Abstract
Purpose
We present a large experience (73 patients) using a standard radiotherapy (RT) protocol to prevent relapse in cranial sites where measurable metastatic neuroblastoma (NB), which is an adverse prognostic marker, is common.
Methods and Materials
High-risk NB patients with measurable cranial disease at diagnosis or residual cranial disease post-induction had those sites irradiated with hyperfractionated 21 Gy; a brain-sparing technique was used for an extensive field. Patients were grouped based on response to systemic therapy. Thus, when irradiated, Group 1 patients were in complete remission and Group 2 patients had primary refractory disease. Follow-up was from the start of cranial RT.
Results
At three years, the 39 Group 1 patients had a progression-free survival (PFS) rate of 51%; control of cranial disease was 79%. Two relapses involved irradiated cranial sites. Two other patients relapsed in irradiated cranial sites 6 and 12 months after a systemic relapse. At three years, the 34 Group 2 patients had a PFS rate of 33%; control of cranial disease was 52%. Group 2 included 19 patients who had residual cranial (+ extracranial) disease. Cranial sites showed major (n=13), minor (n=2), or no response (n=4) to RT; five patients progressed in the cranial RT field at 10-to-27 months. Group 2 also included 15 patients who had persistent NB in extracranial but not cranial sites: two relapsed in irradiated cranial sites and elsewhere at 8 and 14 months. Cranial RT was well tolerated, with no grade ≥2 toxicities.
Conclusions
Hyperfractionated 21 Gy cranial RT may help control NB and is feasible without significant toxicity in children.
Keywords: cranial metastases, hyperfractionated radiotherapy, neuroblastoma, radiotoxicity, prognosis
INTRODUCTION
For high-risk neuroblastoma (NB), chemotherapy dose-intensification has improved response rates,1,2 and myeloablative therapy,3 13-cis-retinoic acid,3 and immunotherapy4 may have decreased relapse rates. Radiotherapy (RT) appears to help prevent relapse in the primary site and is therefore standard of care for that purpose.5,6 In contrast, RT is not routinely used to consolidate regression in sites of measurable metastatic disease when post-induction studies show no evidence of NB. Cranial sites merit special attention, as explained below.
Measurable metastatic involvement of skull bones by NB is common.7–9 Possible reasons include the relatively large size of the head in young children who comprise 90% of NB patients, and the extensive red bone marrow (BM) still present in their cranium; trophic factors may also have a role.10 The prominence of metastatic spread of NB to cranial sites has intrigued investigators for 100 years,11 “raccoon eyes” from ecchymotic proptosis are an ominous hallmark of NB, and large cranial metastases can cause blindness.12
Of 182 patients treated for newly- or recently-diagnosed high-risk NB at Memorial Sloan-Kettering Cancer Center (MSKCC), 1990–2007, 54 (30%) presented with measurable disease in cranial sites. A 21% incidence at diagnosis of “intracranial/orbit” involvement was documented in 567 stage 4 patients (all ages) treated on Children’s Cancer Group (CCG) protocols 1989–1996.9 The true incidence was probably higher given that the patients were not routinely evaluated by computed tomography (CT) or magnetic resonance imaging (MRI) of the head, and only 18% of patients underwent 123I-metaiodobenzylguanidine (MIBG) scintigraphy, which greatly improves disease detection. The cranial sites of metastatic NB almost invariably involve cortical bone; central nervous system parenchymal metastases are exceedingly rare at diagnosis. In contrast, measurable metastatic NB in extracranial sites at initial presentation is common in soft tissue (especially lymph nodes or liver), but very rare in skeleton. In fact, no patient in the MSKCC series mentioned above had extracranial skeletal lesions that were measurable (to be distinguished from the diffuse and widespread osteomedullary MIBG uptake characteristic of NB).
In 1988, a NB patient of ours relapsed periorbitally where a large mass had been present at diagnosis. That tragic occurrence prompted us to adopt a consistent standardized RT approach5 aimed at helping to prevent the lethal scenario of relapse in sites of metastatic NB including in the head. Also in 1988, a 19-month old NB patient with multiple visible calvarial masses at diagnosis received whole brain irradiation (24 Gy); significant hormonal and neurodevelopmental problems ensued. We subsequently applied brain-sparing techniques, exploiting the low penetrance of electron beams, to minimize risks of such toxicities.13
In young NB patients, irradiating cranial sites poses major challenges. These include avoiding all of the following: cosmetic sequelae from impaired bone growth; neurodevelopmental delay from effects on the brain; further ototoxicity already incurred by platinum-based chemotherapy; exacerbation of poor dentition resulting from intensive chemotherapy; and vision loss. An additional challenge is integrating RT into the overall complex treatment program used for this aggressive cancer. The need for general anesthesia in very young children is also an issue. We now describe our results with cranial RT.
METHODS AND MATERIALS
MSKCC patients with high-risk NB (>18 months old at diagnosis or MYCN-amplified NB at any age) plus measurable cranial disease at diagnosis or residual cranial disease after induction receive a standard RT protocol5 to one or more cranial sites. (Measurable disease is distinct from the commonly seen osteomedullary MIBG uptake.) We herein group patients based on response to systemic therapy. The RT is used a) to help consolidate first complete or very good partial remission (CR/VGPR) (Group 1), or b) as part of treatment of primary refractory disease, i.e., incomplete response to induction therapy but no progressive disease (PD) (Group 2).
The standard RT protocol delivers external beam RT, planned with CT: 6 MV photons and 6–16 MeV electrons are used, depending on the specific target and on the critical surrounding normal tissues (e.g., brain). The total dose is 21 Gy, given in 1.5 Gy fractions, two fractions per day (4–6 hours apart), on seven consecutive weekdays, as described.5,13 In a brain-sparing technique for patients with extensive calvarial involvement by NB, right and left lateral 6 MeV electron fields were used to minimize the depth of the radiation dose.13 Consent for RT is obtained in accordance with Institutional Review Board guidelines, with RT as part of MSKCC protocols.
Disease status is assessed every two-to-three months through ≥3 years from diagnosis by CT or MRI of sites that have or had measurable disease (including the primary site and distant sites such as the head and orbits), 131I- or 123I-MIBG scan, urine catecholamine levels, and histochemical studies of bilateral BM biopsies (two-to-four sites) and aspirates (four sites). The International NB Response Criteria14 are used: CR, no evidence of disease; VGPR, volume of primary mass reduced by 90%-99%, no evidence of distant disease (including normal MIBG) except for skeletal residua, catecholamines normal; partial response (PR), >50% decrease in measurable disease and ≤1 positive BM site; mixed response, >50% decrease of any lesion with <50% decrease in any other; no response (NR), <50% decrease but <25% increase in any lesion; and PD, new lesion or >25% increase in an existing lesion.
Toxicity is graded using the National Cancer Institute Common Toxicity Criteria.
Calculating from the start of cranial RT, the probability ± standard error (SE) of failure in the cranial RT field was estimated using the cumulative incidence function,15 and progression-free survival (PFS) was estimated using the Kaplan-Meier method.16
RESULTS
Patient characteristics (Table 1)
Table 1.
Clinical profile
| Group 1 (n=39) |
Group 2 (n=34) |
Total (n=73) |
|
|---|---|---|---|
| Male:female | 19:20 | 16:18 | 35:38 |
| Age (yrs) at Dx: Median (range) | 3.1 (0.9 – 10.1) | 3.9 (1.5 – 20.5) | 3.3 (0.9 –20.5) |
| Age (yrs) at RT: Median (range) | 3.6 (1.4 – 10.7 | 4.6 (2.2 – 20.8) | 4.2 (1.4 – 20.8) |
| Months from Dx to RT: Median (range) | 7.5 (2.8 – 11.3) | 8.2 (3.3 – 23.6) | 7.8 (2.8 – 23.6) |
| MYCN amplification | 17 (50%) of 34 | 6 (23%) of 26 | 23 (38%) of 60 |
| Cranial sites* | |||
| Calvarium, diffuse/localized | 12/6 | 15/12 | 27/18 |
| Orbit, bilateral/unilateral | 11/10 | 3/2 | 15/11 |
| Base of skull | 6 | 4 | 10 |
| Mandible | 6 | 3 | 9 |
| Treatments | |||
| Chemotherapy** induction alone | 1 | 1 | 2 |
| + 3F8 | 7 | 16 | 23 |
| + SCT + 3F8 | 19 | 11 | 30 |
| + 131I-3F8 + 3F8 | 10 | 5 | 15 |
| + SCT | 2 | 1 | 3 |
Dx, diagnosis; RT, radiotherapy; SCT, stem-cell transplantation.
Some patients had more than one site involved and irradiated.
N6/N7 chemotherapy regimen2 in 64 (88%) of the 73 patients.
Among the total of 73 patients (52% female), 39 were treated with cranial RT in first CR/VGPR (Group 1) and 34 had primary refractory disease (Group 2). Group 2 included six adolescents/adults but Group 1 had none. The median time from diagnosis to cranial RT was similar for the two groups (~8 months), but five Group 2 patients were treated >14 months from diagnosis. Amplified MYCN was more frequent in Group 1 (50%) than in Group 2 (23%) patients. There were no early relapses and all patients were followed for >2 months from cranial RT.
The most common targets were calvarial and orbital sites. Thus, calvarial sites were irradiated in 46% of Group 1 and in 79% of Group 2 patients, and one or both orbits were irradiated in 54% of Group 1 and 15% of Group 2 patients. The mandible was irradiated in nine patients. A brain-sparing technique13 was used in 22 patients.
Induction chemotherapy in 88% of the patients consisted of the N6/N7 regimen,2 including 38 (97%) of the 39 Group 1 patients and 27 (79%) of the 34 Group 2 patients. Consolidative systemic treatment varied depending on the era but included immunotherapy with the anti-GD2 3F8 monoclonal antibody4,17,18 in all but five patients, myeloablative chemotherapy with stem-cell transplantation in 33 patients, and targeted RT using 131I-3F818 in 15 patients.
Group 1: disease control
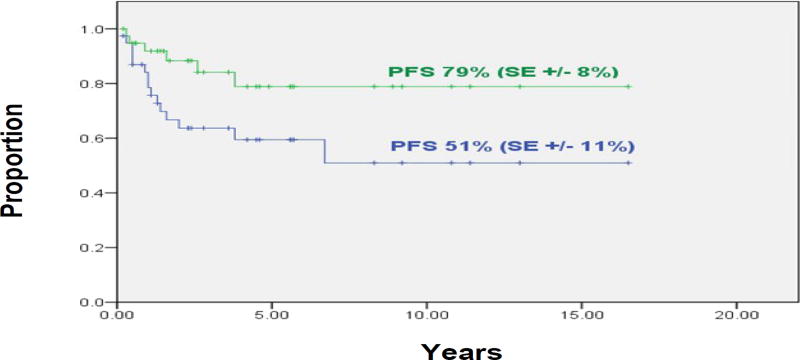
At three years, the 39 patients treated in first CR/VGPR had a PFS rate of 51% (SE±11%) and control of their cranial disease was 79% (SE±8%) (Figure 1). Sixteen patients relapsed at 1-to-79 (median, 12) months. Two of these initial relapses were in the cranial RT field (one was an isolated relapse at 46 months). Two of the other 14 relapses were in sites within the cranial RT field but occurred (at six and 12 months, respectively) after the initial (systemic) relapse.
Figure 1.
Group 1 patients (n=39): Progression-free survival (green line) and probability of local failure in irradiated cranial sites (blue line).
Group 2: disease control
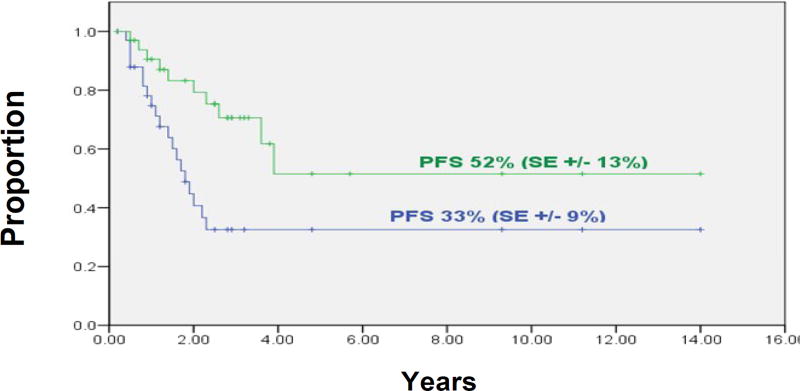
At three years, the 34 patients in Group 2 had a PFS rate of 33% (SE±9%), and control of their cranial disease was 52% (SE±13%) (Figure 2).
Figure 2.
Group 2 patients (n=34): Progression-free survival (green line) and probability of local failure in irradiated cranial sites (blue line).
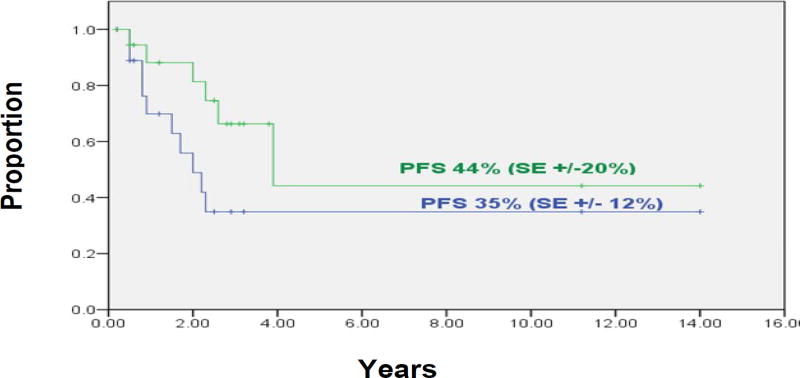
Group 2 included 19 patients who had persistent cranial disease with (n=13) or without (n=6) NB elsewhere. Those sites of cranial disease showed CR (n=12, including one treated concomitantly with irinotecan-temozolomide which may have radiation-sensitizing activity), PR (n=1), less than PR (n=2), and NR (n=4) to RT (Table 2). At three years, the PFS rate of these 19 patients was 35% (±12%) and control of cranial disease was 44% (±20%) (Figure 3). Of these 19 patients, 14 have not progressed in cranial sites at 4+ to 134+ (median, 33+) months, four had PD in the cranial RT field and elsewhere at 10, 14, 20, and 27 months, and one had an isolated relapse in the cranial field at 23 months. Regarding the subset of five patients whose persistent cranial NB was irradiated >14 months from diagnosis, two relapsed in cranium and elsewhere at 14 and 27 months, two relapsed in extracranial sites at five and 23 months but not in cranial sites through 45+ and 55+ months, and one is in an overall CR at 37+ months.
Table 2.
Responses of residual cranial disease* to radiotherapy
| Patient subsets | CR/VGPR | PR | <PR | NR |
|---|---|---|---|---|
| Patients** with no residual extracranial disease (n=6) | 5 | 0 | 0 | 1 |
| Patients with residual extracranial disease | ||||
| Children (n=9) | 5 | 0 | 2 | 2 |
| Adolescents/Adults (n=4) | 2 | 1 | 0 | 1 |
Orbital site alone in one patient; all other patients had residual neuroblastoma in calvarial bones.
Exclusively children
Figure 3.
Group 2 patients (n=19) who had residual cranial disease when treated with cranial radiotherapy: Progression-free survival (green line) and probability of local failure in irradiated cranial sites (blue line).
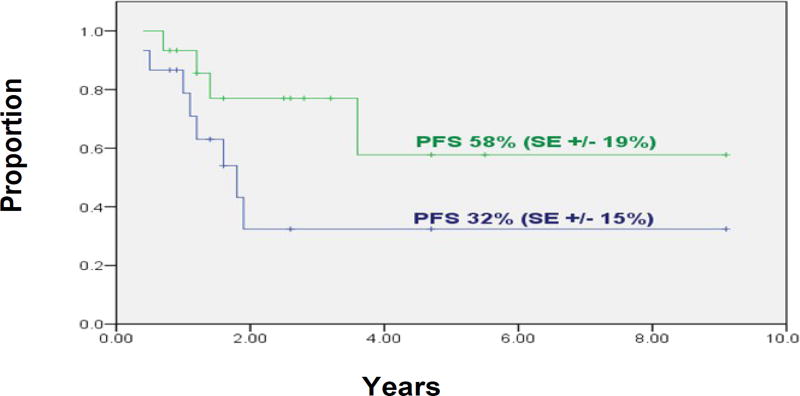
Group 2 also included 15 patients who had primary refractory NB in extracranial sites, but no evidence of persistent NB in cranial sites: 13 have not relapsed in the cranial RT field with a follow-up of 10+ to 104+ (median, 35+) months, and two relapsed within irradiated cranial sites and elsewhere at 8 and 14 months. At three years, the PFS rate of these 15 patients was 32% (+15%) and control of cranial disease was 58% (+19%) (Figure 4).
Figure 4.
Group 2 patients (n=15) who had no evidence of residual cranial disease when treated with cranial radiotherapy: Progression-free survival (green line) and probability of local failure in irradiated cranial sites (blue line).
Among the six adolescents/adults in Group 2, two had no evidence of cranial disease when they received cranial RT but both relapsed in cranial and extracranial sites at 8 and 14 months. Four adolescents/adults had persistent cranial disease that was irradiated (Table 2): one had NR but is progression-free at 12+ months, and three achieved CR or PR, with one relapsing at 20 months in the cranium and elsewhere, one in overall CR at 15+ months, and one relapsing elsewhere but not in the cranial field through 37 months.
Toxicity
The cranial RT procedure was well tolerated. Young patients received propofol for general anesthesia and could drink clear liquids until two hours before each treatment, which was outpatient. (With RT sessions in the morning and early afternoon, children had plenty of time to eat and play normally each day.) Acute side-effects were less than grade 2. Superficial RT using electrons resulted in clinically insignificant erythema, calvarial irradiation caused self-limited alopecia, and one patient developed zoster in an irradiated dermatome. The cranial RT caused no hormonal deficiencies, myelosuppression, cosmetic sequelae, or vision problems. The longest female survivor, treated with a brain-sparing technique, became pregnant. Hearing loss was attributable to the cisplatin used in induction.19 Neurodevelopmental delay in some patients was a preexisting condition or multifactorial in etiology. One patient had mild generalized thinning of hair through 24+ months after RT to an extensive cranial field. Dentition was comparable to patients treated with intensive chemotherapy but no cranial RT; acute and long-term dental side-effects were less than grade 2. There have been no secondary solid tumors.
DISCUSSION
Eradicating all cancer cells is required for cure but is a daunting challenge with high-risk NB in part because of its typically massive metastatic tumor burden. We have used hyperfractionated 21-Gy RT not only for ablating visible residual metastatic NB, but also for preventing relapse in distant sites deemed to be especially worrisome because they contained measurable NB at diagnosis.5,13 We do not rely on chemotherapy to be effective in lysing all cancer cells in those sites, even when follow-up scans show no NB. Thus, RT to previous sites of measurable disease - as well as to sites of persistent disease - has been an integral part of our multimodality treatment program aimed at applying all reasonable measures to kill every malignant neuroblast.
NB patients with measurable cranial involvement who are >18 months old at diagnosis and/or have MYCN-amplified disease constitute a particularly high-risk cohort,9 at least in part due to the considerable extent of disease but also possibly because the lesions might be surrogate markers for aggressive disease. That was one conclusion from the landmark CCG study (567 stage 4 patients) correlating metastatic sites with outcome. In that study, cranial or orbital lesions were significantly associated with an unfavorable prognosis.9 We therefore view as encouraging the three-year PFS rates of 51% for Group 1 and 32% for Group 2 (which included adolescents/adults, an age group associated with NB excessively resistant to cytotoxic therapy). Effective systemic therapy is critical, but a contributory role to those results can be ascribed to cranial RT, given the excellent control of cranial disease in both Group 1 and Group 2, as well as the high response rate of persistent post-induction cranial disease in Group 2. Conversely, ineffective systemic therapy can result in widespread relapse with re-seeding of cranial sites as the reason for recurrence of NB within the cranial RT field. Despite variations in systemic therapies, 68 (93%) of the 73 patients received 3F8 immunotherapy and 64 (88%) received the same dose-intensive induction. The cranial RT protocol was identical in all patients.
As previously explained,5 we decided on hyperfractionated 21-Gy RT because it promised good local control plus less toxicity (compared to standard fractionation), based on the following: 1) doses in the 16-to-35 Gy range produced clinically important anti-NB effects;20,21 2) laboratory studies showed a low repair capacity of NB cell lines,22,23 as well as optimal cytotoxity, with twice-daily fractions of 1.2-to-1.5 Gy delivered six hours apart;23 and 3) the alpha/beta model favored a relatively low fraction size as a way to minimize late toxicities in healthy tissue.24 Fortunately, 21 Gy has a modest impact on bone growth and formation.25 Combined photon/electron energy allows full-dose RT to sites of concern in, for example, calvarial bones and skull base, but spares underlying brain and the pituitary gland.13
It is now generally accepted, based on large studies,5,6 that local RT to the primary site to help consolidate remission is warranted in patients with high-risk NB but it is not routinely prescribed for sites of measurable metastatic disease in CR. A randomized trial might be necessary to prove a disease-control advantage, and an acceptable toxicity risk, of adding local RT to consolidate CR of distant measurable NB metastases, including in cranial sites. We suggest, however, that support for that strategy comes from the experience reported herein, with excellent local control and negligible toxicity to date. In addition, experience elsewhere confirms that the majority of relapses occur in previous sites of distant disease,26–28 including the skull,28 prompting some investigators to question the policy of not irradiating sites of measurable metastatic NB that become MIBG-negative.28 The urgency of the issue is driven home by cases such as the one cited in the Introduction and the case of a child recently referred to us after an isolated relapse in the site of a temporal skull mass visible at diagnosis but not irradiated because of CR. Staging patients using 123I-MIBG is more sensitive than using 131I-MIBG, and is mandatory for accurate assessment of disease status,29 but scintigraphic studies cannot detect very minimal residual disease.
We are deeply concerned about sequelae from cytotoxic therapy, as evidenced by our reports on a) late effects of oncologic treatment,19,30,31 b) our avoidance of chemotherapy and radiotherapy in many NB patients for whom standard guidelines dictate their use,32–34 and c) our efforts to reduce therapy for high-risk NB.2 Yet the findings to date regarding cranial RT indicate a paucity of short-term problems and we view disease control as the priority.
A twice-daily schedule of 21 Gy cranial RT is feasible in young patients. The absence of myelosuppression and mucositis allows concomitant use, if indicated, of chemotherapy which can potentially sensitize NB to RT. The low RT dose, the limited RT penetration with electron energy, and, possibly, the hyperfractionation minimize risks of sequelae such as permanent alopecia, distorted facial growth, hypothalamic/pituitary insufficiency, and neurodevelopmental delay. Dentition remains a concern with mandibular irradiation. Longer follow-up may yet reveal a risk of secondary neoplasms.
Acknowledgments
Supported in part by National Cancer Institute grants no. CA61017 and CA72868, the Robert Steel Foundation, New York, NY, and the Katie’s Find A Cure Fund, New York, NY
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest in this work.
References
- 1.Pearson ADJ, Craft AW, Pinkerton CR, Meller ST, Reid MM. High-dose rapid schedule chemotherapy for disseminated neuroblastoma. Eur J Cancer. 1992;28A:1654–1659. doi: 10.1016/0959-8049(92)90062-7. [DOI] [PubMed] [Google Scholar]
- 2.Kushner BH, Kramer K, LaQuaglia MP, Modak S, Yataghene K, Cheung N-KV. Reduction from seven to five cycles of intensive induction chemotherapy in children with high-risk neuroblastoma. J Clin Oncol. 2004;22:4888–4892. doi: 10.1200/JCO.2004.02.101. [DOI] [PubMed] [Google Scholar]
- 3.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 4.Cheung N-KV, Kushner BH, Cheung I, et al. Anti-GD2 antibody treatment of minimal residual stage 4 neuroblastoma diagnosed at more than 1 year of age. J Clin Oncol. 1998;16:3053–3060. doi: 10.1200/JCO.1998.16.9.3053. [DOI] [PubMed] [Google Scholar]
- 5.Kushner BH, Wolden S, LaQuaglia MP, et al. Hyperfractionated low-dose (21 Gy) radiotherapy for high-risk neuroblastoma following intensive chemotherapy and surgery. J Clin Oncol. 2001;19:2821–2828. doi: 10.1200/JCO.2001.19.11.2821. [DOI] [PubMed] [Google Scholar]
- 6.Hans-Kogan DA, Swift PS, Selch M, et al. Impact of radiotherapy for high-risk neuroblastoma: A Children’s Cancer Group study. Int J Radiat Oncol Biol Phys. 2003;56:28–39. doi: 10.1016/s0360-3016(02)04506-6. [DOI] [PubMed] [Google Scholar]
- 7.Alberts AS, Weich DJV, Anderson JD. [Increased incidence of skull metastases in neuroblastoma] S Afr Med J. 1984;66:62–63. [PubMed] [Google Scholar]
- 8.Taguchi N, Koide R, Tsunematsu Y, et al. Bone metastasis of malignant solid tumors in childhood. Jpn J Cancer Chemother. 1987;14:1723–1728. [PubMed] [Google Scholar]
- 9.DuBois SG, Kalika Y, Lukens JN, et al. Metastatic sites in stage IV and IVS neuroblastoma 16 correlate with age, tumor biology, and survival. J Pediatr Hematol/Oncol. 1999;21:181–189. doi: 10.1097/00043426-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 10.de la Monte SM, Moore GW, Hutchins GM. Nonrandom distribution of metastases in neuroblastic tumors. Cancer. 1983;52:915–925. doi: 10.1002/1097-0142(19830901)52:5<915::aid-cncr2820520529>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Hutchinson R. On suprarenal sarcoma in children with metastases in the skull. Quart J Med. 1907;1:31–38. [Google Scholar]
- 12.Belgaumi AF, Kauffman WM, Jenkins JJ, et al. Blindness in children with neuroblastoma. Cancer. 1997;80:1997–2004. [PubMed] [Google Scholar]
- 13.Wolden SL, Barker CA, Kushner BH, et al. Brain-sparing radiotherapy for neuroblastoma skull metastases. Pediatr Blood Cancer. 2008;50:1163–1168. doi: 10.1002/pbc.21384. [DOI] [PubMed] [Google Scholar]
- 14.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 15.Prentice RL, Kalbfleisch JD, Peterson AV, et al. The analysis of failure time data in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 17.Kushner BH, Kramer K, Cheung N-KV. Phase II trial of the anti-GD2 monoclonal antibody 3F8 and granulocyte-macrophage colony-stimulating factor for neuroblastoma. J Clin Oncol. 2001;19:4189–4194. doi: 10.1200/JCO.2001.19.22.4189. [DOI] [PubMed] [Google Scholar]
- 18.Cheung N-KV, Kushner BH, LaQuaglia M, et al. N7: A novel multi-modality therapy of high risk neuroblastoma (NB) in children diagnosed over 1 year of age. Med Pediatr Oncol. 2001;36:227–230. doi: 10.1002/1096-911X(20010101)36:1<227::AID-MPO1055>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 19.Kushner BH, Budnick A, Kramer K, Modak S, Cheung N-KV. Ototoxicity from high-dose 17 use of platinum compounds in patients with neuroblastoma. Cancer. 2006;107:417–422. doi: 10.1002/cncr.22004. [DOI] [PubMed] [Google Scholar]
- 20.Halperin EC, Cox EB. Radiation therapy in the management of neuroblastoma: The Duke University Medical Center experience 1967–1984. Int J Radiat Oncol Biol Phys. 1986;12:1829–1837. doi: 10.1016/0360-3016(86)90326-3. [DOI] [PubMed] [Google Scholar]
- 21.Rosen EM, Cassady JR, Frantz CN, et al. Neuroblastoma: The Joint Center for Radiation Therapy/Dana-Farber Cancer Institute/Children’s Hospital experience. J Clin Oncol. 1984;2:719–732. doi: 10.1200/JCO.1984.2.7.719. [DOI] [PubMed] [Google Scholar]
- 22.Deacon JM, Wilson PA, Peckham MJ. The radiobiology of human neuroblastoma. Radiother Oncol. 1985;3:201–209. doi: 10.1016/s0167-8140(85)80029-3. [DOI] [PubMed] [Google Scholar]
- 23.Wheldon TE, O’Donoghue J, Gregor A, et al. Radiobiological considerations in the treatment of neuroblastoma by total body irradiation. Radiother Oncol. 1986;6:317–326. doi: 10.1016/s0167-8140(86)80199-2. [DOI] [PubMed] [Google Scholar]
- 24.Thames HD, Withers HR, Peters LJ, et al. Changes in early and late radiation responses with altered fractionation: Implications for dose-survival relationships. Int J Radiat Oncol Biol Phys. 1982;8:219–226. doi: 10.1016/0360-3016(82)90517-x. [DOI] [PubMed] [Google Scholar]
- 25.Willman KY, Cox RS, Donaldson SS. Radiation induced height impairment in pediatric Hodgkin’s disease. Int J Radiat Oncol Biol Phys. 1994;28:85–92. doi: 10.1016/0360-3016(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 26.Matthay KK, Atkinson JB, Stram DO, et al. Patterns of relapse after autologous purged bone marrow transplantation for neuroblastoma: A Childrens Cancer Group pilot study. J Clin Oncol. 1993;11:2226–2233. doi: 10.1200/JCO.1993.11.11.2226. [DOI] [PubMed] [Google Scholar]
- 27.Sibley GS, Mundt AJ, Goldman S, et al. Patterns of failure following total body irradiation and bone marrow transplantation with or without a radiotherapy boost for advanced neuroblastoma. Int J Radiat Oncol Biol Phys. 1995;32:1127–1135. doi: 10.1016/0360-3016(95)00011-m. [DOI] [PubMed] [Google Scholar]
- 28.Sangthawan D, Desrosiers PM, Randall ME, Robertson K, Goebel Fallon R. Relapse in the skull after myeloablative therapy for high-risk neuroblastoma. Pediatr Hematol Oncol. 2003;20:23–30. [PubMed] [Google Scholar]
- 29.Kushner BH, Yeh SDJ, Kramer K, Larson SM, Modak S, Cheung N-KV. Impact of MIBG scintigraphy on assessing response of high-risk neuroblastoma to dose-intensive induction chemotherapy. J Clin Oncol. 2003;21:1082–1086. doi: 10.1200/JCO.2003.07.142. [DOI] [PubMed] [Google Scholar]
- 30.Kushner BH, Cheung N-KV, Kramer K, Heller G, Jhanwar JC. Neuroblastoma and treatment-related myelodysplasia/leukemia: The Memorial Sloan-Kettering experience and a literature review. J Clin Oncol. 1998;16:3880–3889. doi: 10.1200/JCO.1998.16.12.3880. [DOI] [PubMed] [Google Scholar]
- 31.Lavardiere C, Cheung N-KV, Kushner BH, et al. Long-term complications in survivors of advanced stage neuroblastoma. Pediatr Blood Cancer. 2005;45:324–332. doi: 10.1002/pbc.20331. [DOI] [PubMed] [Google Scholar]
- 32.Cheung N-KV, Kushner BH, LaQuaglia MP, et al. Survival from non-stage 4 neuroblastoma without cytotoxic therapy: An analysis of clinical and biological markers. Eur J Cancer. 1997;33:2117–2121. doi: 10.1016/s0959-8049(97)00281-5. [DOI] [PubMed] [Google Scholar]
- 33.Kushner BH, LaQuaglia MP, Kramer K, Cheung N-KV. Radically different treatment recommendations for newly diagnosed neuroblastoma: Pitfalls in assessment of risk. J Pediatr Hematol/Oncol. 2004;26:35–39. doi: 10.1097/00043426-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Kushner BH, Kramer K, LaQuaglia MP, Modak S, Cheung N-KV. Liver involvement in neuroblastoma: The Memorial Sloan-Kettering experience supports treatment reduction in young patients. Pediatr Blood Cancer. 2006;46:278–284. doi: 10.1002/pbc.20564. [DOI] [PubMed] [Google Scholar]