Abstract
Buprenorphine (BPN), a mixed opioid drug with high affinity for mu (MOR) and kappa (KOR) opioid receptors, has been shown to produce behavioral responses in rodents that are similar to those of antidepressant and anxiolytic drugs. Although recent studies have identified KORs as a primary mediator of BPN’s effects in rodent models of depressive-like behavior, the role of MORs in BPN’s behavioral effects has not been as well explored. The current studies investigated the role of MORs in mediating conditioned approach behavior in the novelty-induced hypophagia (NIH) test, a behavioral measure previously shown to be sensitive to chronic treatment with antidepressant drugs. The effects of BPN were evaluated in the NIH test 24 h post-administration in mice with genetic deletion of the MOR (Oprm1−/−) or KOR (Oprk1−/−), or after pharmacological blockade with the non-selective opioid receptor antagonist naltrexone and selective MOR antagonist cyprodime. We found that behavioral responses to BPN in the NIH test were blocked in Oprm1−/− mice, but not in Oprk1−/− mice. Both cyprodime and naltrexone significantly reduced approach latency at doses experimentally proven to antagonize the MOR. In contrast the selective MOR agonist morphine and the selective KOR antagonist nor-BNI were both ineffective. Moreover, antinociceptive studies revealed persistence of the MOR antagonist properties of BPN at 24 h post-administration, the period of behavioral reactivity. These data support modulation of MOR activity as a key component of BPN’s antidepressant-like effects in the NIH paradigm.
Keywords: Buprenorphine, antidepressant, endogenous opioid system, novelty-induced hypophagia
1. Introduction
Opioid receptors and their endogenous ligands are important modulators of the neural pathways involved in the regulation of mood and emotional states (Lutz and Kieffer, 2013). Activation of mesocorticolimbic mu opioid receptors (MOR) facilitates the release of dopamine into central reward pathways, promoting enhanced mood and euphoria (Johnson and North, 1992; Spanagel et al., 1992; Contet et al., 2004). In contrast, the kappa opioid receptor (KOR) and its endogenous ligand dynorphin are thought to mediate behavioral responses to aversive or stressful experiences (Bruchas et al., 2010; Lalanne et al., 2014). Exposure to stress increases dynorphin levels in limbic regions (Shirayama et al., 2004; McLaughlin et al., 2006b) and activation of KORs is reported to elicit dysphoria and depressive-like behavior in rodents (Carlezon et al., 2006; McLaughlin et al., 2006a; McLaughlin et al., 2006b). Notably, several animal studies have shown that treatment with selective KOR antagonists mitigates stress-induced affective behavior (Mague et al., 2003; McLaughlin et al., 2003; Carr et al., 2010). These preclinical findings have lent support to pharmacological modulation of the KOR as a potential target for the development of novel antidepressants and anxiolytics.
Buprenorphine (BPN), an opioid drug with mixed pharmacological effects, has recently emerged as a promising candidate for an antidepressant drug. Clinical studies show low doses of BPN to be effective in alleviating depressive symptoms in treatment-resistant patients and reducing suicidal ideation in severely suicidal patients (Bodkin et al., 1995; Nyhuis et al., 2008; Yovell et al., 2015). BPN has also been found to ameliorate depressive and anxiety-like behavior in rodents (Almatroudi et al., 2015; Browne et al., 2015; Falcon et al., 2015). BPN acts as a high affinity partial agonist at the mu opioid receptor (MOR) and an antagonist at the kappa opioid receptor (KOR), although it interacts with delta and nociceptin receptors at higher concentrations (Lutfy and Cowan, 2004). Due to its complex pharmacology, the mechanisms underlying BPN’s behavioral response has been unclear. Recent studies suggest that KOR antagonism may contribute to BPN’s behavioral effects (Almatroudi et al., 2015; Falcon et al., 2016). However, whether BPN’s activity at the MOR significantly influences its effects on rodent behavioral tests for antidepressant activity is not as well understood.
Our laboratory has previously investigated BPN’s therapeutic effects in the novelty-induced hypophagia test, a conflict-based behavioral task that assesses the impact of environmental stressors on conditioned approach response for a palatable food reward. We demonstrated that a single administration of low dose BPN (0.25 mg/kg) to mice reduces approach latency for palatable food in a novel environment (Falcon et al., 2015), an effect typically observed after chronic treatment with conventional antidepressant drugs or acute treatment with benzodiazepines (Bodnoff et al., 1988; Bodnoff et al., 1989; Dulawa and Hen, 2005). The primary goal of the present study was to discern the role of MOR versus KOR in mediating BPN’s effects on conditioned approach behavior in the NIH test. To that end, we employed two approaches, genetic deletion and selective pharmacological modulation of opioid receptors, to distinguish the MOR from the KOR as the primary mediator of BPN’s effects in this paradigm. The results of this study show that BPN was effective in reducing approach latency in the novel arena in Oprk1−/− mice but not Oprm1−/− mice. Administration of the selective MOR antagonist cyprodime and the blanket opioid antagonist naltrexone, but not the MOR agonist morphine or KOR antagonist nor-BNI, replicated the effects of BPN in the NIH test. These findings suggest a role for the MOR in mediating the antidepressant-like response of BPN in a behavioral measure of motivational conflict.
2. Materials and methods
2.1 Animals
Male C57BL/6J mice, 7 weeks of age upon arrival were purchased from Jackson Laboratories (Bar Harbor, ME) and used for the majority of the studies. Male Oprm1−/− mice and littermate wild-type (WT) controls were generated using heterozygous breeding. Male Oprk1−/− mice used in this study were generated from mating pairs of Oprk1−/− purchased from Jackson Laboratories and maintained for several generations via homozygous breeding. Male C57BL/6J mice, originally from Jackson Laboratories but generated within the colony, were used as their wild type (WT) controls because this was the background strain used in all of the genetic lines. Mice were housed up to 5 per cage (or in pairs for NIH experiments) in polycarbonate cages and maintained under a 12 h light-dark cycle (lights on at 0700 hours) in a temperature (20–22°C) and humidity (44–60%)-controlled environment. Food and water were available ad libitum. All experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and protocols were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania.
2.2 Drugs and treatment
Buprenorphine hydrochloride (Sigma, St. Louis, MO and NIDA) and nor-binaltorphimine dihydrochloride (nor-BNI; Tocris Bioscience, Ellisville, MO) were administered at doses previously found to produce antidepressant effects using the forced swimming test in C57BL/6J mice (Falcon et al., 2015). Doses of morphine sulfate (Spectrum Chemical, New Brunswick, NJ), U50,488 (Sigma), naltrexone hydrochloride (Sigma), and cyprodime hydrochloride (Tocris) were chosen based on results from pilot studies investigating their antinociceptive effects. Buprenorphine and nor-BNI were dissolved in distilled water and injected intraperitoneally (i.p.). Morphine and naltrexone were dissolved in physiological saline and injected i.p. Cyprodime, a selective MOR antagonist originally characterized by Marki et al. (1999), was dissolved in 1% ethanol and delivered by i.p. injection. Mice in the control groups were injected with vehicle or saline where appropriate. All doses were calculated according to the base weight of the drug and administered in a volume of 10 ml/kg.
2.3 Novelty-induced hypophagia (NIH) test
Mice were pair housed and trained to eat a palatable food (three peanut butter chips presented in a small, clear petri dish) in a home feeding cage. Opaque, black, plastic dividers were placed inside each cage to separate the mice during home cage training sessions. Mice were allowed to habituate to the dividers for 1 h before the start of the training session. Animals were trained to consume the chips in 15 min sessions conducted daily until they met the criteria of three consecutive days with approach latencies of 30 sec or below. For novel cage testing, mice were placed in an empty, clear polycarbonate cage (25.5 × 46 × 20 cm) with bright lighting (60 W light bulb) and scented with lemon (20% Lemon Joy solution). There was no food deprivation or habituation period prior to the novel cage test. The novel cage test session was videotaped, and the latency to approach during the 15 min test session was measured. The approach latency was defined by the time it took the mouse to approach the dish in the center of the arena and begin feeding.
In Experiment 1, mice were treated with vehicle or BPN (0.25 mg/kg) immediately after their last training session and tested 24 h later in the novel environment (n = 8–19 per group). Similarly, in Experiment 2, mice were injected with vehicle, morphine (10 mg/kg), or nor-BNI (10 mg/kg) and tested 24 h later (n = 10–22 per group). In Experiment 3, mice were injected with vehicle or cyprodime (3 or 10 mg/kg) and tested 1 h later (n = 10–30 per group). In a separate cohort, mice were injected with vehicle or 10 mg/kg cyprodime and tested 24 h later (n = 10 per group). In Experiment 4, mice were treated with vehicle or naltrexone (1 mg/kg) and tested 1 h later (n = 9–10 per group). In a separate cohort, mice were treated with vehicle or naltrexone (1 mg/kg) and tested 24 h later (n = 9–10 per group).
2.4 Test for analgesia
Mice were individually placed onto a hot plate (Columbus Instruments, Columbus OH) heated to 55°C enclosed by an acrylic container. The latency for the mouse to lick the hindpaw or jump was recorded. An 80 second cut-off was imposed that allowed drug-dependent differences in latency to hind-paw lick or jump to be measured and avoided tissue damage. It was less than the longest latency observed in morphine-treated animals and shorter than cut off- latencies of up to 120 s used in previous studies (Bansinath et al., 1990; Yoshida et al., 2003). No pathological or behavioral changes were observed when animals were examined following hot-plate testing.
To determine the MOR and KOR antagonist properties of each drug, naltrexone (1 mg/kg) (n = 6–7 per group) and cyprodime (10 mg/kg) (n= 8–16 per group) were injected 1 h prior to morphine or U50,488 administration and animals were tested 30 min later. To assess MOR activity 24 h after acute administration of BPN (0.25 mg/kg), animals were first examined for their baseline antinociceptive response and then tested 30 min after morphine (10 mg/kg) administration (n = 9 per group). Data for this experiment is expressed as percentage of baseline (pre-morphine) response.
2.5 Statistical analyses
One-way and two-way ANOVAs were performed to determine the significance of differences between experimental groups. Significant overall main effects or interactions were followed by Holms-Sidak’s post hoc test. Unpaired two-tailed Student’s t-tests were applied where appropriate. For all tests, p < 0.05 was considered statistically significant. Data are expressed as mean ± SEM.
3. Results
3.1 Effects of MOR and KOR disruption on BPN’s behavioral effects in the NIH test
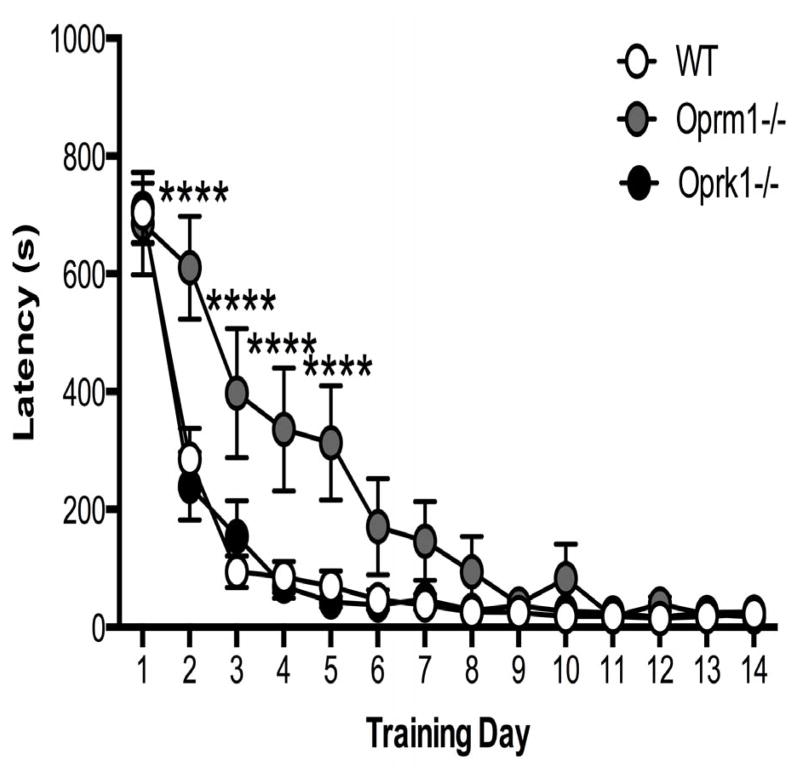
Acquisition of conditioned approach behavior to the palatable food was delayed significantly in Oprm1−/− mice compared with wildtype (WT) and Oprk1−/− mice (Figure 1). Repeated measures two-way ANOVA revealed a significant main effect of genotype [F2,67 = 8.047, p < 0.001] and time [F13,871 = 98.69, p < 0.001], in addition to a significant genotype*time interaction [F26,871 = 4.239, p < 0.001]. Between the second and fifth day of training, approach latencies for Oprm1−/− mice were significantly longer compared to WT and Oprk1−/− mice (p < 0.001).
Figure 1.
Effect of genotype on approach latencies during home cage training. Oprm1−/− mice exhibited significantly higher approach latencies compared to WT mice during training. N = 15–36 per group. (**** p < 0.0001 compared to WT). Data in all figures are depicted as mean ± SEM.
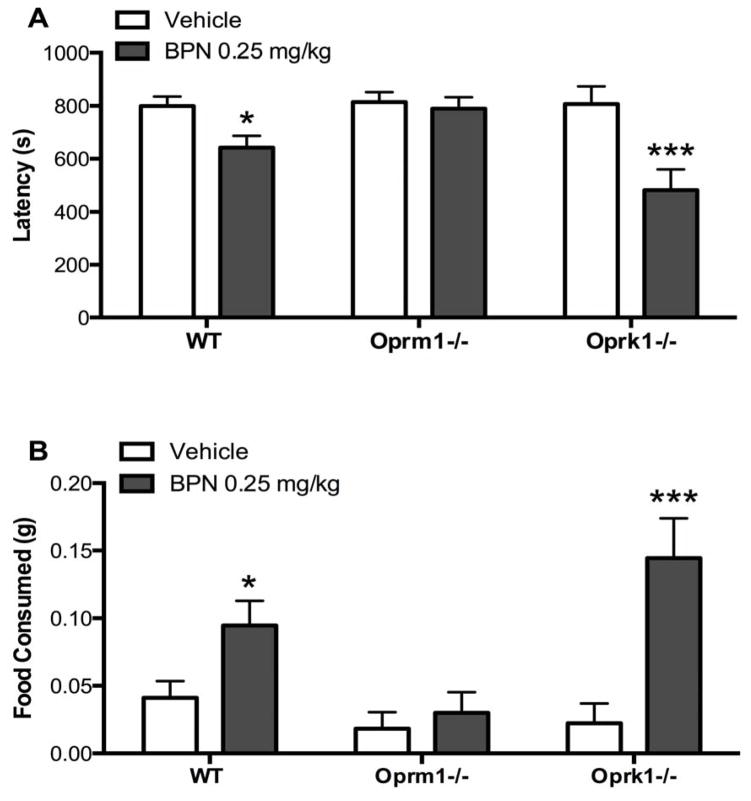
The behavioral effects of BPN (0.25 mg/kg) in the NIH test 24 h post-administration were assessed in Oprm1−/−, Oprk1−/−, and WT mice (Figure 2). A significant genotype*treatment interaction was observed for the latency (Panel A) to approach the food [F2,70 = 3.573, p = 0.033] and amount of food (Panel B) consumed [F2,69 = 3.882, p = 0.025] in the novel arena. Post-hoc tests revealed BPN significantly reduced approach latencies and increased food consumption in WT (p < 0.05) and Oprk1−/− (p < 0.001) mice, but not Oprm1−/− mice.
Figure 2.
BPN’s behavioral effects in the NIH test are blocked in Oprm1−/− mice. A). BPN reduced latency to approach and B) increased food consumed in WT and Oprk1−/− mice but not Oprm1−/− mice. N = 8–19 per group. (*p < 0.05, ***p < 0.001 compared to vehicle).
3.2 Effects of nor-BNI and morphine in the NIH test 24 h post-administration
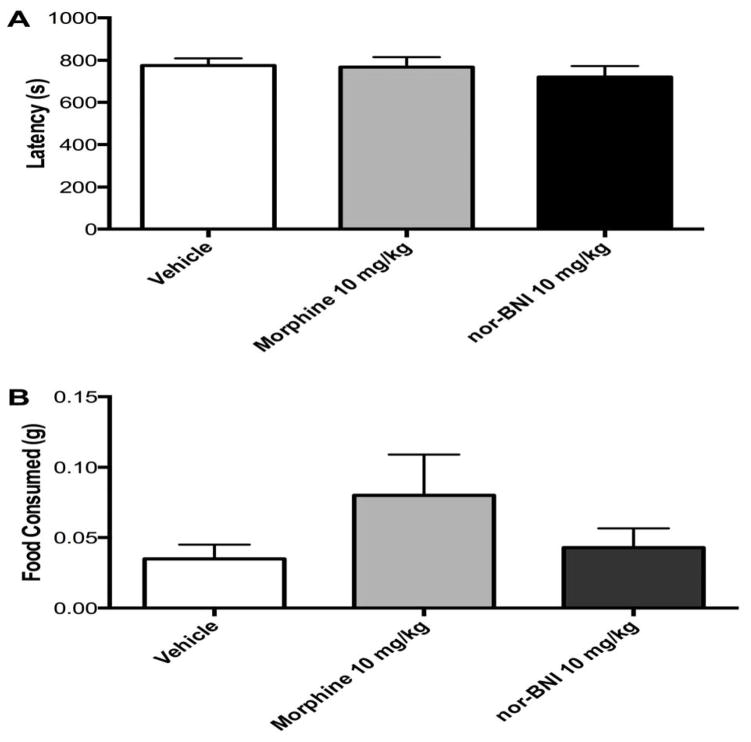
The effects of morphine (10 mg/kg) and nor-BNI (10 mg/kg) treatment on approach behavior in the NIH test 24 h post-administration were assessed in WT animals (Figure 3). One-way ANOVA revealed no significant effects of treatment on approach latencies (Figure 3A) [F2,44 = 0.465, p = 0.631] or food consumption (Figure 3B) [F2,44 = 1.972, p = 0.151] in the novel arena.
Figure 3.
Treatment with the MOR agonist morphine (10 mg/kg, i.p.) or the KOR antagonist nor-BNI (10 mg/kg, i.p.) does not affect behavior in the NIH test 24 h post administration. There were no significant effects on A) approach latency in the NIH test or B) amount of food consumed in the novel arena. N = 10–22 per group.
3.3 Effect of selective MOR antagonist cyprodime in the NIH test
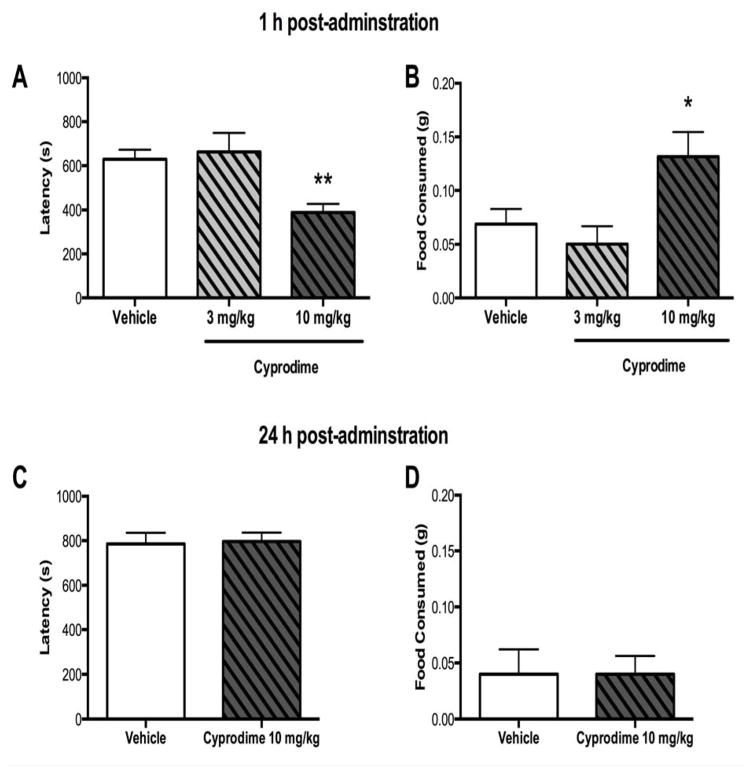
To characterize the effects of cyprodime in the NIH paradigm, we first examined novel arena behavior in WT animals treated with vehicle, 3 mg/kg or 10 mg/kg cyprodime 1 h post-administration (Figure 4A–B). One-way ANOVA revealed a significant effect of treatment on latency to approach [F2,54 = 7.224, p = 0.002] and food consumption [F2,55 = 4.545, p = 0.015] in the novel arena. Specifically, cyprodime administered at a dose of 10 mg/kg, but not 3 mg/kg, 1 h prior to testing significantly reduced latency to approach the food reward (p < 0.01) and increased the amount of food consumed (p < 0.05). When tested 24 h post-administration,10 mg/kg cyprodime did not affect approach latency or food consumed in the novel arena (Figure 4C–D).
Figure 4.
Treatment with the MOR antagonist cyprodime (10 mg/kg, i.p.) reduced approach latency and increased food consumption in the NIH test 1 h post administration. A) A dose response curve revealed that at 1 h post administration 10 mg/kg, but not 3 mg/kg, cyprodime significantly reduced approach latency and B) increased the amount of food consumed. N = 10–30 per group. C) In a separate cohort of animals 10 mg/kg cyprodime tested 24 h later did not exhibit any changes approach latency or D) food consumed compared to vehicle treated animals. N = 10 per group. (*p < 0.05, **p < 0.01 compared to vehicle).
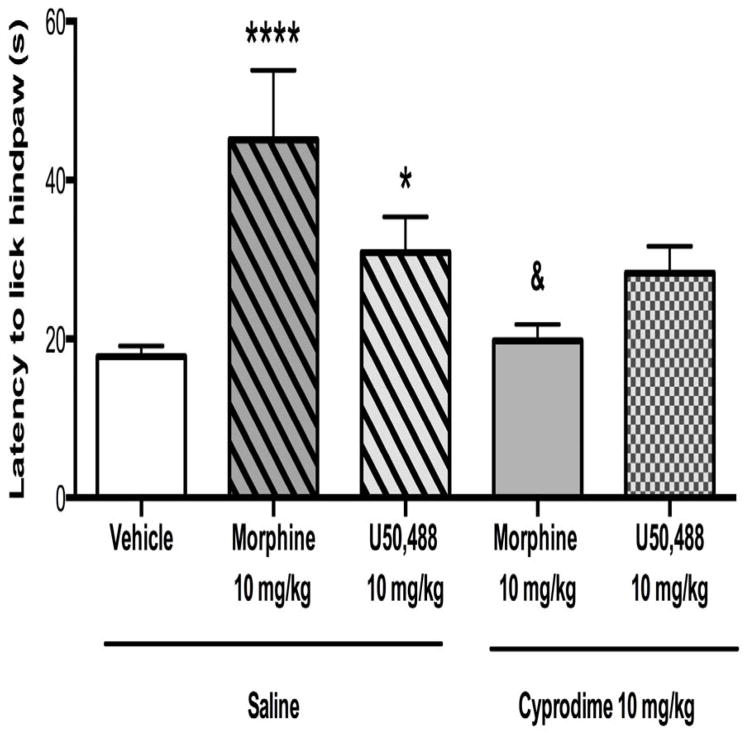
Cyprodime’s selectivity for MOR receptors was confirmed using the hot plate test (Figure 5). One-way ANOVA revealed a significant effect of treatment on antinociceptive response [F4,46 = 7.513, p < 0.0001]. Antinociception was significantly increased in animals treated with morphine (10 mg/kg, p < 0.0001) or U50,488 (10 mg/kg, p < 0.05). Pretreatment with cyprodime (10 mg/kg) significantly blocked antinociception induced by both morphine (p < 0.001), but not U50,488.
Figure 5.
Cyprodime (10 mg/kg, i.p.) selectively blocks MOR but not KOR according to tests of antinociception using the hot plate. Cyprodime pretreatment (1 h) blocked the antinociceptive effects induced by the MOR agonist morphine (10 mg/kg, i.p.) but not the KOR agonist U50,488 (10 mg/kg, i.p.). N = 8–16 per group. (*p < 0.05, ****p < 0.0001 compared to saline + vehicle, &p < 0.05 compared to saline + morphine).
3.4 Effect of non-selective opioid receptor antagonist naltrexone in the NIH test
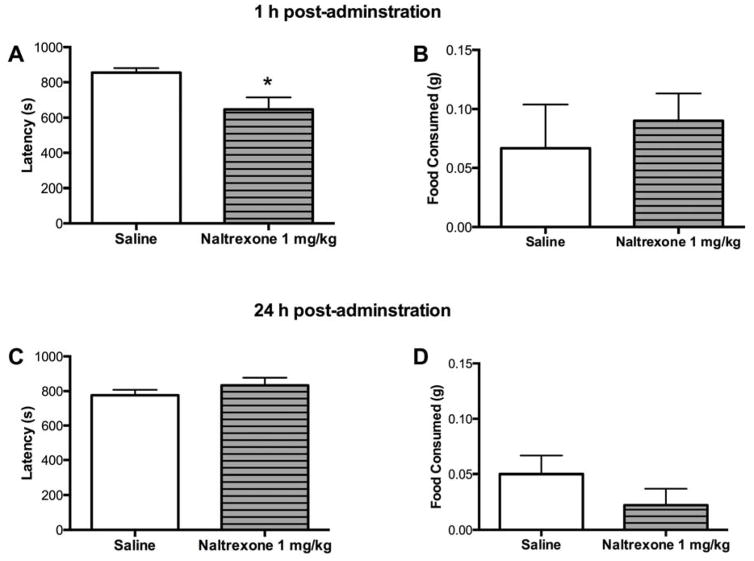
Next, we investigated whether treatment with naltrexone, a non-selective opioid antagonist, would reproduce the effects seen with cyprodime in the NIH test (Figure 6). The effects of naltrexone (1 mg/kg) 1 h post-administration were examined in WT mice. Unpaired two-tailed Student’s t-tests revealed a significant effect of 1 mg/kg naltrexone treatment on approach latency (t17= 2.712, p < 0.05) but not food consumed in the novel arena (Figure 6A–B). In a separate cohort, animals treated with 1 mg/kg naltrexone and tested 24 h later did not differ from vehicle treated animals in approach latency or food consumption (Figure 6C–D).
Figure 6.
Treatment with the non-selective opioid antagonist naltrexone (1 mg/kg) reduced approach latency in the NIH test 1 h post-administration. A) Acute naltrexone treatment reduced approach latency in the novel arena, B) but did not affect the amount of food consumed. N= 9–10 mice per group. C) In a separate cohort, animals treated with naltrexone and tested 24 h later did not exhibit any changes in approach latency or D) food consumed. N=9–10 mice per group. (*p < 0.05 compared to vehicle).
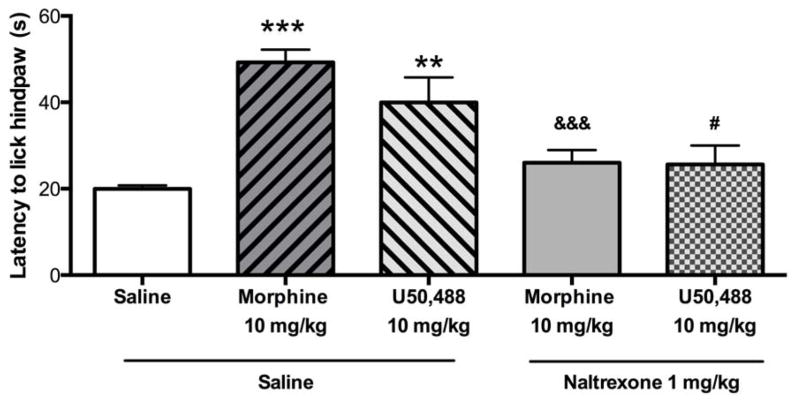
The ability of naltrexone to effectively block MOR and KOR agonist activity was confirmed using the hot plate test (Figure 7). One-way ANOVA revealed a significant effect of treatment on antinociceptive response [F4,28 = 8.892, p < 0.001]. The latency for animals to lick their hindpaw was significantly increased in animals treated with the selective MOR agonist morphine (10 mg/kg, p < 0.001) or the selective KOR agonist U50,488 (10 mg/kg, p < 0.01). Pretreatment with naltrexone (1 mg/kg) significantly blocked antinociception induced by both morphine (p < 0.001) and U50,488.
Figure 7.
Naltrexone (1 mg/kg) blocks both MOR and KOR according to tests of antinociception. Naltrexone pretreatment blocked the antinociceptive effects of the MOR agonist morphine (10 mg/kg, i.p.) and the KOR agonist U50,488 (10 mg/kg, i.p.). N = 6–7 per group. (**p < 0.01, ***p < 0.001 compared to saline + saline, &&&p < 0.001 compared to saline + morphine, #p < 0.05 compared to saline + U50).
3.5 Effect of BPN treatment on morphine-induced antinociception 24 h post-administration
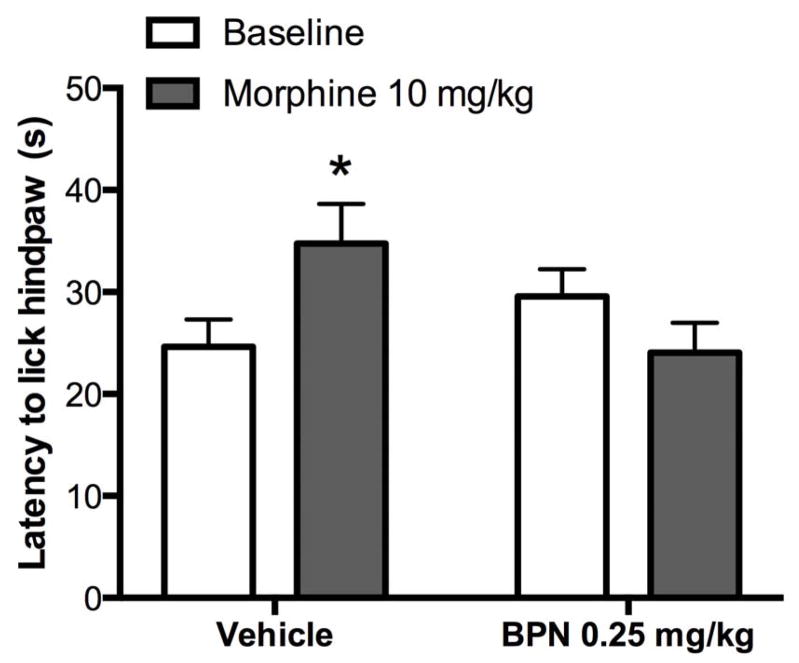
BPN’s activity at the MOR 24 h post-administration was determined using the hot plate test (Figure 8). Repeated measures two-way ANOVA revealed a significant BPN*morphine interaction in the latency for animals to lick their hindpaw [F1, 16 = 6.708, p < 0.05]. Post-hoc tests revealed that BPN (0.25 mg/kg) pretreatment had no effect on baseline latency values, but blocked morphine-induced antinociception compared to vehicle pretreated animals (p < 0.05).
Figure 8.
Pretreatment with BPN (0.25 mg/kg, i.p.) has no effect on baseline latency but blocks morphine (10 mg/kg, i.p.) -induced antinociception when tested 24 h after injection. (*p < 0.05 compared to vehicle ). N = 9 mice per group.
4. Discussion
Two important findings in this study provide information concerning the behavioral role of the opioid system in appetitive approach behavior and the pharmacological effects underlying BPN’s effects in the NIH test, a behavioral paradigm relevant to depression and anxiety. First, utilizing animals with genetic knockdown of individual opioid receptors, a critical role for the MOR in the behavioral effects of BPN on approach latency in the NIH test was established. Second, demonstrating that BPN acts as a MOR antagonist at the time of testing (24 h post-administration) in the NIH test critically supports the hypothesis that selective antagonism of the MOR is sufficient to alleviate the aversive effects of novelty on conditioned approach behavior for palatable food. Taken together, these data provide supportive preclinical evidence for the modulation of MOR activity as an integral component of rodent models of BPN’s therapeutic effects for depression and anxiety.
The present study is the first to show that genetic deletion of the MOR abolishes the behavioral effects of BPN in the NIH test. The MOR is known to be involved in mediating the reinforcing properties of rewarding stimuli. Oprm1−/− mice fail to show conditioned place preference for drugs of abuse (Matthes et al., 1996; Kieffer and Gaveriaux-Ruff, 2002) and exhibit reduced operant and hedonic responding for palatable food rewards (Papaleo et al., 2007). In the current study, Oprm1−/− mice displayed a marked delay in acquiring conditioned approach behavior towards the peanut butter chips during training sessions. However, these animals did eventually develop stable approach latencies, suggesting that attribution of incentive salience to palatable food can occur independently of MOR activity, possibly through adoption of other feeding-related pathways (Menzies et al., 2013). Oprm1−/− and Oprk1−/− mice did not significantly differ from WT mice in their final baseline approach latency in the novel arena, suggesting that the absence of opioid receptors per se does not mitigate the effects of novelty in this paradigm. Previous studies have reported increased behavioral resistance to stress in Oprk1−/− mice (Redila and Chavkin, 2008; Falcon et al., 2016) and reduced anxiogenic behavior in Oprm1−/− mice (Filliol et al., 2000; Ide et al., 2010). However, none have directly examined the effects of opioid receptor deletion in the NIH test. It is possible that a lack of hedonic value for food reward in Oprm1−/− mice may preclude observations of a baseline antidepressant effect in the NIH test. Thus, pharmacological modulation of MOR tone may influence motivational behavior in the NIH paradigm in a manner that cannot be directly replicated in the complete absence of the receptor. Interestingly, BPN’s effect on approach latency in the novel arena was more pronounced in Oprk1−/− compared to wildtype mice. We speculate that this may be due to compensatory upregulation of delta opioid receptors (Slowe et al., 1999), which have been shown to heterodimerize with MORs (Gomes et al., 2000).
Previous characterizations of the antinociceptive effects of BPN over time have shown it to rapidly activate the MOR at the onset of injection but this is followed by a period of prolonged blockade of the MOR lasting hours due to slow receptor dissociation (Cowan et al., 1977; Walker et al., 1995). In the present study we confirmed that pretreatment of BPN at the dose and time used in NIH testing antagonized the antinociceptive effects of morphine. Thus, the antagonism of MORs, as opposed to the brief early period of MOR activation, likely mediates the protracted effects of BPN in the NIH test. This proposition is further supported by our findings that treatment with the selective MOR antagonist cyprodime and blanket opioid antagonist naltrexone is sufficient to reduce approach latency in the NIH test, whereas morphine treatment was ineffective. To our knowledge, only one other study has investigated the effects of selective MOR antagonism in the NIH paradigm. Almatroudi et al. (2015) reported no behavioral effect of the irreversible MOR antagonist CCAM in the NIH test when administrated 48 h prior to testing. However, CCAM may more closely model genetic deletion of MORs rather than the competitive blockade of MOR activity by cyprodime described here. In line with our findings, the same study showed naltrexone to be effective in reducing approach latency in the novel arena. Intriguingly, treatment with the KOR antagonist nor-BNI was ineffective in reducing approach latency in the NIH test. Although this result aligned with our findings with KOR knockout mice, it is contradictory to other studies conducted in mice demonstrating behavioral responses to nor-BNI in the NIH test (Almatroudi et al., 2015; Huang et al., 2016). This discrepancy may be due in part to procedural differences in testing conditions, e.g., strain differences. Alternatively, KOR antagonism may have increased exploratory behavior rather than restore motivational salience for the food reward. Indeed, KOR antagonists have previously been shown to increase exploratory behaviors in the elevated plus maze and open field (Knoll et al., 2007; Bruchas et al., 2009; Wiley et al., 2009; Wittmann et al., 2009).
Given the well-established role of the MOR in mediating hedonic value, it may at first appear counterintuitive that pharmacological blockade of MORs reinstated incentive behavior for palatable food in the NIH paradigm. However, stress-induced activation of MORs in the ventral tegmental area (VTA) has been shown to reduce DA transmission in the nucleus accumbens (NAc) (Latagliata et al., 2014) and local administration of MOR antagonists into the VTA can increase striatal dopamine levels (Devine et al., 1993). Therefore, antagonism of MORs by cyprodime or BPN during stress may restore conditioned incentive behavior (i.e., approach) in the NIH paradigm by counteracting the inhibition of mesoaccumbens DA transmission in response caused by either the stress or anxiety components of the test (Robinson et al., submitted). On the other hand, MOR antagonism may prevent induction of anxiety-like behavior during exposure to novelty stress through alterations in amygdala neuronal activity. Local infusion of a MOR agonist into the central amygdala has been shown to produce anxiolytic effects in the elevated plus maze by reducing time spent in the open arms, whereas treatment with a MOR antagonist oppositely increased open arm time (Wilson and Junor, 2008). Moreover, drug-induced reductions in basolateral amygdala neuronal excitability have been linked with anxiolytic-like behavior in the NIH test (Gamble-George et al., 2016). Thus, activity at the MOR may potentially exert context-specific regulation of circuitries at the intersection of the stress response and reward processing, thereby promoting reward-seeking behavior under familiar conditions and inhibiting reward seeking under stressful conditions in the NIH paradigm.
5. Conclusions
In summary, these studies propose a novel mechanism of action for BPN’s antidepressant like effects in the novelty-induced hypophagia test, a conflict-based behavioral task. Utilizing genetic and pharmacological techniques, we systematically characterized the role of individual opioid receptors in mediating incentive behavior in the NIH paradigm. This work identified the MOR as a critical mediator of approach behavior in the NIH paradigm and a potential pharmacological target for alleviating prodepressive behaviors. These findings have practical significance for identifying neurobiological mechanisms underlying the effects of BPN related to emotional behaviors. BPN has been shown to treat depression in treatment-resistant patients (Bodkin et al., 1995; Nyhuis et al., 2008; Yovell et al., 2015) in small studies and opioid modulation by ALKS 5461, BPN combined with a MOR antagonist samidorphan to block BPN abuse potential, is under clinical trials (Ehrich et al., 2015). The present findings add to the literature by finding that MOR antagonism, in addition to KOR antagonism (Almatroudi et al., 2015; Falcon et al., 2016), may contribute to BPN’s behavioral effects on emotional behavior.
Highlights.
Oprm1−/− mice, but not Oprk1−/− mice, showed slower learning to approach palatable food.
Oprm1−/− mice, but not Oprk1−/− mice, failed to respond to buprenorphine in the novelty-induced hypophagia test
Buprenorphine and cyprodime antagonized mu opioid receptors
Buprenorphine and cyprodime reduced approach latency in the novelty induced hypophagia test
Acknowledgments
This research was supported by USPHS grants T32 MH14652, R01 MH86599 and R01 MH092412 from the National Institutes of Health. The funding sources had no involvement in the study design, data analysis or preparation of the report. We would like to acknowledge the assistance of Dr. Edgardo Falcon and Christian Cazares in conducting some of the behavioral experiments.
Footnotes
Conflict of interest statement
I.L. received research support from Orexigen. The other authors have no conflict to disclose. The research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Almatroudi A, Husbands SM, Bailey CP, Bailey SJ. Combined administration of buprenorphine and naltrexone produces antidepressant-like effects in mice. J Psychopharmacol. 2015;29:812–821. doi: 10.1177/0269881115586937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansinath M, Ramabadran K, Turndorf H, Puig MM. Effects of the benzomorphan kappa-opiate, MR 2266 and its (+) enantiomer MR 2267, on thermonociceptive reactions in different strains of mice. Neurosci Lett. 1990;117:212–217. doi: 10.1016/0304-3940(90)90146-z. [DOI] [PubMed] [Google Scholar]
- Bodkin JA, Zomberg GL, Lukas SE, Cole JO. Buprenorphine treatment of refractory depression. J Clin Psychopharmacol. 1995;15:49–57. doi: 10.1097/00004714-199502000-00008. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Quirion R, Meaney MJ. A comparison of the effects of diazepam versus several typical and atypical anti-depressant drugs in an animal model of anxiety. Psychopharmacology. 1989;97:277–279. doi: 10.1007/BF00442264. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology. 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- Browne CA, van Nest DS, Lucki I. Antidepressant-like effects of buprenorphine in rats are strain dependent. Behav Brain Res. 2015;278:385–392. doi: 10.1016/j.bbr.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Lemos JC, Chavkin C. CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PloS one. 2009;4:e8528. doi: 10.1371/journal.pone.0008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Therap. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I. Antidepressant-like effects of kappa-opioid receptor antagonists in Wistar Kyoto rats. Neuropsychopharmacology. 2010;35:752–763. doi: 10.1038/npp.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C, Kieffer BL, Befort K. Mu opioid receptor: a gateway to drug addiction. Curr Opin Neurobiol. 2004;14:370–378. doi: 10.1016/j.conb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Cowan A, Lewis JW, Macfarlane IR. Aognist and atagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol. 1977;60:537–545. doi: 10.1111/j.1476-5381.1977.tb07532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine DP, Leone P, Wise RA. Mesolimbic dopamine neurotransmission is increased by administration of mu-opioid receptor antagonists. Eur J Pharmacol. 1993;243:55–64. doi: 10.1016/0014-2999(93)90167-g. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29:771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Ehrich E, Turncliff R, Leigh-Pemberton R, Fernandez E, Jones R, Fava M. Evaluation of opioid modulation in major depressive disorder. Neuropsychopharmacology. 2015;40:1448–1455. doi: 10.1038/npp.2014.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon E, Maier K, Robinson SA, Hill-Smith TE, Lucki I. Effects of buprenorphine on behavioral tests for antidepressant and anxiolytic drugs in mice. Psychopharmacology. 2015;232:907–915. doi: 10.1007/s00213-014-3723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon E, Browne CA, Leon RM, Fleites VC, Sweeney R, Kirby LG, Lucki I. Antidepressant-like effects of buprenorphine are mediated by kappa opioid receptors. Neuropsychopharmacology. 2016;41:2344–51. doi: 10.1038/npp.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nature Gen. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Gamble-George JC, Baldi R, Halladay L, Kocharian A, Hartley N, Silva CG, Roberts H, Haymer A, Marnett LJ, Holmes A, Patel S. Cyclooxygenase-2 inhibition reduces stress-induced affective pathology. eLife. 2016;5:e14137. doi: 10.7554/eLife.14137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Yakovleva T, Aldrich JV, Tunis J, Parry C, Liu-Chen LY. Two short-acting kappa opioid receptor antagonists (zyklophin and LY2444296) exhibited different behavioral effects from the long-acting antagonist norbinaltorphimine in mouse anxiety tests. Neurosci Lett. 2016;615:15–20. doi: 10.1016/j.neulet.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide S, Sora I, Ikeda K, Minami M, Uhl GR, Ishihara K. Reduced emotional and corticosterone responses to stress in mu-opioid receptor knockout mice. Neuropharmacology. 2010;58:241–247. doi: 10.1016/j.neuropharm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer BL, Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Therap. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Lalanne L, Ayranci G, Kieffer BL, Lutz PE. The kappa opioid receptor: from addiction to depression, and back. Front Psychiat. 2014;5:170. doi: 10.3389/fpsyt.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latagliata EC, Valzania A, Pascucci T, Campus P, Cabib S, Puglisi-Allegra S. Stress-induced activation of ventral tegmental mu-opioid receptors reduces accumbens dopamine tone by enhancing dopamine transmission in the medial pre-frontal cortex. Psychopharmacology. 2014;231:4099–4108. doi: 10.1007/s00213-014-3549-7. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Cowan A. Buprenorphine: a unique drug with complex pharmacology. Curr Neuropharmacol. 2004;2:395–402. doi: 10.2174/1570159043359477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL. The multiple facets of opioid receptor function: implications for addiction. Curr Opin Neurobiol. 2013;23:473–479. doi: 10.1016/j.conb.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Therap. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Marki A, Monory K, Otvos F, Toth G, Krassnig R, Schmidhammer H, Traynor JR, Roques BP, Maldonado R, Borsodi A. Mu-opioid receptor specific antagonist cyprodime: characterization by in vitro radioligand and [35S]GTPgammaS binding assays. Eur J Pharmacol. 1999;383:209–214. doi: 10.1016/s0014-2999(99)00610-x. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior Activation of Kappa Opioid Receptors by U50,488 Mimics Repeated Forced Swim Stress to Potentiate Cocaine Place Preference Conditioning. Neuropsychopharmacology. 2006a;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social Defeat Stress-Induced Behavioral Responses are Mediated by the Endogenous Kappa Opioid System. Neuropsychopharmacology. 2006b;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies JR, Skibicka KP, Leng G, Dickson SL. Ghrelin, reward and motivation. Endocrine Development. 2013;25:101–111. doi: 10.1159/000346058. [DOI] [PubMed] [Google Scholar]
- Nyhuis PW, Gastpar M, Scherbaum N. Opiate treatment in depression refractory to antidepressants and electroconvulsive therapy. J Clin Psychopharmacol. 2008;28:593–595. doi: 10.1097/JCP.0b013e31818638a4. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Kieffer BL, Tabarin A, Contarino A. Decreased motivation to eat in mu-opioid receptor-deficient mice. Eur J Neurosci. 2007;25:3398–3405. doi: 10.1111/j.1460-9568.2007.05595.x. [DOI] [PubMed] [Google Scholar]
- Redila VA, Chavkin C. Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology. 2008;200:59–70. doi: 10.1007/s00213-008-1122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- Slowe SJ, Simonin F, Kieffer B, Kitchen I. Quantitative autoradiography of mu-, delta- and kappa1 opioid receptors in kappa-opioid receptor knockout mice. Brain Res. 1999;818:335–345. doi: 10.1016/s0006-8993(98)01201-3. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. PNAS. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker EA, Zering G, Woods JH. Buprenorphine antagonism of mu opioids in the rhesus monkey tail-withdrawal procedure. J Pharmacol Exp Therap. 1995;273:1345–1352. [PubMed] [Google Scholar]
- Wiley MD, Poveromo LB, Antapasis J, Herrera CM, Bolanos Guzman CA. Kappa-opioid system regulates the long-lasting behavioral adaptations induced by early-life exposure to methylphenidate. Neuropsychopharmacology. 2009;34:1339–1350. doi: 10.1038/npp.2008.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Junor L. The role of amygdalar mu-opioid receptors in anxiety-related responses in two rat models. Neuropsychopharmacology. 2008;33:2957–2968. doi: 10.1038/sj.npp.1301675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann W, Schunk E, Rosskothen I, Gaburro S, Singewald N, Herzog H, Schwarzer C. Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacology. 2009;34:775–785. doi: 10.1038/npp.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Ohdo S, Takane H, Tomiyoshi Y, Matsuo A, Yukawa E, Higuchi S. Chronopharmacology of analgesic effect and its tolerance induced by morphine in mice. J Pharmacol Exp Therap. 2003;305:1200–1205. doi: 10.1124/jpet.103.049031. [DOI] [PubMed] [Google Scholar]
- Yovell Y, Bar G, Mashiah M, Baruch Y, Briskman I, Asherov J, Lotan A, Rigbi A, Panksepp J. Ultra-low-dose buprenorphine as a time-limited treatment for severe suicidal ideation: A randomized controlled trial. Am J Psychiatr. 2015;173:491–498. doi: 10.1176/appi.ajp.2015.15040535. [DOI] [PubMed] [Google Scholar]