Abstract
Objective
To assess the impact of baseline heart failure (HF) burden on survival with primary implantable cardioverter defibrillator (ICD) among Medicare recipients
Background
Survival after primary ICD implantation may differ between trial and Medicare populations
Methods
Linking data from the CMS ICD registry and the Medicare files (05–09), we identified primary ICD recipients ≥66 years with ejection fraction ≤35%. Number of prior HF hospitalizations (prior-HF-hosp) and length of hospitalization (hospital-day) prior to implantation were used to define HF burden. Crude all-cause mortality was estimated. Adjusted hazard ratios were derived from COX models.
Results
Of 66,974 ICD recipients (73% male, 88% white, mean age 75), 11,876 died (average follow-up=1.4 years), with three year mortality of 31%. Among patients with no prior-HF-hosp, three-year mortality was 27%, compared to 63% in those with ≥3 prior-HF-hosp (adjusted HR: 1.8). Among patients with same-day implantation, three-year mortality was 25%, compared to 53% in those with >1-week hospital-day prior to implantation (adjusted HR: 1.9). Mortality at three-year among the 31,685 ICD recipients with no prior-HF-hosp and same-day implantation (low HF burden) was similar to that in trials (22%).
Conclusion
Nearly one third of Medicare ICD recipients died within three years, reflecting a population with more advanced age and disease than seen in trial populations for primary prevention ICD. Nearly half of Medicare recipients had a low HF burden and had a survival similar to trial ICD recipients. Future research is warranted to understand the effectiveness of primary ICD implantation among Medicare beneficiaries with heavy HF burdens.
Keywords: heart failure, implantable cardioverter-defibrillator, primary prevention, mortality
INTRODUCTION
Several landmark trials have demonstrated the efficacy of implantable cardioverter defibrillators (ICDs) in systolic heart failure (HF)(1–3). As a result, the Centers for Medicare & Medicaid Services (CMS) issued a National Coverage Determination in 2005 that expanded the ICD indication to include primary prevention of sudden cardiac death (SCD) among Medicare beneficiaries with prior myocardial infarction (MI) or chronic HF and left ventricular ejection fraction (EF) < 35% despite receipt of medical therapy(4). Data from the IMPROVE-HF registry has been extrapolated to project that ICD may be indicated to prolong survival for as many as 800,000 additional HF patients, most of whom are over 65 years old(5).
The demographics of patients currently receiving ICDs indicate that they are older with a higher prevalence of non-cardiac comorbidities than their counterparts enrolled in the landmark clinical trials(6). The survival benefit of ICDs is likely to attenuate with age(7–9), since increasing age has been associated with a decreased risk of SCD among HF populations(7–8) and non-cardiac death is more common in older ICD recipients(10). The survival benefit of ICDs also diminishes among patients with non-cardiac comorbidities, such as chronic kidney disease (9,11–12).
The efficacy of ICD implantation for primary prevention (primary ICDs) has been demonstrated among outpatient trial participants with stable mild-moderate HF symptoms, which have informed long-term cost-effectiveness estimates(1,13–14). It is not known how these benefits might translate to older patients with differing HF burdens, as reflected by multiple prior HF hospitalizations or prolonged current hospitalization for acute decompensation of chronic HF. In this study, we aimed to 1) describe long-term survival with primary ICDs in the Medicare population and 2) evaluate the impact of HF and comorbidity burden on survival with ICDs, using the number of previous HF hospitalizations and the length of hospitalization prior to ICD implantation as proxies for chronic and acute HF burden, respectively
METHODS
Data sources
We conducted a retrospective cohort study using the CMS-ICD registry and Medicare Provider Analysis and Review (MedPAR) files from January 1, 2005 through December 31, 2009.
CMS-ICD Registry
The CMS ICD registry is a subset of the American College of Cardiology (ACC)-National Cardiovascular Data Registry (NCDR) ICD Registry, which is the sole repository for ICD implantation data for Medicare beneficiaries(15–19). The registry was developed through a partnership of the Heart Rhythm Society and the American College of Cardiology Foundation, utilizing the expertise of the National Cardiovascular Data Registry. The data is entered by hospital personnel and is only included in the analytic file if hospitals achieve certain completeness on specific data elements(20). In addition, a subset of hospitals is randomly selected for quality control review to evaluate data accuracy. Currently, over 400,000 patients are included in the CMS-ICD registry, which contains 37 out of 170 data elements that the ACC-NCDR collects. These 37 elements include a patient’s identifying information, history and clinical characteristics, medications, facility information, provider information, ICD indications, device information, and in-hospital complications.
MedPAR file
The MedPAR file contains data from claims for services provided to fee-for-service beneficiaries admitted to Medicare-certified inpatient hospitals. It includes information on beneficiary demographics, diagnoses, procedures, and health resource utilization from hospitals or skilled nursing facilities, as well as detailed data on accommodation and departmental charges, days of care, and entitlement.
We linked the ICD registry to MedPAR files by four non-unique identifiers: gender, date of birth, admission date for ICD implantation, and provider ID, similarly to previously described methods(21–22). We validated this linkage by comparing to a linkage using a personal identifier (social security number cross-linked to beneficiary ID) among the subset of the data that had this identifier. We found that our linkage method had 95% specificity, 98% sensitivity, and 98% positive predictive value compared to the linkage method using personal identifiers.
Study population
The study population consisted of Medicare fee-for-service beneficiaries who had a reduced (≤35%) EF and received an ICD for the primary prevention of SCD. Patients who had a history of cardiac arrest or sustained ventricular tachycardia (VT) were excluded. We also required patients to be eligible for Medicare for at least one year prior to the index procedure and to be at least 66 years of age at the time of implantation. We censored patients in the analyses at the earliest occurrence of the following: death or the end of the study period.
Outcome
The study outcome was all-cause mortality. The date of death was obtained from the MedPAR file.
Measures of acute and chronic HF burden and subgroups
We used the number of previous HF hospitalizations and the length of hospitalization prior to ICD implantation as indicators of chronic and acute HF burden. We followed a previously validated algorithm with PPV of 94% to identify HF hospitalizations within one year prior to ICD implantation from MedPAR data(23), and then categorized the number of prior HF hospitalizations into four levels (Table 2). We also classified the interval from admission to ICD implantation to four levels (Table 3). Patient who had no prior HF hospitalization and received ICD on the admission date were considered to have low burden of HF.
Table 2.
Characteristics and outcomes by the number of prior HF hospitalizations
| 0 hospitalizations | 1 hospitalizations | 2 hospitalizations | ≥3 hospitalizations |
|
|---|---|---|---|---|
| N (% total population) | 52,963(79) | 10,247(15) | 2,501(4) | 1,263(1) |
|
| ||||
| Age (y) § | 75(71–80) | 76(71–80) | 76(71–81) | 76(71–80) |
| Age ≤ 70y | 13,005(25) | 2,357(23) | 564(23) | 309(24) |
| Age ≥ 80y | 14,025(26) | 3,016(29) | 733(29) | 346(27) |
| Male | 39,620(75) | 6,906(67) | 1,566(63) | 761(60) |
| White | 47,332(89) | 8,682(85) | 1,968(79) | 934(74) |
| EF(%)§ | 25(20–30) | 25(20–30) | 25(20–30) | 23(20–30) |
| ≤20% | 16,418(31) | 4,406(43) | 1,075(43) | 610(48) |
| HF Duration | 52,705 | 9,816 | 2,422 | 1238 |
| New HF | 7,965(15) | 0(0) | 0(0) | 0(0) |
| 0–3 months | 7,297(14) | 1,587(16) | 348(14) | 157(12) |
| 3–9 months | 6,317(12) | 1,771(18) | 398(16) | 166(13) |
| >9 months | 31,126(59) | 6,458(66) | 1,676(69) | 915(74) |
| NYHA class | 52,663 | 10,211 | 2489 | 1261 |
| I | 3,617(7) | 253(2) | 44(2) | 12(1) |
| II | 17,597(33) | 2,386(23) | 474(19) | 189(15) |
| III | 29,239(56) | 6,824(67) | 1,716(69) | 882(70) |
| IV | 2,210(4) | 748(7) | 255(10) | 178(14) |
| QRS ≥ 120 msec | 32,949(62) | 6,817(67) | 1,651(66) | 852(67) |
| Ischemic cause HF | 42,168(80) | 7,995(78) | 2,020(81) | 1,049(83) |
| Unsustained VT | 12,382(23) | 2,273(22) | 590(24) | 309(24) |
| Prior Hospitalization for any cause§§ | 0·6(1) | 2(1·3) | 3·2(1·4) | 5.5(2.6) |
| ≥ 5 | 506(1) | 498(5) | 402(16) | 720(57) |
| LOS of implantation§§ | 3·9(5·2) | 4·2(5·5) | 5(6) | 5.9(6.2) |
| Charlson Score *§§ | 0·6(1·2) | 1·8(1·6) | 2·5(1·7) | 3.1(1·8) |
|
| ||||
| Outcomes | ||||
|
| ||||
| Death | 8,135 | 2,449 | 769 | 523 |
| Average follow-up(range) | 1·4(0d-4y) | 1·3(0d-4y) | 1·3(0d-4y) | 1·1(0d-4y) |
| Median survival(y) (IQR) | *** | *** | 3(2·9–3·4) | 2·9(1·7–2·1) |
| 1-yr mortality risk(%) (95% CI) | 10(10–11) | 17(16–18) | 24(23–26) | 33(30–36) |
| 2-yr mortality risk(%) (95% CI) | 19(19–20) | 30(29–31) | 37(35–40) | 51(48–55) |
| 3-yr mortality risk(%) (95% CI) | 27(27–28) | 40(38–43) | 52(46–57) | 63(57–68)** |
| Hazard ratio (HR) for death | - | 1·7(1·6–1·7) | 2·2(2·1–2·4) | 3·4(3.2–3·8) |
| Age, sex, race-adjusted HR | - | 1·6(1·6–1·7) | 2·2(2·0–2·3) | 3.3(3.0–3.6) |
| Multivariable adjusted HR+ | - | 1·2(1·1–1·2) | 1·3(1·2–1·5) | 1.8(1·6–2·0) |
Median(IQR);
Mean (SD);
Without counting cardiac conditions (myocardial infarction and heart failure);
The marked survival times are censored observations
Follow-up was not long enough to observe median survival time
Adjusted for age, gender, race, year of implantation, device type, time from admission to implantation, time from implantation to discharge, EF, ischemic HF, QRS duration, NYHA class, HF duration, Presence of unsustained VT, Non-dilated cardiomyopathy, cerebralvascular disease, diabetes, chronic kidney disease, COPD, osteoporosis, dementia, depression, mania, anxiety, psychotic disorder, delirium, metastatic cancer., MI in 40 days, CABG/PCI in 90 days. Prior use of flu vaccine, pneumococcal vaccine, pace maker, Number of prior hospitalizations for any cause, for mi or other cardiac disease, Charlson comorbidity score without counting MI and HF.
Table 3.
Characteristics and outcomes by the number of days from admission to ICD implantation
| 0 days | 1 day | 2–7 days | ≥8 days | |
|---|---|---|---|---|
| N (% total population) | 39,576(59) | 5,636(8) | 16,959(25) | 4,803(6) |
|
| ||||
| Age(y) § | 75(70–80) | 76(71–80) | 76(71–81) | 76(71–81) |
| Age ≤ 70y | 10,005(25) | 1,260(22) | 3,877(23) | 1,093(23) |
| Age ≥ 80y | 9,982(25) | 1,553(28) | 5,151(30) | 1,434(30) |
| Male | 29,145(74) | 4,269(76) | 12,027(71) | 3,412(71) |
| White | 35,815(91) | 5,023(89) | 14,198(84) | 3,880(81) |
| EF(%)§ | 25(20–30) | 25(20–30) | 25(20–30) | 25(20–30) |
| EF ≤ 20% | 11,845(30) | 2,034(36) | 6,743(40) | 2,156(45) |
| HF Duration | 39,071 | 5,570 | 16,766 | 4,774 |
| New HF | 4,919(13) | 748(13) | 1,898(11) | 400(8) |
| 0–3 months | 4,007(10) | 793(14) | 3,379(20) | 1,210(25) |
| 3–9 months | 5,533(14) | 658(12) | 1,928(12) | 533(11) |
| >9 months | 24,612(63) | 3,371(61) | 9,561(57) | 2,631(55) |
| NYHA class | 39,398 | 5,605 | 16,852 | 4,769 |
| I | 2,555(6) | 348(6) | 866(5) | 157(3) |
| II | 13,510(34) | 1,662(30) | 4,449(26) | 1026(21) |
| III | 22,286(57) | 3,321(59) | 10,089(60) | 2,971(62) |
| IV | 1,047(3) | 274(5) | 1,448(9) | 615(13) |
| QRS ≥120 msec | 25,363(64) | 3,752(67) | 10,365(61) | 2,789(58) |
| Ischemic cause HF | 31,111(79) | 4,452(79) | 13,662(81) | 4,007(83) |
| Unsustained VT | 6,384(16) | 1,436(26) | 5,578(33) | 2,156(45) |
| Prior Hospitalization for any cause§§ | 0.9(1.3) | 1.1(1.5) | 1.1(1.6) | 1.4(1.8) |
| ≥ 5 | 919(2) | 202(4) | 738(4) | 267(6) |
| Time from Implantation to discharge (days)§§ | 1.4(1.7) | 2.1(2.7) | 2.8(3.2) | 4.4(5) |
| Charlson Score*§§ | 0.8(1.3) | 1(1.4) | 1.1(1.6) | 1.3(1.7) |
|
| ||||
| Outcome | ||||
|
| ||||
| Death | 5,245 | 972 | 3,999 | 1,660 |
| Average follow-up(range) | 1.5(0d-4y) | 1.4(0d-4y) | 1.3(0d-4y) | 1.2(0d-4y) |
| Median survival(y) (IQR) | *** | *** | 3.8(3.8-.) | 2.7(2.6–3.3) |
| 1-yr mortality risk(%) (95% CI) | 8(8–8) | 12(11–13) | 18(18–19) | 29(28–31) |
| 2-yr mortality risk(%) (95% CI) | 16(16–17) | 22(21–23) | 30(29–31) | 43(41–44) |
| 3-yr mortality risk(%) (95% CI) | 25(24–25) | 32(29–35) | 41(39–42) | 53(50–57) |
| Hazard ratio(HR) for death | - | 1.4(1·3–1·5) | 2.1(2.0–2.2) | 3.4(3.2–3.6) |
| Age, sex, race-adjusted HR | - | 1.4(1·3–1·5) | 2.0(1.9–2.1) | 3.2(3.1–3.4) |
| Multivariable adjusted HR+ | - | 1.1(1·0–1·2) | 1.4(1.4–1.5) | 1.9(1.8–2.0) |
Median(IQR);
Mean (SD);
Without cardiac conditions: myocardial infarction and heart failure;
The marked survival times are censored observations;
Follow-up was not long enough to observe median survival time
Adjusted for age, gender, race, year of implantation, device type, time from implantation to discharge, EF, ischemic HF, QRS duration, NYHA class, HF duration, Presence of unsustained VT, Non-dilated cardiomyopathy, cerebralvascular disease, diabetes, chronic kidney disease, COPD, osteoporosis, dementia, depression, mania, anxiety, psychotic disorder, delirium, metastatic cancer., MI in 40 days, CABG/PCI in 90 days. Prior use of flu vaccine, pneumococcal vaccine, pace maker, Number of prior hospitalizations for any cause, for hf, mi or other cardiac disease, Charlson comorbidity score without counting MI and HF.
Statistical analysis
Baseline characteristics of the overall study population and each subgroup were characterized by percentages for categorical variables and using medians and interquartile ranges or means and standard deviations for continuous variables.
We graphed cumulative mortality over time using Kaplan-Meier estimators for the entire study population and for each subgroup. Differences among subgroups were tested using log-rank tests. We used proportional hazards regression models to derive crude, demographic (age, gender, and race), and multivariable-adjusted HRs among the subgroups. We attempted to account for the impact of other measures of HF severity (such as HF duration and NYHA class) and of comorbidities (such as CAD, un-sustained VT, Non-dilated cardiomyopathy, cerebrovascular disease, diabetes, chronic kidney disease, COPD, osteoporosis, mental disorders, metastatic cancer) on post-implantation mortality in the multivariable-adjusted model (see footnotes for Table 2 and 3 for the complete list of covariates in the model).
All analyses were conducted using SAS for Windows, release 9.2 (SAS Institute Inc., Cary, NC). The study was approved by the Institutional Review Board of Brigham and Women's Hospital.
RESULTS
Characteristics and survival of overall Medicare patients with ICD
The final study cohort consisted of 66,974 Medicare HF patients who underwent ICD implantation for primary prevention of SCD (Appendix I for the description of the final study patients). The median age was 75 years and the majority were male (73%) and white (88%) (Table 1). More than half of the patients (61%) had known HF for more than nine months, with 79% having HF due to an ischemic cause. The median EF was 25%. Over a quarter (28%) of the patients were hospitalized at least once for any cause within one year prior to implantation, among which, 14% had more than five hospitalizations. During the average follow-up of 1·4 years, 11,876 patients died. One-, two-, and three- year mortality after ICD implantation was 12%, 22%, and 31%, respectively (Table 1).
Table 1.
Main characteristic and outcomes among Medicare beneficiaries receiving primary ICD implantations (N=66,974)
| Median Age (y) (IQR) | 75(71–80) |
| Male | 48,853(73) |
| White | 58,916(88) |
| Median EF % (IQR) | 25(20–30) |
| HF Duration | |
| New HF | 7,965(12) |
| 0–3 months | 9,389(14) |
| 3–9 months | 8,652(13) |
| >9 months | 40,175(61) |
| Missing (N) | 793 |
| NYHA class | |
| I | 3,926(6) |
| II | 20,647(31) |
| III | 38,667(58) |
| IV | 3,384(5) |
| Missing (N) | 350 |
| QRS ≥ 120 msec | 42,269(63) |
| Ischemic cause HF | 53,232(79) |
| ≥1 Prior hospitalization for any cause | 18,752 (28) |
| ≥5 Prior hospitalization for any cause | 2,624 (4) |
| Cardiac Resynchronization Therapy - Defibrillators | 32,201(48) |
|
| |
| Outcomes | |
|
| |
| Death (N) | 11,876 |
| In-hospital death (N) | 327 |
| In hospital death risk(%) (95% CI) | 0·49 (0·45–0·55) |
| Average follow-up(range) | 1·4 (0 day–4 yrs) |
| Mortality rate (per 1,000 person-year) | 128 |
| 1-yr mortality risk(%) (95% CI) | 12 (12–13) |
| 2-yr mortality risk(%) (95% CI) | 22 (22–23) |
| 3-yr mortality risk(%) (95% CI) | 31 (30–32) |
Chronic HF burden: number of prior HF hospitalizations
At least one prior hospitalization for HF had occurred in the year preceding ICD implantation for 14,011 patients (21%). Of these, 27% had two or more hospitalizations attributed to HF (Table 2). A higher number of prior HF hospitalizations was associated with lower EF: 31% had EF ≤ 20% among patients with no prior HF hospitalization versus 48% among patients with three or more prior HF hospitalizations). Prior HF hospitalization was also associated with higher NYHA class at time of implant: 59% of patients no prior HF hospitalization had NYHA class III/IV symptoms at implantation compared to 84% among patients with three or more prior HF hospitalizations. Chronic HF burden was also associated with more frequent comorbidities, as reflected by the number of prior all-cause hospitalizations and the Charlson comorbidity score. (Table 2)
Mortality by the number of prior HF hospitalizations
After ICD placement, mortality at three years increased from 27% in patients without prior HF hospitalization to 63% in those with three or more prior HF hospitalizations (Table 2, Figure 1). Among patient who had more than three prior HF hospitalizations, more than half died by two years of implantation (Figure 1). Compared to patients without a prior hospitalization, the crude HR for mortality ranged from 1.7 for patients with one prior HF hospitalization to 3.4 for patients with three or more prior HF hospitalizations. The pattern of HRs across levels of chronic HF burden remained similar after adjusting for demographics. The impact of number of hospitalizations on all-cause mortality suggests that the number of hospitalizations may serve as a surrogate for HF severity and comorbidities, because its effect diminished substantially after adjusting for these variables in multivariable analyses. (Table 2) Although close to a half (48%, Table 1) of the patients received a CRT-D device, the impact of the number of prior HF hospitalization on post-implantation mortality did not vary by device type (data available upon request).
Figure 1. Kaplan-Meier cumulative crude mortality by the number of prior HF hospitalizations.
After ICD placement, mortality at three years increased from 27% in patients without prior HF hospitalization to 63% in those with three or more prior HF hospitalizations. Among patient who had more than three prior HF hospitalizations, more than half died by two years of implantation.
Acute HF burden: number of days from admission to ICD implantation
Of 27,398 patients who had at least one hospital day between the admission day and ICD implantation, 62% had been hospitalized for two to seven days prior to the procedure. Similar to the impact of the number of prior HF hospitalizations, a longer duration from admission to ICD implantation was associated with lower EF, higher NYHA class, and higher comorbidity burden. It was also associated with greater proportion of HF of ischemic origin (79% in the same-day implantation patients versus 83% in those who hospitalized for eight or more days before implantation). (Table 3)
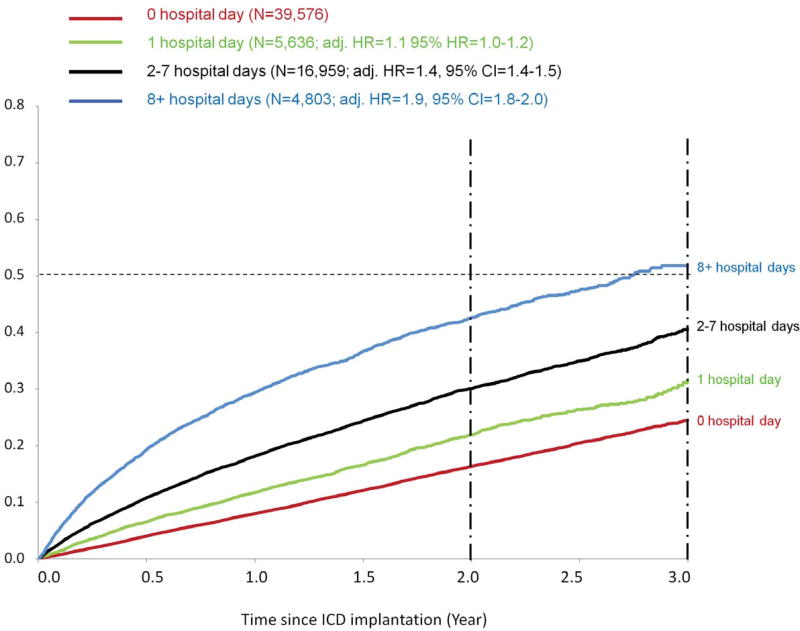
Mortality by the number of days from admission to ICD implantation
Mortality at three years was more than twice as high, from 25% in patients with same-day implantation to 53% in the patients hospitalized for eight or more days before implantation (Table 3, Figure 2). Among patients who had more than eight hospital-days before implantation, more than half died by three years (Figure 2). Compared to the same-day implantation group, the crude HR for mortality ranged from 1.4 in the same-day implantation group to 3.4 in the eight or more days group. The pattern of elevated HRs remained similar after adjusting for demographics. After adjusting for HF severity and comorbidities, the impact of the hospitalization duration on mortality was attenuated, indicating that duration of hospitalization, like number of HF hospitalizations, likely serves as a surrogate for overall disease severity. (Table 3) The impact of the hospitalization duration on post-implantation mortality was also the same between patients received CRT-D and simple ICD (data available upon request).
Figure 2. Kaplan-Meier cumulative crude mortality by the number of days from admission to ICD implantation.
Mortality at three years was more than twice as high, from 25% in patients with same-day implantation to 53% in the patients hospitalized for eight or more days before implantation. Among patients who had more than eight hospital-days before implantation, more than half died by three years.
A Subgroup with Low HF burden
Accumulating acute and chronic HF burden is associated with escalating mortality risk. Conversely, patients who had no HF hospitalization in the year before ICD implantation and received the device on the admission day (47%, N=31, 685) had the lowest risk of mortality. (Table 4) In fact, these patient without a high burden of HF had similar mortality after ICD implantation as those in the major trials (Figure 3). By contrast, a higher mortality was seen for primary ICD recipients was seen in those with both chronic and acute burdens of HF at the time of implantation (N=6,120, three-year mortality=50–69%). (Table 4)
Table 4.
Mortality at three years after ICD implantation among subgroups categorized by acute and chronic HF burdens
| Acute HF burden | 0 Hospital days from admission to implantation |
1–7 Hospital days from admission to implantation |
8+ Hospital days from admission to implantation |
|---|---|---|---|
|
| |||
| Chronic HF burden | |||
| 0 Prior HF hospitalizations | 22% (N=31,685) | 34% (N=17,581) | 50% (N=3,697) |
| 1–2 Prior HF hospitalizations | 35% (N=7,332) | 50% (N=4,459) | 59% (N=957) |
| 3+ Prior HF hospitalizations | 54% (N=559) | 69% (N=555) | 61% * (N=149) |
The survival times are censored observations
Figure 3. Post-implantation Mortality among Medicare Populations with Low Burden of HF (N=31, 685).
Patient who had no HF hospitalization in the year before ICD implantation and received the device on the admission day (47%, N=31, 685) had similar mortality after ICD implantation as those in the major trials (Mortality at three-year in patients with low HF burden=22% versus 16–22% in SCD-HeFT and MADIT II).
DISCUSSION
We assessed mortality after ICD implantation in a large population of Medicare beneficiaries using a national ICD registry linked to Medicare inpatient claims data. Approximately one in three Medicare patients died within three years of implantation in our study. We observed higher mortality among patients with a higher number of prior HF hospitalizations (measure of chronic HF burden) and a longer duration between admission and ICD implantation (measure of acute HF burden). Both measures may be surrogates not only for HF severity but also for other comorbidities, and they have additive effect on post-ICD mortality. Although our analyses did not include a comparison group of non-ICD recipients and could not estimate the relative effect of ICDs in these subgroups, they provided insights on the sub-populations less likely to experience a competing risk of mortality that cannot be prevented by ICD therapy. Specifically, we observed nearly half of the Medicare ICD recipients had low burden of HF at the time of implantation and their survival was similar to what had been demonstrated in the landmark trials.
Saxon and colleagues assessed the long-term mortality after ICD implantation for primary and secondary prevention during the post-ICD coverage expansion period,(24) 21% died at three years for patients receiving an ICD (N=108, 027) and 29% for those receiving CRT-D (N=77, 751). The overall survival after primary ICD implantation appears to be shorter among our Medicare population given that mortality in the Saxon study is likely to have been elevated by including patients receiving the devices for secondary prevention. The shorter survival after ICD implantation among our Medicare patients could reflect more severe HF or comorbidities associated with older age. Compared to primary ICD recipients in a nationwide ICD registry(25), which includes patients younger than 66 years of age, our Medicare ICD recipients had worse HF symptoms by NYHA class.
We attempted to quantify the chronic burden of HF by the number of prior HF hospitalizations, which has been shown to be an independent predictor of mortality among HF patients(26–28). Patients with repeated HF hospitalizations are more likely to die from non-arrhythmic causes of death such as pump failure or non-cardiac causes(29–30). Moreover, previous HF hospitalization has been shown to predict early HF death and lower ICD efficacy in the MADIT-II trial(31), The decision to implant an ICD in patients with prior HF hospitalizations should be made cautiously and with appropriately calibrated expectations of HF survival.
To describe the burden of a prolonged exacerbation of HF, we used the duration from admission to ICD implantation, assuming that prior to implantation patients were likely undergoing evaluation or treatment either for HF or for other serious comorbidities. Increasing the number of days during a HF hospitalization suggests that decompensation is more complicated, more severe, or more refractory to interventions. Although we were not able to investigate what happened during the prolonged hospitalization before the procedure in our study population, our assumption was supported by the observation that those who received an elective ICD procedure had shorter time from admission to implantation (mean=0.3 days, SD=1) than those who received ICDs during an admission for HF, other cardiac conditions or non-cardiac conditions (mean=5 days, SD=4). Indications for HF admission generally include symptoms at rest, which meet the definition of Class IV HF, for which ICD are generally considered to be contra-indicated. Evidence to support ICD use among patients with ongoing Class IV symptoms is limited, as these patients were not included in pivotal trials of ICD efficacy among HF populations (1, 3), although the use of ICD with CRT has been shown to be beneficial in ongoing Class IV patients in COMPANION (2). Prevention of SCD would be expected to have less impact on overall mortality than preventing death due to HF after HF decompensation since the major cause of death among trial patients hospitalized for worsening HF is progressive HF rather than SCD(32), and HF hospitalization is a major risk factor for death in ICD recipients(31). Our findings indicate research is needed on whether ICD implantation during an admission for HF decompensation will have a survival benefit due to competing mortality. This may be particularly important for patients requiring a prolonged hospitalization given our finding that length of hospitalization prior to implantation was associated with shorter survival.
Lastly, the post-implantation mortality among our Medicare primary ICD recipients is higher than that of the patients included in the major primary ICD trials(1,3)—16% in SCD-HeFT and 22% in MADIT II (at three-year), However, we observed the mortality was similar to that of the landmark trials (1, 3) when ICDs were implanted among Medicare patients who had no prior HF hospitalizations and can receive ICDs on the admission day (Table 4), despite the underlying age difference. It is encouraging that we identified this sub-population by using the rough proxies of HF burdens. It is also worth noting that a substantial proportion of the Medicare ICD recipients were in the “low HF burden” category at the time of implantation, which reflects an appropriate selection of Medicare ICD recipients in the current practice. Nevertheless, future research is warranted to refine our measures of baseline HF burden to support clinical decisions on who should receive the device.
Our results should be interpreted in light of inherent limitations. As mentioned earlier, our analyses did not include a comparison group of non-ICD recipients, and could not estimate the relative effect of ICDs by underlying HF burdens. The registry and Medicare data were not always in agreement. For example, 6% of patients who received ICDs with a primary prevention indication were also noted in the registry to have sustained VT noted (suggesting secondary prevention). We excluded these patients in order to avoid mixing primary and secondary ICDs. A review of a tertiary care center’s ICD registry found incomplete coding for indication, further supporting our decision to exclude patients with discrepant indication data(33).
We linked 64% (N=122, 562) of registry records to MedPAR file. The MedPAR file does not contain claims for the majority of non-fee-for-service Medicare beneficiaries, unless those beneficiaries enrolled in a cost-based managed care plan and the plan elects to have CMS to process and pay for the service provided(34). Given the prevalence of Medicare beneficiaries under a non-cost-based managed care arrangement, which ranges from 14% to 22% during the study time frame(34), our matched records represent approximately 79% of the implantations recorded in the registry that would be for fee-for-service Medicare beneficiaries. Incomplete linkage may have also been due to data entry errors or inconsistencies in the information collection policy between the registry and Medicare files on the fields that were used for linkage. For example, when gender is unknown to Medicare, “Female” value is automatically assigned for that claim(35). Also, if arrival to the emergency department is recorded as the patient’s admission date in the registry, it would differ from the date recorded in the claims data. Nevertheless, these errors are likely to be random and unlikely to compromise the validity of our findings.
CONCLUSIONS
Approximately one in three Medicare primary ICD recipients died within three years of implantation. Medicare patients with a low cumulative burden of HF had similar mortality to those in the landmark trials. Further studies are needed to assess effectiveness of primary ICD implantation among patients with heavy burdens of HF and other comorbidities to guide clinical strategies and policy decisions among Medicare beneficiaries.
Acknowledgments
Funding/Support: This project is funded by contract No.HHSA290-2005-0016-I -TO3 from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services (DHHS) as part of the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) program, IAA Contract 500-2010-00001I TO6 and CEA Contract 500-2010-00001I TO2 from the Centers for Medicare & Medicaid Services (CMS), U.S. DHHS.
Dr. Setoguchi is supported by a mid-career development award grant K02-HS017731 from the AHRQ, U.S. DHHS. She also reported receiving research support from Johnson & Johnson and receiving personal income for consulting from Sanofi-Aventis. Dr Setoguchi has made available online a detailed listing of financial disclosures (http://www.dcri.duke.edu/about-us/conflict-of-interest/). Dr. Seeger is a paid consultant to Optum Insight and WHISCON.
Role of the Sponsor: The funding agency had no role in the design and conduct of the study and in the collection, analysis, and interpretation of the data. The manuscript was based on a report done under contract to AHRQ. AHRQ had the draft report reviewed by independent peer reviewers before acceptance of the final report.
Disclaimer: The views expressed in this article are those of the authors and do not represent policies of the AHRQ, CMS or the U.S. DHHS.
Abbreviations List
- CRT
Cardiac resynchronization therapy
- CRT-D
Cardiac resynchronization therapy-Defibrillator
- EF
Ejection fraction
- HF
Heart failure
- ICD
Implantable cardioverter defibrillator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previous Presentation: This article was presented in part at the AHRQ 2011 National Conference on September 21, 2011, in Bethesda, Maryland.
Conflict of Interest Disclosures: We declare that we have no conflicts of interest.
References
- 1.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 2.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 3.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 4.CMS National Coverage Determination for ICD. 2005 [Google Scholar]
- 5.Fonarow GC, Yancy CW, Hernandez AF, Peterson ED, Spertus JA, Heidenreich PA. Potential impact of optimal implementation of evidence-based heart failure therapies on mortality. Am Heart J. 2011;161:1024–1030. e3. doi: 10.1016/j.ahj.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 6.Hammill SC, Kremers MS, Stevenson LW, et al. Review of the registry's fourth year, incorporating lead data and pediatric ICD procedures, and use as a national performance measure. Heart Rhythm. 7:1340–5. doi: 10.1016/j.hrthm.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Lindenfeld J, Shakar S, Zolty R, et al. Abstract 1880: Increasing Age Decreases the Risk of Sudden Death Compared to Progressive Heart Failure in Patient with Advance Heart Failure. Circulation. 2006;114 II_373-a- [Google Scholar]
- 8.Mehta PA, Dubrey SW, McIntyre HF, et al. Mode of death in patients with newly diagnosed heart failure in the general population. Eur J Heart Fail. 2008;10:1108–16. doi: 10.1016/j.ejheart.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Setoguchi S, Nohria A, Rassen JA, Stevenson LW, Schneeweiss S. Maximum potential benefit of implantable defibrillators in preventing sudden death after hospital admission because of heart failure. Cmaj. 2009;180:611–6. doi: 10.1503/cmaj.080769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein AE, Kay GN, Plumb VJ, et al. Implantable cardioverter-defibrillator prescription in the elderly. Heart Rhythm. 2009;6:1136–43. doi: 10.1016/j.hrthm.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Koplan BA, Epstein LM, Albert CM, Stevenson WG. Survival in octogenarians receiving implantable defibrillators. Am Heart J. 2006;152:714–9. doi: 10.1016/j.ahj.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Goldenberg I, Moss AJ, McNitt S, et al. Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol. 2006;98:485–90. doi: 10.1016/j.amjcard.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Mark DB, Nelson CL, Anstrom KJ, et al. Cost-effectiveness of defibrillator therapy or amiodarone in chronic stable heart failure: results from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Circulation. 2006;114:135–42. doi: 10.1161/CIRCULATIONAHA.105.581884. [DOI] [PubMed] [Google Scholar]
- 14.Sanders GD, Hlatky MA, Owens DK. Cost-effectiveness of implantable cardioverter-defibrillators. N Engl J Med. 2005;353:1471–80. doi: 10.1056/NEJMsa051989. [DOI] [PubMed] [Google Scholar]
- 15.Hammill S, Phurrough S, Brindis R. The National ICD Registry: now and into the future. Heart Rhythm. 2006;3:470–3. doi: 10.1016/j.hrthm.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 16.Hammill SC, Kremers MS, Kadish AH, et al. Review of the ICD Registry's third year, expansion to include lead data and pediatric ICD procedures, and role for measuring performance. Heart Rhythm. 2009;6:1397–401. doi: 10.1016/j.hrthm.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Hammill SC, Kremers MS, Stevenson LW, et al. Review of the registry's fourth year, incorporating lead data and pediatric ICD procedures, and use as a national performance measure. Heart Rhythm. 2010;7:1340–5. doi: 10.1016/j.hrthm.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Hammill SC, Kremers MS, Stevenson LW, et al. Review of the Registry's second year, data collected, and plans to add lead and pediatric ICD procedures. Heart Rhythm. 2008;5:1359–63. doi: 10.1016/j.hrthm.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Hammill SC, Stevenson LW, Kadish AH, et al. Review of the registry's first year, data collected, and future plans. Heart Rhythm. 2007;4:1260–3. doi: 10.1016/j.hrthm.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 20.American College of Cardiology Foundation. National Cardiovascular Data Registry Program Requirements. 2012 [Google Scholar]
- 21.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. doi: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Q, Glynn RJ, Dreyer NA, Liu J, Mogun H, Setoguchi S. Validity of claims-based definitions of left ventricular systolic dysfunction in Medicare patients. Pharmacoepidemiol Drug Saf. 2011;20:700–8. doi: 10.1002/pds.2146. [DOI] [PubMed] [Google Scholar]
- 23.Lee DS, Donovan L, Austin PC, et al. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care. 2005;43:182–8. doi: 10.1097/00005650-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Saxon LA, Hayes DL, Gilliam FR, et al. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation. 122:2359–67. doi: 10.1161/CIRCULATIONAHA.110.960633. [DOI] [PubMed] [Google Scholar]
- 25.Al-Khatib SM, Hellkamp A, Curtis J, et al. Non-evidence-based ICD implantations in the United States. Jama. 2011;305:43–9. doi: 10.1001/jama.2010.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pocock SJ, Wang D, Pfeffer MA, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 27.Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154:260–6. doi: 10.1016/j.ahj.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 28.Solomon SD, Dobson J, Pocock S, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–7. doi: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 29.Setoguchi S, Stevenson LW. Hospitalizations in patients with heart failure: who and why. J Am Coll Cardiol. 2009;54:1703–5. doi: 10.1016/j.jacc.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Wang NC, Piccini JP, Konstam MA, et al. Implantable cardioverter-defibrillators in patients hospitalized for heart failure with chronically reduced left ventricular ejection fraction. Am J Ther. 17:e78–87. doi: 10.1097/MJT.0b013e3181e70a65. [DOI] [PubMed] [Google Scholar]
- 31.Goldenberg I, Moss AJ, Hall WJ, et al. Causes and consequences of heart failure after prophylactic implantation of a defibrillator in the multicenter automatic defibrillator implantation trial II. Circulation. 2006;113:2810–7. doi: 10.1161/CIRCULATIONAHA.105.577262. [DOI] [PubMed] [Google Scholar]
- 32.O'Connor CM, Miller AB, Blair JE, et al. Causes of death and rehospitalization in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction: results from Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) program. Am Heart J. 159:841–849. e1. doi: 10.1016/j.ahj.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 33.Zweibel S, Clyne C, Crespo E. ICD Implantation and Evidence-Based Patient Selection. JAMA. 2011;305:1538–1539. doi: 10.1001/jama.2011.458. [DOI] [PubMed] [Google Scholar]
- 34.Research Data Assistance Center UoM, Minneapolis, MN. Medicare Managed Care Enrollees and the Medicare Utilization Files. Technical Brief, Publication Number TN-009. 2009 Mar; 206, Updated July 2009. [Google Scholar]
- 35.van Rees JB, de Bie MK, Thijssen J, Borleffs CJ, Schalij MJ, van Erven L. Implantation-related complications of implantable cardioverter-defibrillators and cardiac resynchronization therapy devices: a systematic review of randomized clinical trials. J Am Coll Cardiol. 2011;58:995–1000. doi: 10.1016/j.jacc.2011.06.007. [DOI] [PubMed] [Google Scholar]