Abstract
Purpose
While obesity is considered a prognostic factor in colorectal cancer (CRC), there is increasing evidence that not only body-mass-index (BMI) matters, but specifically abdominal fat distribution. As part of the ColoCare study, this study measured the distribution of adipose tissue compartments in CRC patients and aimed to identify the body metric that best correlates with these measurements as a useful proxy for adipose tissue distribution.
Materials and methods
In 120 newly-diagnosed CRC patients who underwent multi-detector-CT, densitometric quantification of total(TFA), visceral(VFA), intraperitoneal(IFA), retroperitoneal(RFA) and subcutaneous fat area(SFA), M.erector spinae and psoas was performed to test the association with gender, age, tumor stage, metabolic equivalents, BMI, Waist-to-Height (WHtR) and Waist to-Hip ratio (WHR).
Results
VFA was 28.8% higher in men (pVFA<0.0001) and 30.5% higher in patients older than 61 years (pVFA<0.0001). WHtR correlated best with all adipose tissue compartments (rVFA=0.69, rTFA=0.84, p<0.0001) and visceral-to-subcutaneous-fat-ratio(VFR, rVFR=0.22, p=<0.05). Patients with tumor stages III/IV showed significantly lower overall adipose tissue than I/II. Increased M. erector spinae mass was inversely correlated with all compartments.
Conclusion
Densitometric quantification on CT is a highly reproducible and reliable method to show fat distribution across adipose tissue compartments. This distribution might be best reflected by WHtR, rather than BMI or WHR.
Keywords: Multidetector computed tomography, obesity, colorectal neoplasms, Body Mass Index, Waist-to-Height Ratio
Introduction
Overweight and obesity, defined as a BMI ≥ 25 kg/m2 and as a BMI ≥ 30 kg/m2 by the WHO, respectively, are an increasing health burden in many countries, as their numbers have nearly doubled worldwide since 1980 [1]. Recently, the Global Burden of Disease Study 2010 reported a global increase in BMI and stated that obesity is the leading risk factor for mortality as well as increased disability-adjusted life years (DALYs) in Australasia, Latin America, and one of the major risk factors in the remaining high-income countries [2]. Aside from the increased incidence of high blood pressure and diabetes mellitus, the rising number of overweight and obese individuals is also associated with higher cancer incidence and mortality rates of multiple tumor types [3; 4], including colorectal cancer (CRC). CRC is one of the most common cancers and numerous studies have observed an obesity-related increase of CRC incidence, which was independent of gender [3; 5-9]. These findings were confirmed by a recent meta-analysis of prospective studies with a total of nine million participants from different countries, which showed a pooled relative risk (RR) of 1.33 (95% CI: 1.25-1.42) of CRC for obese compared with normal weight individuals [10]. A BMI ≥ 30 kg/m2 was associated with worse outcome, increased overall mortality, disease recurrence, the occurrence of a second primary tumor [11] as well as perioperative morbidity due to increased wound infections [11-13]. High pre-diagnosis BMI exhibited a stronger predictive value than high post-diagnosis BMI for CRC survival, and had stronger effects on overall mortality, CRC-related mortality and mortality from cardiovascular diseases in CRC patients [14].
However, BMI does not capture all dimensions of obesity adequately. More relevant seem to be differences in the distribution of abdominal adipose tissue across several compartments. These can be distinguished into total fat area (TFA), subcutaneous fat area (SFA) and visceral fat area (VFA), which can be further divided into intraperitoneal and retroperitoneal fat areas (IFA and RFA, respectively). Visceral adipose tissue is more strongly associated with obesity-related morbidities, such as metabolic syndrome, than is subcutaneous adipose tissue [15-17]. VFA is also associated with an unfavorable inflammatory adipokine profile, which also supports the hypothesis that it has a specific pathogenic role [3; 9; 18]. As obesity is also relevant in carcinogenesis, particularly of CRC, it is important to understand the impact of specific adipose tissue compartments, especially VFA, on the development and prognosis of colorectal cancer.
The ColoCare study is an international cohort study designed to identify factors of colorectal cancer prognosis in patients with newly diagnosed CRC. Adipose tissue compartments of CRC patients were characterized as part of the ColoCare study. The aims of the present study were first to establish a precise, easy and reproducible method of measuring adipose tissue compartments and second, to compare the measured compartments with body metrics (BMI, WHR and WHtR) in order to identify which body metric is most strongly associated with the individual adipose tissue distribution in CRC patients and potentially define easier measures of adipose tissue distribution to facilitate risk assessment.
Materials and Methods
The ColoCare study is approved by the local institutional review board. All patients gave written informed consent.
Patient characteristics
From October 2010 to February 2012, 205 patients were enrolled in the ColoCare study at the study site at time of surgery. Eligibility criteria were: primary diagnosis of colorectal cancer and prior to surgery, age > 18 years, German language proficient and able to provide consent. We retrospectively retrieved abdominal CT scans using Centricity RIS 4.1i and GE PACS (GE Medical Systems, Buckinghamshire, UK). Data on diagnosis, date of surgery, UICC classification and location of tumor, age, height, and weight were abstracted from the hospital information system I.S.-H.*med. (SAP, Walldorf, Germany). Information on metabolic equivalents of daily activity was retrieved from patient questionnaires (VITAL) [19].
Out of the 205 patients enrolled in ColoCare, CT scans of 142 patients were available (112 at the University Clinic and 30 from referring physicians). In 11 of these 142 patients, quantification of adipose tissue compartments was not possible because the body circumference was not within the field-of-view (FOV) of the CT scanner. Out of these 11 patients, four were not positioned in the center of FOV and seven were severely obese (BMI 33.5 - 40 kg/m2). There was one additional case of a burst abdomen, which prevented proper evaluation of the adipose tissue compartments. In six other cases, the scans were not compatible with the viewer or the quality of the scans was too poor for evaluation. Four cases were excluded because no data on weight and/or height were available. Thus, 120 of 142 patients with available CT scans were included for quantification of adipose tissue compartments with following scan parameters: mean slice thickness: 3 mm (min: 1mm; max: 6.5mm); in 100 scans intravenous contrast media was administered, 20 without contrast media. Time of scan was between August 2010 and August 2012, 79 scans were conducted before surgery and 41 after surgery, with 10 days as the median interval between CT-scan and surgery. For detailed patient characteristics, see Table 1. For characteristics of excluded patients, refer to Table 11.
Table 1.
Characteristics of the study population, n = 120.
| Categories | N (%) |
|---|---|
|
| |
| Age (y) | |
| Mean (+/- SD) | Mean 60.7 (+/- 11.6 SD) |
|
| |
| ≤61 years | 60 (50.0) |
|
| |
| >61 years | 60 (50.0) |
|
| |
| Sex | |
| Men | 84 (70.0) |
|
| |
| Women | 36 (30.0) |
|
| |
| BMI (kg/m2) | |
| Mean (+/- SD) | Mean 26.2 (+/- 4.2 SD) |
|
| |
| Underweight (< 18.5) | 5 (4.2) |
|
| |
| Normal weight (18.5 – 24.9) | 41 (34.2) |
|
| |
| Overweight (25.0 – 29.9) | 54 (45.0) |
|
| |
| Obese Class I (30.0 – 34.9) | 14 (11.7) |
|
| |
| Obese Class II and III (≥ 35.0) | 6 (5.0) |
|
| |
| Location of Tumor | |
| Colon | 39 (32.5) |
|
| |
| Recto sigmoid | 8 (6.7) |
|
| |
| Rectum | 73 (60.8) |
|
| |
| Stage | |
| I | 19 (15.8) |
|
| |
| II | 35 (29.2) |
|
| |
| III | 31 (25.8) |
|
| |
| IV | 34 (28.3) |
|
| |
| Unknown | 1 (0.8)* |
|
| |
| Time of CT scan | |
| Pre-surgery | 79 (65.8) |
|
| |
| Post-surgery | 41 (34.2)** |
|
| |
| Metabolic Equivalents (METs, n=77)*** | |
| Mean (+/- SD) | Mean 17.9 (+/- 18.8 SD) |
One patient underwent radiation before surgery and was tumor free afterwards.
median (1/3 quartile) scan time after surgery (days): 13.0 (6.8/72.6).
Vital questionnaire, time frame: 1 year before baseline [19].
Quantification of adipose tissue compartments and muscles on CT
Quantification based on CT data was performed using a volume tool (Syngo Volume tool, Siemens Healthcare, Munich, Berlin, Germany). We performed an area-based quantification of adipose tissue compartments on two representative levels of the abdomen. Level L3/4 reportedly showed the best correlation with volume-based quantification of adipose tissue compartments and cardio-metabolic risk factors, including subgroups with varying age and gender of the Framingham Heart Study [20]. Level L4/5 has been observed to be strongly correlated with diabetes and hypertension [21]. By manually determining specific regions of interest (ROI) at L3/4 and L4/5, the TFA (whole circumference, see Figure 1), the VFA (along the fascial plane tracing the abdominal wall, see Figure 1) and the RFA (defining the retroperitoneum, see Figure 1) were measured (volumetric quantification of selected slice, divided by slice thickness). The adipose tissue was selected by limiting the measurements to a lower attenuation limit of -190 HU and an upper attenuation limit of -30 HU [22; 23]. The remaining adipose tissue compartments were calculated as follows: SFA was determined by subtracting VFA from TFA, and IFA by subtracting RFA from VFA. The visceral to subcutaneous fat ratio (VFR) was calculated as VFA/SFA.
Figure 1.
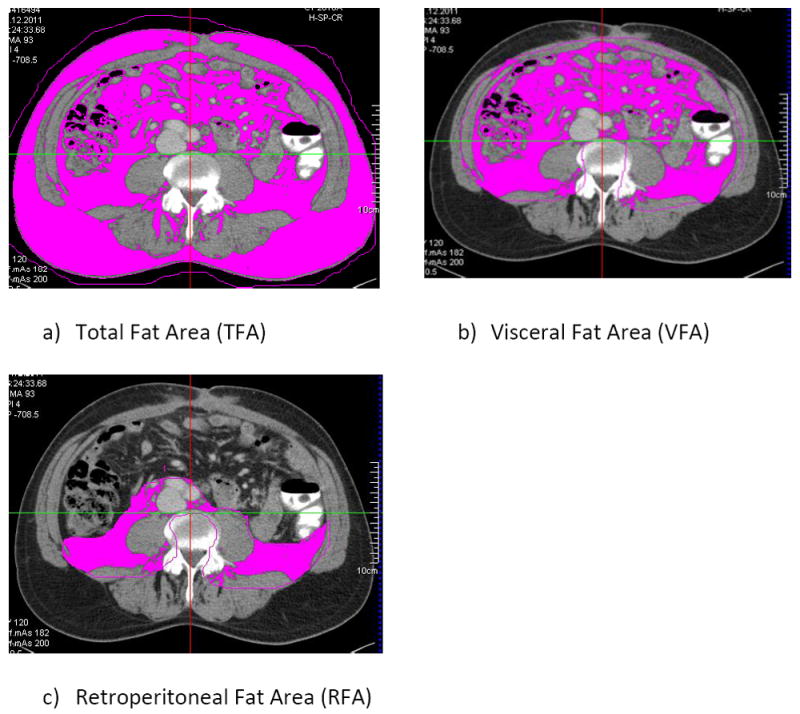
Example of quantification of adipose tissue area in CT scans via specific ROIs: a) TFA, b) VFA and c) RFA
On both levels (L 3/4 and L 4/5), specific ROIs of muscle-area (volumetric quantification of selected slice, divided by slice thickness) were manually selected using the Syngo Volume Tool: M. erector spinae (by tracing the Fascia thoracolumbalis) and M. psoas major (Figure 2). The muscle tissue was selected by limiting the measurements to a lower attenuation limit of 40 HU and an upper attenuation limit of 100 HU. These thresholds were chosen to be narrower than other commonly used ranges (0-100 HU or -29-150 HU) based on visual controls that we conducted as a plausibility test within our study cohort. By choosing a lower attenuation limit of 40 HU we avoid measuring errors that could otherwise occur due to the application of contrast media and the lipid content of the muscle.[24-28] The skeletal muscle index was calculated (SMI = area(cm2) M.psoas + M. erector spinae/(height2(m2)).[28]
Figure 2.
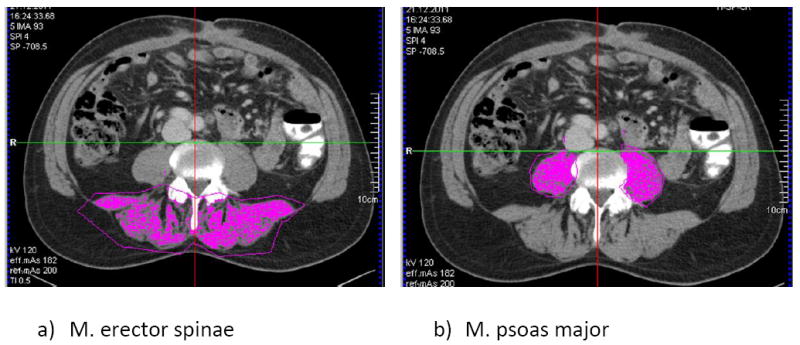
Example of quantification of muscle area: a) M. erector spinae and b) M. psoas
Inter-observer agreement
For quality control, TFA, VFA, RFA, M. erector spinae and M. psoas major at levels L3/4 and L4/5 of CT scans of n = 10 patients were analyzed by two blinded readers (one experienced radiologist and one trained medical student) to assure measurement validity and reproducibility.
By utilizing the Aquarius Intuition software (TeraRecon, Foster City, USA) in the mode “Abdomen Tech/ Fat Analysis” the waist circumference on the CT scans was measured at the level of the navel [29; 30] and the hip measurement was taken at the level of the spina iliaca anterior superior. As this method is not prone to mistakes we did not conduct a blinded analysis with two different raters.
The manual WHR and WHtR measurements were taken by a trained research assistant with the patient standing upright and measured to the closest mm, three independent times. Waist circumference was measured at the level of the navel and the narrowest part of the waist (mean of three measurements). Hip circumference was determined at the level of the spina iliaca anterior superior and the widest part of the hip.
Type and duration of physical activity during the past 12 months were assessed with questionnaires (VITAL) and converted to metabolic equivalents (METs) with 1 Met ⩠ 3.5 ml oxygen per kg body weight per minute in men (women: 3.15 ml/kg/min) [19; 31-33].
Statistical analysis
Statistical analyses were performed using SAS 9.3 (2008, SAS Institute, Cary, USA). Continuous data were tested for normal distribution performing the Shapiro-Wilk test and observing q-q plot distributions of the data. Non-normal data (VFA, IFA, RFA and VFR) were square-root transformed to achieve normality. Bivariate analyses were performed using the t-test (paired and unpaired) for comparing means (time of scan, gender, age). Pearson correlations for continuous variables as well as Spearman correlations for discrete variables were assessed (gender, BMI categories, stage, age categories and scan time). Finally, adipose tissue compartments from CT-scans were regressed on BMI, WHR and WHtR in separate analyses of variance (ANOVA) models and potential effect modifiers and confounders were then added into multivariable regression models [gender, age (in years), pre / post-surgery scan, level of scan (“L3/4, L4/5”), stage (“I, II, III, IV”) metabolic equivalents (continuous), area M. psoas and erector spinae (continuous)]. All correlation analyses had a statistical power of ~90% and were considered significant at α= 0.05. The inter-observer agreement of adipose tissue compartment assessment was tested using the Bland-Altman test.
Time point of CT scan
There were 78 CT scans before and 42 CT scans after surgery available. There was no difference in adipose tissue distribution or body metrics (BMI, WHR, WHtR) between assessments taken from patients before or after surgery (results not shown). This suggests that pre-and post-surgery CT scans provide similar information and can be combined for statistical analysis.
Gender
Men and women had an identical median age (61.5 years) and nearly identical mean age (women: 60.6 +/-11.4; men: 60.7 +/-11.6).
Results
Distribution of adipose tissue compartments
The area-based quantification of the TFA, VFA, RFA, IFA, SFA and VFR at levels L3/4 is provided in Table 2. As there was no relevant difference between level L3/4 and L4/5, following results are shown for level L3/4.
Table 2.
Distribution (area) and density of adipose tissue across compartments, n = 120.
| TFA | VFA | SFA | IFA | RFA | VFR | |
|---|---|---|---|---|---|---|
|
| ||||||
| L3/4 | ||||||
| Mean area (cm2) | 384.1 | 173.6 | 209.4 | 109.9 | 63.9 | 0.89 |
| SD | 167.4 | 104.4 | 96.5 | 67.3 | 47.7 | 0.5 |
| Mean Density (HU) | -87.7 | -80.5 | - | - | -84.5 | - |
| SD | 19.4 | 24.6 | - | - | 19.5 | - |
SD= standard deviation; HU= Hounsfield Units, TFA= total fat area, VFA= visceral fat area, SFA= subcutaneous fat area, IFA= intraperitoneal fat area, RFA= retroperitoneal fat area and VFR= visceral fat ratio VFA/SFA.
Gender
Men had significantly higher transformed VFA (p=0.0001), RFA (p=<0.0001), IFA (p=0.0016) and VFR (p=<0.0001) values at level L3/4 compared to women. Women, however, tended to have higher SFA values than men, but not significantly different (Table 3).
Table 3.
T-test of gender and age, n = 120
| TFA | VFAa | SFA | IFAa | RFAa | VFRa | |
|---|---|---|---|---|---|---|
|
| ||||||
| Gender (Men vs. Womenr) | ||||||
| RPD | 12.5% | 28.8%* | -9.7% | 24.2%* | 35.6%* | 36.9%* |
| P | 0.19 | 0.0001* | 0.26 | 0.0016* | <0.0001* | <0.0001* |
|
| ||||||
| Age Group (≤61r vs. >61) | ||||||
| RPD | 29.5%* | 30.5%* | 8.6% | 29.8%* | 29.1%* | 25.5%* |
| P | 0.001* | <0.0001* | 0.33 | 0.0001* | 0.0001* | <0.0001* |
RPD=relative percent difference,
= square root transformed variable;
=indicates the reference group, TFA= total fat area, VFA= visceral fat area, SFA= subcutaneous fat area, IFA= intraperitoneal fat area, RFA= retroperitoneal fat area and VFR= visceral fat ratio VFA/SFA.
=significant (p<0.05)
Age
We compared patients older than 61 years vs. younger (median split). Older patients had significantly higher TFA values, as well as VFA, IFA, RFA and VFR values than patients 61 years of age or younger at level L3/4 (p=0.001, p=<0.0001, p=<0.0001, p<0.0001 and p=<0.0001 respectively). SFA, however, was not significantly different between both age groups (Table 3). No age difference was observed for BMI or WHR, but patients older than 61 years collectively had higher WHtR measures than their younger counterparts (RPD=6.5%; p=0.0029).
Stage
Patients with higher tumor stages (III and IV) showed significantly lower adipose tissue in all compartments in equal measure compared with the group of patients with lower stage tumors (I and II) (Table 4).
Table 4.
A) T-test of patient stage group 1 (stages I, II; n = 54) versus patient stage group 2 (stages III, IV; n = 65).
| A) | ||||
|---|---|---|---|---|
| Compartment | Stage Group 1r Mean (SD) | Stage Group 2 Mean (SD) | RPD | P |
| TFA | 434.9 (153.2) | 342.9 (169.4) | -21.2%* | 0.003* |
| VFAa | 13.8 (3.6) | 11.5 (4.15) | -16.8%* | 0.002* |
| SFA | 231.7 (89.3) | 192.0 (99.5) | -17.1%* | 0.025* |
| IFAa | 11.0 (3.05) | 9.1 (3.32) | -17.6%* | 0.001* |
| RFAa | 8.1 (2.6) | 6.9 (2.85) | -14.9%* | 0.019* |
| VFRa | 0.95 (0.27) | 0.86 (0.27) | -8.8% | 0.098 |
| B) | |||||
|---|---|---|---|---|---|
| TFA | VFAa | SFA | IFAa | RFAa | |
| Stage | IV from I-III | IV from I-II | IV from I-II | IV from I-II | IV from I-II |
| P | 0.003 | 0.005 | 0.023 | 0.006 | 0.029 |
SD= standard deviation, RPD=relative percent difference,
= square root transformed version of the variable was used;
=indicates the reference group, TFA= total fat area, VFA= visceral fat area, SFA= subcutaneous fat area, IFA= intraperitoneal fat area, RFA= retroperitoneal fat area and VFR= visceral fat ratio VFA/SFA.
= significant (p < 0.05) and B) ANOVA using the Duncan test for identifying significant differences of mean fat compartment measures between stages.
Body metrics: Waist-to-Height-Ratio (WHtR), Body-Mass-Index (BMI) and Waist-to- Hip-Ratio (WHR)
Overall, we noted a strong, statistically significant correlation of the WHtR with all adipose tissue compartments. In particular, the correlations of VFA and IFA with WHtR were stronger than with BMI and WHR. WHtR was moderately correlated with VFR (Table 5). For both genders there were strong correlations of the different compartments with WHtR. Furthermore in women WHtR correlated significantly with VFR, while men did not (Table 6).
Table 5.
Pearson correlations of body metrics with adipose tissue compartments, α = 0.5, N(total) = 120, N(women)=84, N(women)=36.
| BMI r(p) | WHR r (p) | WHtR r (p) | |
|---|---|---|---|
| TFA L3/L4 | 0.85 (<.0001) * | 0.31 (<0.01) * | 0.84 (<.0001) * |
| VFA L3/L4a | 0.65 (<.0001) * | 0.51 (<.0001) * | 0.69 (<.0001) * |
| IFA L3/L4a | 0.65 (<.0001) * | 0.47 (<.0001) * | 0.71 (<.0001) * |
| RFA L3/L4a | 0.52 (<.0001) * | 0.45 (<.0001) * | 0.51 (<.0001) * |
| SFA L3/L4 | 0.77 (<.0001) * | 0.04 (0.7) | 0.71 (<.0001) * |
| VFR L3/L4a | 0.15 (0.11) | 0.51 (<.0001) * | 0.22 (<0.05) * |
: square root transformed version of the variable was used, TFA= total fat area, VFA= visceral fat area, SFA= subcutaneous fat area, IFA= intraperitoneal fat area, RFA= retroperitoneal fat area and VFR= visceral fat ratio VFA/SFA.
= significant (p<0.05).
Table 6.
Pearson correlations of body metrics with adipose tissue compartments in A) men and B) women, α=0.5, N=84.
| A) | |||
|---|---|---|---|
| BMI r(p) | WHR r (p) | WHtR r (p) | |
| TFA L3/L4 | 0.85 (<0.001)* | 0.27 (<0.05)* | 0.85 (<0.001)* |
| VFA L3/L4a | 0.63 (<0.001)* | 0.36 (<0.01)* | 0.76 (<0.001)* |
| IFA L3/L4a | 0.59(<0.001)* | 0.34 (<0.01)* | 0.74 (<0.001)* |
| RFA L3/L4a | 0.52(<0.001)* | 0.30 (<0.01)* | 0.58 (<0.001)* |
| SFA L3/L4 | 0.81(<0.001)* | 0.14 (0.21) | 0.70 (<0.001)* |
| VFR L3/L4a | -0.02 (0.84) | 0.24 (<0.05)* | 0.21 (0.06) |
| B) | |||
| TFA L3/L4 | 0.85 (<0.001)* | 0.33 (0.05) | 0.91 (<0.001)* |
| VFA L3/L4a | 0.70 (<0.001)* | 0.50 (<0.01)* | 0.86 (<0.001)* |
| IFA L3/L4a | 0.73 (<0.001)* | 0.47 (<0.01)* | 0.86 (<0.001)* |
| RFA L3/L4a | 0.49 (<0.01)* | 0.41 (<0.05)* | 0.65 (<0.001)* |
| SFA L3/L4 | 0.75 (<0.001)* | 0.05 (0.76) | 0.71 (<0.001)* |
| VFR L3/L4a | 0.33 (<0.05)* | 0.58 (<0.01)* | 0.51 (<0.01)* |
: square root transformed version of the variable was used, TFA= total fat area, VFA= visceral fat area, SFA= subcutaneous fat area, IFA= intraperitoneal fat area, RFA= retroperitoneal fat area and VFR= visceral fat ratio VFA/SFA.
=significant (p<0.05).
BMI correlated positively with all compartments at both levels. The correlations of TFA, SFA, and RFA with BMI were stronger than with WHtR and WHR; BMI however, was not significantly correlated with the VFR (Table 5). In the gender subgroup analysis, BMI and the VFR were positively correlated among women, but not men (Table 6).
WHR was most weakly, but still significantly correlated with most of the different compartments and VFR, but excluding SFA (Table 5). In the gender subgroup analysis, WHR was not correlated significantly with SFA men and woman and with TFA in women (Table 6).
WHR and WHtR-Measurement
As there is no standardized method of WHR and WHtR measurement, we evaluated which manually-collected measure was most strongly correlated with the CT-collected measures and the adipose tissue compartments. WHR (manually) as the ratio of narrowest waist circumference and widest hip circumference on a standing patient correlated most strongly with the WHR calculated from CT data (r=0.64, p=0.014). Both manually collected WHtR measures were strongly correlated with CT data (navel: r=0.93, p<.0001; narrowest waist level: r= 0.92, p<.0001).
Physical activity and adipose tissue compartments
At baseline, patients reported a mean of 17.9 (median 11.5) metabolic equivalents per week (METs) during the past 12 months. Overall, there was no significant correlation between the adipose tissue compartments and physical activity levels (results not shown). In multivariate linear regression analyses, only a modest association between physical activity and the SFA compartment at level L3/4 was seen, such that, per unit increase in METs, SFA increased by about 0.98 cm2 while adjusting for age, sex, scan time and stage (p=0.042).
Physical activity vs. muscle
There was a significant positive correlation of physical activity and the M. psoas (r=0.267, p=0.019), while there was no significant correlation with M. erector spinae. However, in male and in younger patients there was a significant positive correlation with both muscles M. psoas and erector spinae, while in women physical activity was negatively correlated with M. erector spinae. Regarding tumor stage, physical activity was positively correlated with the M. erector spinae in patients with stage III /IV, while there was no correlation with either muscle in patients with stage I / II (Table 7).
Table 7.
Pearson correlations of physical activity (METs) with M. psoas and M. erector spinae, α=0.5, N=77.
| M. Psoas r(p) | M. erector spinae r(p) | |
|---|---|---|
| METs | 0.267 (0.019)* | 0.17 (0.13) |
| METs female | -0.29 (0.19) | -0.49 (0.0216)* |
| METs male | 0.37 (0.0055)* | 0.35 (0.0080)* |
| METs ≤ 61 | 0.48 (0.0014)* | 0.44 (0.0039)* |
| METS >61 | 0.02 (0.93) | -0.12 (0.50) |
| METs Stage 1/2 | 0.27 (0.13) | -0.03 (0.85) |
| METs Stage 3/4 | 0.26 (0.09) | 0.30 (0.0496)* |
=significant (p<0.05).
Muscle vs. adipose tissue compartments
Generally, reduced adipose tissue at multiple adipose tissue compartments was associated with greater M. erector spinae (Table 8). No significant correlations between any of the compartments and M. psoas were observed.
Table 8.
Correlation of M. erector spinae and adipose tissue compartments; N = 120, r (p-value).
| TFA r(p) | VFAa r(p) | SFA r(p) | IFAa r(p) | RFAa r(p) | VFRa r(p) | |
|---|---|---|---|---|---|---|
| M. erector spinae-L3/4 | -0.37 (<.0001)* | -0.30 (0.001)* | -0.31 (0.0006)* | -0.27 (0.003)* | -0.28 (0.002)* | -0.10 (0.29) |
: square root transformed version of the variable was used, TFA= total fat area, VFA= visceral fat area, SFA= subcutaneous fat area, IFA= intraperitoneal fat area, RFA= retroperitoneal fat area and VFR= visceral fat ratio VFA/SFA.
=significant.
Body metrics vs. muscle
BMI was not correlated with either M. psoas or M. erector spinae, or the muscle index. WHR was positively correlated with the M. psoas, but not with M. erector spinae and the muscle index. However, WHtR was negatively correlated with both M. psoas and M. erector spinae, as well as the muscle index (Table 9).
Table 9.
Pearson correlations of BMI, WHR and WHtR with M. psoas, M. erector spinae and SMI (skeletal muscle index); N = 120, r (p-value).
| M. Psoas r(p) | M. erector spinae r(p) | SMI r(p) | |
|---|---|---|---|
| BMI | 0.14 (0.13) | -0.17 (0.06) | -0.08 (0.36) |
| WHR | 0.19 (0.043)* | 0.06 (0.50) | 0.05 (0.58) |
| WHtR | -0.23 (0.0112)* | -0.51 (<.0001)* | -0.4 (<.0001)* |
SMI = skeletal muscle index.
=significant.
Inter-observer agreement
The Bland-Altman analysis of TFA, VFA and SFA as well as the M. psoas and dorsal muscles showed a high concordance between the two independent, blinded readers in a preliminary investigation with 10 CT-data sets (Table 10). Therefore, the remaining quantifications of adipose tissue compartments were performed by one reader.
Table 10.
Bland-Altman analysis of 10 CT data sets read by 2 independent observer shows following equation: ‘Observer2 = y + m*Observer1’. A slope ‘m’ of 1 indicates no difference between observer 1 and 2.
| TFA | VFA | RFA | M. Psoas | M. erector spinae | |
|---|---|---|---|---|---|
| Slope | 0.999 | 1.193 | 0.962 | 1.006 | 1.019 |
TFA= total fat area, VFA= visceral fat area, SFA= subcutaneous fat area, IFA= intraperitoneal fat area, RFA= retroperitoneal fat area and VFR= visceral fat ratio VFA/SFA.
Discussion
In this study, we assessed body fat distribution in CRC patients based on diagnostic CT scans, with the aim to explore the association between adipose tissue distribution and gender, age, disease stage, muscle mass, physical activity and body metrics. The area-based quantification of adipose tissue compartments on the basis of CT scans proved to be reliable and reproducible. In the literature, the levels L3/4 and L 4/5 are deemed comparable with volume-based measurements, and are strongly correlated with obesity-related mortality, such as with diabetes or hypertension [20; 21]. Men showed higher VFA values and VFR in comparison to women. Furthermore, older patients (>61 years) had higher VFA and VFR than younger patients. This is important as a specific pathogenic role is attributed to the visceral adipose tissue, and therefore patients at risk for obesity-related morbidities might be identified by quantification of adipose tissue compartments.
Notably, with increasing tumor stage we found a depletion of adipose tissue in all compartments, which affected all compartments to a similar extent and might reflect beginning cachexia.
In contrast to BMI and WHR, only the WHtR showed a strong correlation with nearly all compartments and VFR at both levels of measurement. Also, only the WHtR correlated negatively with the muscle mass. However, similar to BMI and WHR, WHtR did not reflect the differences of adipose tissue distribution by age or gender. These results indicate that WHtR might be a better marker than BMI or WHR for the distribution of adipose tissue and could replace BMI in clinical routines as an initial tool for the evaluation of adipose tissue distribution. Intriguingly, several studies have shown that WHtR is more strongly correlated with obesity-associated mortality in patients with diabetes, hypertension and dyslipidemia than WHR or BMI [34-36]. Furthermore, WHtR is more applicable to children and different ethnicities [37]. Our results support the use of this variable in further research and potentially in clinical decision making.
There was no association between self-reported physical activity and the different adipose tissue compartments in our patient group. This, however, could be explained by the generally low level of physical activity among our patient population, and therefore should be further investigated in future studies, especially in patients with a wider range of physical activity.
Regarding measurements of muscle distribution, the M. erector spinae area was inversely correlated with nearly all adipose tissue compartments at both levels, while M. psoas area was less predictive. However, physical activity was positively correlated with muscle mass, especially in men and younger patients, which might be an effect of more muscle mass and a higher level of physical activity in these subgroups.
As obesity is associated with incidence of and mortality from CRC [2; 4; 12; 15; 18; 22; 36], our future aim is to evaluate which measurement of body composition and adipose tissue distribution or specific compartment is the strongest predictor of morbidity (e.g. perioperative complications, tolerance of chemotherapy), recurrence and mortality. Another topic of research will be the correlation of adipose tissue compartments and muscle area with cachexia and sarcopenia in CRC patients.
This study has several limitations.
As we only included ColoCare patients, our number of recruited patients is relatively small. As a result, in a larger sample size certain not yet detected differences among subgroups might be revealed. Only ColoCare participants with available CT data from the process of routine staging or preoperative planning were included in this study. Patients who were not entirely positioned within the field of view of the CT scanner were excluded (n=11), as were patients with unknown weight or height. Patients with lower stages are underrepresented in this study, as they often did not receive a routine CT scan. For reasons of radiation protection, we did not conduct CT scans solely for study purposes.
As we could not find statistical differences regarding the muscle and adipose tissue distribution between CT-scans of patients before and after surgery we included both into one group. However, as the major group were rectal cancer patients, we think that the surgery in mainly pelvic location did not influence the more cranial situated measurements, especially L3/4.
As we performed a retrospective analysis of preexisting CT scans, various CT scanners with different protocols were used. Nevertheless, this had no influence on the quantification of adipose tissue or muscle as we adjusted for differences between the scans regarding slice thickness. Furthermore, we observed no difference in adipose tissue measurements concerning the application of contrast media. Regarding muscle measurements, we narrowed the attenuation range to 40-100 HU in comparison to other commonly used ranges (0-100 HU or -29-150 HU) to avoid measuring errors that could otherwise occur due to the application of contrast media and the lipid content of the muscle [24-27] [38; 39] Also, we tested an upper attenuation limit of 150 HU with our CT-Data, and found no further increase in muscle area. All decisions regarding these thresholds were based on visual controls that we conducted as a plausibility test to make sure we measure the correct muscle area.
To our knowledge, this is the first investigation of adipose tissue compartments in comparison with body metrics including BMI, WHR and WHtR in patients with CRC, which contains a thorough evaluation of clinical information. There were previous studies in CRC patients evaluating adipose tissue compartments in comparison with BMI with slightly different emphasis, which also showed the low prognostic value of BMI and a weak correlation of BMI with the prognostically relevant visceral adipose tissue. [40-42]
Conclusion
Regional densitometric quantification of adipose tissue on CT at levels L3/4 and L4/5 is a highly reproducible and reliable method for obtaining accurate data on different adipose tissue compartments. Male patients had significantly more VFA and a higher VFR than women. The fat distribution among CRC patients appears to change with age, with more VFA and a higher VFR among older patients (> 61 years). WHtR was a better predictor of adipose tissue compartments and muscle mass than BMI and WHR, but did not adequately capture differences by age and gender, compared to the CT-based measurement. Our study illustrates the utility of both CT scan-based adipose tissue compartment assessment and WHtR in both outcomes research and possibly clinical practice.
Supplementary Material
Key points.
Densitometric quantification of adipose tissue on CT is highly reproducible and reliable
Waist-to-Height-Ratio better correlates with adipose tissue compartments and VFR than BMI or Waist-to-Hip-Ratio
Men have higher a higher visceral fat area than women
Patients older than 61 years have higher visceral fat area
Patients with tumor stages III/IV have significantly lower adipose tissue than I/II
Footnotes
Conflict of Interest Statement:
Regarding this study there is no conflict of interest for all contributing authors.
References
- 1.WHO. http://wwwwhoint/mediacentre/factsheets/fs311/en/
- 2.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 4.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 5.Martinez ME, Giovannucci E, Spiegelman D, Hunter DJ, Willett WC, Colditz GA. Leisure-time physical activity, body size, and colon cancer in women. Nurses’ Health Study Research Group. J Natl Cancer Inst. 1997;89:948–955. doi: 10.1093/jnci/89.13.948. [DOI] [PubMed] [Google Scholar]
- 6.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 7.Dai Z, Xu YC, Niu L. Obesity and colorectal cancer risk: a meta-analysis of cohort studies. World J Gastroenterol. 2007;13:4199–4206. doi: 10.3748/wjg.v13.i31.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Marchand L, Wilkens LR, Kolonel LN, Hankin JH, Lyu LC. Associations of sedentary lifestyle, obesity, smoking, alcohol use, and diabetes with the risk of colorectal cancer. Cancer Res. 1997;57:4787–4794. [PubMed] [Google Scholar]
- 9.Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013;62:933–947. doi: 10.1136/gutjnl-2013-304701. [DOI] [PubMed] [Google Scholar]
- 10.Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One. 2013;8:e53916. doi: 10.1371/journal.pone.0053916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dignam JJ, Polite BN, Yothers G, et al. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. 2006;98:1647–1654. doi: 10.1093/jnci/djj442. [DOI] [PubMed] [Google Scholar]
- 12.Meyerhardt JA, Catalano PJ, Haller DG, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98:484–495. doi: 10.1002/cncr.11544. [DOI] [PubMed] [Google Scholar]
- 13.Balentine CJ, Wilks J, Robinson C, et al. Obesity increases wound complications in rectal cancer surgery. J Surg Res. 2010;163:35–39. doi: 10.1016/j.jss.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Campbell PT, Newton CC, Dehal AN, Jacobs EJ, Patel AV, Gapstur SM. Impact of body mass index on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2012;30:42–52. doi: 10.1200/JCO.2011.38.0287. [DOI] [PubMed] [Google Scholar]
- 15.Kim LJ, Nalls MA, Eiriksdottir G, et al. Associations of visceral and liver fat with the metabolic syndrome across the spectrum of obesity: the AGES-Reykjavik study. Obesity (Silver Spring) 2011;19:1265–1271. doi: 10.1038/oby.2010.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koster A, Stenholm S, Alley DE, et al. Body fat distribution and inflammation among obese older adults with and without metabolic syndrome. Obesity (Silver Spring) 2010;18:2354–2361. doi: 10.1038/oby.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 18.Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114:71–83. doi: 10.1080/13813450801954303. [DOI] [PubMed] [Google Scholar]
- 19.White E, Patterson RE, Kristal AR, et al. VITamins And Lifestyle cohort study: study design and characteristics of supplement users. Am J Epidemiol. 2004;159:83–93. doi: 10.1093/aje/kwh010. [DOI] [PubMed] [Google Scholar]
- 20.Irlbeck T, Massaro JM, Bamberg F, O’Donnell CJ, Hoffmann U, Fox CS. Association between single-slice measurements of visceral and abdominal subcutaneous adipose tissue with volumetric measurements: the Framingham Heart Study. Int J Obes (Lond) 2010;34:781–787. doi: 10.1038/ijo.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balentine CJ, Marshall C, Robinson C, et al. Validating quantitative obesity measurements in colorectal cancer patients. J Surg Res. 2010;164:18–22. doi: 10.1016/j.jss.2010.05.048. [DOI] [PubMed] [Google Scholar]
- 22.Sjostrom L, Kvist H, Cederblad A, Tylen U. Determination of total adipose tissue and body fat in women by computed tomography, 40K, and tritium. Am J Physiol. 1986;250:E736–745. doi: 10.1152/ajpendo.1986.250.6.E736. [DOI] [PubMed] [Google Scholar]
- 23.Yoshizumi T, Nakamura T, Yamane M, et al. Abdominal fat: standardized technique for measurement at CT. Radiology. 1999;211:283–286. doi: 10.1148/radiology.211.1.r99ap15283. [DOI] [PubMed] [Google Scholar]
- 24.Goodpaster BH, Thaete FL, Kelley DE. Composition of skeletal muscle evaluated with computed tomography. Ann N Y Acad Sci. 2000;904:18–24. doi: 10.1111/j.1749-6632.2000.tb06416.x. [DOI] [PubMed] [Google Scholar]
- 25.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985) 2000;89:104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 26.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985) 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 27.Idoate F, Cadore EL, Casas-Herrero A, et al. Adipose tissue compartments, muscle mass, muscle fat infiltration, and coronary calcium in institutionalized frail nonagenarians. Eur Radiol. 2015;25:2163–2175. doi: 10.1007/s00330-014-3555-5. [DOI] [PubMed] [Google Scholar]
- 28.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 29.Mason C, Katzmarzyk PT. Waist circumference thresholds for the prediction of cardiometabolic risk: is measurement site important? Eur J Clin Nutr. 2010;64:862–867. doi: 10.1038/ejcn.2010.82. [DOI] [PubMed] [Google Scholar]
- 30.WHO. Waist Circumference and Waist-Hip Ratio Report of a WHO Expert Consultation. World Health Organisation; Geneva: 2008. [Google Scholar]
- 31.Littman AJ, White E, Kristal AR, Patterson RE, Satia-Abouta J, Potter JD. Assessment of a one-page questionnaire on long-term recreational physical activity. Epidemiology. 2004;15:105–113. doi: 10.1097/01.ede.0000091604.32542.97. [DOI] [PubMed] [Google Scholar]
- 32.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 34.Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008;61:646–653. doi: 10.1016/j.jclinepi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012;13:275–286. doi: 10.1111/j.1467-789X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 36.Hsieh SD, Ashwell M, Muto T, Tsuji H, Arase Y, Murase T. Urgency of reassessment of role of obesity indices for metabolic risks. Metabolism. 2010;59:834–840. doi: 10.1016/j.metabol.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 37.Ashwell M. Obesity risk: importance of the waist-to-height ratio. Nurs Stand. 2009;23:49–54. doi: 10.7748/ns2009.06.23.41.49.c7050. quiz 55. [DOI] [PubMed] [Google Scholar]
- 38.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 39.Strandberg S, Wretling ML, Wredmark T, Shalabi A. Reliability of computed tomography measurements in assessment of thigh muscle cross-sectional area and attenuation. BMC Med Imaging. 2010;10:18. doi: 10.1186/1471-2342-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto N, Fujii S, Sato T, et al. Impact of body mass index and visceral adiposity on outcomes in colorectal cancer. Asia Pac J Clin Oncol. 2012;8:337–345. doi: 10.1111/j.1743-7563.2011.01512.x. [DOI] [PubMed] [Google Scholar]
- 41.Tsujinaka S, Konishi F, Kawamura YJ, et al. Visceral obesity predicts surgical outcomes after laparoscopic colectomy for sigmoid colon cancer. Dis Colon Rectum. 2008;51:1757–1765. doi: 10.1007/s10350-008-9395-0. discussion 1765-1757. [DOI] [PubMed] [Google Scholar]
- 42.Rickles AS, Iannuzzi JC, Mironov O, et al. Visceral obesity and colorectal cancer: are we missing the boat with BMI? J Gastrointest Surg. 2013;17:133–143. doi: 10.1007/s11605-012-2045-9. discussion p 143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


