Abstract
Background
Several guidelines have advocated the need for adequate cancer-related pain (CRP) management. The pain management index (PMI) has been proposed as an auditable measure of the appropriateness for analgesic therapy.
Objectives
To determine the adequacy of CRP management based on the PMI status and its patient-related predictors at the point of referral to a pain clinic (PC).
Methods
Consecutive patients referred to a PC had standardized initial assessments and status documentation on the Brief Pain Inventory (BPI) ratings; pain mechanism, using a neuropathic pain diagnostic questionnaire (the Douleur Neuropathique 4 tool); episodic pain; oral morphine equivalent daily dose; the Hospital Anxiety Depression Scale and the Emotion Thermometer scores; and cancer diagnosis, metastases, treatment, and pain duration. Predictors of “negative PMI status” [PMI(−)] were examined in logistic regression models. Variables with p<0.25 in an initial bivariable analysis were entered into a multivariable model.
Results
Of 371 participants, 95 (25.6%) had PMI(−), suggesting undertreatment of CRP. Both female sex and higher scores on the BPI’s “interference with general activity” more strongly predicted PMI(−). Patients who received either radiotherapy or one or more adjuvant analgesics prior to the initial consultation at the PC, those who had neuropathic pain, those who had a greater need for emotional help, and those with higher BPI’s “relief ” scores were all less likely to be PMI(−).
Conclusion
The potential burden of patient and family distress associated with suboptimal CRP management in one in four patients should generate major public health concern and prompt appropriate educational and health policy measures to address the deficit.
Keywords: cancer pain, pain management, opioid analgesics, pain measurement
Introduction
Cancer-related pain (CRP) is a burdensome symptom with the potential to negatively impact patients’ and their families’ quality of life.1 A recent meta-analysis reported pooled prevalence rates of 55% for CRP in patients who were receiving disease modifying treatment and 64% in those with advanced metastatic or terminal disease.2
CRP may be controlled in up to 90% cases with appropriate analgesic therapies.3 Several authors have highlighted the need for adequate control of CRP and many published guidelines advocate a standardized strategic approach to its management.4–9 The World Health Organization (WHO) pain ladder forms the basis of most CRP management guidelines.4 This advocates the use of analgesic medication in a three-step approach to achieve CRP relief. Step 1 involves the use of non-opioids such as paracetamol for mild pain. Step 2 advises the use of a weak opioid for persistent pain or pain increasing to moderate level. Step 3 for moderate to severe pain advocates the use of a strong opioid such as morphine when moderate pain is persisting or increasing to a severe level.
Although control of CRP is attainable in most patients with treatment modalities that are available and relatively safe, many cancer patients suffer unnecessarily due to inadequate control of CRP.10 This inadequacy is multifactorial; it is associated in particular with negative perceptions and various barriers that originate from among patients, families, informal caregivers, health care providers, institutions, and society in general.1
As pharmacological therapy is the mainstay of CRP management,4 adequacy of pain management can be reflected by the appropriateness of the analgesic prescribed.11 Based on the assumption that the goal of cancer pain control is to enable patients to function at an optimal level, and ultimately in the terminal phase to die relatively free of pain, CRP treatment is inadequate when substantial pain persists, regardless of the reason for this failure, such as insufficient use of pain medication and other procedures.12
The pain management index (PMI) is an auditable measure of the appropriateness of analgesic therapy.13 It is a composite measure reflecting the patient’s pain severity and the appropriateness of the strength of the analgesic used in relation to the reported pain severity.14
A 1993 study, based on the WHO cancer pain management guidelines, found that 42% of outpatients with CRP received inadequate analgesic therapy.15 A systematic review which included 26 studies, published between 1994 and 2007, reported that potential undertreatment, based on PMI status, varied in prevalence from 8% to 82%.16 In a more recent review of 20 studies, from the 2007 to 2013 period, there was a decrease in the prevalence of undertreatment but it remains significantly high at 31.8%.17
The epidemiology of CRP and its treatment in Portugal are not well documented, but undertreatment is to be expected as strong opioids are only prescribed to a small proportion of eligible patients.18 A population-based study of chronic pain, including 49 patients with cancer pain, reported that opioids were used in only 10.13% of those with CRP.19 Given the limited published data in the Portuguese context, the aim of this study was to determine the adequacy of CRP management based on PMI status and identify its patient-related predictors at the point of referral to a cancer pain clinic (PC). The collection of these data may help to focus future educational, research, and health policy development in the management of CRP.
Methods
Setting and design
The present study was conducted from June 1, 2009 to April 30, 2010 in the specialist PC of the Portuguese Cancer Institute, a national tertiary-level cancer center in Lisbon, Portugal. The study was cross-sectional in design, reflecting assessments that were conducted at subjects’ first consultation in the PC.
Study population and eligibility criteria
Consecutive new patient referrals to an outpatient PC were approached for consent to participate in both an initial cross-sectional and a related longitudinal study of CRP characteristics and management. The following eligibility criteria were applied: adult patients (>18 years of age) were included if they had a cancer diagnosis, provided informed consent to participate in the study, and had the cognitive capacity to rate their current pain on a numerical rating scale (0 meaning no pain, 10 meaning the worst pain imaginable); patients were excluded if they had no evidence of active cancer or had non-CRP. CRP was defined as pain directly related to malignant involvement or pain related to anticancer treatment, such as chemotherapy, radiotherapy, or surgery. Ethics approval for the present study was obtained from the Research Ethics Board of the Portuguese Cancer Institute (Lisbon, Portugal).
Assessment data and tools
Patients underwent standardized assessments and documentation of clinical data. Translated Portuguese versions of standard tools previously validated in English and also, in most cases, in Portuguese were used.
Recorded demographic data included sex, education, marital status, income per month (euros), and socioeconomic status. Regarding education, primary referred to 0–4 years of education; secondary referred to 5–12 years; and tertiary referred to university or >12 years. For ease of reporting, the Portuguese socioeconomic groupings of 1, 2, and 3; 4, 5, and 6; 7 and 8; and 9 were transformed in a similar descending order to groups 1, 2, 3, and 4, respectively.20
Scores ≥4 on a Portuguese-translated version (unpublished) of the original Short Portable Mental Status Questionnaire were used to screen for cognitive impairment.21 Performance status (0–4: 0, fully active and able to carry on all predisease performance tasks without restriction; 4, indicating that the patient is unable to provide self-care and confined to bed or chair) was rated using the Eastern Cooperative Oncology Group (ECOG) scale.22 Scores >7 on the anxiety and depression subscales of the Hospital Anxiety and Depression Scale (HADS) and >4 on the Emotion Thermometer (ET) tool were used to screen for anxiety, depression, and emotional distress, respectively.23,24
The recording of cancer characteristics included primary cancer diagnosis, metastases, cancer disease modifying treatments ≤30 days before the first PC consultation (cytotoxic chemotherapy, radiotherapy, or surgery), and documentation of palliative status (in relation to the goals of treatment).
Pain data included pain mechanisms (nociceptive and/or neuropathic), episodic pain, analgesic drugs prescribed before the first PC consultation, pain duration, and the Brief Pain Inventory (BPI) score.25,26 A score of ≥4 of the “Douleur Neuropathique 4” (DN4) was used to designate a positive DN4 status, meaning that CRP mechanism screened positive for a neuropathic component (NeuCP).26,27 Episodic pain, defined as a transitory exacerbation of pain that occurs in addition to otherwise stable persistent pain,28 was recorded and subdivided into episodic incident pain (when a trigger or incident activity was identifiable) and episodic breakthrough pain (when no trigger was identified). The oral morphine equivalent daily dose (MEDD) was calculated according to standard recommendations29 and recorded along with the number of current adjuvant (pharmacological) analgesic treatments (grouped as none and one or more).
PMI
The PMI reflects a level of congruence between a patient’s reported pain intensity and the potency of their analgesia at the point of initial assessment in the PC. BPI average scores in the none (0), mild (1–3), moderate (4–6), and severe (7–10) pain intensity categories were graded as 0, 1, 2, and 3, respectively, as “pain severity ratings” for computing the PMI values. The grading of “analgesic drug class” for PMI computation was determined by the most potent level of analgesic drug prescribed to the patient before their first PC consultation and categorized as 0=no analgesic drugs; 1=nonopioid drugs (eg, paracetamol); 2=weak opioids (eg, codeine); and 3=strong opioids (eg, fentanyl). The PMI value for each patient was then computed by subtracting the patient’s pain severity rating from the grade of analgesic drug class.
The PMI values range from −3 (a patient with severe pain, but receiving no analgesic drugs) to +3 (a patient reporting no pain and having been prescribed morphine or an equivalent strong opioid). Negative PMI values [PMI(−)] are considered to indicate pain undertreatment (inadequate CRP management), and positive PMI values of 0–3 [PMI(+)] are considered a conservative indicator of acceptable treatment.13,14
Data analyses
Data were analyzed using the software Stata® (version 14; StataCorp LP, College Station, TX, USA). Means are expressed with standard deviations (SDs). Continuous and categorical variables were compared using Student’s t-tests, and chi-square tests or Fisher’s exact tests, respectively. The initial MEDD was highly skewed and underwent logarithmic transformation for further analysis.
Predictors of PMI(−) were examined in logistic regression models, generating odds ratios (ORs) and 95% confidence intervals (CIs). The specification of independent variables for multivariable logistic regression analyses was based on a combination of clinical plausibility, the need to avoid multicollinearity, review of the literature, and the results of initial bivariable analyses comparing the groups with negative and positive PMI status. Variables with p<0.25 in the bivariable analyses were considered for entry in multivariable models. A backward elimination approach was used to derive a final model. The Hosmer–Lemeshow test and the area under receiver operating characteristic curve (AU-ROC) were used to assess each model’s goodness of fit and discrimination threshold. Statistical significance was set at p<0.05.
Results
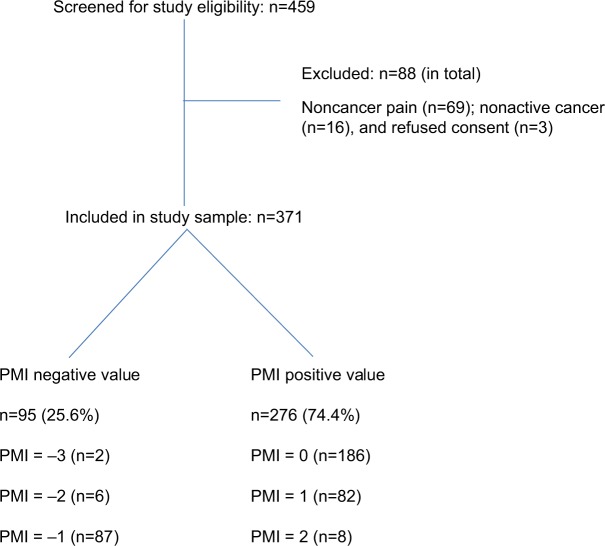
The derivation of the study sample and a summary of patients’ PMI status is presented in Figure 1. Of 459 individuals who were screened for the study, 88 were excluded because of non-CRP, nonactive cancer, or failure to consent, resulting in a final study sample of 371 patients. Of these, 95 (25.6%) had PMI(−), suggesting undertreatment of their cancer pain. Most of the PMI(−) values were in the −1 category (n=87). The derivation and summary of both pain severity grades and analgesic drug class grades are presented in Table 1. None of the study samples had a BPI average rating of zero. Although 77 patients (20.8%) rated their pain as severe, only nine (11.7%) of these were prescribed a strong opioid.
Figure 1.
Derivation of study sample and PMI status.
Abbreviation: PMI, pain management index.
Table 1.
Derivation and summary of PMI grades for pain severity and analgesic drug classes
| Pain severity
|
Analgesic drug class prescribed PMI grade
|
Total (%) | ||||
|---|---|---|---|---|---|---|
| Categories* | PMI grade | 0 | 1 | 2 | 3 | |
| None (0) | 0 | 0 | 0 | 0 | 0 | 0 |
| Mild (1–3) | 1 | 18 | 13 | 42 | 8 | 81 (21.8) |
| Moderate (4–6) | 2 | 6 | 3 | 164 | 40 | 213 (57.4) |
| Severe (7–10) | 3 | 2 | 0 | 66 | 9 | 77 (20.8) |
| Total (%) | 26 (7.0) | 16 (4.3) | 272 (73.3) | 57 (15.4) | 371 (100) | |
Notes:
Descriptive categories (mild, moderate, and severe) are based on patients’ Brief Pain Inventory average pain intensity rating (0–10).
Abbreviation: PMI, pain management index.
The demographic, cognitive and functional, and clinical and psychological variables, as well as their associations with the PMI status are shown in Table 2. The mean age of the entire study sample was 62.1±14.3 years and 199 (54%) were female. Most of the patients had completed only primary education (69.8%); most were married (64.1%); approximately half of the sample had a monthly income of ≤485 euros; 46 (12.4%) had a mild cognitive deficit; and 62 (16.7%) were rated in the ECOG 3–4 categories. An addictive history screen was positive in 86 (23.2%) patients. The HADS screening scores were positive for depression and anxiety in 280 (75.5%) and 262 (70.6%) patients, respectively. Screening on the ET tool for “distress” and “need for help” was positive in 170 (45.8%) and 167 (45.0%) cases, respectively. The median (Q1–Q3) MEDD values in mg for males and females were 30 (20–60) and 30 (15–60), respectively. The logarithmically transformed MEDD values (mean±SD) for males and females were 3.39±1.25 and 3.19±1.42 (p=0.053). More detailed demographic findings have been described in a previous publication.20 Female sex and not needing help (to manage emotional problems) had statistically significant associations with PMI(−) status.
Table 2.
Demographic, cognitive and functional, and clinical and psychological characteristic variables and their associations with PMI status (n=371)
| Characteristics | PMI positive
|
PMI negative
|
p-value | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Demographic | ||||||
| Sex | Male | 136 | 49.3 | 36 | 37.9 | 0.05 |
| Female | 140 | 50.7 | 59 | 62.1 | ||
| Education | Primary | 194 | 70.3 | 65 | 68.4 | 0.78 |
| Secondary | 67 | 24.3 | 23 | 24.2 | ||
| Tertiary | 15 | 5.4 | 7 | 7.4 | ||
| Marital status | Single | 34 | 12.3 | 9 | 9.5 | 0.71 |
| Married | 173 | 62.7 | 65 | 68.4 | ||
| Divorced | 31 | 11.2 | 8 | 8.4 | ||
| Widow | 38 | 13.8 | 13 | 13.7 | ||
| Income per month | ≤485 euros | 127 | 46.0 | 49 | 51.6 | 0.35 |
| >485 euros | 149 | 54.0 | 46 | 48.4 | ||
| Socioeconomic statusa | Group 1 | 43 | 15.6 | 10 | 10.5 | 0.56 |
| Group 2 | 104 | 37.7 | 42 | 44.2 | ||
| Group 3 | 62 | 22.4 | 21 | 22.1 | ||
| Group 4 | 67 | 24.3 | 22 | 23.2 | ||
| Cognitive and functional | ||||||
| Cognitive function | Normal | 244 | 88.4 | 81 | 85.3 | 0.42 |
| Mild deficit | 32 | 11.6 | 14 | 14.7 | ||
| ECOG | 0 | 88 | 31.9 | 32 | 33.7 | 0.83 |
| 1 | 96 | 34.8 | 32 | 33.7 | ||
| 2 | 46 | 16.7 | 15 | 15.8 | ||
| 3 | 24 | 8.7 | 11 | 11.6 | ||
| 4 | 22 | 7.9 | 5 | 5.2 | ||
| Clinical and psychological | ||||||
| Drug/alcohol abuse | No | 210 | 76.1 | 75 | 78.9 | 0.57 |
| Yes | 66 | 23.9 | 20 | 21.1 | ||
| HADS anxiety >7 | No | 78 | 28.3 | 31 | 32.6 | 0.42 |
| Yes | 198 | 71.7 | 64 | 67.4 | ||
| HADS depression >7 | No | 64 | 23.2 | 27 | 28.4 | 0.30 |
| Yes | 212 | 76.8 | 68 | 71.6 | ||
| ET distress >4 | No | 147 | 53.3 | 54 | 56.8 | 0.54 |
| Yes | 129 | 46.7 | 41 | 43.2 | ||
| ET need help >4 | No | 143 | 51.8 | 61 | 64.2 | 0.03 |
| Yes | 133 | 48.2 | 34 | 35.8 | ||
Note:
Socioeconomic groups (1, managers and liberal professionals; 2, clerical and services workers; 3, craft and machines workers; 4, unskilled laborers).
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ET, Emotion Thermometer; HADS, Hospital Anxiety and Depression Scale; PMI, pain management index.
The associations of both cancer and pain characteristics with PMI status are shown in Table 3. Only 10 (2.7%) patients had a lung cancer diagnosis; 263 (71%) had metastatic disease; and 176 (47.4%) had their treatment goal as palliative. In the month prior to their first PC consultation, 167 (45.0%), 112 (30.2%), and 176 (47.4%) underwent chemotherapy, surgery, and radiotherapy, respectively. Screening for NeuCP was positive in 161 (43.4%) patients. For analgesia, no opioid was prescribed for 42 (11.3%) patients; 210 (56.6%) were prescribed one or more adjuvant medications. Pain duration was over 3 months in 179 (48.2%) patients. Absence of head and neck metastatic disease, no exposure to recent radiotherapy, and no prescription of opioids at PC referral all had a statistically significant association with PMI(−) status.
Table 3.
Cancer and pain characteristics variables and their associations with PMI status (n=371)
| Characteristics | PMI positive
|
PMI negative
|
p-value | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Cancer | ||||||
| Primary cancer | Head and neck | 70 | 25.4 | 22 | 23.2 | 0.95 |
| Lung | 7 | 2.5 | 3 | 3.2 | ||
| Gastrointestinal | 61 | 22.1 | 21 | 22.1 | ||
| Breast | 31 | 11.2 | 12 | 12.6 | ||
| Genitourinary | 61 | 22.1 | 18 | 18.9 | ||
| Other | 46 | 16.7 | 19 | 20.0 | ||
| Bone metastases | No | 182 | 65.9 | 60 | 63.2 | 0.62 |
| Yes | 94 | 34.1 | 35 | 36.8 | ||
| Head/neck metastases | No | 254 | 92.0 | 93 | 97.9 | 0.04 |
| Yes | 22 | 8.0 | 2 | 2.1 | ||
| Central nervous system metastases | No | 258 | 93.5 | 92 | 96.8 | 0.22 |
| Yes | 18 | 6.5 | 3 | 3.2 | ||
| Chemotherapya | No | 150 | 54.3 | 54 | 56.8 | 0.67 |
| Yes | 126 | 45.7 | 41 43.2 | |||
| Surgerya | No | 189 | 68.5 | 70 | 73.7 | 0.34 |
| Yes | 87 | 31.5 | 25 | 26.3 | ||
| Radiotherapya | No | 134 | 48.6 | 61 | 64.2 | 0.008 |
| Yes | 142 | 51.4 | 34 | 35.8 | ||
| Palliative goal | No | 140 | 50.7 | 55 | 57.9 | 0.23 |
| Yes | 136 | 49.3 | 40 | 42.1 | ||
| Pain | ||||||
| Nociceptive visceral | No | 195 | 70.7 | 64 | 67.4 | 0.55 |
| Yes | 81 | 29.3 | 31 | 32.6 | ||
| Nociceptive bone pain | No | 165 | 59.8 | 57 | 60.0 | 0.97 |
| Yes | 111 | 40.2 | 38 | 40.0 | ||
| DN4 positive | No | 151 | 54.7 | 59 | 62.1 | 0.21 |
| Yes | 125 | 45.3 | 36 | 37.9 | ||
| Breakthrough pain | No | 157 | 56.9 | 55 | 57.9 | 0.86 |
| Yes | 119 | 43.1 | 40 | 42.1 | ||
| Incident pain | No | 105 | 38.0 | 46 | 48.4 | 0.07 |
| Yes | 171 | 62.0 | 49 | 51.6 | ||
| No opioids | No | 263 | 95.3 | 66 | 69.5 | <0.001 |
| Yes | 13 4.7 | 29 | 30.5 | |||
| Adjuvant use | No | 112 | 40.6 | 49 | 51.6 | 0.06 |
| ≥1 | 164 | 59.4 | 46 | 48.4 | ||
| Pain duration | ≤3 months | 147 | 53.3 | 45 | 47.4 | 0.32 |
| >3 months | 129 | 46.7 | 50 | 52.6 | ||
Note:
Within the last 30 days before the initial consultation at the pain clinic; DN4 – neuropathic pain diagnostic questionnaire (positive: total score≥4).
Abbreviations: DN4, “Douleur Neuropathique 4”; PMI, pain management index.
Continuous variables (age, the BPI main items, and the DN4 scores) and their associations with the PMI status are shown in Table 4. A statistically significant association with PMI(−) status was noted for higher pain intensity ratings, greater interference of pain with general activity, and reported lesser pain relief.
Table 4.
Continuous variables and their associations with PMI status (n=371)
| Variables | PMI | Mean | SD | 95% CI | p-value |
|---|---|---|---|---|---|
| Age | Positive | 62.46 | 14.08 | 60.79–64.13 | 0.45 |
| Negative | 61.18 | 15.08 | 58.12–64.24 | ||
| BPI_worst pain | Positive | 7.09 | 2.46 | 6.80–7.39 | <0.001 |
| Negative | 8.15 | 2.81 | 7.58–8.72 | ||
| BPI_least pain | Positive | 2.64 | 1.61 | 2.45–2.83 | <0.001 |
| Negative | 4.03 | 2.21 | 3.58–4.48 | ||
| BPI_average pain | Positive | 4.54 | 1.53 | 4.36–4.72 | <0.001 |
| Negative | 6.05 | 2.41 | 5.56–6.54 | ||
| BPI_pain right now | Positive | 5.02 | 2.42 | 4.73–5.31 | <0.001 |
| Negative | 6.41 | 2.90 | 5.82–7.00 | ||
| BPI_pain relief | Positive | 49.57 | 26.65 | 46.41–52.72 | <0.001 |
| Negative | 30.42 | 31.59 | 23.99–36.86 | ||
| BPI_general activitya | Positive | 5.17 | 2.59 | 4.87–5.48 | 0.008 |
| Negative | 5.99 | 2.54 | 5.47–6.51 | ||
| BPI_mooda | Positive | 7.53 | 2.43 | 7.24–7.82 | 0.48 |
| Negative | 7.33 | 2.43 | 6.83–7.82 | ||
| BPI_walkinga | Positive | 5.20 | 2.62 | 4.89–5.51 | 0.12 |
| Negative | 5.67 | 2.42 | 5.18–6.17 | ||
| BPI_worka | Positive | 4.45 | 2.92 | 4.09–4.79 | 0.47 |
| Negative | 4.69 | 2.89 | 4.10–5.28 | ||
| BPI_relationsa | Positive | 2.97 | 1.88 | 2.75–3.20 | 0.87 |
| Negative | 3.01 | 1.90 | 2.62–3.40 | ||
| BPI_sleepa | Positive | 4.97 | 1.91 | 4.74–5.19 | 0.23 |
| Negative | 4.69 | 1.92 | 4.30–5.09 | ||
| BPI_enjoyment lifea | Positive | 7.37 | 2.44 | 7.08–7.66 | 0.37 |
| Negative | 7.63 | 2.37 | 7.15–8.11 | ||
| DN4 score | Positive | 3.88 | 2.49 | 3.59–4.17 | 0.14 |
| Negative | 3.44 | 2.54 | 2.93–3.96 |
Note:
Pain interference with the given variable; DN4 – neuropathic pain diagnostic questionnaire (positive: total score≥4).
Abbreviations: BPI, Brief Pain Inventory; CI, confidence interval; DN4, “Douleur Neuropathique 4”; PMI, pain management index; SD, standard deviation.
The logistic regression analyses are summarized in Table 5. The final multivariable model contained seven statistically significant independent predictors of PMI(−). In the final model, female sex was arguably an independent predictor (OR=1.7; 95% CI 0.99–2.92, p=0.05) of PMI(−). Similarly, higher BPI scores for “interference with general activity” predicted PMI(−) status (OR=1.14; 95% CI 1.02–1.27, p=0.02). Negative predictors of PMI(−) included recent radiotherapy treatment, adjuvant analgesic use, positive NeuCP screening, a reported higher need for emotional help, and higher BPI “pain relief ” scores. The Hosmer–Lemeshow goodness-of-fit test for the final model had a chi-square test value=6.33 (p=0.61) and the AU-ROC was 0.782.
Table 5.
Bivariable and multivariable logistic regression results for predictors of negative PMI (n=371)
| Predictors variables | Bivariable analyses
|
Multivariable modela,b
|
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age | 0.99 | 0.98–1.01 | 0.45 | 0.99 | 0.97–1.00 | 0.14 |
| Female sex | 1.59 | 0.99–2.57 | 0.05 | 1.70 | 0.99–2.92 | 0.05 |
| Drug/alcohol abuse | 0.58 | 0.07–5.00 | 0.62 | |||
| Palliative care goal | 0.75 | 0.47–1.20 | 0.23 | |||
| Head/neck metastases | 0.25 | 0.06–1.08 | 0.06 | 0.26 | 0.05–1.32 | 0.10 |
| CNS metastases | 0.47 | 0.13–1.62 | 0.23 | |||
| Radiotherapy in last 30 days | 0.53 | 0.32–0.85 | 0.009 | 0.45 | 0.26–0.79 | 0.006 |
| DN4 positive | 0.74 | 0.46–1.19 | 0.21 | 0.54 | 0.30–0.97 | 0.04 |
| Incident pain | 0.65 | 0.41–1.05 | 0.07 | |||
| No opioids | 8.89 | 4.38–18.04 | <0.001 | |||
| Adjuvants≥1 | 0.64 | 0.40–1.02 | 0.06 | 0.57 | 0.32–0.99 | 0.05 |
| Pain duration>3 months | 1.27 | 0.79–2.02 | 0.32 | |||
| ET_need help>4 | 0.60 | 0.37–0.97 | 0.04 | 0.55 | 0.32–0.96 | 0.03 |
| BPI_interference with walking | 1.08 | 0.98–1.18 | 0.12 | 1.09 | 0.98–1.21 | 0.13 |
| BPI_interference with general activity | 1.14 | 1.03–1.25 | 0.009 | 1.14 | 1.02–1.27 | 0.02 |
| BPI_pain relief | 0.96 | 0.95–0.97 | <0.001 | 0.97 | 0.96–0.98 | <0.001 |
| Intercept | 1.28 | 0.20–8.09 | 0.79 | |||
Notes:
Model’s goodness of fit: Hosmer–Lemeshow chi-square=6.33 (p=0.61).
Model’s discrimination: area under ROC=0.782.
Abbreviations: BPI, Brief Pain Inventory; CI, confidence interval; CNS, central nervous system; DN4, “Douleur Neuropathique 4” (neuropathic pain diagnostic questionnaire); ET, Emotion Thermometer; OR, odds ratio; PMI, pain management index; ROC, receiver operating characteristic curve.
Discussion
Adequacy of CRP management
In this study, patients’ PMI status suggests that CRP management was inadequate in 25.6% of individuals: about one in four patients were likely undertreated at initial consultation in our outpatient cancer PC. This finding was corroborated by an almost 20% difference in mean percentage pain relief between those with the PMI(−) and the PMI(+) status. Overall, the study’s findings are largely consistent with literature data in indicating that CRP is still inadequately managed. However, it is important to acknowledge contextual and methodological differences when comparing this study to previously published data.
In the original PMI study by Cleeland et al, 60% of patients with moderate to severe CRP were not prescribed an analgesic medication that was appropriate to their level of pain.13 Comparatively, our study had two important methodological differences. First, we used the BPI “average pain” rating as opposed to the “worst pain” rating used by Cleeland et al. We could have therefore underestimated the prevalence of PMI(−). Second, we categorized moderate and severe pain as numerical ratings in the 4–6 and 7–10 range, respectively, whereas the corresponding ranges in the Cleeland et al study were 4–7 and 8–10. This difference suggests that we could have comparatively overestimated the prevalence of PMI(−). The degree to which these two differences (acting in opposing directions) impacted our final estimate is unknown. The numerical cut points used for the severity categorization of pain have been keenly debated.30,31 A study based on CRP interference determined categorical ranges of 1–4 for mild, 5–6 for moderate, and 7–10 for severe pain.32 A study based on the impact of pain on quality of life measures suggested that pain scores should be classified as 1–5 for mild, 6 for moderate, and 7–10 for severe pain.33 There is therefore some inconsistency in the published literature regarding the categorization of pain intensity and in the overall evaluation of CRP management.14,34 In previous palliative care studies, stable pain was defined as a pain intensity score of ≤3 for three consecutive days and the categorization for mild, moderate, and severe was 0–3, 4–6, and 7–10, respectively.35,36 A similar categorization was used in the current study and “average pain” was chosen as it was more reflective of the overall analgesia achieved in the week preceding initial PC consultation than an isolated single “worst pain” rating.
Despite the definitional challenges, valid comparisons can be made with some studies. Mitera et al used the PMI to retrospectively assess CRP management in an outpatient palliative radiotherapy clinic using categories of 1–4 (mild), 5–6 (moderate), and 7–10 (severe) for numerical “pain now” ratings; they reported a PMI(−) prevalence of 25.8%, comparable with our estimate of 25.6%.32 A culturally more comparable cross-sectional survey of patients in a variety of Portuguese palliative care settings included 151/164 (92%) patients with a cancer diagnosis.37 The median oral MEDD was 84 mg, whereas our study had a value of 30 mg. The PMI status was reported for 136 of these patients and was negative in only 4%. This lower prevalence estimate for PMI(−) suggests better CRP management, which probably reflects the involvement of a palliative care team in their care. Overall, cross-sectional studies are limited to a narrow window of time and do not capture the dynamic changes in regard to pain intensity and its treatment.
From the perspective of potential CRP undertreatment, it is important to also evaluate our study results in the context of published longitudinal data and aggregate data. Fisch et al performed a prospective, longitudinal study of pain and analgesics prescribed in 2,026 medical oncology outpatients with pain.38 Pain intensity scores were categorized according to the original Cleeland et al method and used to compute PMI values; 33% of patients had PMI(−) at initial assessment and no significant change occurred in follow-up 4–5 weeks later. Our 25.6% PMI(−) rate compares favorably with aggregate estimates from systematic reviews.16,17 In an initial systematic review published in 2008,16 43.4% of patients had PMI(−), reflecting inadequate CRP treatment; in a 2014 update with 20 new studies,17 there was ~25% decrease in this figure to 31.8%. Both of these studies used the original Cleeland et al criteria to categorize pain. The substantive degree of inadequate CRP management in our and other studies prompts us to question what factors underpin this from a broader perspective.
Study’s prediction model of inadequate cancer pain treatment
In the bivariable logistic regression analysis, “no opioid” status was strongly associated with PMI(−) status. However, this proved not to be an independent predictor in the multivariable model, suggesting that confounding was an issue that led to this variable being dropped. Of the 42/371 (11.3%) patients who were not prescribed an opioid, 29/95 (30.5%) were in the PMI(−) group and 13/276 (4.7%) were in the PMI(+) group. Exclusion of the no opioid category in the final multivariable model would suggest that the prescription of too weak an analgesic (incongruent with the pain intensity reported) was perhaps more consistently associated with undertreatment. We would have expected that having a documented palliative goal of treatment might have generated a more pain focused approach and led to a negative association with undertreatment, but this was not the case. However, documentation of a palliative goal of treatment is probably prone to inconsistency. Although socioeconomic factors have been consistently associated with undertreatment,17 our study failed to demonstrate such an association. The seven independent predictors of undertreatment were in the sex, psychosocial, and pain or pain management domains.
Sex association
We previously reported no sex difference in ratings of pain intensity in this study sample.20 Although female sex was not strictly a statistically significant independent predictor of undertreatment in the current study, it clearly was borderline with p=0.05. The MEDD in females was lower and the mean sex difference of logarithmically transformed MEDD was also borderline in terms of statistical significance (p=0.053). This suggests that being female had an association with CRP undertreatment and that relative opioid under prescribing in females occurred.
Literature data regarding the association of female sex with CRP undertreatment are conflicting: some studies using the PMI have demonstrated an association,13,34,39 whereas others have not.10,16 Although female sex is associated with higher risk of chronic noncancer pain, the picture is less clear in relation to CRP, in which complex clustering with other symptoms such as depression and fatigue may compound the interpretive challenges.40 Regardless of sex association with CRP risk, this does not necessarily explain the tendency toward inadequate treatment in females, for whom a negative prescriber bias may exist.
Psychological variables
Among the psychological variables, a greater patient perceived need for help (based on ET scores >4) had a negative association with PMI(−) status. Although this finding might seem to be generally counterintuitive, a speculative interpretation is that the smaller proportion of those with ET scores >4 in the PMI(−) or undertreated group compared to the PMI(+) group, 35.8% versus 48.2%, respectively, might reflect a degree of denial and possibly an associated failure to acknowledge a need for help in those with PMI(−) status. We previously reported no relationship between psychological screening scores and pain intensity in this study sample.20 Apart from the ET score difference in the current study, we found no association between other measures of anxiety, depression, or distress in this analysis.
Radiotherapy association
Recent treatment with radiotherapy had an independent negative association with PMI(−) status: having radiation treatment lessened the likelihood of undertreatment of CRP. Although patients with higher pain intensity are probably more likely to be considered for radiation treatment, we postulate that exposure to specialist physicians in radiation oncology may result in more appropriate use of opioids to manage pain.
Neuropathic pain and adjuvant use
The presence of a NeuCP reduces the odds of being under-treated. The use of adjuvant medications is associated with PMI(−) status. Their use for NeuCP, which is known to be less opioid sensitive,41 is consistent with published guidelines.6 For patients with NeuCP and severe pain, adding an adjuvant drug may be more appropriate rather than changing a prescribed opioid or increasing its dosage.3
BPI findings
The BPI ratings for pain interference with general activities had a positive independent association with PMI(−) status. Clinically, it is perhaps easier to interpret this BPI rating as an outcome rather than a statistical predictor. Similarly, the same could be said for the BPI rating of pain relief: the more a patient is undertreated for pain, the lower will be their rating for pain relief.
Strengths and limitations of the study
Although our consecutive study sample consisted of 71% with metastatic disease, it included those who had CRP in association with recent surgery, chemotherapy, and radiotherapy; consequently, it was relatively heterogeneous and representative of the spectrum of patients with cancer pain. The proportion with ECOG status in the 0–2 range was 83.3%, which would be higher than most palliative care settings and thus more representative of the cancer disease population than many palliative care-based studies.
There are some significant study limitations. First, the cross-sectional design limits assessment to a narrow temporal window of patients’ pain intensity experience. Second, referral bias is very likely with a PC, as reflected by 290 patients (78.2%) having moderate or severe pain, and we cannot exclude the possibility of the same set of physicians referring undertreated patients with CRP. Also, the proportion of study patients with lung cancer (2.7%) and head and neck cancer (24.8%) diagnoses are disproportionately low and high, respectively. Third, most patients had metastatic disease which arguably confines the generalization of the study findings to the more advanced cancer stages. Fourth, the PMI has several limitations as a tool, especially in relation to its fixed temporal assessment. It does not capture the dynamic changes in relation to pain intensity and the reactive speed and willingness of the prescriber to changing the dose of the analgesic in accordance with expressed pain level. It does not validly capture the impact of the multiple nonpharmacological and pharmacological (other than opioid prescribing) therapeutic interventions for pain management; it does not capture the complex multidimensional nature of CRP. Fifth, prescriber characteristics and attitudes were not examined. Similarly, we did not assess how patient’s attitudes and knowledge influenced their willingness to seek care, nor did we assess individual preferences or compliance with the prescribed analgesic regimen. Furthermore, we did not assess the impact of analgesic tolerance or drug tolerability on the adequacy of CRP treatment.
Study implications
Despite some limitations, the study findings have important implications for clinical practice, education, health policy, and research. We are concerned that because this study was conducted in one of the leading cancer institutions in Portugal, these findings could reflect the “tip of the iceberg” at a national level. Further studies, including surveys, are needed to determine the true extent of the problem and its underpinnings. Nevertheless, given the study results, it is important to complete routine pain assessments in cancer patients to ensure that they receive appropriate analgesics and dosages are adjusted in a timely manner for effective CRP management. A broader approach is therefore needed in CRP management, including better education to ensure pain guidelines implementation; early palliative care referral for pain management; auditing and the maintenance of minimum practice standards.3,42 For instance, a case-based pain management approach and a palliative care curriculum are effective in improving the opioid prescribing practices of medical residents.43 There are few studies that have investigated the characteristics/profile of physicians who have difficulty in controlling CRP. Such information would be useful in detecting those physicians who specifically need educational training to increase their CRP management skills.44
It is likely that a better integration between oncologic and palliative/supportive care, as advocated by the European Society of Medical Oncology, may produce better outcomes for patients and families.45 Palliative care specialists may positively influence the cultural barriers – existing among physicians working at oncology centers – about the use of opioids. The brand new “National Plan on Palliative Care” billed in December 2016 in Portugal is a promising turning point for those suffering from advanced life-limiting diseases.
Conclusion
Using the PMI(−) status to reflect undertreatment of cancer pain, approximately one in four patients suffering from CRP received inadequate analgesic treatment at the point of referral to a PC. We identified seven variables that were independently associated with undertreatment: female sex and patient ratings of greater interference with general activity were positively associated; radiation treatment, presence of neuropathic pain, use of one or more adjuvant analgesics, desire for emotional help, and perceived pain relief with current managements had negative associations with undertreatment.
Future prospective studies across other care settings are warranted to further examine inadequate analgesic use in CRP management and the various predictors identified in association with this. Both patient and prescriber surveys are needed to determine barriers and enablers for optimal CRP management in the Portuguese context.
Acknowledgments
This study was funded by Grunenthal S.A. Portugal. The funders have had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
This research has been presented in part at the 8th World Research Congress of the European Association for Palliative Care, Lleida, Spain, June 5–7, 2014.
Footnotes
Author contributions
PR-P conceived the study and was responsible for recruitment and data collection. PGL performed the statistical analysis. PR-P also drafted the manuscript with contributions from the other authors. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
At the time the study was carried out, Dr Reis-Pina was a member of the clinical staff at the PC of the Portuguese Cancer Institute (Lisbon, Portugal). In the past 5 years, Dr Reis-Pina has received honoraria from Laboratórios Vitória, S.A. Portugal; Grünenthal, S.A. Portugal; and Angelini Farmacêutica, Lda. The other authors report no conflicts of interest in this work.
References
- 1.Reis-Pina P, Lawlor PG, Barbosa A. Cancer-related pain management and the optimal use of opioids. Acta Med Port. 2015;28(3):376–381. doi: 10.20344/amp.5801. [DOI] [PubMed] [Google Scholar]
- 2.van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 2016;51(6):1070–1090. doi: 10.1016/j.jpainsymman.2015.12.340. [DOI] [PubMed] [Google Scholar]
- 3.Apolone G, Corli O, Caraceni A, et al. Pattern and quality of care of cancer pain management. Results from the Cancer Pain Outcome Research Study Group. Br J Cancer. 2009;100:1566–1574. doi: 10.1038/sj.bjc.6605053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . Cancer Pain Relief with a Guide to Opioid Availability. 2nd ed. Geneva, Switzerland: World Health Organization; 1996. [Accessed March 1, 2017]. Available from: http://apps.who.int/iris/bit-stream/10665/37896/1/9241544821.pdf. [Google Scholar]
- 5.Paice JA, Portenoy R, Lacchetti C, et al. Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(27):3325–3345. doi: 10.1200/JCO.2016.68.5206. [DOI] [PubMed] [Google Scholar]
- 6.Scottish Intercollegiate Guidelines Network . Control of Pain in Adult Patients with Cancer. A National Clinical Guideline. Edinburgh, UK: SIGN; 2008. [Accessed March 1, 2017]. (SIGN Guideline 106). Available from: www.sign.ac.uk/pdf/SIGN106.pdf. [Google Scholar]
- 7.Ripamonti CI, Bandieri E, Roila F, Berti M, Roila F, ESMO Guidelines Working Group Management of cancer pain: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22(Suppl 6):69–77. doi: 10.1093/annonc/mdr390. [DOI] [PubMed] [Google Scholar]
- 8.Caraceni A, Hanks G, Kaasa S, et al. European Palliative Care Research Collaborative (EPCRC) European Association for Palliative Care (EAPC) Use of opioids in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13(2):e58–e68. doi: 10.1016/S1470-2045(12)70040-2. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network . Guidelines for Supportive Care. Fort Washington, PA: NCCN; 2015. [Accessed March 1, 2017]. Adult cancer pain. Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Google Scholar]
- 10.Shvartzman P, Friger M, Shani A, Barak F, Yoram C, Singer Y. Pain control in ambulatory cancer patients: can we do better? J Pain Symptom Manage. 2003;26(2):716–722. doi: 10.1016/s0885-3924(03)00220-3. [DOI] [PubMed] [Google Scholar]
- 11.Hakonsen GD, Strelec P, Campbell D, Hudson S, Loennechen T. Adherence to medication guideline criteria in cancer pain management. J Pain Symptom Manage. 2009;37(6):1006–1018. doi: 10.1016/j.jpainsymman.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 12.de Wit R, van Dam F, Loonstra S, et al. The Amsterdam Pain Management Index compared to eight frequently used outcome measures to evaluate the adequacy of pain treatment in cancer patients with chronic pain. Pain. 2001;91(3):339–349. doi: 10.1016/S0304-3959(00)00455-3. [DOI] [PubMed] [Google Scholar]
- 13.Cleeland C, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med. 1994;330:592–596. doi: 10.1056/NEJM199403033300902. [DOI] [PubMed] [Google Scholar]
- 14.Russell PB, Aveyard SC, Oxenham DR. An assessment of methods used to evaluate the adequacy of cancer pain management. J Pain Symptom Manage. 2006;32(6):581–588. doi: 10.1016/j.jpainsymman.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 15.Zenz M, Willweber-Strumpf A. Opiophobia and cancer pain in Europe. Lancet. 1993;341(8852):1075–1076. doi: 10.1016/0140-6736(93)92425-s. [DOI] [PubMed] [Google Scholar]
- 16.Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985–1991. doi: 10.1093/annonc/mdn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greco M, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149–4154. doi: 10.1200/JCO.2014.56.0383. [DOI] [PubMed] [Google Scholar]
- 18.De Conno F, Ripamonti C, Brunelli C. Opioid purchases and expenditure in nine western European countries: are we killing off morphine? Palliat Med. 2005;19(3):179–184. doi: 10.1191/0269216305pm1002oa. [DOI] [PubMed] [Google Scholar]
- 19.Azevedo L, Costa-Pereira A, Mendonça L, Dias CC, Castro-Lopes JM. A population-based study on chronic pain and the use of opioids in Portugal. Pain. 2013;154(12):2844–2852. doi: 10.1016/j.pain.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 20.Pina P, Sabri E, Lawlor PG. Characteristics and associations of pain intensity in patients referred to a specialist cancer pain clinic. Pain Res Manag. 2015;20(5):249–254. doi: 10.1155/2015/807432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 22.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 23.Pais-Ribeiro J, Silva I, Ferreira T, Martins A, Meneses R, Baltar M. Validation study of a Portuguese version of the Hospital Anxiety and Depression Scale. Psychol Health Med. 2007;12(2):225–235. doi: 10.1080/13548500500524088. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell AJ, Baker-Glenn EA, Granger L, Symonds P. Can the distress thermometer be improved by additional mood domains? Part I. Initial validation of the Emotion Thermometers tool. Psychooncology. 2010;19(2):125–133. doi: 10.1002/pon.1523. [DOI] [PubMed] [Google Scholar]
- 25.Cleeland C, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 26.Azevedo LF, Costa-Pereira A, Dias C, et al. Tradução, adaptação cultural e estudo multicêntrico de validação de instrumentos para rastreio e avaliação do impacto da dor crónica. Pain (Portugal) 2007;15:6–56. Portuguese. [Google Scholar]
- 27.Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4) Pain. 2005;114(1–2):29–36. doi: 10.1016/j.pain.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Mercadante S, Radbruch L, Caraceni A, et al. Episodic (breakthrough) pain: consensus conference of an expert working group of the European Association for Palliative Care. Cancer. 2002;94(3):832–839. doi: 10.1002/cncr.10249. [DOI] [PubMed] [Google Scholar]
- 29.Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose ratios for opioids. A critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22(2):672–687. doi: 10.1016/s0885-3924(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 30.Paul SM, Zelman DC, Smith M, Miaskowski C. Categorizing the severity of cancer pain: further exploration of the establishment of cutpoints. Pain. 2005;113(1–2):37–44. doi: 10.1016/j.pain.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 32.Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the pain management index. J Pain Symptom Manage. 2010;39(2):259–267. doi: 10.1016/j.jpainsymman.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 33.McDonald R, Ding K, Chow E, et al. Classification of painful bone metastases as mild, moderate, or severe using both EORTC QLQ-C15-PAL and EORTC QLQ-BM22. Support Care Cancer. 2016;24(12):4871–4878. doi: 10.1007/s00520-016-3341-9. [DOI] [PubMed] [Google Scholar]
- 34.de Wit R, van Dam F, Abu-Saad HH, et al. Empirical comparison of commonly used measures to evaluate pain treatment in cancer patients with chronic pain. J Clin Oncol. 1999;17(4):1280–1287. doi: 10.1200/JCO.1999.17.4.1280. [DOI] [PubMed] [Google Scholar]
- 35.Fainsinger R, Nekolaichuk C, Lawlor P, et al. An international multicentre validation study of a pain classification system for cancer patients. Eur J Cancer. 2010;46(16):2896–2904. doi: 10.1016/j.ejca.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 36.Fainsinger RL, Fairchild A, Nekolaichuk C, Lawlor P, Lowe S, Hanson J. Is pain intensity a predictor of the complexity of cancer pain management? J Clin Oncol. 2009;27(4):585–590. doi: 10.1200/JCO.2008.17.1660. [DOI] [PubMed] [Google Scholar]
- 37.Gonçalves F, Almeida A, Antunes C, et al. A cross-sectional survey of pain in palliative care in Portugal. Support Care Cancer. 2013;21(7):2033–2039. doi: 10.1007/s00520-013-1746-2. [DOI] [PubMed] [Google Scholar]
- 38.Fisch MJ, Lee JW, Weiss M, et al. Prospective observational study of pain and analgesic prescribing in medical oncology outpatients with breast, colorectal, lung or prostatic cancer. J Clin Oncol. 2012;30(16):1980–1988. doi: 10.1200/JCO.2011.39.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uki J, Mendoza T, Cleeland SC, Nakamura Y, Takeda F. A brief cancer pain assessment tool in Japanese: the utility of the Japanese Brief Pain Inventory–BPI-J. J Pain Symptom Manage. 1998;16(6):364–373. doi: 10.1016/s0885-3924(98)00098-0. [DOI] [PubMed] [Google Scholar]
- 40.Fillingim RB, King CD, DaSilva MCR, Rahim-Williams B, Riley JL., III Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10(5):447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McNicol ED, Midbari A, Eisenberg E. Opioids for neuropathic pain. Cochrane Database Syst Rev. 2013;8:CD006146. doi: 10.1002/14651858.CD006146.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maltoni M. Opioids, pain, and fear. Ann Oncol. 2008;19(1):5–7. doi: 10.1093/annonc/mdm555. [DOI] [PubMed] [Google Scholar]
- 43.Ury WA, Rahn M, Tolentino V, et al. Can a pain management and palliative care curriculum improve the opioid prescribing practices of medical residents? J Gen Intern Med. 2002;17(8):625–631. doi: 10.1046/j.1525-1497.2002.10837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okuyama T, Wang XS, Akechi T, et al. Adequacy of cancer pain management in a Japanese cancer hospital. Jpn J Clin Oncol. 2004;34(1):37–42. doi: 10.1093/jjco/hyh004. [DOI] [PubMed] [Google Scholar]
- 45.Mercadante S, Roila F, Berretto O, Labianca R, Casilini S, DOMAIN-AIOM Study Group Prevalence and treatment of cancer pain in Italian oncological wards centres: a cross-sectional survey. Support Care Cancer. 2008;16(11):1203–1211. doi: 10.1007/s00520-008-0456-7. [DOI] [PubMed] [Google Scholar]