Abstract
Background
CBL0137 is a novel drug that modulates FACT (Facilitates Chromatin Transcription), resulting in simultaneous Nuclear factor-κB suppression, Heat shock factor 1 suppression and p53 activation. CBL0137 has demonstrated antitumor effects in animal models of several adult cancers and neuroblastoma.
Procedures
CBL0137 was tested against the Pediatric Preclinical Testing Program (PPTP) in vitro cell line panel at concentrations from 1.0 nM to 10.0 μM and against the PPTP in vivo solid tumor xenograft and acute lymphocytic leukemia (ALL) panels at 50 mg/kg administered intravenously weekly for 4 weeks.
Results
The median relative IC50 (rIC50) value for the PPTP cell lines was 0.28 μM (range: 0.13 μM to 0.80 μM). There were no significant differences in rIC50 values by histotype. CBL0137 induced significant differences in event-free survival (EFS) distribution compared to control in 10 of 31 (32%) of the evaluable solid tumor xenografts and in 8 of 8 (100%) evaluable ALL xenografts. Significance differences in EFS distribution were observed in 4 of 6 osteosarcoma lines, 3 of 3 rhabdoid tumor lines, and 2 of 6 rhabdomyosarcoma lines. No objective responses were observed among the solid tumor xenografts. For the ALL panel, 1 xenograft achieved CR and 4 achieved PR.
Conclusions
The most consistent in vivo activity for CBL0137 was observed against ALL xenografts, with some solid tumor xenograft lines showing tumor growth delay. It will be important to relate the drug levels in mice at 50 mg/kg to those in humans at the recommended phase 2 dose.
Keywords: Preclinical Testing, Developmental Therapeutics, curaxin CBL0137
INTRODUCTION
CBL0137 is a member of a new class of anticancer drugs, named curaxins, that modulate important pathways involved in pathogenesis of cancer. CBL0137 is reported to activate TP53 and inhibit cellular stress pathways mediated by nuclear factor-κB (NF-κB) and Heat Shock Factor-1 (HSF-1) 1–3. It is considered that apoptosis induced by CBL0137 is mediated through inhibition of FACT (FAcilitates Chromatin Transcription), a chromatin remodeling complex composed of SSRP1 and SUPT16H subunits 1. This complex is involved in the transcription of genes with highly ordered chromatin structure that are essential for replication, and mitosis 4–6. Curaxins bind DNA without inducing damage or activating DNA damage sensitive pathways, but alter DNA topology sequestering FACT on DNA making it unable to bind histones that regulate normal chromatin remodeling 1.
FACT is overexpressed in several tumor types (e.g., pancreatic ductal carcinoma and lung carcinoma) relative to paired normal tissue, and is associated with a more aggressive phenotype with overall poor survival 7. Less is known regarding FACT expression in pediatric cancer. Overexpression of the SSRP1 subunit was associated with an 11q12 amplicon in an anaplastic medulloblastoma 8, and Carter et al. 9 observed down regulation of MYCN when either SSRP1 or SUPT16H subunits of FACT were suppressed, suggesting that FACT may regulate expression of MYCN in neuroblastoma cell lines. FACT is expressed in early embryogenesis and in undifferentiated progenitors and stem cells of adult tissues. In contrast FACT subunit proteins are poorly detected in differentiated tissues 10.
The role of NFκB in cancer is well established 11. This transcription factor is constitutively activated in acute lymphoblastic leukemia (ALL) 12, and activation of the classical pathway is considered as one factor in suppression of differentiation in rhabdomyosarcoma 13 and in suppression of apoptosis in murine B lymphocytes, Hodgkins lymphoma cells and some breast cancer cell lines 14–16. NFκB is also associated with drug resistance in neuroblastoma cells 17, possibly through upregulation of MDR1 18 that encodes the ABC drug transporter ABCB1.
CBL0137 has been shown to reduce colony formation in several pancreatic tumor cell lines at sub-micromolar concentrations, suppress growth of PANC-1 tumors and to have additive activity when combined with gemcitabine in this orthotopic xenpograft model. CBL0137 also retarded growth of several patient derived pancreatic ductal carcinoma xenografts, the sensitivity of which correlated with level of FACT by immunohistochemistry 19. Oral CBL0137, significantly extended survival (median survival time from start of treatment = 31.0±9.9 days versus controls = 3.0±0.2 days; P<0.0001), and was as effective as cisplatin or cyclophosphamide single-agent treatment when evaluated in the TH-MYCN transgenic model of neuroblastoma, and the combination of CBL0137 cyclophosphamide/topotecan resulted in more than doubling of lifespan against the BE(2)-C neuroblastoma xenograft model 9. CBL0137 was selected for testing against the in vitro and in vivo PPTP panels because of its novel mechanism of action.
MATERIALS AND METHODS
In vitro testing
In vitro testing was performed using DIMSCAN [20], as previously described in a characterized panel of 24 cell lines 20. Cells were incubated in the presence of CBL0137 for 96 hours at concentrations from 1.0 nM to 10 µM and analyzed as previously described 21.
In vivo tumor growth inhibition studies
CB17SC scid−/− female mice (Taconic Farms, Germantown NY), were used to propagate subcutaneously implanted kidney/rhabdoid tumors, sarcomas (Ewing, osteosarcoma, rhabdomyosarcoma), neuroblastoma, and non-glioblastoma brain tumors, while BALB/c nu/nu mice were used for glioma models, as previously described 22. Human leukemia cells were propagated by intravenous inoculation in female non-obese diabetic (NOD)/scid−/− mice as described previously 23. Female mice were used irrespective of the patient gender from which the original tumor was derived. All mice were maintained under barrier conditions and experiments were conducted using protocols and conditions approved by the institutional animal care and use committee of the appropriate consortium member. Eight to ten mice were used in each control or treatment group. Tumor volumes (cm3) [solid tumor xenografts] or percentages of human CD45-positive [%hCD45+] cells [ALL xenografts] were determined and responses were determined using three activity measures as previously described 22. An in-depth description of the analysis methods is included in the Supplemental Response Definitions section.
Statistical Methods
The exact log-rank test, as implemented using Proc StatXact for SAS®, was used to compare event-free survival distributions between treatment and control groups. P-values were two-sided and were not adjusted for multiple comparisons given the exploratory nature of the studies.
Drugs and Formulation
CBL0137 was provided to the Pediatric Preclinical Testing Program by Cleveland Biolabs Inc., through the Cancer Therapy Evaluation Program (NCI). CBL0137 was formulated in 5% dextrose with a working solution of 10 mg/ml and with dosing at 0.05 ml per 10 gm body weight. CBL0137 was administered by intravenous injection, weekly for 4 consecutive weeks. The total planned treatment and observation period was 6 weeks. Both dose and schedule were proposed by the drug supplier, based upon mouse toxicology studies.
RESULTS
In vitro testing
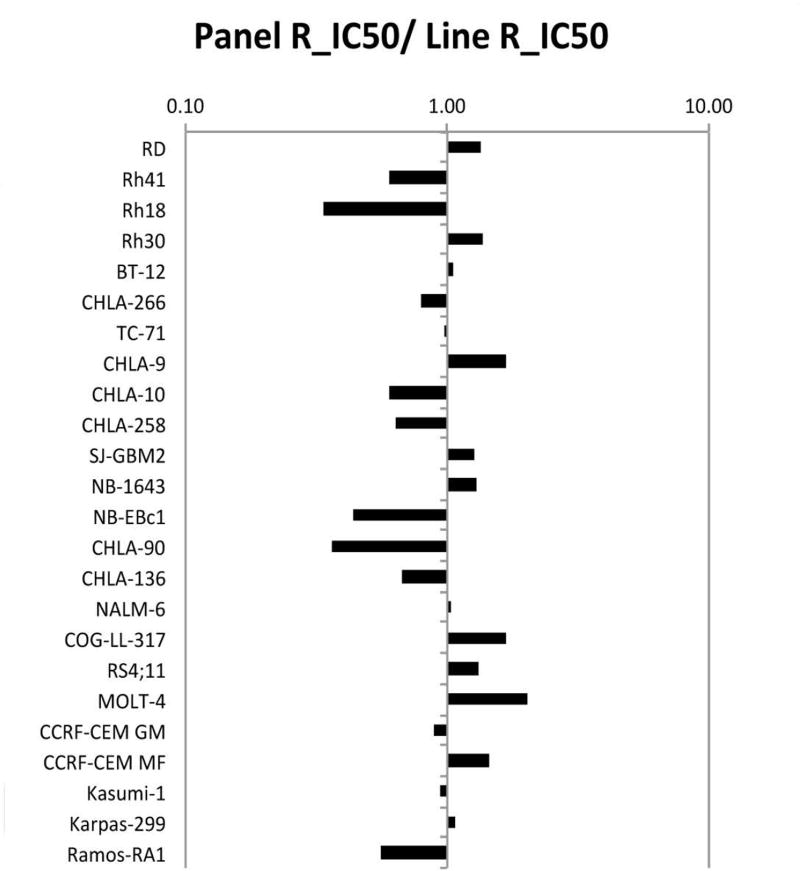
CBL0137 was tested against the PPTP’s in vitro cell line panel at concentrations ranging from 1.0 nM to 10.0 μM using the PPTP’s standard 96 hour exposure period. The median relative IC50 (rIC50) value for the PPTP cell lines was 0.28 μM, with a range from 0.13 μM to 0.80 μM (Table I). The most sensitive cell line, MOLT-4, is an ALL cell line. A metric used to compare the relative responsiveness of the PPTP cell lines to CBL0137 is the ratio of the median rIC50 of the entire panel to that of each cell line. Higher ratios are indicative of greater sensitivity to CBL0137 and are shown in Figure 1 by bars to the right of the midpoint line.
Table 1.
In Vitro Activity of IVT CBL0137 against PPTP Cell Lines
| Cell Line | Histotype | rIC50 (µM) |
Panel rIC50 /Line rIC50 |
T/C Ymin % (Observed) |
Relative In/Out (Observed Ymin)1 |
|---|---|---|---|---|---|
| RD | Rhabdomyosarcoma | 0.20 | 1.33 | 0.00 | −100.0% |
| Rh41 | Rhabdomyosarcoma | 0.45 | 0.60 | 0.00 | −100.0% |
| Rh18 | Rhabdomyosarcoma | 0.80 | 0.34 | 0.19 | −99.6% |
| Rh30 | Rhabdomyosarcoma | 0.20 | 1.35 | 0.00 | −100.0% |
| BT-12 | Rhabdoid | 0.26 | 1.06 | 0.00 | −100.0% |
| CHLA-266 | Rhabdoid | 0.34 | 0.80 | 0.77 | −97.1% |
| TC-71 | Ewing sarcoma | 0.28 | 0.98 | 0.00 | −100.0% |
| CHLA-9 | Ewing sarcoma | 0.16 | 1.69 | 0.00 | −100.0% |
| CHLA-10 | Ewing sarcoma | 0.45 | 0.60 | 0.00 | −100.0% |
| CHLA-258 | Ewing sarcoma | 0.43 | 0.63 | 0.00 | −100.0% |
| SJ-GBM2 | Glioblastoma | 0.21 | 1.27 | 0.01 | −99.9% |
| NB-1643 | Neuroblastoma | 0.21 | 1.28 | 0.01 | −100.0% |
| NB-EBc1 | Neuroblastoma | 0.61 | 0.44 | 0.00 | −100.0% |
| CHLA-90 | Neuroblastoma | 0.74 | 0.36 | 0.02 | −99.9% |
| CHLA-136 | Neuroblastoma | 0.40 | 0.67 | 0.00 | −100.0% |
| NALM-6 | ALL | 0.26 | 1.02 | 0.00 | −100.0% |
| COG-LL-317 | ALL | 0.16 | 1.67 | 0.00 | −100.0% |
| RS4;11 | ALL | 0.20 | 1.33 | 0.17 | −98.9% |
| MOLT-4 | ALL | 0.13 | 2.04 | 0.00 | −100.0% |
| CCRF-CEM (1) | ALL | 0.31 | 0.88 | 0.00 | −100.0% |
| Kasumi-1 | AML | 0.29 | 0.94 | 0.00 | −100.0% |
| Karpas-299 | ALCL | 0.25 | 1.07 | 0.00 | −100.0% |
| Ramos-RA1 | NHL | 0.49 | 0.56 | 0.00 | −100.0% |
| Median | 0.28 | 1.00 | 0.00 | −100.0% | |
| Minimum | 0.13 | 0.34 | 0.00 | −100.0% | |
| Maximum | 0.80 | 2.04 | 0.77 | −97.1% |
The Relative In/Out (I/O)% values compare the relative difference in final cell number compared with the starting cell number for treated cells and for control cells calculated as follows: (Observed Ymin−Y0)/(100−Y0) if Observed Ymin>Y0; and (Observed Ymin−Y0)/(Y0) if Observed Ymin<Predicted Ymin). Y0 is an estimate of the starting cell number derived from determinations of the doubling time for each cell line. Relative I/O% values range between 100% (no treatment effect) to −100% (complete cytotoxic effect), with a Relative I/O% value of 0% being observed for a completely effective cytostatic agent.
Figure 1.
CBL0137 in vitro activity: The median rIC50 ratio graph shows the relative rIC50 values for the cell lines of the PPTP panel. Each bar represents the ratio of the panel rIC50 to the rIC50 value of the indicated cell line. Bars to the right represent cell lines with higher sensitivity, while bars to the left indicate cell lines with lesser sensitivity.
In vivo testing
CBL0137 was tested against the PPTP solid tumor xenografts at 50 mg/kg administered by the intravenous route weekly for 4 weeks. Toxicity testing was performed prior to efficacy testing using doses of 90 mg/kg, 68 mg/kg and 50 mg/kg. At 90 mg/kg, weight loss > 10% was observed, as was mortality in 2 of 5 treated animals. The dose selected for efficacy testing was 50 mg/kg, based upon excessive tail necrosis at 68 mg/kg. CBL0137 at 50 mg/kg was generally well tolerated, with a 3.0% mortality rate in the treated groups (12/403), compared to a 0.7% rate for control animals (3/401).
Forty of 42 tested xenograft models were considered evaluable for efficacy. Results from two glioblastoma lines, propagated in athymic nude mice, were excluded from efficacy analysis as toxicity rates exceeded 25%. Complete details of testing are provided in Supplemental Table I, including total numbers of mice, number of mice that died, or were otherwise excluded, numbers of mice with events and average times to event, tumor growth delay, as well as numbers of responses and T/C values.
CBL0137 induced significant differences in EFS distribution compared to control in 10 of 31 (32%) of the solid tumor xenografts evaluable for this measure and in 8 of 8 (100%) of the evaluable ALL xenografts (Table II). BT-41 was not evaluable for differences in EFS distribution because most of the treated and control animals did not experience an event (relative tumor volume > 4) during the treatment/observation period. For those xenografts with a significant difference in EFS distribution between treated and control groups, the EFS T/C activity measure additionally requires an EFS T/C value of > 2.0 for intermediate activity and indicates a substantial agent effect in slowing tumor growth. High activity further requires a reduction in final tumor volume compared to the starting tumor volume. CBL0137 induced tumor growth inhibition meeting criteria for intermediate EFS T/C activity in 0 of 30 (0%) evaluable solid tumor xenografts. For the ALL panel, 7 of 8 (88%) xenografts met criteria for intermediate activity. Partial responses were observed in ALL B-precursor (ALL-2, ALL-17), T-cell ALL (ALL-8, ALL-31) and a CR was observed in and infant MLL (MLL-7).
Table 2.
Summary of in Vivo Activity of CBL0137
| Line | Tumor Type | Median Time to Event |
P- value |
EFS T/C1 |
Median RTV/CD45 at End of Study |
Tumor Volume T/C |
EFS Activity |
Median Group Response2 |
|---|---|---|---|---|---|---|---|---|
| BT-29 | Rhabdoid | 36.7 | <0.001 | 1.9 | >4 | 0.43 | Low | PD2 |
| KT-14 | Rhabdoid | 26.9 | 0.040 | 1.7 | >4 | 0.67 | Low | PD2 |
| KT-16 | Rhabdoid | 13.8 | <0.001 | 1.8 | >4 | 0.51 | Low | PD2 |
| KT-10 | Wilms | 16.4 | 0.164 | 1.4 | >4 | 0.74 | Low | PD1 |
| KT-11 | Wilms | 18.4 | 0.056 | 1.2 | >4 | 0.59 | Low | PD1 |
| KT-13 | Wilms | 17.3 | 0.169 | 1.6 | >4 | 0.56 | Low | PD1 |
| SK-NEP-1 | Ewing | 14.6 | 0.130 | 1.3 | >4 | 0.83 | Low | PD1 |
| EW5 | Ewing | 11.0 | 0.803 | 1.1 | >4 | 1.10 | Low | PD1 |
| EW8 | Ewing | 12.8 | 0.568 | 1.2 | >4 | 0.77 | Low | PD1 |
| TC-71 | Ewing | 14.2 | 0.762 | 1.1 | >4 | 0.82 | Low | PD1 |
| CHLA258 | Ewing | 7.1 | 0.376 | 0.7 | >4 | 1.00 | Low | PD1 |
| Rh10 | Alveolar RMS | 34.1 | <0.001 | 1.8 | >4 | 0.34 | Low | PD2 |
| Rh28 | Alveolar RMS | 25.2 | 0.954 | 0.9 | >4 | 1.23 | Low | PD1 |
| Rh30 | Alveolar RMS | 17.7 | 0.101 | 1.3 | >4 | 0.76 | Low | PD1 |
| Rh30R | Alveolar RMS | 20.7 | <0.001 | 1.6 | >4 | 0.48 | Low | PD2 |
| Rh41 | Alveolar RMS | 12.9 | 0.236 | 1.1 | >4 | 0.83 | Low | PD1 |
| Rh18 | Embryonal RMS | 22.7 | 0.148 | 1.1 | >4 | 0.90 | Low | PD1 |
| BT-28 | Medulloblastoma | 18.5 | 0.093 | 1.4 | >4 | 0.67 | Low | PD1 |
| BT-50 | Medulloblastoma | 36.9 | 0.046 | 1.2 | >4 | 0.86 | Low | PD1 |
| BT-36 | Ependymoma | 25.1 | 0.010 | 0.7 | >4 | 1.37 | Low | PD1 |
| BT-41 | Ependymoma | > EP | NE | . | 2.5 | 0.86 | NE | PD2 |
| NB-SD | Neuroblastoma | 8.2 | 0.950 | 0.9 | >4 | 1.03 | Low | PD1 |
| NB-1691 | Neuroblastoma | 5.6 | 0.290 | 0.9 | >4 | 1.13 | Low | PD1 |
| NB-EBc1 | Neuroblastoma | 5.9 | 0.071 | 1.2 | >4 | 0.74 | Low | PD1 |
| CHLA-79 | Neuroblastoma | 9.7 | 0.345 | 1.1 | >4 | 0.86 | Low | PD1 |
| NB-1643 | Neuroblastoma | 6.4 | 0.465 | 1.0 | >4 | 0.96 | Low | PD1 |
| OS-1 | Osteosarcoma | > EP | <0.001 | > 1.5 | 3.8 | 0.73 | NE | PD2 |
| OS-2 | Osteosarcoma | 32.1 | <0.001 | 1.4 | >4 | 0.72 | Low | PD1 |
| OS-17 | Osteosarcoma | 16.9 | 0.196 | 1.2 | >4 | 0.86 | Low | PD1 |
| OS-9 | Osteosarcoma | 28.4 | 0.058 | 1.3 | >4 | 0.77 | Low | PD1 |
| OS-33 | Osteosarcoma | 31.7 | 0.003 | 1.4 | >4 | 0.75 | Low | PD1 |
| OS-31 | Osteosarcoma | 21.3 | 0.045 | 1.2 | >4 | 0.84 | Low | PD1 |
| ALL-2 | ALL B-precursor | 39.9 | <0.001 | 2.4 | >25 | . | Int | PR |
| ALL-4 | ALL B-precursor | 15.3 | 0.002 | 3.3 | >25 | . | Int | PD2 |
| ALL-7 | ALL B-precursor | 13.2 | <0.001 | 1.7 | >25 | . | Low | PD2 |
| ALL-8 | ALL T-cell | 34.6 | <0.001 | 3.5 | >25 | . | Int | PR |
| ALL-17 | ALL B-precursor | 32.9 | <0.001 | 3.2 | >25 | . | Int | PR |
| ALL-19 | ALL B-precursor | 15.1 | <0.001 | 2.4 | >25 | . | Int | PD2 |
| ALL-31 | ALL T-cell | 31.9 | <0.001 | 3.5 | >25 | . | Int | PR |
| MLL-73 | ALL Infant MLL | 34.2 | <0.001 | 3.8 | >25 | . | Int | CR |
EFS T/C value is defined by the ratio of the median time to event of the treatment group and the median time to event of the respective control group. High activity requires: a) an EFS T/C > 2; b) a significant difference in EFS distributions, and c) a net reduction in median tumor volume for animals in the treated group at the end of treatment as compared to at treatment initiation. Intermediate activity demonstrates criteria a) and b) above, but not having a net reduction in median tumor volume for treated animals at the end of the study. Low activity demonstrates EFS T/C < 2.
Objective response measures are described in detail in the Supplemental Response Definitions. PD1 = progressive disease with EFS T/C ≤ 1.5, and PD2 = progressive disease with EFS T/C > 1.5.
Tumor Volume T/C value: Relative tumor volumes (RTV) for control (C) and treatment (T) mice were calculated at day 21 or when all mice in the control and treated groups still had measurable tumor volumes (if less than 21 days). The T/C value is the mean RTV for the treatment group divided by the mean RTV for the control group. High activity = T/C ≤ 0.15; Intermediate activity = T/C ≤ 0.45 but > 0.15; and Low activity = T/C > 0.45.
KMT2A (MLL) rearranged B-ALL.
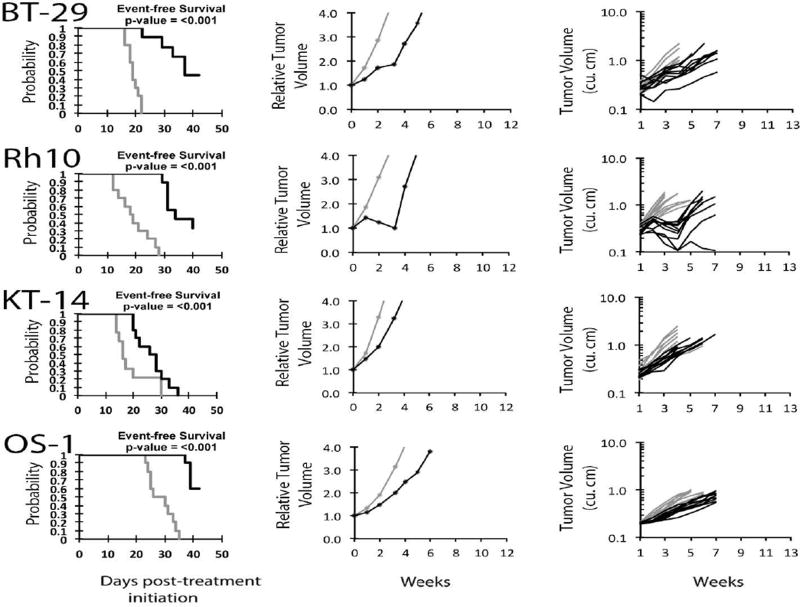
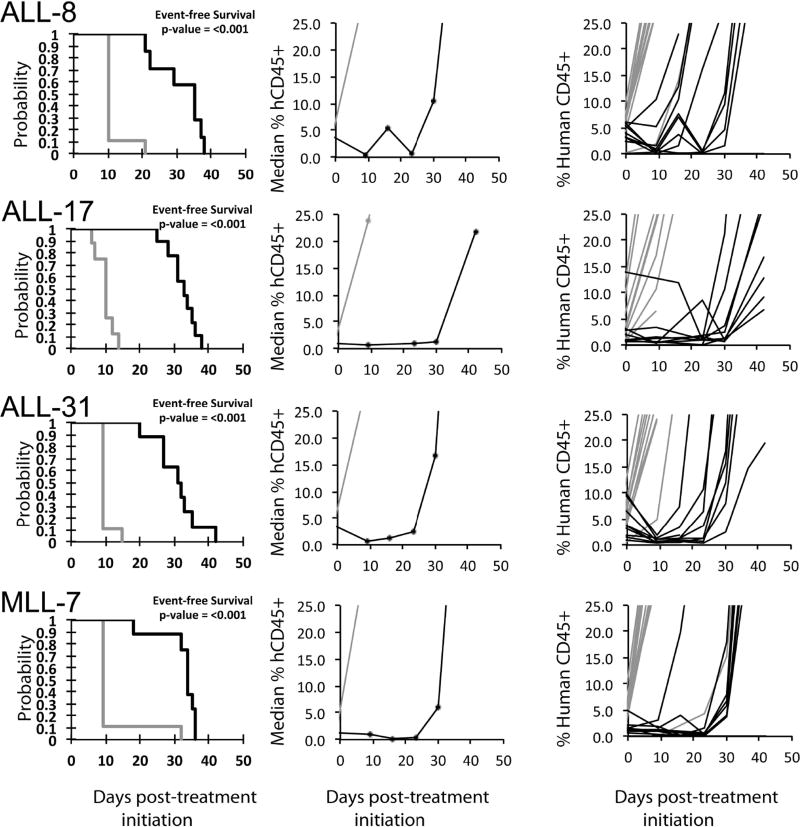
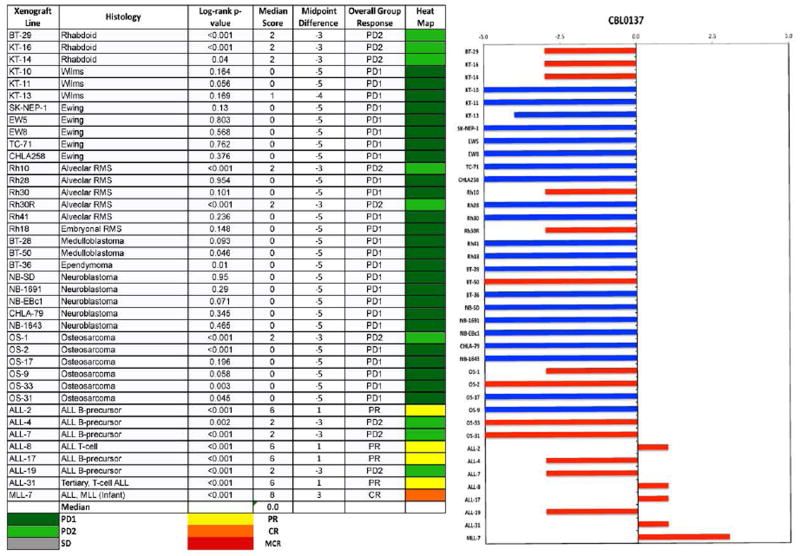
No objective responses were observed among the solid tumor xenografts. Six of 31 showed PD2 responses, indicating progressive disease with tumor growth delay greater than 1.5-fold in time to event. Of these six models 5 were wild type for TP53. However, 12 other solid tumor models wild type for TP53 were unresponsive (i.e., PD1 responses) to CBL0137. Figure 2 shows examples of tumor growth curves and Kaplan-Meier EFS curves for solid tumor xenografts with PD2 responses. For the ALL panel, 1 xenograft achieved CR and 4 achieved PR, with Figure 3 showing examples of these responses. For these responding xenograft lines, the human CD45% in peripheral blood was kept at low levels during the treatment period (day 1 to day 22), but increased rapidly after treatment was stopped. Each of the ALL xenograft lines was wildtype for TP53. The objective response results for both solid tumor and leukemia models are represented in Figure 4 using a ‘COMPARE’ format, based on the objective response scoring criteria centered around the midpoint score of 0 that represents stable disease. In the figure, xenografts with PD2 are indicated by a score of −3, and xenografts with regression (PR or CR) are indicated by bars to the right of the midpoint line. Red bars indicate xenografts with significant differences in EFS distribution between the treated and control groups. Figure 4 shows also the objective response activity in a “heat map” format. The relative expression of FACT subunits SSRP1 and SUPT16H for the PPTP in vitro and in vivo panels are shown in Supplemental Figure 1.
Figure 2.
CBL0137 in vivo objective response activity for more sensitive solid tumors (rhabdoid, BT-28, KT-14), rhabdomyosarcoma (Rh10) and Osteosarcoma (OS-1): Kaplan-Meier curves for EFS (left), median relative tumor volume graphs (center), and individual tumor volume graphs (right) are shown for selected lines. Controls (gray lines); Treated (black lines), statistical significance (p values) of the difference between treated and control groups are included.
Figure 3.
CBL0137 in vivo objective response activity for leukemia models: Kaplan-Meier curves for EFS (left), median relative tumor volume graphs (center), and individual tumor volume graphs (right) are shown for selected lines. Controls (gray lines); Treated (black lines), statistical significance (p values) of the difference between treated and control groups are included.
Figure 4.
Left: The colored heat map depicts group response scores. A high level of activity is indicated by a score of 6 or more, intermediate activity by a score of ≥2 but <6, and low activity by a score of <2. Right: representation of tumor sensitivity based on the difference of individual tumor lines from the midpoint response (stable disease). Bars to the right of the median represent lines that are more sensitive, and to the left are tumor models that are less sensitive. Red bars indicate lines with a significant difference in EFS distribution between treatment and control groups, while blue bars indicate lines for which the EFS distributions were not significantly different. BT-41 is not included because it is not evaluable for the response measure.
DISCUSSION
Curaxins represent novel anticancer agents that simultaneously activate TP53 and suppress survival pathways such as NF-κB. CBL0137 has been reported to have significant activity against various preclinical models of adult carcinoma, and in the TH-MYCN transgenic mouse model of neuroblastoma 9.
The median relative IC50 value for the PPTP cell lines was 0.28 μM, with a fairly narrow range range from 0.13 μM to 0.80 μM (Table 1). The most sensitive cell line, MOLT-4, is an ALL cell line. There were no significant differences in rIC50 values by histotype. Of note, the median rIC50 for the neuroblastoma cell lines exceeded that of the non-neuroblastoma cell lines (0.51 μM vs 0.26 μM, resepctively, p=0.16), while the median rIC50 for the ALL cell lines was less than that of the non-ALL cell lines (0.20 μM vs 0.31 μM, respectively, p=0.06). At the highest concentrations used (10 µ M) CBL0137 was clearly cytotoxic with Ymin values at or approaching −100% for all cell lines.
At present no human pharmacokinetic data are available for CBL0137. However, following a single dose of 30 or 60 mg/kg to CD-1 mice, Cmax levels in plasma reached 6 and 13 µM, respectively. At these doses the plasma half-life was 5.62 and 6.47 Hr, respectively (Cleveland Biolabs data, unpublished). Thus, the rIC50 concentrations determined in vitro are below the Cmax and AUC achieved in mice.
In vivo, CBL0137 was well tolerated at the dose and schedule used. Against solid tumor models the activity of CBL0137 was modest with some delay in tumor growth (PD2) in 6 models, but overall exerted low activity. These data are consistent with results using pancreatic carcinoma xenograft models where tumor growth inhibition varied from approximately 20–60% 19. Of note, none of five neuroblastoma xenografts responded significantly to CBL0137 treatment in contrast to the TH-MYCN transgenic and BE(2)-C xenograft models previously reported 9. In the latter report, a higher dose of CBL0137 (60 mg/kg) with more frequent dosing (every 4 days) was employed compared to that used by the PPTP (50 mg/kg every 7 days). CBL0137 demonstrated greater activity against leukemia models with partial responses (PR) measured in 3 B-precursor ALL, 1 T-cell and a complete response (CR) in an infant MLL model. Of note, the responding ALL models had relatively low level expression of both SSRP1 and SUPT16H (that encodes SPT16), considered as markers for sensitivity when expressed at high levels 19. In contrast, KT-13 (Wilms tumor) expressed the highest level of SUPT16 and had high-level expression of SSRP1, but had a poor response (PD1, T/C EFS = 1.6). Thus, the major activity against these models appears to be in the leukemia panel, although the duration of response was short suggesting that the depth of remisson was not deep.
Of importance in considering next steps is how the drug levels in mice at the dose used for testing relate to the systemic exposures to CBL0137 observed in patients. Until human pharmacokinetic data are available for comparison, it is not possible to determine whether the results presented here are likely an under- or over-estimate of the likely efficacy of CBL0137 against pediatric malignancies. Carter et al. reported that CBL0137 enhanced the activity of cytotoxic agents (cyclophosphamide, topotecan) that are used in treatment of neuroblastoma and other solid tumors of childhood 9. If systemic exposure in mice and humans is reasonably comparable, then further combination studies with standard cytotoxic agents in solid tumor and leukemia models may be valuable in informing pediatric development of CBL0137.
Supplementary Material
Response definitions
Supplemental Figure 1. Relative expression of SSRP1 and SUPT16H (encoding SPT16) in PPTP in vitro and in vivo models.
Supplemental Table I. Efficacy of CBL0137 against PPTP Xenograft Models
Acknowledgments
This work was supported by NO1-CM-42216, CA21765, and CA108786 from the National Cancer Institute and used CBL0137 supplied by Cleveland Biolabs, Inc.. In addition to the authors this paper represents work contributed by the following: Sherry Ansher, Jennifer Richmond, Joshua Courtright, Kathryn Evans, Edward Favours, Henry S. Friedman, Danuta Gasinski, Melissa Sammons, Joe Zeidner, Jianrong Wu, Ellen Zhang, and Jian Zhang. Children’s Cancer Institute Australia for Medical Research is affiliated with the University of New South Wales and the Sydney Children’s Hospitals Network.
Glossary
- PPTP
Pediatric Preclinical Testing Program
- EFS T/C
Ratio of Event Free Survival Treated/Control
- Cmax
Maximum plasma concentration
- (NOD)/scid−/−
Non-Obese diabetic/severe combined immune deficient
- FACT
Facilitates Chromatin Transcription
- rIC50
median relative IC50
- %hCD45+
percentages of human CD45-positive
Footnotes
Conflict of interest statement: Andrei Gudkov was previously Cleveland BioLabs' Chief Scientific Officer and continues to receive compensation from Cleveland Biolabs. Andrei Purmal is an employee of Incuron LLC, which is a joint subsidiary of the Bioprocess Capital Ventures closed-end investment fund and Cleveland BioLabs. The remaining authors consider that there are no actual or perceived conflicts of interest.
References
- 1.Gasparian AV, Burkhart CA, Purmal AA, et al. Curaxins: anticancer compounds that simultaneously suppress NF-kappaB and activate p53 by targeting FACT. Science translational medicine. 2011 Aug 10;3(95):95ra74. doi: 10.1126/scitranslmed.3002530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neznanov N, Gorbachev AV, Neznanova L, et al. Anti-malaria drug blocks proteotoxic stress response: anti-cancer implications. Cell cycle. 2009 Dec;8(23):3960–3970. doi: 10.4161/cc.8.23.10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neznanov N, Komarov AP, Neznanova L, Stanhope-Baker P, Gudkov AV. Proteotoxic stress targeted therapy (PSTT): induction of protein misfolding enhances the antitumor effect of the proteasome inhibitor bortezomib. Oncotarget. 2011 Mar;2(3):209–221. doi: 10.18632/oncotarget.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orphanides G, Wu WH, Lane WS, Hampsey M, Reinberg D. The chromatin-specific transcription elongation factor FACT comprises human SPT16 and SSRP1 proteins. Nature. 1999 Jul 15;400(6741):284–288. doi: 10.1038/22350. [DOI] [PubMed] [Google Scholar]
- 5.Yarnell AT, Oh S, Reinberg D, Lippard SJ. Interaction of FACT, SSRP1, and the high mobility group (HMG) domain of SSRP1 with DNA damaged by the anticancer drug cisplatin. The Journal of biological chemistry. 2001 Jul 13;276(28):25736–25741. doi: 10.1074/jbc.M101208200. [DOI] [PubMed] [Google Scholar]
- 6.Zeng SX, Li Y, Jin Y, et al. Structure-specific recognition protein 1 facilitates microtubule growth and bundling required for mitosis. Molecular and cellular biology. 2010 Feb;30(4):935–947. doi: 10.1128/MCB.01379-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia H, Miecznikowski JC, Safina A, et al. Facilitates chromatin transcription complex is an "accelerator" of tumor transformation and potential marker and target of aggressive cancers. Cell reports. 2013 Jul 11;4(1):159–173. doi: 10.1016/j.celrep.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo KC, Rossi MR, Eberhart CG, Cowell JK. Genome wide copy number abnormalities in pediatric medulloblastomas as assessed by array comparative genome hybridization. Brain pathology. 2007 Jul;17(3):282–296. doi: 10.1111/j.1750-3639.2007.00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter DR, Murray J, Cheung BB, et al. Therapeutic targeting of the MYC signal by inhibition of histone chaperone FACT in neuroblastoma. Science translational medicine. 2015 Nov 4;7(312):312ra176. doi: 10.1126/scitranslmed.aab1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia H, Fleyshman D, Kolesnikova K, et al. Expression of FACT in mammalian tissues suggests its role in maintaining of undifferentiated state of cells. Oncotarget. 2011 Oct;2(10):783–796. doi: 10.18632/oncotarget.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harbor perspectives in biology. 2009 Nov;1(5):a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kordes U, Krappmann D, Heissmeyer V, Ludwig WD, Scheidereit C. Transcription factor NF-kappaB is constitutively activated in acute lymphoblastic leukemia cells. Leukemia. 2000 Mar;14(3):399–402. doi: 10.1038/sj.leu.2401705. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Garzon R, Sun H, et al. NF-kappaB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer cell. 2008 Nov 4;14(5):369–381. doi: 10.1016/j.ccr.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu M, Lee H, Bellas RE, et al. Inhibition of NF-kappaB/Rel induces apoptosis of murine B cells. The EMBO journal. 1996 Sep 2;15(17):4682–4690. [PMC free article] [PubMed] [Google Scholar]
- 15.Bargou RC, Emmerich F, Krappmann D, et al. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin's disease tumor cells. The Journal of clinical investigation. 1997 Dec 15;100(12):2961–2969. doi: 10.1172/JCI119849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sovak MA, Bellas RE, Kim DW, et al. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. The Journal of clinical investigation. 1997 Dec 15;100(12):2952–2960. doi: 10.1172/JCI119848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong MB, Bian X, Liu Y, et al. Signaling from p53 to NF-kappaB determines the chemotherapy responsiveness of neuroblastoma. Neoplasia. 2006 Nov;8(11):967–977. doi: 10.1593/neo.06574. [DOI] [PubMed] [Google Scholar]
- 18.Bentires-Alj M, Barbu V, Fillet M, et al. NF-kappaB transcription factor induces drug resistance through MDR1 expression in cancer cells. Oncogene. 2003 Jan 9;22(1):90–97. doi: 10.1038/sj.onc.1206056. [DOI] [PubMed] [Google Scholar]
- 19.Burkhart C, Fleyshman D, Kohrn R, et al. Curaxin CBL0137 eradicates drug resistant cancer stem cells and potentiates efficacy of gemcitabine in preclinical models of pancreatic cancer. Oncotarget. 2014 Nov 30;5(22):11038–11053. doi: 10.18632/oncotarget.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frgala T, Kalous O, Proffitt RT, Reynolds CP. A fluorescence microplate cytotoxicity assay with a 4-log dynamic range that identifies synergistic drug combinations. Mol Cancer Ther. 2007 Mar 1;6(3):886–897. doi: 10.1158/1535-7163.MCT-04-0331. 2007. [DOI] [PubMed] [Google Scholar]
- 21.Kang MH, Smith MA, Morton CL, Keshelava N, Houghton PJ, Reynolds CP. National Cancer Institute Pediatric Preclinical Testing Program: Model description for in vitro cytotoxicity testing. Pediatr Blood Cancer. 2011 Feb;56(2):239–249. doi: 10.1002/pbc.22801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houghton PJ, Morton CL, Tucker C, et al. The pediatric preclinical testing program: Description of models and early testing results. Pediatr Blood Cancer. 2006 Oct 25; doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 23.Liem NL, Papa RA, Milross CG, et al. Characterization of childhood acute lymphoblastic leukemia xenograft models for the preclinical evaluation of new therapies. Blood. 2004 May 15;103(10):3905–3914. doi: 10.1182/blood-2003-08-2911. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Response definitions
Supplemental Figure 1. Relative expression of SSRP1 and SUPT16H (encoding SPT16) in PPTP in vitro and in vivo models.
Supplemental Table I. Efficacy of CBL0137 against PPTP Xenograft Models