as one of the key signaling pathways in the process of tumorigenesis.19,20 DKK-1 was reported to be a downstream target of β-catenin/T-cell factor and participates in a negative feedback loop in the Wnt signaling in colon cancer cells.8,21 High expression of DKK-1 in PDAC has been reported in limited cohort while the the clinical significance and prognostic value of the expression of DKK-1 in PDAC remained to be investigate.
In this study, we initially analyzed the expression of DKK-1 in PDAC tissue and cell line at mRNA and protein level. Three Oncomine databases collectively showed that the mRNA expression of DKK-1 was upregulated in PDAC tissues compared with adjacent non-tumor tissues or normal pancreatic tissues. Consistently, in our 35 pairs of freshly-frozen tissues, we found mRNA levels of DKK-1 upregulated obviously in PDAC tissues compared with the matched adjacent non-tumor tissues (P = 0.0082) in 17/35(70.83%) of the cases. In addition, protein level as analyzed by Western blot in cell lines and by IHC in PDAC tissues. Consistent with previous studies,13 we observed that DKK-1 expression was increased in 100% (6/6) of PDAC cell lines compared with non-malignant hTERT-HPNE cells and 65.9% (205/311) of paired PDAC and adjacent non-tumor tissues.
Then we evaluate the clinical significance of tissue DKK-1 protein expression levels as a tumor prognostic biomarker. Our result suggested that the median survival time of PDAC patients was significantly shorter in accordance with the higher expression levels of DKK-1. Furthermore, we found that high-level expression of DKK-1 was significantly associated with aggressive features of PDAC, including T stage, lymph node metastasis. And this is in coincidence with previous research containing 279 non–small cell lung cancers and 280 esophageal squamous cell carcinomas.10 Univariate analysis demonstrated that increased expression of DKK-1 was significantly associated with the overall survival rate in PDAC patients. While multivariate analysis confirmed that DKK-1 expression was an independent risk factor for poor prognosis of PDAC patients. In summary, this study demonstrated that DKK-1 was significantly upregulated in PDAC tissues and cell lines. DKK-1 as a secreted tumor marker could serve as an important prognostic factor and may serve as a novel molecular therapeutic target in PDAC.
Materials and methods
Clinical specimen collection and tissue microarray construction
32 freshly-frozen PDAC tissues and matched adjacent non-tumor tissues (Ren Ji cohort 1) were acquired between January 2014 and December 2014 from Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, China. Tissue microarrays (TMA) containing 311 PDAC specimens and corresponding non-tumor tissues (Ren Ji cohort 2) obtained from Ren Ji Hospital from January 2002 to April 2015 consecutively, were constructed using diameter of 1.5-mm cores. In Ren Ji cohort 2, there were 176 males and 135 females, aging from 38 to 90 y with a median age of 65 y. Clinicopathologic characteristics of patients are available in Table 1. All the specimens were collected from the patients who underwent surgical resection in Ren Ji Hospital and all the pathological data was retrieved from the Pathology Department. This study was approved by the Research Ethics Committees of Ren Ji Hospital, and written informed consent was received from all the patients. None of the cases had received tumor-related therapy before diagnosis. For all cases enrolled in this study, clinical data was available. Follow-up was conducted by office visit, telephone call, or outpatient clinic visit, and overall survival (OS) information was acquired from all patients in Ren Ji cohort 1 and 266 patients in Ren Ji cohort 2. OS was defined as the time from the date of surgery until death from any cause or last follow-up visit deadline, June 19, 2016.
PDAC data sets acquisition and process
PDAC microarray data sets GSE15471 (Affymetrix Human Genome U133 Plus 2.0 Array), GSE28735 (Affymetrix Human Gene 1.0 ST Array), GSE71729 (Agilent-014850 Whole Human Genome Microarray 4 × 44K), TCGA-PAAD (UNC_IlluminaHiSeq_RNASeqV2, level 3) and corresponding clinical data in this study were directly downloaded from Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) and the open access tiers of the Cancer Genome Atlas (TCGA) data portal (https://gdc-portal.nci.nih.gov/), respectively. GSE15471 contained 78 samples, including 39 PDAC tumors and 39 matching adjacent noncancerous tissue samples that were obtained from resected pancreas of 36 pancreatic cancer patients. 3 pairs were performed replicate microarray hybridizations. GSE28735 consisted of 45 PDAC samples and 45 paired adjacent non-tumor tissue samples. 3 specimens without follow-up information were excluded from Kaplan-Meier analysis. GSE71729 analysis used data from 145 primary PDAC tumors and 46 pancreas. TCGA-PAAD RNA-Seq analysis used data from 183 PDAC tumors, among which 166 cases with OS information and 141 cases with relapse-free survival (RFS) information were retrieved for downstream survival analysis. Expression data extraction was performed with R 3.2.5 software.
Cell culture
Human PDAC cell lines SW1990, Capan-2, PANC-1, MIA PaCa-2, BxPC-3, AsPC-1 and CFPAC-1 were purchased from Shanghai Institute of Biochemistry and Cell Biology at the Chinese Academy of Sciences (Shanghai, China) and an immortalized normal human pancreatic ductal epithelial cell line HPDE6-C7 was kindly provided by Professor ZG Zhang (Shanghai Cancer Institute, Shanghai, China). Cells were cultured in complete growth medium containing 10% (v/v) fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin (100 µg/ml) at 37°C in 5% CO2 atmosphere.
Total RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from PDAC tissues (Ren Ji cohort 1) using Trizol reagent (Takara, Cat. #9108). 1μg of total RNA was reverse-transcribed using the PrimeScript RT Reagent Kit (Takara, Cat. #RR037A), and real-time PCR was detected using SYBR Premix Ex Taq II(Takara, Cat. #RR420A) and StepOne Real-Time PCR System (Applied Biosystems, Grand Island, NY, USA) according to the manufactuer's instruction. The relative mRNA expression level was calculated by the 2−ΔΔCt method and the amplified transcript level of each specific gene was normalized to the expression of the internal control 18S. Primer sequences in this study are listed as follows: DKK-1 forward, 5′-CCTTGAACTCGGTTCTCAATTCC-3′, reverse, 5′- CAATGGTCTGGTACTTATTCCCG-3′; 18S forward, 5′-TGCGAGTACTCAACACCAACA-3′, reverse: 5′-GCATATCTTCGGCCCACA-3′.
Western blot
Western blot was performed using standard techniques as described previously.22 β-actin antibody was used as a control for whole-cell lysates. The DKK-1 antibody was purchased from Abcam (Abcam, Cat. #ab109416). Anti-rabbit or anti-mouse IgG secondary antibody was purchased from Cell Signaling Technology Inc. (CST, Cat # 5151S, # 5470S).
Immunohistochemistry
The human PDAC tissue microarray sections (Ren Ji cohort 2) were deparaffinized using xylene and rehydrated with graded alcohol. Activity of endogenous peroxidase was quenched with 3% hydrogen peroxide at room temperature for 15 min. Then antigen retrieval was achieved under high temperature and high pressure. After being blocked with 10% BSA for 30 min, sections were incubated with DKK-1 antibody (1:400, Abcam, Cat. #ab109416) at 4°C overnight. The next day, the sections were incubated with corresponding peroxidase-labeled secondary antibody for 30 min at room temperature and washed with PBS for 3 times. Finally, Diaminobenzidine tetrahydrochloride (DAB; BOSTER, SA1022) was used for the color-reaction and hematoxylin was used for nucleus counterstaining.
Protein expression was assessed according to the extent and intensity of staining. The extent of positive cells was measured on a scale of 0–3: 0, 0–5%; 1, 6–35%; 2, 36–70%; 3, 71–100%. The intensity of staining was evaluated on a scale of 0–3: 0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining. A final score was obtained by using grades of the intensity staining × grades of extent. The tissues with a final score < 4 were sorted into “DKK-1 low expression” and those with a final score ≥ 4 were classified as “DKK-1 high expression.”
Statistical analysis
Data were presented as the means ± SD. SPSS 22.0 software (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Graphical representations were performed with GraphPad Prism 6 (San Diego, CA, USA) software. Correlation of DKK-1 expression with categorical clinical variables in patients with PDAC was evaluated by χ2 test or Fisher's exact test. Survival curves were constructed using the Kaplan-Meier method and analyzed by the log-rank test. Univariate and multivariate Cox regression analysis were performed to identify the factors that had a significant influence on survival by Cox proportional hazards model. The student's t-test or Mann–Whitney U test was used for comparison between 2 groups depending on distribution. P values (2-sided) less than 0.05 were considered statistically significant.
List of abbreviations
- DKK-1
Dickkopf-1
- PDAC
pancreatic ductal adenocarcinoma
- PC
Pancreatic cancer
- OS
overall survival
- RFS
relapse-free survival
- HCC
hepatocellular carcinoma
- TMA
Tissue microarrays
- GEO
Gene Expression Omnibus
- TCGA
Cancer Genome Atlas
Declarations
Ethics approval and consent to participate: This study was approved by the Ethics Committee of Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University. And all patients involved in this study provided written informed consent.
Availability of data and materials
PDAC microarray data sets GSE15471, GSE28735, GSE71729, TCGA-PAAD and corresponding clinical data in this study were directly downloaded from Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) and the open access tiers of the Cancer Genome Atlas (TCGA) data portal (https://gdc-portal.nci.nih.gov/).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from National Natural Science Foundation of China (Project 81401931).
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30. https://doi.org/ 10.3322/caac.21332. PMID:26742998 [DOI] [PubMed] [Google Scholar]
- [2].Stotz M, Eisner F, Szkandera J, Absenger G, Kornprat P, Lackner C, Samonigg H, Gerger A, Pichler M. Clinico-pathological characteristics and clinical outcome of different histological types of pancreatic cancer in a large Middle European series. J Clin Pathol. 2013;66(9):753-7. Epub 2013/06/12. https://doi.org/ 10.1136/jclinpath-2012-201394. PMID:23750038 [DOI] [PubMed] [Google Scholar]
- [3].Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039-49. Epub 2014/09/11. https://doi.org/ 10.1056/NEJMra1404198. PMID:25207767 [DOI] [PubMed] [Google Scholar]
- [4].Ying JE, Zhu LM, Liu BX. Developments in metastatic pancreatic cancer: Is gemcitabine still the standard? World J Gastroenterol. 2012;18(8):736-45. Epub 2012/03/01. https://doi.org/ 10.3748/wjg.v18.i8.736. PMID:22371633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baarsma HA, Konigshoff M, Gosens R. The WNT signaling pathway from ligand secretion to gene transcription: Molecular mechanisms and pharmacological targets. Pharmacol Ther. 2013;138(1):66-83. Epub 2013/01/19. https://doi.org/ 10.1016/j.pharmthera.2013.01.002. PMID:23328704 [DOI] [PubMed] [Google Scholar]
- [6].Jiang H, Li Q, He C, Li F, Sheng H, Shen X, Zhang X, Zhu S, Chen H, Chen X, et al.. Activation of the Wnt pathway through Wnt2 promotes metastasis in pancreatic cancer. Am J Cancer Res. 2014;4(5):537-44. Epub 2014/09/19. PMID:25232495 [PMC free article] [PubMed] [Google Scholar]
- [7].Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391(6665):357-62. Epub 1998/02/05. https://doi.org/ 10.1038/34848. PMID:9450748 [DOI] [PubMed] [Google Scholar]
- [8].Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23(52):8520-6. Epub 2004/09/21. https://doi.org/ 10.1038/sj.onc.1207892. PMID:15378020 [DOI] [PubMed] [Google Scholar]
- [9].Tao YM, Liu Z, Liu HL. Dickkopf-1 (DKK1) promotes invasion and metastasis of hepatocellular carcinoma. Dig Liver Dis. 2013;45(3):251-7. Epub 2012/12/26. https://doi.org/ 10.1016/j.dld.2012.10.020. PMID:23266194 [DOI] [PubMed] [Google Scholar]
- [10].Yamabuki T, Takano A, Hayama S, Ishikawa N, Kato T, Miyamoto M, Ito T, Ito H, Miyagi Y, Nakayama H, et al.. Dikkopf-1 as a novel serologic and prognostic biomarker for lung and esophageal carcinomas. Cancer Res. 2007;67(6):2517-25. Epub 2007/03/17. https://doi.org/ 10.1158/0008-5472.can-06-3369. PMID:17363569 [DOI] [PubMed] [Google Scholar]
- [11].Li S, Qin X, Liu B, Sun L, Zhang X, Li Z, Shan B, You J, Zhou Q. Dickkopf-1 is involved in invasive growth of esophageal cancer cells. J Mol Histol. 2011;42(6):491-8. Epub 2011/09/13. https://doi.org/ 10.1007/s10735-011-9347-1. PMID:21909757 [DOI] [PubMed] [Google Scholar]
- [12].Yu B, Yang X, Xu Y, Yao G, Shu H, Lin B, Hood L, Wang H, Yang S, Gu J, et al.. Elevated expression of DKK1 is associated with cytoplasmic/nuclear beta-catenin accumulation and poor prognosis in hepatocellular carcinomas. J Hepatol. 2009;50(5):948-57. Epub 2009/03/24. https://doi.org/ 10.1016/j.jhep.2008.11.020. PMID:19303159 [DOI] [PubMed] [Google Scholar]
- [13].Takahashi N, Fukushima T, Yorita K, Tanaka H, Chijiiwa K, Kataoka H. Dickkopf-1 is overexpressed in human pancreatic ductal adenocarcinoma cells and is involved in invasive growth. Int J Cancer. 2010;126(7):1611-20. https://doi.org/ 10.1002/ijc.24865. PMID:19711349 [DOI] [PubMed] [Google Scholar]
- [14].Shen Q, Fan J, Yang XR, Tan Y, Zhao W, Xu Y, Wang N, Niu Y, Wu Z, Zhou J, et al.. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol. 2012;13(8):817-26. Epub 2012/06/29. https://doi.org/ 10.1016/s1470-2045(12)70233-4. PMID:22738799 [DOI] [PubMed] [Google Scholar]
- [15].Han SX, Zhou X, Sui X, He CC, Cai MJ, Ma JL, Zhang YY, Zhou CY, Ma CX, Varela-Ramirez A, et al.. Serum dickkopf-1 is a novel serological biomarker for the diagnosis and prognosis of pancreatic cancer. Oncotarget. 2015;6(23):19907-17. Epub 2015/06/24. https://doi.org/ 10.18632/oncotarget.4529. PMID:26101916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fedi P, Bafico A, Nieto Soria A, Burgess WH, Miki T, Bottaro DP, Kraus MH, Aaronson SA. Isolation and biochemical characterization of the human Dkk-1 homologue, a novel inhibitor of mammalian Wnt signaling. J Biol Chem. 1999;274(27):19465-72. Epub 1999/06/26. PMID:10383463 [DOI] [PubMed] [Google Scholar]
- [17].Krupnik VE, Sharp JD, Jiang C, Robison K, Chickering TW, Amaravadi L, Brown DE, Guyot D, Mays G, Leiby K, et al.. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999;238(2):301-13. Epub 1999/11/26. PMID:10570958 [DOI] [PubMed] [Google Scholar]
- [18].Qian J, Yi Q. DKK1 as a novel target for myeloma immunotherapy. Oncoimmunology. 2012;1(5):756-8. Epub 2012/08/31. https://doi.org/ 10.4161/onci.19655. PMID:22934273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1):11-26. Epub 2012/12/22. https://doi.org/ 10.1038/nrc3419. PMID:23258168 [DOI] [PubMed] [Google Scholar]
- [20].Berndt JD, Moon RT. Cell biology. Making a point with Wnt signals. Science. 2013;339(6126):1388-9. Epub 2013/03/23. https://doi.org/ 10.1126/science.1236641. PMID:23520097 [DOI] [PubMed] [Google Scholar]
- [21].Gonzalez-Sancho JM, Aguilera O, Garcia JM, Pendas-Franco N, Pena C, Cal S, Garcia de Herreros A, Bonilla F, Munoz A. The Wnt antagonist DICKKOPF-1 gene is a downstream target of beta-catenin/TCF and is downregulated in human colon cancer. Oncogene. 2005;24(6):1098-103. Epub 2004/12/14. https://doi.org/ 10.1038/sj.onc.1208303. PMID:15592505 [DOI] [PubMed] [Google Scholar]
- [22].Yan TT, Fu XL, Li J, Bian YN, Liu DJ, Hua R, Ren LL, Li CT, Sun YW, Chen HY, et al.. Downregulation of RPL15 may predict poor survival and associate with tumor progression in pancreatic ductal adenocarcinoma. Oncotarget. 2015;6(35):37028-42. Epub 2015/10/27. https://doi.org/ 10.18632/oncotarget.5939. PMID:26498693 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
PDAC microarray data sets GSE15471, GSE28735, GSE71729, TCGA-PAAD and corresponding clinical data in this study were directly downloaded from Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) and the open access tiers of the Cancer Genome Atlas (TCGA) data portal (https://gdc-portal.nci.nih.gov/).