Abstract
Physical activity (PA) and vitamin D are thought to affect colorectal cancer prognosis. The present study investigates associations between 25(OH)D3 and PA in prospectively followed colorectal cancer patients in the ColoCare study.
At 6, 12 and 24 months after surgery patients donated a blood sample, wore an accelerometer for 10 consecutive days and completed a PA questionnaire. Plasma 25(OH)D3 levels were measured by high performance liquid chromatography. We tested associations using partial correlations and multivariate linear regression analysis, adjusted for season, age and BMI. A total of 137 assessments of 25(OH)D3 levels and physical activity were conducted (58 at 6 months, 51 at 12 months and 28 at 24 months). More than 60% of the patients were vitamin D deficient (25(OH)D3 ≤20ng/mL), independent of study time point. At 6 months follow-up, accelerometry-based vigorous and moderate-to-vigorous physical activity were positively associated with 25(OH)D3 levels (P=0.04; P=0.006,). Physical activity together with season were significant predictors of elevated 25(OH)D3 levels.
Our results suggest that the majority of colorectal cancer patients may suffer from vitamin D deficiency. Engaging in physical activity may be an effective approach to increase their 25(OH)D3 levels.
Keywords: vitamin D, 25(OH)D3, accelerometry, physical activity, colorectal cancer
Introduction
Vitamin D (cholecalciferol) is a prohormone that can be synthesized in the skin by UV-B radiation or taken up via food, especially fortified dairy products and fish or meat (1, 2). 25-hydroxyvitamin D3 (25(OH)D)3 is the major circulating form of vitamin D and is regarded as a reliable surrogate marker for vitamin D status (3, 4). Vitamin D has been associated with decreased risk or improved survival for a number of cancers such as breast, prostate, and colon cancer (5–10). McDonnell et al. reported 25(OH)D levels of ≥40ng/mL to be associated with a substantial risk reduction for invasive cancers in general (11). A consensus statement by the Institute of Medicine (12) and a more recent report by the American Geriatrics Society Workgroup (AGSW) on vitamin D supplementation (13) defined serum 25(OH)D3 levels of ≤ 20 ng/mL (50 nmol/L) as deficient. The AGSW further determined vitamin D insufficiency as serum levels between 21 and 29 ng/mL (72 nmol/L) and levels of ≥30 ng/mL (75 nmol/L) as sufficient (13).
Colorectal cancer is the third most common cancer in men and the second most common cancer diagnosed in women worldwide (14). Findings from observational studies suggest that higher vitamin D levels (measured as 25(OH)D3) may be beneficial for colorectal cancer prognosis. A study conducted in Norway observed 20–30% lower death rates among colon cancer patients diagnosed in autumn, when 25(OH)D3 levels were higher, compared with patients diagnosed in Winter months, with lower 25(OH)D3 (15). Ng et al. investigated the influence of 25(OH)D3 levels at least 2 years prior to diagnosis on mortality in 304 colorectal cancer patients from the Nurses’ Health Study and the Health Professionals Follow-Up Study (16). Their results showed a significantly lower mortality among patients with higher 25(OH)D3 levels. Patients in the highest quartile had an adjusted hazard ratio (HR) of 0.52 (95% confidence interval (CI), 0.29 to 0.94) for overall mortality compared to patients in the lowest quartile, while a non-significant trend was reported for colorectal-cancer-specific mortality. A meta-analysis investigating the associations between serum 25(OH)D3 levels and survival in colorectal and breast cancer patients reported significantly reduced mortality for patients with sufficient levels (>75 nmol/L) (17). Specifically, elevated levels of circulating 25(OH)D3 were associated with a decreased risk of dying from any cause by 29% and the risk of colorectal-cancer-specific mortality by 35%.
Due to the close relationship between plasma 25(OH)D3 concentrations in blood and UV-B exposure, physical activity, especially outdoors, is strongly associated with increased 25(OH)D3 levels. For example, the Third National Health and Nutrition Examination Survey (NHANES) showed, that mean 25(OH)D3 levels increased with rising frequency of indoor and outdoor physical activity levels compared to inactive participants; outdoor leisure physical activity was significantly associated with higher 25(OH)D3 levels compared to inactive participants (p=0.0005) (18). Jaaskelainen et al. reported similar findings with positive associations between serum 25(OH)D3 concentrations and leisure-time physical activity, especially outdoor activities (19). However, not only sun exposure, but physical activity itself may influence 25(OH)D3 levels. As suggested by Abboud et al. skeletal muscles may serve as an extravascular 25(OH)D3 storage and physical exercise is suggested to promote 25(OH)D3 release from skeletal muscles (20, 21). This is in line with the observation that elderly individuals who do not engage in vigorous physical activity have a threefold increase in risk (95% CI= 1.0–9.7) for marginal 25(OH)D3 deficiency compared to elderly with some vigorous physical activity (22). Thus, it is not entirely clear, whether elevated 25(OH)D3 levels are simply a biomarker for physical activity. This is of relevance for studies of colorectal cancer prognosis, because higher levels of physical activity themselves have been consistently associated with improved survival among colorectal cancer patients. Conversely, physical activity may serve as a potential biological mechanism to explain the observed associations with elevated 25(OH)D3 levels.
The aim of this study was to examine 25(OH)D3 levels among colorectal cancer patients of the prospective cohort study ColoCare, at 6, 12 and 24 months post-surgery and to investigate the associations between 25(OH)D3 levels with physical activity assessed by accelerometry and questionnaires.
Methods
The ColoCare study
The present study is nested in the prospective ColoCare study (ClinicalTrials.gov Identifier: NCT02328677), an international cohort of newly-diagnosed stage I-IV colorectal cancer patients (ICD-10 C18-C20). The ColoCare Consortium is a multicenter initiative of interdisciplinary research on colorectal cancer outcomes and prognosis, and comprises patients recruited at the Fred Hutchinson Cancer Research Center, Seattle (Washington, USA), the National Center for Tumor Diseases, Heidelberg (Germany), the H. Lee Moffitt Cancer Center and Research Institute, Tampa (Florida, USA) and the Huntsman Cancer Institute, Salt Lake City (Utah, USA). ColoCare inclusion criteria are: patients first-diagnosed with colon or rectal cancer (stages I–IV), age >18 years, English (US sites) or German (German site)-speaking, and mentally/physically able to consent and participate. Subjects meeting the inclusion criteria are recruited to the ColoCare study prior to tumor surgery. Baseline examination includes anthropometric measurements, biospecimens collection (blood, urine, feces, and fresh frozen tissue), and self-administered questionnaires on symptoms, health behaviors and health-related quality-of-life. Participants are followed-up (1) passively by retrieving medical data from hospital records, and (2) actively at 3, 6, 12, 24 and 36 months post-surgery with collection of blood, stool, urine and questionnaires on symptoms, health behaviors, health-related quality-of-life, and dietary assessment by food frequency questionnaire. All analyses in this manuscript are based on data collected between 2010 and 2014 at the ColoCare site in Heidelberg, Germany.
Physical activity was assessed by accelerometry, pedometry and by standardized questionnaires. Accelerometers and pedometers were offered to participants as an optional study assessment at 6, 12 and 24 months post-diagnosis. During the initial phase of the physical activity assessment (September 2011 to July 2012), only participants invited to the NCT in Heidelberg for their follow-up visit were invited to participate. Subsequently, (July 2012 to February 2014), participants were also invited to take part in the physical activity assessment, either in person or via telephone if their follow-up was conducted at their local general practitioners. The last chemotherapy cycle had to be finished at least 2 weeks before each follow-up visit for a patient to be eligible to participate. Implementation and feasibility of the physical activity assessment has been published (23).
The present study included 137 physical activity assessments by accelerometry from 102 patients enrolled in the ColoCare study site in Heidelberg, with some participants taking part multiple times at their different follow-up time points. The study was approved by the Ethics Committee of the University of Heidelberg and all participants provided written informed consent.
Physical activity data collection
The Actigraph GT3x+ (Actigraph, Pensacola, FL) accelerometers are small, light-weight devices that assess physical activity in three axes. It can record raw acceleration data, activity counts, step counts, energy expenditure, amount of sleep, and has an integrated light sensor. The device was attached on an elastic belt and patients were instructed to wear it below their chests (23). The chest was chosen as attachment site in order not to interfere with the surgical scars or stomas. Data were collected in 30 Hz intervals. Raw data were downloaded and processed using the ActiLife software (ActiGraph, Pensacola, FL, version 6.6.3). Accelerometer data were then summed up into 10 second epochs. Data were considered valid if the devices were worn for at least 4 days and for at least 6 hours per day. Non-wear time was defined as at least 60 minutes of consecutive zero counts with a 2 minute interruption tolerance (23). A modified version of the Community Healthy Activities Model Program For Seniors (CHAMPS) Physical Activity Questionnaire for Older Adults was used to subjectively assess participants’ physical activity at 6, 12 and 24 months post-surgery (24). This questionnaire assesses quantity (frequency and hours per week) and type of activity during the past four weeks. The CHAMPS questionnaire was validated in English and Spanish language (24). For this study we translated the questionnaire into German.
Blood processing and 25(OH)D3 assays
Blood samples (EDTA) were taken from patients at 6, 12 and 24 months post- surgery at the NCT or taken at the patient’s general practitioners and shipped on ice overnight. Preparation of plasma was performed within 4h after blood-draw by retaining supernatant after centrifugation (2500g; 15min) and was stored in aliquots at −80°C. Plasma levels of 25(OH)D3 were assessed by reverse-phase high performance liquid chromatography (HPLC) in the Biochemistry and Biomarkers Laboratory of the Division of Preventive Oncology, German Cancer Research Center, DKFZ (Dr. Owen). The assay was run including 5% blinded duplicate samples. In addition, two internal laboratory controls (high and low concentrations) were included within each batch. The intra-assay and inter-assay coefficients of variation (CV) of 25(OH)D3 at concentrations of 50ng/mL were 2.3% and 3.1% respectively.
Data analysis
Accelerometry and questionnaire data on physical activity were calculated as minutes of total, light, moderate, vigorous and moderate to vigorous (MVPA) per week. Cut-points for the different physical activity levels were defined as follows: light activity (100–1,951 counts per minute), moderate activity (1,952–5,724 counts per minute), vigorous activity (5,725–20,000 counts per minute) and moderate-to-vigorous activity (1,952–20,000 counts per minute) (23, 25). Accelerometry data exceeding 20,000 counts per minute were considered spuriously high data and, thus, excluded from analyses [21]. Moderate-to-vigorous physical activity included both moderate and vigorous physical activities. To obtain minutes per week in the different intensity classes, the total amount of minutes in the respective physical activity intensity was divided by the number of measurement days and then multiplied by 7.
Seasons were categorized as Spring (March, April, May), Summer (June, July, August), Fall (September, October, November) and Winter (December, January, February). To account for the small sample sizes within each season, we combined Summer and Fall, and Winter and Spring (n=73 in Summer/Fall, n=64 in Winter/Spring). 25(OH)D3 levels were comparable between Summer and Fall (20.71 ng/mL and 17.53 ng/mL, respective medians) and Winter and Spring (12.44 ng/mL and 10.59 ng/mL, respective medians). 25(OH)D3 categories were determined as deficient (≤20 ng/mL), insufficient (21–<30 ng/mL) and sufficient (≥30 ng/mL), based on a consensus statement by the American Geriatrics Society Workgroup on vitamin D supplementation and the opinion of international experts (13).
Differences in 25(OH)D3 levels across seasons at each follow-up time point were determined using the Kruskal Wallis Test, which accounted for skewed distributions. This test was also performed to compare 25(OH)D3 levels in patients, who received adjuvant chemotherapy versus those who did not, at the 6 and 12 month time points.
Spearman partial correlations were calculated to assess the relationship between minutes per week of physical activity at each intensity, daily minutes spent in outdoor physical activity during the past 2 months and 25(OH)D3 levels accounting for season, body mass index (BMI; kg/m2), age and sex. For the 24 month time point correlations without adjustment were calculated, because of the small sample size. For the multivariate linear regression analysis, where necessary, variables were square-root transformed to examine the associations between 25(OH)D3 levels and age, sex, season, BMI, moderate and vigorous physical activity. All statistical tests were two-tailed and statistical significance was set at the p<0.05 level. Statistical analyses were performed using SAS (version 9.3, SAS Institute Cary, North Carolina).
Results
One hundred and fifty-six physical activity measurements at 6 (n=58), 12 (n=51) or 24 (n=28) follow-up time points were completed with at least 4 days of wear time. Participant characteristics by each follow-up time point are shown in Table 1. At each time point, mean age was > 60 years, mean BMI was in the overweight range, and over 60% of participants were male. Twelve percent of participants at the 6 month follow-up time point met the recommendations to engage in at least 150 minutes of at least moderate physical activity in bouts of at least 10 minutes per week. At the 12 month time point 14% of the participants met the recommendations and at the 24 month time point 12% of participants. Blood was collected at 137 time points because collections were not feasible due to bad vein conditions in 19 cases, predominantly a result of chemotherapy. The mean level of measured 25(OH)D3 was 17.51 ng/mL at 6 months, 17.80 at 12 months and 18.11 ng/mL at 24 months.
Table 1.
Study patient characteristics at follow-up time points
| Study patient characteristics
| |||
|---|---|---|---|
| Characteristics N=137 |
6 months follow-up N=58 |
12 months follow-up N=51 |
24 months follow-up N=28 |
| Sex | |||
| Male | 37 (64%) | 35 (69%) | 20 (71%) |
| Female | 21 (36%) | 16 (31%) | 8 (29%) |
| Age (years) | 60.4 ± 13.1 | 61.3 ± 11.6 | 63.0 ± 11.6 |
| BMI (kg/m2) | 25.3±3.5 | 26.1±3.2 | 27.7±4.1 |
| Stage | |||
| 0+I | 7 (12%) | 9 (18%) | 4 (14%) |
| II | 18 (31%) | 16 (31%) | 11 (39%) |
| III | 18 (31%) | 15 (29%) | 11 (39%) |
| IV | 6 (10%) | 4 (7%) | 1 (4%) |
| Unknown | 9 (16%) | 7 (14%) | 1 (4%) |
| Previous adjuvant chemotherapy (n, %) | 15 (26%) | 16 (31%) | 7 (25%) |
| 25(OH)D3 (ng/mL) | 17.51 ± 10.63 | 17.80 ± 11.55 | 18.11 ± 8.80 |
| Deficient status, <20ng/mL, (n, %) | 38 (66%) | 34 (67%) | 17 (61%) |
| Total PA/week (mins) | |||
| Accelerometer | 1,320 ± 387 | 1,304 ± 399 | 1,268 ± 343 |
| Questionnaire | 551 ± 384 | 530 ± 505 | 596 ± 406 |
| Light PA/week (mins) | |||
| Accelerometer | 1,082 ± 299 | 1,056 ± 321 | 1,056 ± 281 |
| Questionnaire | 168 ±158 | 68 ± 112 | 74 ± 119 |
| Moderate PA/week (mins) | |||
| Accelerometer | 222 ± 156 | 236 ± 165 | 197 ± 131 |
| Questionnaire | 325 ± 266 | 246 ± 285 | 249 ± 182 |
| Vigorous PA/week (mins) | |||
| Accelerometer | 16 ± 47 | 12 ± 23 | 15 ± 39 |
| Questionnaire | 27 ± 95 | 50 ± 102 | 23 ± 68 |
| Moderate-to-vigorous PA/week (mins) | |||
| Accelerometer | 238 ± 176 | 248 ± 165 | 212 ± 148 |
| Questionnaire | 352 ± 255 | 296 ± 324 | 272 ± 182 |
Values are means ± SD
Abbreviations: BMI, body mass index; PA, physical activity; mins, minutes; MVPA, moderate-to-vigorous physical activity
Figure 1 shows 25(OH)D3 status by time points. Within each follow-up time point more than 60% of the participants were vitamin D deficient (25(OH)D3≤20ng/mL) and 16% or fewer were vitamin D sufficient (25(OH)D3≥30ng/mL). Figure 2 shows the 25(OH)D3 status by season (Summer/Fall vs. Winter/Spring) within the three follow-up time points. At 6 and 12, but not at 24 months there were statistically significant differences in 25(OH)D3 levels between Summer/Fall and Winter/Spring (p=0.04, p=0.004, respectively). At both 6 and 12 months post-surgery there were no statistically significant differences in 25(OH)D3 levels between participants who received adjuvant chemotherapy and those who did not (yes vs. no: 17.22 ng/mL vs. 13.55 ng/mL and 14.21 ng/mL vs. 14.85 ng/mL, respective medians). At 6 months post-surgery, we observed significant positive correlations between accelerometry-derived moderate-to-vigorous and vigorous physical activity with 25(OH)D3 levels after adjustment for season, BMI and age (ρ=0.27, P=0.04; ρ=0.37, P=0.006, respectively) (Table 2). A trend for a positive correlation between moderate physical activity and 25(OH)D3 levels (ρ=0.24, p=0.08) was also observed. No statistically significant correlations between 25(OH)D3 levels and accelerometry-derived physical activity were seen at 12 or 24 months post-surgery or with questionnaire-derived physical activity at any time point.
Figure 1.
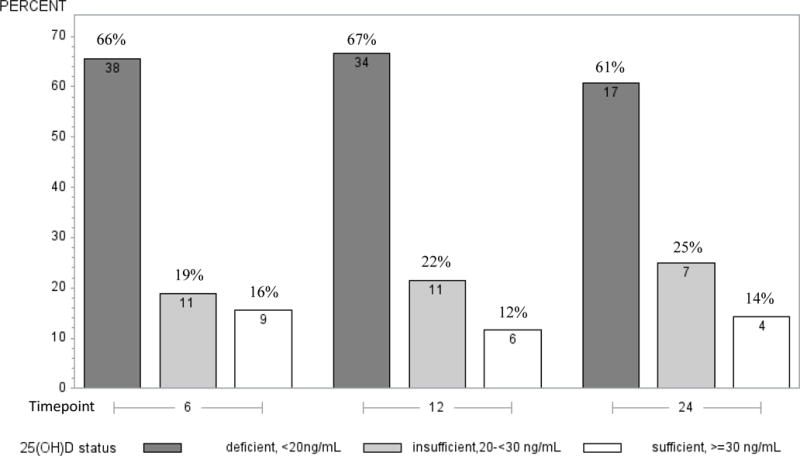
Description of 25(OH)D3 status by time points according to deficiency definitions by the American Geriatrics Society Workgroup on vitamin D supplementation
Figure 2.
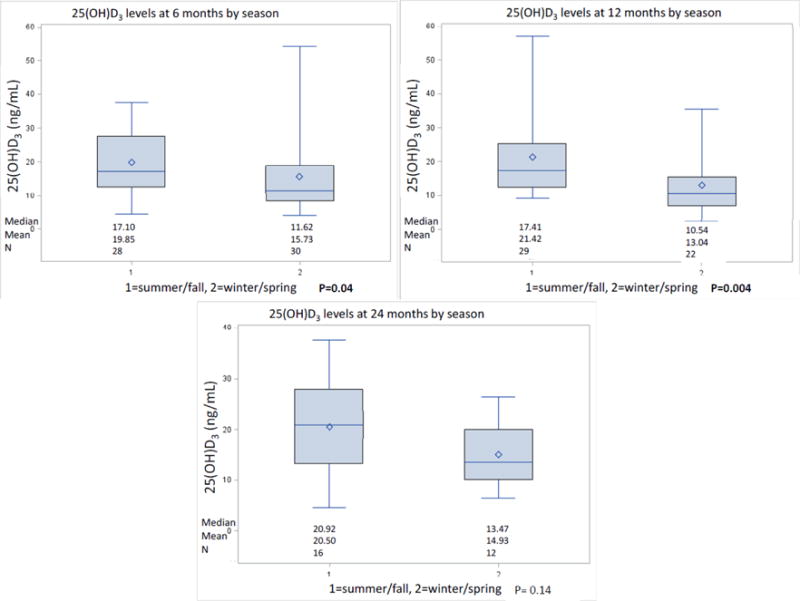
Differences in 25(OH)D3 levels by season at each time point
Table 2.
Partial correlation coefficients between 25(OH)D3 levels (ng/mL) and weekly minutes of physical activity, including participation in outdoor physical activity within the past 2 months, derived from accelerometry and questionnaires, adjusted for season, age and BMI.
| Spearman correlation coefficient | P-value | |
|---|---|---|
| 6 months n=58 | ||
|
| ||
| Accelerometry | ||
| Light PA (min) | 0.05 | 0.74 |
| Moderate PA (min) | 0.23 | 0.08 |
| Vigorous PA (min) | 0.37 | 0.006 |
| Moderate-to-vigorous PA (min) | 0.27 | 0.04 |
| Total PA (min) | 0.17 | 0.22 |
| Steps | 0.15 | 0.29 |
| Questionnaire | ||
| Light PA (min) | 0.13 | 0.33 |
| Moderate PA (min) | −0.15 | 0.27 |
| Vigorous PA (min) | 0.08 | 0.56 |
| Moderate-to-vigorous PA (min) | −0.15 | 0.27 |
| Total PA (min) | 0.01 | 0.92 |
| Outdoor PA (min) n=19* | 0.11 | 0.68 |
|
12 months n=51 | ||
| Accelerometry | ||
| Light PA(min) | −0.09 | 0.57 |
| Moderate PA (min) | 0.08 | 0.60 |
| Vigorous PA (min) | 0.12 | 0.44 |
| Moderate-to-vigorous PA (min) | 0.06 | 0.68 |
| Total PA (min) | −0.01 | 0.96 |
| Steps | −0.08 | 0.59 |
| Questionnaire | ||
| Light PA(min) | 0.13 | 0.38 |
| Moderate PA (min) | 0.09 | 0.53 |
| Vigorous PA (min) | 0.03 | 0.83 |
| Moderate-to-vigorous PA (min) | 0.18 | 0.22 |
| Total PA (min) | 0.17 | 0.26 |
| Outdoor PA (min) n=22* | 0.35 | 0.15 |
|
24 months n=28** | ||
| Accelerometry | ||
| Light PA(min) | 0.21 | 0.29 |
| Moderate PA (min) | 0.04 | 0.83 |
| Vigorous PA (min) | 0.001 | 0.99 |
| Moderate-to-vigorous PA (min) | 0.02 | 0.92 |
| Total PA (min) | 0.09 | 0.66 |
| Steps | 0.20 | 0.31 |
| Questionnaire | ||
| Light PA (min) | −0.16 | 0.42 |
| Moderate PA(min) | −0.21 | 0.28 |
| Vigorous PA (min) | 0.05 | 0.81 |
| Moderate-to-vigorous PA (min) | −0.17 | 0.38 |
| Total PA (min) | 0.02 | 0.94 |
data not available for all participants and time points, because outdoor PA question was added subsequently to the ColoCare questionnaire
no adjustments due to the small sample size, no evaluation of outdoor PA
Abbreviations: PA, physical activity; min, minutes
Multiple linear regression analyses are shown in Table 3. The results suggest, that season and accelerometry-derived minutes per week of vigorous physical activity were significantly associated with 25(OH)D3 levels at 6 months (β=0.18, 95% CI 0.07 to 0.29). At 12 months the only significant association with 25(OH)D3 levels was season (β=−1.14, 95%CI −1.82 to −0.46).
Table 3.
Multivariate linear regression for square-root-transformed 25(OH)D3 concentrations with season, BMI and weekly minutes of accelerometry-derived physical activity.
| Variable | β-estimate, standard error | p-value | 95% confidence interval |
|---|---|---|---|
|
6 months n=58
| |||
| Intercept | +2.27 ±0.73 | 0.003 | +0.82 to 3.73 |
|
| |||
| Age | +0.02±0.01 | 0.22 | −0.01 to 0.04 |
|
| |||
| Sex | +0.23±0.34 | 0.51 | −0.46 to 0.91 |
| BMI (kg/m2) | +0.04 ±0.05 | 0.47 | −0.07 to 0.14 |
| Moderate PAa) | +0.01 ±0.03 | 0.76 | −0.06 to 0.08 |
| Vigorous PAb) | +0.18 ±0.05 | 0.002 | +0.07 to 0.29 |
| Seasonc) | −0.71 ±0.31 | 0.02 | −1.33 to −0.10 |
|
| |||
|
12 months n=51
| |||
| Intercept | +4.18 ±0.77 | <0.0001 | +2.64 to 5.73 |
|
| |||
| Age | −0.00012±0.02 | 0.97 | −0.03 to 0.03 |
|
| |||
| Sex | −0.72±0.38 | 0.07 | −1.49 to 0.57 |
| BMI (kg/m2) | −0.08 ±0.05 | 0.13 | −0.19 to 0.03 |
| Moderate PAa) | +0.04 ±0.04 | 0.33 | −0.04 to 0.13. |
| Vigorous PAb) | −0.02 ±0.09 | 0.84 | −0.20 to 0.16 |
| Seasonc) | −1.14 ±0.34 | 0.002 | −1.82 to −0.46 |
|
| |||
|
24 months n=28
| |||
| Intercept | +3.25 ±0.94 | 0.002 | +1.31 to 5.19 |
|
| |||
| Age | −0.02±0.02 | 0.38 | −0.07 to 0.03 |
|
| |||
| Sex | −0.59±0.55 | 0.29 | −1.74 to 0.55 |
| BMI (kg/m2) | −0.01 ±0.05 | 0.85 | −0.12 to 0.10 |
| Moderate PAa) | +0.02 ±0.07 | 0.80 | −0.12 to 0.15 |
| Vigorous PAb) | −0.04 ±0.09 | 0.67 | −0.22 to 0.15 |
| Seasonc) | −0.48 ±0.48 | 0.33 | −1.49 to 0.53 |
Minutes of moderate physical activity measured by accelerometry, square-root transformed
Minutes of vigorous physical activity measured by accelerometry, square-root transformed
1=summer/fall, 2=winter/spring
Variable features: BMI, moderate-to-vigorous PA, moderate PA: continuous; Season: binary
Abbreviations: min, minutes; BMI, body mass index; kg, kilogram; PA, physical activity; MVPA, moderate-to-vigorous physical activity
Discussion
In this study, only 16% of the participants had sufficient 25(OH)D3 levels, 33% were characterized as vitamin D insufficient, and 60% were vitamin D deficient. At each timepoint, only 12–14% met the physical activity recommendations of engaging in at least 150 minutes of MVPA per week. Physical activity and season were associated with significantly higher 25(OH)D3 levels in colorectal cancer patients, although the associations were not as strong as in healthy populations (18, 19).
The majority of colorectal cancer patients at all three follow-up time points were characterized by a deficient vitamin D status based on cut points defined by most experts, including the American Geriatrics Society Workgroup on vitamin D supplementation(13). Similar results were also reported in a review investigating the prevalence of vitamin D deficiency and insufficiency in cancer patients with a prevalence of vitamin D deficiency of over 70% (26). Within the three follow-up time points in this study, 25(OH)D3 levels were significantly higher in Summer/Fall compared to those in Winter/Spring. Because only a limited proportion of vitamin D originates from dietary intake, especially fortified dairy products and fatty fish intake (which are both not commonly consumed in Germany) (27, 28), the subcutaneous synthesis of vitamin D through UV-B exposure plays a significant role in achieving adequate vitamin D levels (3). The seasonal variation of 25(OH)D3 levels seen in this study is consistent with our prior study (29) and most likely based on the fact that UV-B exposure in the Spring and Summer months is more frequent compared to the Winter months. Although we observed seasonal variations, 25(OH)D3 levels stayed low over time. 25(OH)D3 is involved in expression of genes affecting the regulation of angiogenesis, proliferation, differentiation and apoptosis. In addition, the vitamin D receptor (VDR) is abundantly present in intestinal epithelial cells of the colon (30–33). This leads to the hypothesis, that vitamin D plays an important role in the development of colorectal cancer. However, its role in colorectal cancer incidence and survival is not well understood.
In a pooled analysis with over 2,000 study participants the authors reported a statistically significant association between 25(OH)D3 levels of ≥30 ng/mL and recurrence of ≥3 colorectal adenomas with an odds ratio of 1.68 (1.09–2.58 95% CI) (34). However, a recent clinical trial of vitamin D supplementation did not show any reduced risk of adenoma recurrence (35). The authors of a meta-analysis of prospective cohort studies reported associations of higher levels of 25(OH)D3 with a decreased risk for mortality in colorectal cancer patients (17). Alternatively, in a systematic review on the effect of 25(OH)D3 levels on non-skeletal health outcomes, Autier and colleagues did not observe any beneficial effect of vitamin D supplementation on cancer, diabetes or cardiovascular disease incidences, severity or clinical course. They concluded that low 25(OH)D3 may be a marker for diseases rather than a cause (36). In a review by Cannell et al. the authors report that in 6 out of 7 randomized controlled trials vitamin D3 supplementation resulted in significantly lowered levels of inflammatory markers (37). Based on that observations, low levels of 25(OH)D3 may be seen as a cause rather than a marker for disease. Thus, future studies are needed to determine whether 25(OH)D3 by itself is bioactive and involved in carcinogenesis or whether it is a surrogate for healthy behavior, specifically physical activity.
In general we observed positive correlations between physical activity and 25(OH)D3; at the 6 month follow-up, correlations between 25(OH)D3 levels and accelerometry-derived moderate-to-vigorous and vigorous physical activity (ρ=0.27, P=0.04, ρ=0.37, P=0.006, respectively) were significant. This association was also retained for vigorous physical activity in the multivariate model (β=0.18, 95% CI 0.07 to 0.29). Previous studies among a diverse range of healthy individuals have consistently observed associations between physical activity and 25(OH)D3 levels with correlations in the range of 0.40 (18, 38–40) (5). De Rui and colleagues observed a 50% reduction of the likelihood of inadequate vitamin D levels in elderly Caucasians engaging in cycling or gardening (41). The associations reported may be attributable to sun exposure during physical activity. Nevertheless, some investigations reported persisting relationships between vigorous physical activity and vitamin D even after adjusting for sun exposure (22, 40). No correlations between 25(OH)D3 levels and questionnaire-derived data on physical activity were observed. This could be explained by the fact, that questionnaires collect subjective data which are liable to recall bias (42, 43). Furthermore, the majority of participants did not report any vigorous physical activity in their questionnaires, whereas the accelerometers measured vigorous physical activity in nearly all patients, although in small amounts (Table 1).
To our knowledge, this is the first study to repeatedly assess 25(OH)D3 levels over time in colorectal cancer patients and to assess associations between 25(OH)D3 levels and physical activity levels. A further strength of this study is the objective assessment of physical activity by accelerometry. However, no information on whether the measured activities were performed indoors or outdoors was available and neither were the individual UV-B exposures. We recognize that the sample size at the 24 month time point was limited, which may have precluded us from observing statistical significant associations.
We observed that a large proportion of colorectal cancer patients had deficient or insufficient 25(OH)D3 levels over extended periods of cancer survivorship. As expected, 25(OH)D3 levels varied by season. 25(OH)D3 levels were significantly related to accelerometry-based physical activity 6 months after surgery. Although, the associations among the colorectal cancer patients studied here, were less prominent than those observed in healthy individuals (18, 19), 25(OH)D3 levels were significantly correlated with physical activity at 6 months. These results suggest that 25(OH)D3 is, to some extent, a marker of moderate-to-vigorous physical activity in colorectal cancer patients. However, the frequently observed improved survival of colorectal cancer patients with higher physical activity may also be mediated in parts by the biologic effects of 25(OH)D3 on gene expression affecting the regulation of angiogenesis, proliferation, differentiation, and apoptosis.
Acknowledgments
The authors thank all ColoCare study participants, the entire ColoCare study team, and David Liesenfeld, who assisted in recruitment for the physical activity assessment. Furthermore, we would like to thank Dr. Yesilda Balavarca for her statistical advice.
Funding: This study was funded by the Lackas Foundation, the Division of Preventive Oncology (Prof. Dr. Ulrich) and the German Consortium of Translational Cancer Research.
Disclosure of funding
German Consortium of Translational Cancer Research (DKTK) and Division of Preventive Oncology Funding (Dr. Ulrich)
Footnotes
Compliance with ethical standards
Conflict of interest:
The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the ethics committee Heidelberg and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689S–96S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 2.Crowe FL, Steur M, Allen NE, Appleby PN, Travis RC, et al. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: results from the EPIC-Oxford study. Public Health Nutr. 2011;14:340–6. doi: 10.1017/S1368980010002454. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 4.Seamans KM, Cashman KD. Existing and potentially novel functional markers of vitamin D status: a systematic review. Am J Clin Nutr. 2009;89:1997S–2008S. doi: 10.3945/ajcn.2009.27230D. ajcn.2009.27230D [pii]10.3945/ajcn.2009.27230D. [DOI] [PubMed] [Google Scholar]
- 5.Wanner M, Richard A, Martin B, Linseisen J, Rohrmann S. Associations between objective and self-reported physical activity and vitamin D serum levels in the US population. Cancer Causes Control. 2015;26:881–91. doi: 10.1007/s10552-015-0563-y. [DOI] [PubMed] [Google Scholar]
- 6.Ma Y, Zhang P, Wang F, Yang J, Liu Z, et al. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol. 2011;29:3775–82. doi: 10.1200/JCO.2011.35.7566. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert R, Metcalfe C, Fraser WD, Donovan J, Hamdy F, et al. Associations of circulating 25-hydroxyvitamin D with prostate cancer diagnosis, stage and grade. Int J Cancer. 2012;131:1187–96. doi: 10.1002/ijc.27327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–91. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 9.Bolland MJ, Grey A, Gamble GD, Reid IR. Calcium and vitamin D supplements and health outcomes: a reanalysis of the Women’s Health Initiative (WHI) limited-access data set. Am J Clin Nutr. 2011;94:1144–9. doi: 10.3945/ajcn.111.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant WB. 25-hydroxyvitamin D and breast cancer, colorectal cancer, and colorectal adenomas: case-control versus nested case-control studies. Anticancer Res. 2015;35:1153–60. [PubMed] [Google Scholar]
- 11.McDonnell SL, Baggerly C, French CB, Baggerly LL, Garland CF, et al. Serum 25-Hydroxyvitamin D Concentrations >/=40 ng/ml Are Associated with >65% Lower Cancer Risk: Pooled Analysis of Randomized Trial and Prospective Cohort Study. PLoS One. 2016;11:e0152441. doi: 10.1371/journal.pone.0152441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): 2011. [Google Scholar]
- 13.American Geriatrics Society Workgroup on Vitamin D. Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for Prevention of Falls and Their Consequences. J Am Geriatr Soc. 2014;62:147–52. doi: 10.1111/jgs.12631. [DOI] [PubMed] [Google Scholar]
- 14.Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 15.Moan J, Porojnicu AC, Robsahm TE, Dahlback A, Juzeniene A, et al. Solar radiation, vitamin D and survival rate of colon cancer in Norway. J Photochem Photobiol B. 2005;78:189–93. doi: 10.1016/j.jphotobiol.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Ng K, Meyerhardt JA, Wu K, Feskanich D, Hollis BW, et al. Circulating 25-hydroxyvitamin d levels and survival in patients with colorectal cancer. J Clin Oncol. 2008;26:2984–91. doi: 10.1200/JCO.2007.15.1027. doi: 26/18/2984 [pii]10.1200/JCO.2007.15.1027. [DOI] [PubMed] [Google Scholar]
- 17.Maalmi H, Ordonez-Mena JM, Schottker B, Brenner H. Serum 25-hydroxyvitamin D levels and survival in colorectal and breast cancer patients: systematic review and meta-analysis of prospective cohort studies. Eur J Cancer. 2014;50:1510–21. doi: 10.1016/j.ejca.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Scragg R, Camargo CA., Jr Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2008;168:577–86. doi: 10.1093/aje/kwn163. discussion 587-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaaskelainen T, Knekt P, Marniemi J, Sares-Jaske L, Mannisto S, et al. Vitamin D status is associated with sociodemographic factors, lifestyle and metabolic health. Eur J Nutr. 2013;52:513–25. doi: 10.1007/s00394-012-0354-0. [DOI] [PubMed] [Google Scholar]
- 20.Abboud M, Gordon-Thomson C, Hoy AJ, Balaban S, Rybchyn MS, et al. Uptake of 25-hydroxyvitamin D by muscle and fat cells. J Steroid Biochem Mol Biol. 2014;144(Pt A):232–6. doi: 10.1016/j.jsbmb.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Abboud M, Puglisi DA, Davies BN, Rybchyn M, Whitehead NP, et al. Evidence for a specific uptake and retention mechanism for 25-hydroxyvitamin D (25OHD) in skeletal muscle cells. Endocrinology. 2013;154:3022–30. doi: 10.1210/en.2012-2245. [DOI] [PubMed] [Google Scholar]
- 22.Brock K, Cant R, Clemson L, Mason RS, Fraser DR. Effects of diet and exercise on plasma vitamin D (25(OH)D) levels in Vietnamese immigrant elderly in Sydney, Australia. J Steroid Biochem Mol Biol. 2007;103:786–92. doi: 10.1016/j.jsbmb.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 23.Skender S, Schrotz-King P, Bohm J, Abbenhardt C, Gigic B, et al. Repeat physical activity measurement by accelerometry among colorectal cancer patients–feasibility and minimal number of days of monitoring. BMC Res Notes. 2015;8:222. doi: 10.1186/s13104-015-1168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hekler EB, Buman MP, Haskell WL, Conway TL, Cain KL, et al. Reliability and validity of CHAMPS self-reported sedentary-to-vigorous intensity physical activity in older adults. J Phys Act Health. 2012;9:225–36. doi: 10.1123/jpah.9.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777–81. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Gupta D, Vashi PG, Trukova K, Lis CG, Lammersfeld CA. Prevalence of serum vitamin D deficiency and insufficiency in cancer: Review of the epidemiological literature. Exp Ther Med. 2011;2:181–193. doi: 10.3892/etm.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flynn A, Hirvonen T, Mensink GB, Ocke MC, Serra-Majem L, et al. Intake of selected nutrients from foods, from fortification and from supplements in various European countries. Food Nutr Res. 2009;53 doi: 10.3402/fnr.v53i0.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhn T, Kaaks R, Teucher B, Hirche F, Dierkes J, et al. Dietary, lifestyle, and genetic determinants of vitamin D status: a cross-sectional analysis from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Germany study. Eur J Nutr. 2014;53:731–41. doi: 10.1007/s00394-013-0577-8. [DOI] [PubMed] [Google Scholar]
- 29.Ulrich CM, Toriola AT, Siegel E, Brenner H, Chang-Claude J, et al. Plasma 25-hydroxyvitamin D3, folate and vitamin B12 biomarkers among international colorectal cancer patients: a pilot study. Journal of Nutritional Science. 2013;2:1–6. doi: 10.1017/jns.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003;3:601–14. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- 31.Diaz GD, Paraskeva C, Thomas MG, Binderup L, Hague A. Apoptosis is induced by the active metabolite of vitamin D3 and its analogue EB1089 in colorectal adenoma and carcinoma cells: possible implications for prevention and therapy. Cancer Res. 2000;60:2304–12. [PubMed] [Google Scholar]
- 32.Palmer HG, Sanchez-Carbayo M, Ordonez-Moran P, Larriba MJ, Cordon-Cardo C, et al. Genetic signatures of differentiation induced by 1alpha,25-dihydroxyvitamin D3 in human colon cancer cells. Cancer Res. 2003;63:7799–806. [PubMed] [Google Scholar]
- 33.Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys. 2012;523:123–33. doi: 10.1016/j.abb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs ET, Hibler EA, Lance P, Sardo CL, Jurutka PW. Association between circulating concentrations of 25(OH)D and colorectal adenoma: a pooled analysis. Int J Cancer. 2013;133:2980–8. doi: 10.1002/ijc.28316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baron JA, Barry EL, Mott LA, Rees JR, Sandler RS, et al. A Trial of Calcium and Vitamin D for the Prevention of Colorectal Adenomas. N Engl J Med. 2015;373:1519–30. doi: 10.1056/NEJMoa1500409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76–89. doi: 10.1016/S2213-8587(13)70165-7. [DOI] [PubMed] [Google Scholar]
- 37.Cannell JJ, Grant WB, Holick MF. Vitamin D and inflammation. Dermatoendocrinol. 2014;6:e983401. doi: 10.4161/19381980.2014.983401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ha CD, Cho JK, Lee SH, Kang HS. Serum vitamin D, physical activity, and metabolic risk factors in Korean children. Med Sci Sports Exerc. 2013;45:102–8. doi: 10.1249/MSS.0b013e31826c6956. [DOI] [PubMed] [Google Scholar]
- 39.Brock K, Huang WY, Fraser DR, Ke L, Tseng M, et al. Low vitamin D status is associated with physical inactivity, obesity and low vitamin D intake in a large US sample of healthy middle-aged men and women. J Steroid Biochem Mol Biol. 2010;121:462–6. doi: 10.1016/j.jsbmb.2010.03.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scragg R, Holdaway I, Jackson R, Lim T. Plasma 25-hydroxyvitamin D3 and its relation to physical activity and other heart disease risk factors in the general population. Ann Epidemiol. 1992;2:697–703. doi: 10.1016/1047-2797(92)90014-h. [DOI] [PubMed] [Google Scholar]
- 41.De Rui M, Toffanello ED, Veronese N, Zambon S, Bolzetta F, et al. Vitamin D deficiency and leisure time activities in the elderly: are all pastimes the same? PLoS One. 2014;9 doi: 10.1371/journal.pone.0094805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neilson HK, Robson PJ, Friedenreich CM, Csizmadi I. Estimating activity energy expenditure: how valid are physical activity questionnaires? Am J Clin Nutr. 2008;87:279–91. doi: 10.1093/ajcn/87.2.279. [DOI] [PubMed] [Google Scholar]
- 43.Ferrari P, Friedenreich C, Matthews CE. The role of measurement error in estimating levels of physical activity. Am J Epidemiol. 2007;166:832–40. doi: 10.1093/aje/kwm148. [DOI] [PubMed] [Google Scholar]


