Abstract
Background
“Complete Extrapolation” of efficacy from adult or other pediatric data, to the pediatric population, is an important scientific tool that reduces the need for pediatric efficacy trials. Dose finding and safety studies in pediatrics are still needed. “No Extrapolation” requires 2 pediatric efficacy trials. “Partial Extrapolation” eliminates the need to conduct 2 pediatric efficacy trials; 1 efficacy or exposure/response study may be sufficient. We examined pediatric extrapolation from 2009 to 2014 evaluating any changes in extrapolation assumptions and the causes for these changes since a prior analysis published in 2011.
Methods
We reviewed all 157 products with 388 pediatric studies submitted to the FDA from 2009 through 2014. We assessed whether efficacy was extrapolated from adult or other pediatric data and categorized extrapolation as Complete, Partial, or No, and identified the reasons for the changes.
Results
Partial extrapolation decreased, whereas use of No and Complete extrapolation noticeably increased. Complete, Partial, or No extrapolations changed from 14%, 68%, and 18% in the 2011 study to 34%, 29%, and 37% respectively in the current study. The changes were mostly due to a better understanding of pediatric pathophysiology, why trials have failed, and improved endpoints.
Conclusions
Evolving science and data obtained from clinical trials increases the certainty of extrapolation assumptions and drives decisions to utilize extrapolation. Lessons learned from the conduct of these trials are critical to improving evidence-based medicine. Extrapolation of Efficacy is a powerful scientific tool that streamlines pediatric product development. Increased knowledge and evolving science inform utilization of this tool.
Keywords: Pediatric Efficacy Trial, Extrapolation of Efficacy, Drug Development
Background
Despite tremendous efforts over the past decades, off-label medicine use in pediatric populations is still at an undesirable level of 40% and up to 90% in neonates.1 Evidence-based medicine is required for adults and nothing less so for the dynamic, growing, and developmentally changing pediatric population. Appropriate product labeling and subsequent prescribing are the derivatives of evidence-based medicine. Pediatric trials are unique for several reasons: small number of patients, limited physiologic data, and ethical complexity increase the difficulty and costs of pediatric trials. Compounding these issues are the broad physiologic changes inherent in the pediatric population.
Extrapolation is defined as “to infer unknown data from known data.” Extrapolation of Efficacy can be used to increase the efficiency and successful completion of pediatric trials. Extrapolation of Efficacy from adult or other pediatric data has facilitated the conduct of pediatric product development trials, subsequent marketing approval, and labeling.2 This approach reduces the number of children that need to be enrolled and the type of clinical trials that need to be conducted for pediatric product marketing approval.
In 1994, the Food and Drug Administration (FDA) finalized a set of rules for the extrapolation of efficacy to the pediatric population from adequate, well-controlled studies with adults.3 Such extrapolation depends on a series of evidence-based fundamental assumptions that the course of the disease and the expected response to therapy in the adult and pediatric populations are similar.2 Another important component, after extrapolation has been deemed possible, is the determination of dose and exposure response parameters in pediatrics, as compared to the reference adult or other population. Safety assessments are almost always required to be obtained in the pediatric population.3–6
Based on data from 1998 to 2009, we found that 60% of pediatric drug development programs required at least one adequate and well-controlled efficacy trial. FDA identified the need for a consistent approach to define and establish disease and treatment response similarity.2 In our current analysis, we examined the use of extrapolation from 2009 through 2014, and any changes in the scientific assumptions forming the basis of extrapolation.
Methods
We focused on pediatric studies submitted from March 2009 through 2014. For the studies conducted, and for which there was subsequent pediatric labeling, we retrieved study information from FDA’s Document Archiving, Reporting & Regulatory Tracking System (DARRTS), or the FDA Document Room. We reviewed and tabulated the contents of the submitted studies and the final labeling according to drug and therapeutic indication.
We assessed the use of Extrapolation of Efficacy (ie, No Extrapolation, Partial Extrapolation, or Complete Extrapolation) based upon the same criteria and algorithm as previously described.2 Briefly, the approach would be classified as No Extrapolation when disease progression and treatment response are assumed to be different in adult and pediatric patients. Although 2 adequate and well-controlled trials are usually required to demonstrate efficacy in the pediatric population, alternative approaches may be utilized in certain situations such as rare disease.7 Pharmacokinetic (PK) studies are also conducted to establish the correct dose, along with safety assessments.
However, a few exceptions (ie, pediatric oncology products for solid tumor treatment), owing to the nature of the disease, are classified as “No Extrapolation,” even though only one efficacy study was required. Solid tumors in pediatric patients are biologically distinct from solid tumors in adults. Effective therapies also are limited. Therefore, activity of the therapy is usually assessed in one trial with a limited number of pediatric patients, by measuring disease-specific surrogates or clinically relevant end points.2 In other indications, one adequate and well-controlled trial in the appropriate pediatric population is usually considered necessary to establish efficacy when “Partial Extrapolation” is being used.
“Complete Extrapolation” involves robust data to support the assumptions that adult and pediatric disease progressions and responses to the intervention are similar, or the exposure-response relationship in adult and pediatric populations is established. Safety data in the pediatric population are still necessary. The effective dose is identified by matching systemic exposures between adult and pediatric populations. Complete Extrapolation of pediatric efficacy is supported by pediatric PK and safety data or, in certain cases, pediatric safety data exclusively.2
Partial Extrapolation is utilized when disease progression and treatment response are similar, but there is uncertainty about the degree of similarity of disease progression and/or treatment exposure-response relationship between adult and pediatric patients. The pediatric evidence required to support Partial Extrapolation ranges from a single adequate, well-controlled trial to confirm efficacy, to a PK/pharmacodynamic (PD) exposure-response study to confirm response in the pediatric population. The latter can be used to confirm the similarity of the exposure-response relationship when sufficient clinical evidence exists to support the assumptions that disease progressions and responses to intervention are similar in the adult and pediatric populations, and when there is a PD measurement that can predict efficacy in the pediatric population.2
Results
We reviewed 388 pediatric studies, including studies submitted in response to 97 FDA-issued Written Requests (WR), representing all of the 157 drug products that had a pediatric claim. Because a WR is issued for a particular active moiety, more than one drug product may be involved. Figure 1 shows the landscape of using an extrapolation approach across different disease areas and indications. Table 1 compares our data to those published in 2011, and shows the change in extrapolation approach over time. Our current data demonstrate a shift from a predominance of Partial to a doubling of both Complete and No Extrapolation. Complete, Partial, or No Extrapolation were used in the following percentage (number) of products: 34% (53/157), 29% (46/157), and 37% (58/157), respectively. The corresponding percentages (number) of products for the 2011 data were 14% (24/166), 68% (113/166), and 18% (29/166), respectively.
Figure 1.
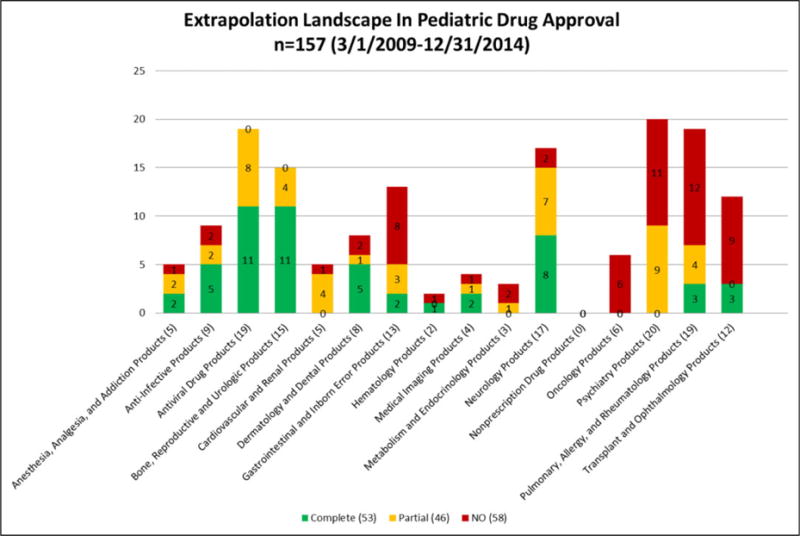
Extrapolation Landscape In Pediatric Drug Approval.
Table 1.
Overview of Extrapolation Assessment Changes.
| Extrapolation Category | Current Data Numbers of Products (%) |
Dunne’s Reference Numbers of Products (%) |
|---|---|---|
| Complete | 53 (34) | 24 (14) |
| Partial | 46 (29) | 113 (68) |
| No | 58 (37) | 29 (18) |
Evolving science and knowledge have driven the changes in the extrapolation approach. Table 2 provides examples where the Extrapolation of Efficacy has shifted over time due to increased certainty of extrapolation assumptions from evolving science and knowledge. For example, the extrapolation approach for products to treat children with partial-onset seizure (POS) in age 4 years and older has shifted from Partial to Complete Extrapolation. In contrast, the extrapolation approach for products indicated for some conditions (infantile gastroesophageal reflux disease [GERD], pediatric type 2 diabetes mellitus, intraocular hypertension, etc.) has shifted from Partial to No Extrapolation. Additionally, the efficacy of topical nasal sprays for rhinitis was initially extrapolated completely from adults and older age groups to children less than 6 years of age. Studies with children ≤6 years of age are now required because the disease is not considered sufficiently similar to that in older children or adults to permit complete extrapolation.
Table 2.
Examples of Extrapolation Approaches Driven by Evolving Science.
| Disease/Indications | Extrapolation
|
Major Reason for Shifting | Present Approach | |
|---|---|---|---|---|
| Current Data (n) | Dunne’s Reference (n) | |||
| GERD in infancy | No (n = 3) | Partial (n = 3) | Advance in understanding disease pathophysiology and progression | Requires adequate and well-controlled pediatric efficacy trials |
| Neonatal bacterial conjunctivitis | No (n = 1) | Partial (n = 3) | Multiple previous failed pediatric trials | Requires adequate and well-controlled efficacy study for neonates |
| T2DM | No (n = 5)a | Partial (n = 4) | Multiple previously failed pediatric trials resulting in better understanding of pediatric disease and its differences from adult disease | Requires 2 adequate and well-controlled efficacy trials in pediatric patients. |
| Intraocular hypertension | No (n = 1)b | Partial (n = 6) | Evolving science and confidence in similarity of disease will permit moving from No to Complete for pediatric patients | Will be Complete Extrapolation of Efficacy |
| Exocrine pancreatic insufficiency | No (n = 6) | N/A (n = 0) | New Regulatory Approach | Require pediatric trials for marketing |
| Rhinitis | No (n = 5) | Complete (n = 2) | Initially extrapolated completely from adults and older age groups to children ≤6 years of age. Disease is considered sufficiently different to require pediatric trials. | Require two adequate and well-controlled efficacy studies. SAR indication granted if PAR trials enroll adequate number of patients with concomitant SAR and efficacy is demonstrated in this subgroup analysis. |
| POS in children aged ≥4 y | Complete (n = 5) | Partial (n = 3) | The advance, based on numerous pediatric trials, in understanding disease pathophysiology and progression is similar between adults and children for this subset of seizures | Complete Extrapolation for POS pts ≥4 years of age |
| Birth control | Complete (n = 11) | N/A (n = 0) | Newly included in assessment | Complete Extrapolation of Efficacy |
Abbreviations: BBB, brain-blood barrier; GERD, gastroesophageal reflux disorder; T2DM, type 2 diabetes mellitus; POS, partial-onset seizure;
Numbers of the Written Requests issued, not numbers of products approved from 2009 through 2014.
Although the current/recent approach was no extrapolation, a recent review of pediatric trials will result in the change to complete extrapolation.
Furthermore, pediatric dominant diseases or evolution in marketing requirements for pediatrics has contributed to the increase in No Extrapolation. Products for the treatment of pediatric rare diseases with no adult counterparts, such as osteogenesis imperfecta and McCune-Albright syndrome, increased from 2% in the previous analysis, to 6% in our current analysis. This contributed to the increased numbers of No Extrapolation. An FDA approved treatment for exocrine pancreatic insufficiency was not available until April 2004. At that time, the FDA declared that all orally administered pancreatic enzyme products are considered new drugs and will require the submission and approval of a New Drug Application (NDA) if manufacturers wished to continue marketing their products. Subsequently, the efficacy of the six enzyme replacement therapy for pancreatic insufficiency, submitted to the FDA from 2009 to 2014, had to be demonstrated in two adequate and well-controlled trials in pediatric patients.
Discussion and Conclusions
Extrapolation of Efficacy plays a major role in pediatric drug development, subsequent marketing approval, and labeling to reduce off-label medicine use in children. The use of Complete, Partial, or No Extrapolation has shifted over time. Extrapolation of Efficacy needs to be implemented using the best knowledge available to support similarity and differences between pediatric and adult populations and should be routinely reevaluated as knowledge increases over time. As noted in this paper, decisions of whether or not to use and how to use Extrapolation of Efficacy in pediatric drug development evolve with the new evolving science and knowledge.
The evidence to support disease similarity and therapeutic response similarity are the fundamental questions that must be answered to utilize the approach of Extrapolation of Efficacy. Consistent with our previous publication, these similarities were assessed based on the data from human and nonclinical studies associated with disease pathogenesis, criteria for disease definition, clinical classification, measures of disease progression, pathophysiologic, histopathologic, and pathobiological characteristics. Advances in understanding of data derived from multiple sources (eg, sponsor clinical study data submissions) have increased the degree of certainty in determining the similarities or differences between pediatric and adult patients in disease progression and treatment response over time. Knowledge gained from failed pediatric trials that did not result in a new pediatric indication has also contributed to this increase in knowledge.8
Impact of Evolving Science and Knowledge on the Changes in the Extrapolation Approach
Changes in the approach to extrapolation in Table 2 are based on current scientific data and experience from human and nonclinical studies.
First, the extrapolation approach shift has been driven by the advance in understanding disease pathophysiology and progression. This can be illustrated by the change in extrapolation strategy for POS in children age 4 years and older. After 15 years of collective efforts among the FDA, academia, and industry, a total of 30 POS trials have been analyzed.9–11 Based on this analysis, the Agency concluded that POS in children 4 years of age and older, when compared to adults, has a similar disease progression, response to intervention, and exposure-response. Thus, the FDA issued a Policy Letter in 2015 to allow Complete Extrapolation of efficacy to be applied in this situation. Additional safety and pediatric dosing data are required from the pediatric population.
Another example, as shown in Table 2, is the shift over time from Partial to No Extrapolation in the extrapolation approach for products to treat infantile GERD. This evolved because the accumulated information on GERD in pediatrics has suggested that the course of GERD in adults is not sufficiently similar to the course of pathologic gastroesophageal reflux in infants to permit extrapolation.12–16 As stated in the Results section, the extrapolation approach for intraocular hypertension treatment products changed from Partial Extrapolation in Dunne’s report to No Extrapolation in our current analysis. The pediatric indication for pilocarpine was approved in 2010 based on the reported evidence from more than two adequate and controlled prospective efficacy studies in the pediatric population.17–19 This would fit in the No Extrapolation category. However, given that the course and pathophysiology of intraocular hypertension and the treatment effect are currently considered sufficiently similar in adults and pediatric patients,17–21 it would be reasonable to take a future approach of Complete Extrapolation of efficacy for the pediatric indication, if it is proven efficacious in adults and safe to use in pediatric patients. In the future, based on submitted pediatric efficacy studies, complete extrapolation will be implemented.
Secondly, another pattern shift was driven by multiple previous failures. For pediatric type 2 diabetes mellitus (T2DM), the Partial Extrapolation approach was used in all 4 products to demonstrate efficacy in pediatric patients in the past based on the assumed continuity between the adult and pediatric population.2 Yet only 1 drug, metformin, demonstrated efficacy and was approved for use in children with T2DM; the other 3 products failed to demonstrate efficacy in pediatric patients. Additionally, some notable differences in pathophysiology and clinical manifestations between adults and children were reported.8 First, children have a higher body mass index and insulin resistance than their adult counterparts.22 The progression from obesity and insulin resistance to T2DM appears faster in children than adults.23 Second, children have a shorter latency period and lower glycosylated hemoglobin, in part because there is less time for the development of glucose dysregulation.22,24–27 Finally, children have a higher incidence of ketoacidosis and glucose toxicity at diagnosis.22,24–26,28 Taken together, for current drug development programs, the FDA changed the approach to No Extrapolation and requires two adequate and well-controlled trials to demonstrate efficacy independently in pediatric patients, as shown in Table 2.
Thirdly, extrapolation pattern shifts were driven by evolution in market requirements and an increase in products for pediatric only or predominantly pediatric rare diseases. Conduct of clinical trials for disease/conditions that occur predominantly in pediatric patients contributed to the increase in numbers of No Extrapolation.
Additionally, inclusion of previously excluded indications: advances in drug labeling approaches have also increased the total number of products for Complete Extrapolation of efficacy. For example, earlier analysis of pediatric extrapolation did not include birth control products, while this current assessment does. FDA considers the postpubertal adolescent as physiologically similar to young adults, and certain birth control products (n = 11) were approved for the postpubertal pediatric population based on Complete Extrapolation of efficacy from adults. The inclusion of this indication contributed to the increased numbers of Complete Extrapolation in our current analysis. Furthermore, we have also included some examples in Table 3 that extrapolation approaches have not changed over time while the number of products being studied appears different.
Table 3.
Examples of Extrapolation Approaches With No Change Over Time.
| Disease/Indications | Extrapolation
|
Present Approach | |
|---|---|---|---|
| Current Data (n) | Dunne’s Reference (n) | ||
| Oncology products | No (n = 7) | No (n = 10) | Remains the same |
| ADHD (6–12 y) | No (n = 6) | No (n = 2) | Remains the same |
| ADHD (13–17 y) | Partial (n = 1) | Partial (n = 2) | Remains the same |
| MDD | No (n = 1) | No (n = 1) | Remains the same |
| HSV and VZV infections | Complete (n = 3) | Complete (n = 1) | Remains the same |
| HBV infection | Partial (n = 2) | Partial (n = 1) | Remains the same |
| HIV infection | Partial (n = 3) | Partial (n = 1) | Remains the same |
| HCV infection | Partial (n = 1) | Partial (n = 1) | Remains the same |
Abbreviations: ADHD, attention-deficit hyperactivity disorder; MDD, Major Depression Disorder; HSV, herpes simplex virus; VZV, varicella zoster virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
The Role of Modeling and Simulation in Extrapolation
The role of modeling and simulation in extrapolation has been discussed over the years. Modeling and simulation allow one to utilize data from other sources, including adults and other pediatric populations, to model/simulate data responses in pediatric population.29 These approaches have proven useful in dose finding and defining the degree of the similarity in exposure-response relationship between adult and pediatric patients. The reliability of these prior data needs to be ensured by the clinicians for the purpose of modeling and simulation. The role of modeling and simulation in Extrapolation of Efficacy is potentially a powerful tool, but requires further validation and exploration. These data currently include no example where this effort changed the approach to extrapolation.
Extrapolation Framework for the Path Forward
Finally, we would like to offer a few thoughts on the extrapolation framework for the path forward. The determination of an extrapolation approach toward a particular indication/disease is an evolving process informed by experience and new science. To increase the impact of extrapolation, some questions may need to be explored, such as whether or not Extrapolation of Efficacy could be used across indications (ie, antibiotics) and across formulations (ie, extrapolates data from intravenous to subcutaneous). To further enhance the application of pediatric Extrapolation of Efficacy, we need to leverage prior data, including those from all previous adult and pediatric clinical trials. Innovative approaches such as modeling/simulation and Bayesian statistics, built on the human and nonclinical data, may enhance understanding of disease biology and assist in minimizing uncertainties in pediatric extrapolation assumptions. However, the potential roles and functions of the modeling and simulation in Extrapolation of Efficacy still require further exploration and must be based on clinical input. Modeling is a tool that can help inform decision making, but it is new science or new understanding of the pathophysiology or expression of the disease in children that will drive change in Extrapolation activities.
Acknowledgments
The authors would like to express gratitude to Alex Gorovets, MD, for the helpful discussion and expertise regarding medical imaging products. The authors also acknowledge Amy Odegaard, MPH, and Ms Mary DeCelle for their assistance in data extraction and Yeruk (Lily) Mulugeta, PharmD, for her assistance with preliminary data sharing in the early phase of the project. The authors would also like to acknowledge Ms Suzanne Malli, BA, BSN, for participation in the scientific discussions and Pamela J. Weinel, MS, MBA, RN, for her editorial assistance in preparing the final submission draft of the manuscript.
Funding
No financial support of the research, authorship, and/or publication of this article was declared.
Footnotes
Author Note
Views expressed in this manuscript are those of the authors and do not necessarily reflect the official positions or policies of the FDA.
Declaration of Conflicting Interests
No potential conflicts were declared.
References
- 1.Sachs AN, Avant D, Lee CS, Rodriguez W, Murphy MD. Pediatric information in drug product labeling. JAMA. 2012;307:1914–1915. doi: 10.1001/jama.2012.3435. [DOI] [PubMed] [Google Scholar]
- 2.Dunne J, Rodriguez WJ, Murphy MD, et al. Extrapolation of adult data and other data in pediatric drug-development programs. Pediatrics. 2011;128:e1242–e1249. doi: 10.1542/peds.2010-3487. [DOI] [PubMed] [Google Scholar]
- 3.FDA. Specific requirements on content and format of labeling for human prescription drugs; revision of “pediatric use” subsection in the labeling; final rule. Fed Regist. 1994;59:64240. https://www.federalregister.gov/documents/1994/12/13/94-30238/specific-requirements-on-content-and-format-of-labeling-for-human-prescription-drugs-revision-of. [Google Scholar]
- 4.The Pediatric Research Equity Act 2007. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM049870.pdf.
- 5.Best Pharmaceuticals for Children Act 2007. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM049870.pdf.
- 6.FDA. Guidance for industry: the content and format for pediatric use supplements. FDA; http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071957.pdf. Published 1996. [Google Scholar]
- 7.FDA. Guidance for industry: pediatric oncology studies in response to a written request (Draft Guidance) https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071976.pdf. Published 2000.
- 8.Christensen ML, Franklin BE, Momper JD, Reed MD. Pediatric drug development programs for type 2 diabetes: a review. J Clin Pharmacol. 2015;55:731–738. doi: 10.1002/jcph.497. [DOI] [PubMed] [Google Scholar]
- 9.Holmes GL. Data related to disease similarity—a case study: PEACE initiative in pediatric epilepsy Paper presented at: Quantitative Assessment of Assumptions to Support Extrapolation of Efficacy in Pediatrics. FDA/University of Maryland CERSI Workshop; June 1, 2016; Silver Spring, MD. http://www.pharmacy.umaryland.edu/media/SOP/wwwpharmacyumarylandedu/centers/cersievents/pedsextrapolation/holmes-presentation-notes.pdf. [Google Scholar]
- 10.Mehrotra S. Quantitative analysis to support full extrapolation of efficacy in children for partial onset seizures in adjunctive setting: FDA-PEACE initiative Paper presented at: Quantitative Assessment of Assumptions to Support Extrapolation of Efficacy in Pediatrics. FDA/University of Maryland CERSI Workshop; June 1, 2016; Silver Spring, MD. http://www.pharmacy.umaryland.edu/media/SOP/wwwpharmacyumarylandedu/centers/cersievents/pedsextrapolation/mehrotra-presentation-notes.pdf. [Google Scholar]
- 11.Sheridan P. Pediatric extrapolation of efficacy in partial seizures: regulatory perspective Paper presented at: Quantitative Assessment of Assumptions to Support Extrapolation of Efficacy in Pediatrics. FDA/University of Maryland CERSI Workshop; June 1, 2016; Silver Spring, MD. http://www.pharmacy.umaryland.edu/media/SOP/wwwpharmacyumarylandedu/centers/cersievents/pedsextrapolation/sheridan-presentation-notes.pdf. [Google Scholar]
- 12.Lightdale JR, Gremse DA. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics. 2013;131:e1684–e1695. doi: 10.1542/peds.2013-0421. [DOI] [PubMed] [Google Scholar]
- 13.Orenstein SR, Izadnia F, Khan S. Gastroesophageal reflux disease in children. Gastroenterol Clin North Am. 1999;28:947–969. doi: 10.1016/s0889-8553(05)70099-6. [DOI] [PubMed] [Google Scholar]
- 14.Vandenplas Y, Belli D, Benhamou P, et al. A critical appraisal of current management practices for infant regurgitation—recommendations of a working party. Eur J Pediatr. 1997;156:343–357. doi: 10.1007/s004310050613. [DOI] [PubMed] [Google Scholar]
- 15.Vandenplas Y, Hauser B. An updated review on gastroesophageal reflux in pediatrics. Expert Rev Gastroenterol Hepatol. 2015;9:1511–1521. doi: 10.1586/17474124.2015.1093932. [DOI] [PubMed] [Google Scholar]
- 16.Vandenplas Y, Rudolph CD, Di Lorenzo C, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESP-GHAN) J Pediatr Gastroenterol Nutr. 2009;49:498–547. doi: 10.1097/MPG.0b013e3181b7f563. [DOI] [PubMed] [Google Scholar]
- 17.Bussieres JF, Therrien R, Hamel P, Barret P, Prot-Labarthe S. Retrospective cohort study of 163 pediatric glaucoma patients. Can J Ophthalmol. 2009;44:323–327. doi: 10.3129/i09-065. [DOI] [PubMed] [Google Scholar]
- 18.Papadopoulos M, Cable N, Rahi J, Khaw PT. The British Infantile and Childhood Glaucoma (BIG) Eye Study. Invest Ophthalmol Vis Sci. 2007;48:4100–4106. doi: 10.1167/iovs.06-1350. [DOI] [PubMed] [Google Scholar]
- 19.Plager DA, Whitson JT, Netland PA, et al. Betaxolol hydrochloride ophthalmic suspension 0.25% and timolol gel-forming solution 0.25% and 0.5% in pediatric glaucoma: a randomized clinical trial. J AAPOS. 2009;13:384–390. doi: 10.1016/j.jaapos.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Fung DS, Roensch MA, Kooner KS, Cavanagh HD, Whitson JT. Epidemiology and characteristics of childhood glaucoma: results from the Dallas Glaucoma Registry. Clin Ophthalmol. 2013;7:1739–1746. doi: 10.2147/OPTH.S45480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitson JT, Roarty JD, Vijaya L, et al. Efficacy of brinzolamide and levobetaxolol in pediatric glaucomas: a randomized clinical trial. J AAPOS. 2008;12:239–246 e3. doi: 10.1016/j.jaapos.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Elder DA, Herbers PM, Weis T, Standiford D, Woo JG, D’Alessio DA. Beta-cell dysfunction in adolescents and adults with newly diagnosed type 2 diabetes mellitus. J Pediatr. 2012;160:904–910. doi: 10.1016/j.jpeds.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gungor N, Arslanian S. Progressive beta cell failure in type 2 diabetes mellitus of youth. J Pediatr. 2004;144:656–659. doi: 10.1016/j.jpeds.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 24.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90:493–500. doi: 10.1210/jc.2004-1133. [DOI] [PubMed] [Google Scholar]
- 25.Goran MI, Ball GD, Cruz ML. Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. J Clin Endocrinol Metab. 2003;88:1417–1427. doi: 10.1210/jc.2002-021442. [DOI] [PubMed] [Google Scholar]
- 26.Elder DA, Hornung LN, Herbers PM, Prigeon R, Woo JG, D’Alessio DA. Rapid deterioration of insulin secretion in obese adolescents preceding the onset of type 2 diabetes. J Pediatr. 2015;166:672–678. doi: 10.1016/j.jpeds.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gungor N, Saad R, Janosky J, Arslanian S. Validation of surrogate estimates of insulin sensitivity and insulin secretion in children and adolescents. J Pediatr. 2004;144:47–55. doi: 10.1016/j.jpeds.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 28.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CERSI. Quantitative Assessment of Assumptions to Support Extrapolation of Efficacy in Pediatrics. at http://www.pharmacy.umaryland.edu/centers/cersievents/pedsextrapolation/. [Public Workshop]. Published 2016.


