Abstract
Background
The molecular pathogenesis of clear cell endometrial cancer (CCEC), a tumor type with a relatively unfavorable prognosis, is not well defined. We searched exome-wide for novel somatically mutated genes in CCECs, and assessed the mutational spectrum of known and candidate driver genes in a large cohort of cases.
Methods
We whole exome sequenced paired tumor-normal DNAs from 16 CCECs (12 CCECs and the CCEC components of four mixed histology tumors). Twenty-two genes-of-interest were Sanger sequenced from another 47 CCECs. Microsatellite instability and stability (MSI and MSS) were determined by genotyping five mononucleotide repeats.
Results
Two tumor exomes had relatively high mutational loads and MSI. The other 14 tumor exomes were MSS and had 236 validated nonsynonymous or splice junction somatic mutations among 222 protein-encoding genes. Among the 63 CCECs in this study, we identified frequent somatic mutations in TP53 (39.7%), PIK3CA (23.8%), PIK3R1 (15.9%), ARID1A (15.9%), PPP2R1A (15.9%), SPOP (14.3%), and TAF1 (9.5%), as well as MSI (11.3%). Five of eight mutations in TAF1, a gene with no known role in CCEC, localized to the putative histone acetyltransferase domain and included two recurrently mutated residues. Based on patterns of MSI and mutations in seven genes, subsets of CCECs molecularly resembled serous ECs (SECs) or endometrioid ECs (EECs).
Conclusions
Our findings demonstrate molecular similarities between CCECs and SECs and EECs, and implicate TAF1 as a novel candidate CCEC driver gene.
Keywords: Endometrial cancer, endometrial carcinoma, adenocarcinoma, clear cell, mutation, exome, uterine cancer
Introduction
Endometrial carcinomas (ECs) account for approximately 76,000 deaths worldwide annually 1. They can be categorized into several histological subtypes, including endometrioid, serous, and clear cell ECs (EECs, SECs, and CCECs). EECs represent ~80% of all newly diagnosed ECs. They are generally low-grade tumors, and are usually diagnosed at an early clinical stage 2. The 5-year relative survival rate for patients with EEC is favorable overall (91%) 3, although patients with high-grade EEC have a less favorable prognosis. SECs and CCECs account for 3–10.5% and 1–6% of newly diagnosed ECs, respectively 4–6. In contrast to most EECs, SECs and CCECs tend to be diagnosed at late clinical stages and are, by definition, high-grade tumors. Five-year relative survival rates are 45% for SEC and 65% for CCEC, compared with 91% for EEC 3.
The mutational landscape of SEC and EEC exomes has been reported by several groups 7–13, and an integrated genomic analysis of SECs and EECs has been conducted by The Cancer Genome Atlas (TCGA) 12. In contrast, the molecular etiology of CCECs remains poorly understood. One primary CCEC has been exome sequenced but the mutational repertoire was not reported 13. Other studies have Sanger sequenced limited numbers of genes in relatively small numbers of CCECs 7, 14–18. The most frequently mutated cancer genes reported in CCEC thus far are TP53, PPP2R1A, PIK3R1, SPOP, PIK3CA, ARID1A, and FBXW7 7, 14–18; 11%–14% of CCECs exhibit microsatellite instability (MSI) 14, 18. The presence of both TP53 and PTEN mutations in CCEC led to speculation of an overlapping molecular etiology with SECs and EECs 14, an idea that has gained support by a subsequent study of several additional genes 17. However, these observations were made in small cohorts (14 and 16 cases/study) 14, 17.
Here we used a combination of exome sequencing and targeted Sanger sequencing to interrogate the mutational landscape of a relatively large series of CCECs. Our findings implicate TAF1 as a novel candidate driver gene in a subset of cases. Moreover, we demonstrate that a substantial number of CCECs molecularly resemble either SECs or EECs.
Methods
Clinical specimens
All tumor specimens were fresh-frozen tissues resected before treatment from patients clinically diagnosed with CCEC (n=59) or EC with clear cell components (n=4). The NIH Office of Human Subjects Research Protections determined that this research was not “human subjects research” per the Common Rule (45 CFR 46), and therefore IRB review was not required for sequencing of these samples; providing institutions received appropriate IRB approval. Our study included 59 cases of CCEC and four cases with a histopathological diagnosis of more than one histology for which only the CCEC components were analyzed.
Genomic DNA extraction
The neoplastic cellularity of tumors is provided in Supplementary Table 1. DNA isolation from regions of tumor tissue, and identity testing of tumor-normal DNA pairs to confirm origination from the same patient, has been described elsewhere 7, 19, 20. Prior to whole exome sequencing, tumor-normal DNAs were further purified by phenol-chloroform extraction. Four non-tumor DNAs (32N, 59N, 64N, and 82N) were whole genome amplified using the REPLI-g amplification service (Qiagen) prior to exome capture; corresponding unamplified DNAs were used for somatic mutation validation.
Library construction and sequencing
Sequencing libraries of 16 paired tumor-normal DNAs were generated using Illumina Truseq (13 pairs) or Agilent SureSelect (3 pairs) capture kits (Supplementary Table 2).
Read mapping, variant calling, and variant filtering
Sequence reads were aligned to NCBI’s Build 36 (hg18) reference using CASAVA v1.8.0 for initial placement followed by a banded Smith-Waterman algorithm (cross_match, http://www.phrap.org), and variants and their associated genotypes were called with bam2mpg, filtering bases with <Q20 Phred score and using the score_variant option, as described previously 7, 21. VarSifter 22 was used to identify coding and splice somatic variants with an MPG score ≥10 in the normal sample, an MPV score ≥10 in the tumor sample, and at least 5 reads covering the site in each sample. Private SNPs detected in any normal sample, probable false-positive variants consistent with sequencing artifacts or misaligned reads as determined by manual data curation, and variants annotated as non-clinical SNPs in dbSNP v135 were filtered out.
PCR amplification and Sanger sequencing
Primer sequences and PCR conditions are available on request. Sanger sequencing and analysis were performed as previously described 7.
Predicting functional significance of missense mutations
Nonsynonymous missense mutations were evaluated using SIFT (May 2011 release) (http://sift.jcvi.org/), Mutation Assessor (Release 2) (http://mutationassessor.org/), and PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/). We classified variants as likely to impact function if they were scored by ≥ 2 algorithms as “damaging” or “damaging_low confidence” (SIFT), “medium impact” or “high impact” (Mutation Assessor), or “deleterious” (PolyPhen).
MSI analysis
MSI analysis was performed as previously described 7. MSI-high tumor genotypes (≥2 unstable mononucleotide markers) are reported here as MSI. MSI-low and -negative tumor genotypes are reported as microsatellite stable (MSS).
Immunoblotting
Cells were lysed in a 1% Triton X-100 buffer containing 1 mM sodium orthovanadate, 10 mM sodium fluoride and 1× protease inhibitor cocktail (Roche). Protein was quantified using the Quick Start™ Bradford reagent (Bio-Rad), denatured, separated by SDS-PAGE, and transferred to PVDF membranes (Turbo™ Transfer, Bio-Rad). TAF1 (Cell Signaling Technology, 12781) and β-actin (Sigma-Aldrich, A2228) were visualized with Clarity™ ECL substrate (Bio-Rad) and SuperSignal™ West Pico chemiluminescent substrate (Thermo Scientific™), respectively.
Accession code for whole exome sequencing data
dbGAP, accession code phs000967.v1.p1.
Results
Discovery screen
We whole exome sequenced tumor-normal pairs from 12 cases of CCEC and the CCEC components of four additional cases: two EECs with focal CCEC (T46 and T60), a mixed papillary serous and clear cell adenocarcinoma (T59), and a mixed histology tumor (T61) described as clear cell and endometrioid with squamous differentiation. Exome sequencing of the 16 paired samples resulted in a mean depth of coverage for aligned sequence reads of 75x; on average 89.3% of targeted bases had sufficient coverage and quality for variant calling (Supplementary Table 2). After filtering, there were 1,685 exonic and 24 splice junction somatic variant calls among the 16 tumors (Supplementary Tables 3 and 4). Two tumors (T61 and T77) had MSI 7 and relatively high mutational loads (662 and 472 somatic variant calls) (Supplementary Table 4). The other 14 tumors were MSS 7, and had relatively low mutational loads (7–85 somatic variant calls) (Supplementary Table 3). Among the 14 MSS tumors, we identified 575 somatic variants in exons (nonsynonymous and synonymous) or at splice junctions. We were able to assess 306 variants (nonsynonymous and splice junction) by Sanger sequencing; 77.1% (236 of 306) of assessed variants validated as somatic mutations (233 nonsynonymous and 3 splice junction) among 222 protein-encoding genes (Supplementary Table 3).
Prevalence screen
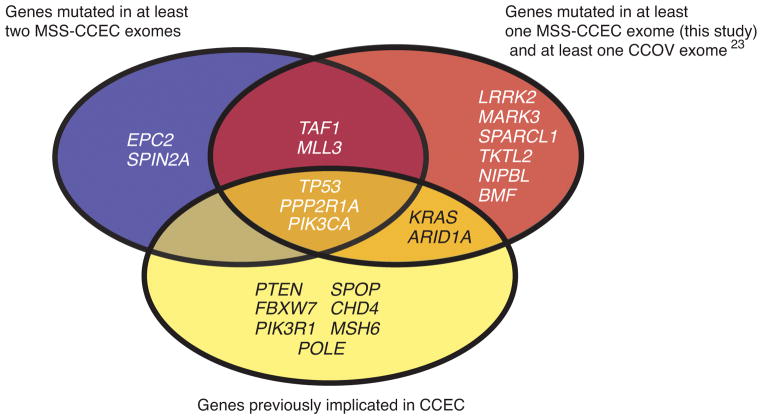
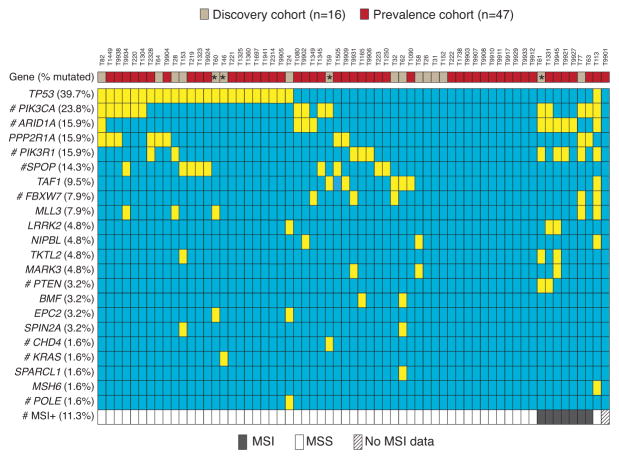
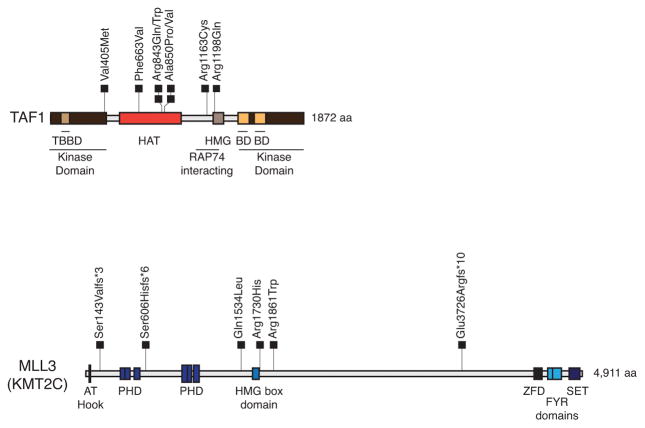
For the mutation prevalence screen we Sanger sequenced 22 genes-of-interest from another 47 CCECs. The genes-of-interest reflected genes that were validated as somatically mutated in (1) at least two MSS CCEC exomes, (2) at least one MSS CCEC exome and at least one clear cell ovarian cancer exome 23, and (3) known cancer genes previously implicated in CCEC (Figure 1). We also determined the MSI status of prevalence screen tumors. Considering all 63 tumors in the combined discovery and prevalence screens, the most frequently mutated genes-of-interest were TP53 (39.7%), PIK3CA (23.8%), PIK3R1 (15.9%), ARID1A (15.9%), PPP2R1A (15.9%), SPOP (14.3%), TAF1 (9.5%), FBXW7 (7.9%), and MLL3 (KMT2C) (7.9%) (Figure 2); MSI was detected in 11.3% of cases. Mutations in TAF1 and MLL3 have not previously been reported in CCEC. Here, we show that TAF1 is expressed at variable levels in EC cell lines (Supplementary Figure 1) and was mutated in 6 of 63 (9.5%) CCECs (Figure 2). All eight TAF1 mutations were missense mutations (Supplementary Table 5). Five TAF1 mutations localized to the putative histone acetyltransferase (HAT) domain, including two recurrently mutated residues (Arginine-843 and Alanine-850) (Figure 3). The three TAF1 mutations (Val405Met, Arg1163Cys, and Arg1198Gln) that localized to regions other than the HAT domain occurred within a single tumor (T113) that has somatically mutated MSH6 and appears to be hypermutated (Figure 2). All eight TAF1 mutations are predicted to impact function by at least two of three in silico algorithms (Supplementary Table 5). For MLL3 we identified six mutations in five tumors (Figure 2 and Supplementary Table 5). Of the six MLL3 mutations, three were frameshifts and three were missense mutations (Supplementary Table 5 and Figure 3). Each of the frameshift mutations occurred amino terminal to the SET (Su(var), Enhancer of zeste, Trithorax) domain, which is a domain commonly found among lysine methyltransferases 24.
Figure 1. Venn diagram displaying criteria used to select genes-of-interest for the follow-up mutation prevalence screen.
Twenty-two genes (shown) met one or more of the three criteria indicated. The clear cell ovarian cancer (CCOV) exome data were reported by Jones et al 23.
Figure 2. Somatic mutational status of 22 genes-of-interest, and the MSI status, in the discovery and prevalence screen CCECs.
(*) indicates four tumors with heterogeneous histology for which the CCEC component was sequenced. Genes and somatic mutation frequencies (% cases mutated) are shown. Somatic mutations (yellow) and MSI (gray) are indicated. (#) denotes genes for which a subset of cases (≤23 tumors) was previously analyzed for mutations and MSI as reported elsewhere 7, 15, 16, 27.
Figure 3. Location of somatic mutations in TAF1 and MLL3 relative to functional domains.
Schematic representation of the TAF1 and MLL3 proteins indicating the relative locations of functional domains. Somatic mutations identified among the 16 discovery screen tumors and 47 prevalence screen tumors are represented by squares. The amino acid (aa) length of the protein is indicated. Domain abbreviations: TBBD (TATA box-binding protein binding), HAT (Histone acetyltransferase), HMG (High Mobility Group), BD (Bromodomain), RAP74 (RNA polymerase II-associating protein 74), AT hook (Adenine Thymine hook), PHD (Plant Homeodomain), ZFD (Zinc Finger Domain), FYR (F/Y rich), SET (Su(var)3–9 and Enhancer-of-zeste and Trithorax).
Comparison of the molecular features of CCEC to those of SECs and EECs
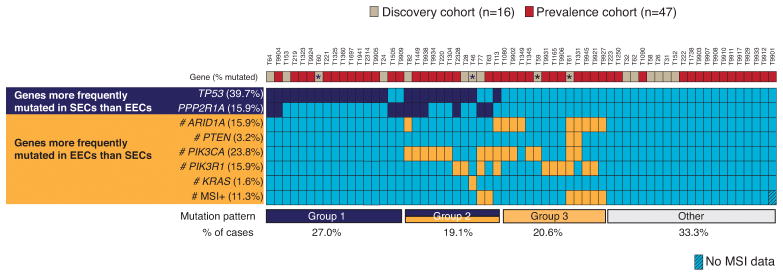
We next sought to compare the molecular profiles of the 63 CCECs in this study to those of SECs and EECs. Seven genes in our prevalence screen (TP53, PPP2R1A, PTEN, ARID1A, KRAS, PIK3R1 and PIK3CA) are mutated at differential frequencies between SECs and EECs: TP53 and PPP2R1A mutations are enriched in SECs whereas PTEN, ARID1A, KRAS, PIK3R1 and PIK3CA mutations are enriched in EECs 12, 15, 16, 25. Likewise, the rate of MSI is higher in EECs than SECs 12, 26. We previously assessed MSI and Sanger sequenced all seven genes in 45 SECs 7, 15, 16 and five of the seven genes in 40 EECs 7, 15, 16, 27; here we Sanger sequenced TP53 and ARID1A in EECs (Supplementary Table 6). Overall, 66.7% of CCECs, 82.2% of SECs, and 97.5% of EECs had at least one detectable alteration across the seven genes and MSI. Next, we classified tumors according to whether they had mutations exclusively in SEC-enriched genes (group 1), exclusively in EEC-enriched genes (group 3), or in a combination of both gene sets (group 2). Whereas the majority (82.5%) of EECs had group 3 mutation profiles, the majority (75.5%) of SECs had either group 1 or group 2 mutation profiles (Supplementary Figure 2). By comparison, the mutation profiles of CCECs had an intermediate distribution to those of SECs and EECs (Figure 4) with 27.0% of cases in group 1, 19.1% in group 2, and 20.6% in group 3; another 33.3% of CCECs had no detectable alterations within this panel of markers.
Figure 4. CCECs resemble both SECs and EECs based on the mutational status of seven genes and MSI.
Vertical columns represent 63 individual CCEC tumors (T) in this study. Frequencies of somatic mutations (% tumors mutated) and MSI are provided (left). CCECs are grouped according to mutation patterns (bottom), into those with mutations exclusively in serous-enriched genes (group 1; dark blue), exclusively in endometrioid-enriched genes (group 3; orange), or in both gene-sets (group 2; orange and dark blue); tumors that had no detectable alterations in this set of markers are indicated as a separate group (gray). The percentage of tumors in each group is indicated (bottom). (#) denotes genes for which a subset of cases (≤23 tumors) was previously analyzed for mutations and MSI as reported elsewhere 7, 15, 16.
Discussion
CCEC is a rare histological EC subtype that is associated with a relatively poor prognosis. A recent study of CCECs within the National Cancer Database noted 5-year overall survival rates of 74.9% for stage I, 64.3% for stage II, and 40.2% for stage III disease 28. Here we used whole exome sequencing to systematically search for somatically mutated genes in 12 cases of CCEC and in the CCEC components of four cases that had other coexisting histologies. Our mutation discovery screen together with targeted gene sequencing in a larger cohort of clinically diagnosed CCECs implicated TAF1 and to a lesser extent MLL3, as novel candidate driver genes of some CCECs. TAF1 encodes a subunit of the TFIID basal transcription factor complex and, although it has been nominated as a candidate driver gene in SECs 11, to our knowledge this is the first report of somatic mutations of TAF1 in CCEC. We speculate that the frameshift mutations we uncovered in MLL3, which encodes a histone lysine methyltransferase, may be pathogenic loss-of-function mutations, based on the prevailing dogma in other solid tumors 29, and on recent observations that truncating mutations in trr, the Drosophila ortholog of MLL3/MLL4, result in tissue overgrowth 30.
Several of our findings in CCECs and the CCEC components of mixed histology tumors are of potential clinical interest. The PIK3CA-PIK3R1-PTEN axis of the druggable PI3-Kinase pathway was mutated in 34.9% of cases. MSI was present in 11.3% of cases and is noteworthy given the clinical responses of two MSI EC patients to the anti-PD-1 antibody pembrolizumab in a phase 2 trial 31. Also noteworthy is our observation that POLE exonuclease domain hotspot mutations, which are associated with a favorable outcome in EECs, were not present among the CCECs in our study; the single case (T24) that had a non-hotspot POLE exonuclease domain mutation (Phe367Leufs*15) was exome sequenced but did not exhibit an ultramutated phenotype.
We found CCECs to be more mutationally heterogenous than SECs or EECs across a panel of seven genes (TP53, PPP2R1A, PIK3CA, PTEN, PIK3R1, ARID1A, KRAS) and MSI as evidenced by shared patterns of alterations between CCECs and SECs, and CCECs and EECs, in addition to CCECs that could not be characterized as such. Similarily, shared patterns of alteration are also observed when comparing the molecular features of CCECs in this study to those of SECs and EECs in the TCGA study. Specifically, TCGA classified SECs and EECs into four molecular subgroups: POLE-mutated (ultramutated), MSI (hypermutated), copy number low-MSS, and copy number high (serous-like) 12. All but one SEC in TCGA’s study were in the copy number high subgroup, and were frequently TP53-mutated, whereas EECs were distributed among the four subgroups. It has recently been proposed that the status of the POLE exonuclease domain, MSI, and TP53/p53 can serve as surrogate molecular markers for this classification 32. Our finding that 39.7% of 63 CCECs in our study were TP53-mutated and another 11.3% had MSI further demonstrates that the molecular features of CCECs are heterogeneous and overlap with those of SECs and EECs. Collectively, these observations support those of three smaller studies noting the molecular heterogeneity of CCECs and their similarities to SECs and EECs 14, 17, 18.
Several studies have noted considerable interobserver variability in the histologic classification of high-grade ECs, including high-grade EECs, SECs, and CCECs, based on morphology alone 33–39. For example, in a retrospective review by 11 gynecologic pathologists of 35 tumors identified from an electronic records search that included the term “clear cell” as well as “uterine” or “endometrial carcinoma”, Fadare et al., noted a moderate level of interobserver agreement ( “k values between any pair of observers ranged from 0.18 to 0.69 (combined: 0.46; SD 0.08)”) in histological classification 36. Similarly, Han et al., noted moderate interobserver agreement in a review by five gynecologic pathologists of 44 tumors originally diagnosed as CCEC or EC with clear cell component (“k scores between any pair of observers ranged from 0.34 to 0.62 (overall: 0.57)) 35. A limitation of our study is that we did not include a systematic pathology review of the discovery or prevalence screen tumors. Therefore, we cannot exclude the possibility that some of the molecular heterogeneity observed in this study may reflect inaccurate histological classification 34–39, a known challenge in EC pathology. However, the aim of our study was not to provide a morphologic or molecular reclassification of CCEC. Rather, our findings reflect the molecular profiles of tumors from patients who were clinically diagnosed with CCEC, or EC with a CCEC component.
In summary, we describe the largest exome sequencing of CCECs, and a comprehensive mutational analysis of known cancer genes in this rare but clinically aggressive subtype. Our study implicates TAF1 as a novel candidate driver gene in a subset of CCECs. Moreover, our findings indicate that some CCECs molecularly resemble SECs and others molecularly resemble EECs. Collectively, our study sheds new insights into the mutational repertoire of CCEC.
Supplementary Material
Acknowledgments
Funding: Supported by the Intramural Program of the National Human Genome Research Institute, National Institutes of Health (HG200379 to D.W.B and HG200330 to J.C.M); Helse Vest, the University of Bergen, the Norwegian Cancer Society, the Research Council of Norway (to H.B.S.); the Arnold Chavkin and Laura Chang Charitable Fund, Cycle for Survival of Memorial Sloan Kettering Cancer Center (to D.A.L); the NIH SPORE in Uterine Cancer (NIH 2P50 CA098258-08 to R. R. B. and K. H. L.); and by Fundació La Marató de TV3 (2/C2013), CB16/12/00231 and PT 13/0010/0014 (to X.M-G.).
We thank Dr. Erling Hoivik for facilitating specimen transfer and critical reading of the manuscript, and Britt Edvardsen for technical support.
Footnotes
The authors have no conflicts of interest to disclose.
Author Contributions: D.W.B., designed and directed the study, and wrote the first draft of the manuscript; M.E.U., P.J.G., and R.R.B., provided critical comments and revisions. X.M-G., K.H.L., R.R.B., D.A.L., D.G.M., P.J.G., and H.B.S., contributed clinical specimens. X.M.-G., A.V.B., and D.C.S., conducted histopathological review of subsets of tumor specimens. M.L.R., prepared DNA, performed identity testing and MSI analysis. NISC performed library construction and exome sequencing. NISC and N.F.H. performed variant calling. M.L.G. curated and validated exome sequencing data. M.L.G., M.L.R., M.E.U., and D.W.B., reviewed validation screen sequencing. M.L.G., M.L.R., and D.W.B., interpreted exome data and established filtering criteria. M.L.G., M.L.R., M.E.U., S.Z., and D.W.B. designed, performed, analyzed, and/or interpreted mutation prevalence screens. F.L., and M.E.U., performed immunoblotting. All authors provided edits and/or comments on the manuscript.
References
- 1.Devilee FATaP., editor. World Health Organization Classification of Tumours: Pathology and genetics of tumors of the breast and female genital organs. IARCPress-WHO; 2003. Tumors of the uterine corpus; pp. 218–257. [Google Scholar]
- 2.Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. FIGO 26h annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S105–143. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- 3.Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M-J. Patient and Tumor Characteristics. Vol. 2007. National Cancer Institute; Bethesda, MD: 2007. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988–2001. SEER Program, NIH Pub. No. 07-6215. [Google Scholar]
- 4.Hasegawa K, Nagao S, Yasuda M, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for clear cell carcinoma of the uterine corpus and cervix. Int J Gynecol Cancer. 2014;24:S90–95. doi: 10.1097/IGC.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 5.Abeler VM, Kjorstad KE. Clear cell carcinoma of the endometrium: a histopathological and clinical study of 97 cases. Gynecol Oncol. 1991;40:207–217. doi: 10.1016/0090-8258(90)90279-t. [DOI] [PubMed] [Google Scholar]
- 6.Dedes KJ, Wetterskog D, Ashworth A, Kaye SB, Reis-Filho JS. Emerging therapeutic targets in endometrial cancer. Nat Rev Clin Oncol. 2011;8:261–271. doi: 10.1038/nrclinonc.2010.216. [DOI] [PubMed] [Google Scholar]
- 7.Le Gallo M, O’Hara AJ, Rudd ML, et al. Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nat Genet. 2012;44:1310–1315. doi: 10.1038/ng.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinde I, Bettegowda C, Wang Y, et al. Evaluation of DNA from the Papanicolaou test to detect ovarian and endometrial cancers. Sci Transl Med. 2013;5:167ra164. doi: 10.1126/scitranslmed.3004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhn E, Wu RC, Guan B, et al. Identification of molecular pathway aberrations in uterine serous carcinoma by genome-wide analyses. J Natl Cancer Inst. 2012;104:1503–1513. doi: 10.1093/jnci/djs345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang H, Cheung LW, Li J, et al. Whole-exome sequencing combined with functional genomics reveals novel candidate driver cancer genes in endometrial cancer. Genome Res. 2012;22:2120–2129. doi: 10.1101/gr.137596.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao S, Choi M, Overton JD, et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc Natl Acad Sci U S A. 2013;110:2916–2912. doi: 10.1073/pnas.1222577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kandoth C, Schultz N, Cherniack AD, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson WJ, Hoivik EA, Halle MK, et al. The genomic landscape and evolution of endometrial carcinoma progression and abdominopelvic metastasis. Nat Genet. 2016;48:848–845. doi: 10.1038/ng.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.An HJ, Logani S, Isacson C, Ellenson LH. Molecular characterization of uterine clear cell carcinoma. Mod Pathol. 2004;17:530–537. doi: 10.1038/modpathol.3800057. [DOI] [PubMed] [Google Scholar]
- 15.Rudd ML, Price JC, Fogoros S, et al. A unique spectrum of somatic PIK3CA (p110alpha) mutations within primary endometrial carcinomas. Clin Cancer Res. 2011;17:1331–1340. doi: 10.1158/1078-0432.CCR-10-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urick ME, Rudd ML, Godwin AK, Sgroi D, Merino M, Bell DW. PIK3R1 (p85alpha) is somatically mutated at high frequency in primary endometrial cancer. Cancer Res. 2011;71:4061–4067. doi: 10.1158/0008-5472.CAN-11-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoang LN, McConechy MK, Meng B, et al. Targeted mutation analysis of endometrial clear cell carcinoma. Histopathology. 2015;66:664–674. doi: 10.1111/his.12581. [DOI] [PubMed] [Google Scholar]
- 18.Stelloo E, Bosse T, Nout RA, et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol. 2015;28:836–844. doi: 10.1038/modpathol.2015.43. [DOI] [PubMed] [Google Scholar]
- 19.Jelinic P, Mueller JJ, Olvera N, et al. Recurrent SMARCA4 mutations in small cell carcinoma of the ovary. Nat Genet. 2014;46:424–426. doi: 10.1038/ng.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salvesen HB, Carter SL, Mannelqvist M, et al. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc Natl Acad Sci U S A. 2009;106:4834–4839. doi: 10.1073/pnas.0806514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teer JK, Bonnycastle LL, Chines PS, et al. Systematic comparison of three genomic enrichment methods for massively parallel DNA sequencing. Genome Res. 2010;20:1420–1431. doi: 10.1101/gr.106716.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teer JK, Green ED, Mullikin JC, Biesecker LG. VarSifter: visualizing and analyzing exome-scale sequence variation data on a desktop computer. Bioinformatics. 2012;28:599–600. doi: 10.1093/bioinformatics/btr711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones S, Wang TL, Shih Ie M, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dillon SC, Zhang X, Trievel RC, Cheng X. The SET-domain protein superfamily: protein lysine methyltransferases. Genome Biol. 2005;6:227. doi: 10.1186/gb-2005-6-8-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McConechy MK, Ding J, Cheang MC, et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol. 2012;228:20–30. doi: 10.1002/path.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Price JC, Pollock LM, Rudd ML, et al. Sequencing of candidate chromosome instability genes in endometrial cancers reveals somatic mutations in ESCO1, CHTF18, and MRE11A. PLoS One. 2013;8:e63313. doi: 10.1371/journal.pone.0063313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudd ML, Mohamed H, Price JC, et al. Mutational analysis of the tyrosine kinome in serous and clear cell endometrial cancer uncovers rare somatic mutations in TNK2 and DDR1. BMC Cancer. 2014;14:884. doi: 10.1186/1471-2407-14-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGunigal M, Liu J, Kalir T, Chadha M, Gupta V. Survival differences among uterine papillary serous, clear cell and grade 3 endometrioid adenocarcinoma endometrial cancers: A National Cancer Database analysis. Int J Gynecol Cancer. 2017;27:85–92. doi: 10.1097/IGC.0000000000000844. [DOI] [PubMed] [Google Scholar]
- 29.Parsons DW, Li M, Zhang X, et al. The genetic landscape of the childhood cancer medulloblastoma. Science. 2011;331:435–439. doi: 10.1126/science.1198056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanda H, Nguyen A, Chen L, Okano H, Hariharan IK. The Drosophila ortholog of MLL3 and MLL4, trithorax related, functions as a negative regulator of tissue growth. Mol Cell Biol. 2013;33:1702–1710. doi: 10.1128/MCB.01585-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Talhouk A, McConechy MK, Leung S, et al. A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer. 2015;113:299–310. doi: 10.1038/bjc.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoang LN, Kinloch MA, Leo JM, et al. Interobserver Agreement in Endometrial Carcinoma Histotype Diagnosis Varies Depending on The Cancer Genome Atlas (TCGA)-based Molecular Subgroup. Am J Surg Pathol. 2017;41:245–252. doi: 10.1097/PAS.0000000000000764. [DOI] [PubMed] [Google Scholar]
- 34.Thomas S, Hussein Y, Bandyopadhyay S, et al. Interobserver variability in the diagnosis of uterine high-grade endometrioid carcinoma. Arch Pathol Lab Med. 2016;140:836–843. doi: 10.5858/arpa.2015-0220-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han G, Soslow RA, Wethington S, et al. Endometrial carcinomas with clear cells: A study of a heterogeneous group of tumors including interobserver variability, mutation analysis, and immunohistochemistry with HNF-1beta. Int J Gynecol Pathol. 2015;34:323–333. doi: 10.1097/PGP.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 36.Fadare O, Parkash V, Dupont WD, et al. The diagnosis of endometrial carcinomas with clear cells by gynecologic pathologists: an assessment of interobserver variability and associated morphologic features. Am J Surg Pathol. 2012;36:1107–1118. doi: 10.1097/PAS.0b013e31825dd4b3. [DOI] [PubMed] [Google Scholar]
- 37.Gilks CB, Oliva E, Soslow RA. Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am J Surg Pathol. 2013;37:874–881. doi: 10.1097/PAS.0b013e31827f576a. [DOI] [PubMed] [Google Scholar]
- 38.Han G, Sidhu D, Duggan MA, et al. Reproducibility of histological cell type in high-grade endometrial carcinoma. Mod Pathol. 2013;26:1594–1604. doi: 10.1038/modpathol.2013.102. [DOI] [PubMed] [Google Scholar]
- 39.Clarke BA, Gilks CB. Endometrial carcinoma: controversies in histopathological assessment of grade and tumour cell type. J Clin Pathol. 2010;63:410–415. doi: 10.1136/jcp.2009.071225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.