Abstract
We aimed to determine if toll-like receptor (TLR) expression is modulated in response to dry eye-associated conditions and in Dry Eye Syndrome (DES).Primary human corneal epithelial cells (HCEC), a SV40 HCEC cell line or a normal human conjunctival epithelial cell line (IOBA-NHC) were cultured under hyperosmolar stress (HOS) (400-500 mOsm/kg) or with DES associated cytokines (IL-1α/β, TNFα or TGFβ) at concentrations ranging from 1-1000 ng/ml for up to 24 hrs. Epithelial cells were harvested from a human cornea organ culture model following 24 hrs of desiccation. Conjunctival impression cytology samples were harvested from subjects with DES and age and gender-matched normal subjects. TLR4, TLR5 or TLR9 mRNA or protein was examined by quantitative RT-PCR, western blotting or flow cytometry. TLR functionality was evaluated in terms of addition of TLR agonists and quantitation of secreted inflammatory cytokines by the use of ELISA and Luminex assays. In SV40 HCEC, HOS significantly increased TLR4 by 8.18 fold, decreased TLR9 by 0.58 fold, but had no effect on TLR5 mRNA expression. TLR4 and TLR9 protein were decreased by 67.7% and 72% respectively. TLR4 mRNA was also significantly up-regulated by up to 9.70 and 3.36 fold in primary HCEC and IOBA-NHC respectively. DES associated cytokines had no effect on TLR4, 5 and 9 expression. In response to desiccation, TLR4 and TLR5 mRNA were significantly up-regulated by 4.81 and 2.51 fold respectively, while TLR9 mRNA was down-regulated by 0.86 fold in HCEC. A similar trend for TLR4 and TLR9 protein was observed. TLR9 mRNA was significantly down-regulated by almost 59.5% in DES subjects. In conclusion, changes in TLR expression occur in dry eye and could have an important role in ocular surface susceptibility to inflammation and infection.
Keywords: Dry eye, toll-like receptors, hyperosmolar stress, inflammation
1. Introduction
Dry Eye Syndrome (DES) is an ocular surface condition that affects millions of individuals every year and is one of the leading causes for visits to the eye doctor. Subjects with severe DES have an increased risk for corneal ulceration and melting (Vivino et al., 2001) and ocular infection (Jhanji et al., 2009) which may result in vision loss. Although DES typically does not result in blindness, subjects often report a decreased quality of life and reduced ability to perform daily activities (Miljanovic et al., 2007) leading to loss of job productivity.
Inflammation plays a pivotal role in DES pathogenesis, and it has been demonstrated to be driven by tear film hyperosmolarity (Bron et al., 2002; Farris, 1994; Gilbard et al., 1978) and instability which stimulate an increase in pro-inflammatory cytokines (Afonso et al., 1999; Pflugfelder et al., 1999; Solomon et al., 2001) at the ocular surface, leading to the disruption of the ocular surface epithelium and exacerbation of the disease. To date, no studies have investigated if in DES there is a change in the expression of innate immune receptors that can stimulate inflammation, such as toll-like receptors (TLRs), on the ocular surface.
Toll-like receptors are a family of highly conserved glycoprotein receptors that recognize conserved motifs on pathogen associated molecular patterns on microbes and as suggested by some studies, host endogenous ligands (Medzhitov et al., 1997; Takeda et al., 2003). The activation of TLRs leads to the production of various pro-inflammatory cytokines and chemokines (Akira and Takeda, 2004; Takeda et al., 2003). Ten functional human TLRs have been identified (TLR1-TLR10), each binding a distinct microbial ligand. Toll-like receptor 4 (TLR4), the most extensively studied of the TLRs is activated by lipopolysaccharide (LPS) (Beutler, 2002) and endogenous host ligands such as heat shock proteins [HSP60 (Ohashi et al., 2000), HSP70 (Vabulas et al., 2002)] and hyaluronic acid fragments (Gariboldi et al., 2008). TLR5 is activated by bacterial flagellin (Hayashi et al., 2001), and TLR9 responds to the unmethylated CpG motifs found in bacterial and viral DNA (Hemmi et al., 2000; Tabeta et al., 2004). With the exception of TLR8, all TLRs are reported to be commonly expressed in the cornea and conjunctiva although some discrepancies remain regarding their subcellular localization. For a detailed review on ocular surface TLRs, the reader is referred to two review articles (Lambiase et al., 2011; Redfern and McDermott, 2010).
Studies have shown topical application of TLR agonists on the corneal epithelium can produce extensive ocular surface inflammation(Adhikary et al., 2008; Johnson et al., 2005; Kumar et al., 2006; Zhang et al., 2003). In particular, the activation of TLR2, 4 and 9 in the murine corneal epithelium has been shown to induce sight-threatening keratitis (Johnson et al., 2005) while the application of eritoran tetrasodium, a TLR4 antagonist, can significantly inhibit corneal inflammation in response to stimulation with LPS (Sun and Pearlman, 2009) suggesting a potential therapeutic role for TLR antagonists in modulating corneal inflammation.
TLR expression has also been shown to be increased in dry eye and its most severe form, Sjögren's syndrome (SS), an autoimmune disorder that causes functional impairment of the salivary and lacrimal glands. In the parotid gland in subjects with SS, TLR7 and TLR9 were expressed throughout the gland on the epithelial islands, lymphocytes, and ductal epithelial cells, while in control subjects, TLR7 and TLR9 expression was limited to the ductal epithelial cells (Zheng et al., 2010). In a SS mouse model, TLR4 and TLR5 mRNA was up-regulated in the cornea and TLR4 was up-regulated in the lacrimal gland (Christopherson PL, 2005). Together these data suggest that TLRs may be involved in the pathogenesis of dry eye inflammation. Considering this, TLR expression was examined in subjects with DES and in various ocular surface cells in response to dry eye associated conditions, such as hyperosmolar stress (HOS), desiccation and cytokines. This study focuses on TLR4, TLR5 and TLR9 which are known to be expressed by ocular surface cells and have been implicated in ocular surface inflammation (Adhikary et al., 2008; Johnson et al., 2005; Kumar et al., 2006; Sun and Pearlman, 2009; Zhang et al., 2003).
2. Methods
2.1. Cell Cultures
Primary human corneal epithelial cell (HCEC) cultures were prepared from human corneas unsuitable for transplantation obtained from eye banks within 3 to 5 days of death with a mean age and standard deviation of 71.5 ± 8.9 years. The tissue was obtained in accordance with the guidelines of the Declaration of Helsinki regarding research involving human tissue. Cells were isolated as previously described (Redfern et al., 2011) and were maintained in EpiLife medium (Invitrogen; Portland, OR). Normal human conjunctival (IOBA NHC) epithelial cells (Diebold et al., 2003) were cultured in DMEM-F12 (1:1 vol/vol), containing 10% fetal bovine serum (FBS) as previously described (Narayanan et al., 2006b). SV40-transformed HCEC were a gift from Dr. Kaoru Araki-Sasaki (Tane Memorial Eye Hospital, Osaka, Japan). The cells were maintained in SHEM (DMEM-Ham's F12, 1:1 vol/vol) supplemented with 10% FBS as previously described (Redfern et al., 2011). All cultured cells were maintained at 37°C in 5% CO2.
2.2. Cell Treatment
Cells were cultured to 60-70% confluence, washed three times with phosphate buffered saline (PBS) to remove floating dead cells as well as residual serum and growth factors and placed in supplement-free (primary HCEC) or serum-free (cell lines) media (SFM) overnight. Cells were cultured for an additional 24 hrs in SFM or SFM with either osmolarity ranging from 400 to 500 mOsm/kg, which was achieved by adding various amounts of sodium chloride (Li et al., 2006); or with 1-1000 ng/ml of IL-1α, IL-1β, TNFα or TGFβ for up to 24 hrs. In some samples treated with HOS, the hyperosmolar media was removed; the cells were washed three times with phosphate buffered saline (PBS), and cultured with normal growth media for an additional 6 or 24 hrs. At the end of the incubation period, the cells were either harvested in RLT lysis buffer (Qiagen; Valencia, CA) or pelleted, snap frozen and stored at -80°C until RNA extraction or western blotting for either TLR mRNA and protein or human beta defensin (hBD)-2 mRNA expression.
2.3. Bacterial DNA
Pseudomonas aeruginosa 19660 (PA 19660) was grown in Difco nutrient broth (BD, Franklin Lakes, NJ) at 37°C to stationary phase. Bacteria were diluted to OD260nm =0.2 in phosphate-buffered saline (PBS), determined to be 1×107 cfu/ml. Bacterial DNA was prepared suspending the bacteria in 100 mM NaCl-10 mM Tris-HCl-25 mM EDTA (pH=8.0) with proteinase K (0.2 mg/ml) and sodium dodecyl sulfate (0.5%) and shaking at 150 rpm, 50°C overnight. DNA was precipitated from the lysate with iso-propanol, washed with 70% ethanol, air dried, dissolved in Tris-EDTA (TE) buffer and stored at 4°C.
2.4. Cytokines secretion
SV40 HCEC were seeded in 12-well plates at a density of 2×105 cells/well and allowed to adhere for 24 hrs. Following adherence, cells were washed two times with PBS and SHEM complete media was replaced by serum-free SHEM for 16 hrs. Cells were then incubated in iso-osmolar (374-377mOs/kg) or hyperosmolar (450mOs/kg) media in the presence of TLR4 agonist, LPS (1ug/ml) or TLR9 agonist, PA 19660 DNA (10 μg/ml) for 24 hrs. After treatment, the supernatant from each well was transferred into a 1.5 ml Eppendorf tube. All supernatant samples were snap frozen in liquid nitrogen and stored at -80°C for later quantitation of IL-8 by an ELISA assay (BioLegend, San Diego, CA); in addition supernatant samples were interrogated for a panel of 13 inflammatory cytokines (which included IL-6) by the use of a Luminex assay (EMD Millipore, Billerica, MA) following the manufacturer's instructions.
2.5. Desiccation Organ Culture Model
Human corneas were obtained from eye banks within 3-5 days of death. The mean age and standard deviation of the donors was 59 ± 1.7 years. Corneas with intact epithelium were stabilized with an agar mold as previously described (McDermott et al., 2001), then placed epithelial side up into 35 mm culture dishes which were filled with M199 media up to the limbal conjunctiva (desiccation model) and maintained as previously described (McDermott et al., 2001) or completely submerged (control). After 24 hrs, the epithelium was collected for analysis of TLR mRNA and protein expression.
2.6. Human Subjects
All procedures involving human subjects were in accordance with the Tenets of the Declaration of Helsinki and were approved by the University of Houston's and Genoa's Institutional Review Board. Written informed consent was obtained from all subjects before participation in the study. Subjects for mRNA analysis were evaluated at the University of Houston and categorized as normal or DES by their subjective responses to the ocular surface disease index (OSDI) questionnaire (Schiffman et al., 2000) and objective clinical signs were also compared between DES and normal subjects. Corneal and conjunctival epithelial staining (graded 1-4 as per the Cornea and Contact Lens Research Unit grading scale with fluorescein and lissamine green respectively; School of Optometry, University of New South Wales, Sydney, Australia), tear production (phenol red thread test), tear film osmolarity (Vapor pressure osmometer, Vapro 5520); and tear stability (fluorescein tear break-up time, Dry Eye Test; Akorn, Chicago, IL) were obtained. Subjects for TLR protein analysis were evaluated at the University of Genoa and were categorized as having DES based on the presence of cornea fluorescein staining greater than three, tear break-up time less than five seconds and a Schirmer's I test less than 8 mm at 5 min.
2.7. Conjunctival Impression Cytology (CIC)
Following the completion of all the objective clinical assessments, a single drop of 0.5% proparacaine hydrochloride anesthetic (Bausch and Lomb; Rochester, NY) was instilled onto each eye. Two to three 6.5 × 13 mm sterile polyether sulfone membranes (Supor; Pall Gellman Sciences; East Hills, NY) were placed on the superior or inferior bulbar conjunctiva without applying pressure to the eye. The membranes from both eyes of one subject were removed and placed either directly into one tube containing 350 μl of lysis buffer (Qiagen) and stored at -80°C until quantitative RT-PCR analysis for TLR mRNA expression or processed for flow cytometry to quantitate TLR4 protein levels.
2.8. Quantitative RT-PCR
Total RNA from CIC samples was extracted using a RNeasy Micro Kit (Qiagen) and all other samples were extracted using a RNeasy Mini Kit (Qiagen). RNA elution columns were DNAse treated prior to RNA elution to avoid genomic DNA contamination. Quantitative RT-PCR was used to quantitate relative TLR mRNA expression. cDNA was generated using SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA). Reverse transcription was performed at 50°C for 60 min. Samples containing no reverse transcriptase or water in place of RNA (no template control) served as negative controls. PCR amplification of cDNA was performed with Brilliant SYBR Green QPCR Master Mix (Stratagene; La Jolla, CA) using specific primers for TLR4, TLR5, TLR9 and GAPDH as previously described (Redfern et al., 2011). Reactions were performed in triplicate using an Mx3005P QPCR System (Stratagene). Amplified gene products were normalized to GAPDH and calibrated to age and gender-matched controls or non-treated culture samples. The relative change of DES subjects or HOS treated samples versus the respective control samples was determined with the value of control samples being normalized to one. For each experiment, the samples were analyzed in triplicate and the mean relative quantity of TLR expression was calculated. Data derived from a minimum of two-three experiments were analyzed using an unpaired Student's t-test where P ≤ 0.05 was considered a significant difference.
2.9. Flow Cytometry
Impression cytology specimens were collected from 19 subjects with DES and analyzed in a masked manner by flow cytometry as previously described (Barabino et al., 2010). Antibodies directed against TLR4 (Santa Cruz Biotechnology; Santa Cruz, CA) were used in intact and permeabilized cells to evaluate membrane and cytoplasmic TLR4 expression. The percentage of positive cells was calculated and compared with those obtained in six normal (control) subjects. Data were analyzed using the Mann Whitney test. P ≤ 0.05 was considered to be significant.
2.10. Western Blotting
Cell pellets were processed for western blotting as previously described (Redfern et al., 2011). The membranes were probed with anti-TLR4 (0.2 μg/ml, Santa Cruz Biotechnology) or anti-TLR9 (1 μg/ml, AbCam; Cambridge, MA) antibodies, then incubated with horseradish peroxidase-conjugated secondary antibody, and visualized with ECL Plus (GE Healthcare; Piscataway, NJ). The membranes were stripped and reprobed using a GAPDH antibody as previously described (Giddabasappa et al., 2011). Densitometry measurements were obtained from non-saturated blots and the pixel intensity was normalized to GAPDH. Data are representative of a minimum of three experiments and were analyzed using an unpaired Student's t-test, with P ≤ 0.05 considered as statistically significant.
3. Results
Changes in TLR4, TLR5 and TLR9 expression at the mRNA and protein levels are summarized in Table 1.
Table 1. Differential expression of TLR4, TLR5 and TLR 9 mRNA and protein.
| TLR Expression in Response to Dry Eye Conditions | ||||
|---|---|---|---|---|
| TLR | In Vitro | Cadaver Corneas or Human Subjects | ||
| Hyperosmolar Stress | Dry Eye Associated Cytokines | Organ Desiccation Model | Conjunctival Impression Cytology Dry Eye Subjects | |
| TLR4 |
↑ TLR4 mRNA ↓TLR4 Protein |
No Effect on TLR4 |
↑ TLR4 mRNA #NS ↑TLR4 protein |
#NS ↑TLR4 mRNA |
| TLR5 | No Effect on TLR5 | No Effect on TLR5 | ↑ TLR5 mRNA | No Effect on TLR5 |
| TLR9 |
↓ TLR 9 mRNA ↓ TLR 9 protein |
No Effect on TLR9 |
↓ TLR 9 mRNA #NS↓ TLR 9 protein |
↓ TLR 9 mRNA |
Up-regulation
Down-regulation
Non-significant (NS) change in expression
3.1. Hyperosmolar stress modulates TLR expression and function
In response to 400, 450, and 500 mOsm/kg HOS stress, TLR4 mRNA was up-regulated by 1.40, 2.72 and 8.18 fold , respectively in SV40 HCEC, whereas TLR9 mRNA was down-regulated by 0.38, 0.58 and 0.16 fold compared to the control (P ≤ 0.05 Student's t-test, n=3). To determine if the change in TLR4 and TLR9 mRNA expression would return to baseline upon withdrawal of HOS, SV40 HCEC were allowed to recover for 6 or 24 hrs in normal growth media. TLR4 expression returned to baseline after a 6 hrs incubation in normal growth media (n=4) while TLR9 mRNA expression remained down-regulated at 6 hrs (n=3) and returned to baseline compared to the untreated control after 24 hrs (n=2) (Fig. 1A). TLR5 mRNA expression was not significantly modulated in response to HOS (data not shown).Since HOS dramatically changed TLR4 mRNA expression in SV40 HCEC, TLR4 mRNA expression was then examined in primary corneal epithelial cells and conjunctival epithelial cells (Fig. 1B) to determine whether this effect was only observed in SV40 HCEC or if it was also present in other ocular surface cells. As with the SV40 HCEC, HOS significantly increased the mRNA expression of TLR4 by 1.35, 3.56, and 9.70 fold in primary HCEC and in IOBA NHC cells by 2.41, 3.55, 3.36 fold in response to 400, 450, and 500 mOsm/kg stress respectively, n=3.
Figure 1. TLR mRNA and protein expression is modulated in response to hyperosmolar stress (HOS) in ocular surface cells.
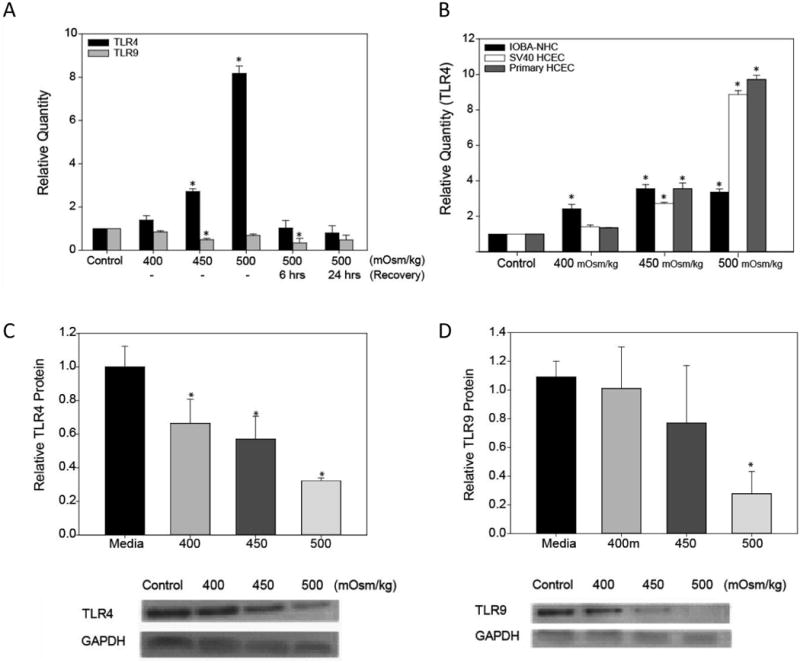
SV40 HCEC were cultured under HOS (400-500 mOsm/kg) or media alone (control) for 24 hrs and following HOS of 500 mOsm/kg, the cells were allowed to recover for 6 and 24 hrs in normal growth media. TLR4 (n=4) and TLR9 (n=3) mRNA expression was determined by quantitative RT-PCR (A). Corneal (primary and SV40 HCEC) and conjunctival epithelial cells (IOBA-NHC) were cultured under HOS for 24 hrs then TLR4 mRNA expression was determined by real-time PCR (B). To confirm a change in protein expression, cell lysates from SV40 HCEC cultured under HOS were analyzed by western blotting for TLR4 (C), TLR9 (D) and GAPDH (n=3). Data were analyzed using an unpaired Student's t-test where P ≤ 0.05 was considered to be statistically significant when compared to control (*).
Unexpectedly, semi-quantitative western blotting revealed that TLR4 protein levels decreased by 33.5%, 42.8%, and 67.7% in response to 400, 450, and 500 mOsm/kg stress, n=3 (Fig. 1C). TLR9 protein was decreased in response to HOS but only at 500 mOsm/kg under which condition TLR9 expression was reduced by 72% (Fig. 1D). No significant changes were observed for TLR5 protein (data not shown).
To determine a functional consequence of reduced TLR protein expression, SV40 HCEC were subjected to HOS to reduce TLR4 expression and then stimulated with LPS (TLR4 ligand) in the presence or absence of IFN-γ. Under iso-osmolar conditions, SV40 HCEC responded to LPS by secretion of IL-8 (P ≤ 0.05 Student's t-test, n=3), however when media osmolarity was changed to 450 mOsm/kg, secretion of IL-8 in the presence of LPS or LPS and IFN-γ did not significantly changed when compared to untreated media (Fig. 2A). Similarly, SV40 HCEC cells were stimulated with Pseudomonas aeruginosa DNA (TLR9 ligand), under HOS for 24 hrs and IL-6 was found decreased under HOS when compared to iso-osmolar conditions (Fig. 2B).
Figure 2. Hyperosmolar stress (HOS) decreases secretion of IL-6 and IL-8 in ocular surface cells.
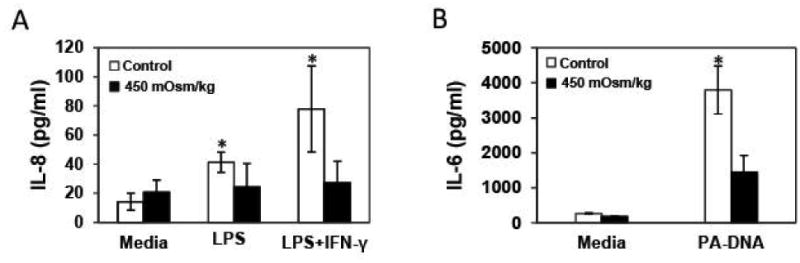
Secreted IL-8 was measured by ELISA in cell culture supernatants of SV40 HCEC cultures under HOS in the presence of LPS (TLR4 ligand) and LPS with IFN-γ. Increased production of IL-8 in the presence of LPS and/or IFN-γ was not observed under HOS but only at iso-osmolar conditions (A). SV40 HCEC were stimulated with Pseudomonas aeruginosa DNA (TLR9 ligand, 10 μg/ml) under HOS for 24 hrs and supernatants were examined for a panel of 13 inflammatory cytokines; among all interrogated cytokines only IL-6 secretion was significantly decreased under HOS when compared to iso-osmolar conditions (B). Data were analyzed using an unpaired Student's t-test where P ≤ 0.05 was considered to be statistically significant when compared to control (*).
3.2 Dry eye associated cytokines do not modulate TLR4, 5, and 9 expression in SV40 HCEC
IL-1α , IL-1β, TNFα, and TGFβ did modulate the expression levels of neither TLR4 mRNA (Fig. 3A) nor TLR9 mRNA (data not shown). Likewise, at the protein level, TLR4 and TLR9 protein did not change (Fig. 3A and Fig. 3B). No significant changes were observed for TLR5 mRNA and protein (data not shown). All treatments were done in SV40 HCEC after 24 hrs of treatment, n=3. Additional concentrations and time points were then examined to ensure that a more optimal testing condition was not overlooked. Again, IL-1β did not significantly modulate the expression of TLR4, TLR5, and TLR9 mRNA in SV40 HCEC at concentrations ranging from 1-1000 ng/ml (n=2) after 24 hrs or after 3, 9, and 12 hrs of treatment using 10 ng/ml (Table 2). IL-1β however was able to modulate the expression of hBD-2 at 10 ng/ml after 24 hrs in SV40 HCEC suggesting efficacy of this cytokine.
Figure 3. Dry eye associated cytokines do not modulate TLR4 and TLR9 mRNA expression.
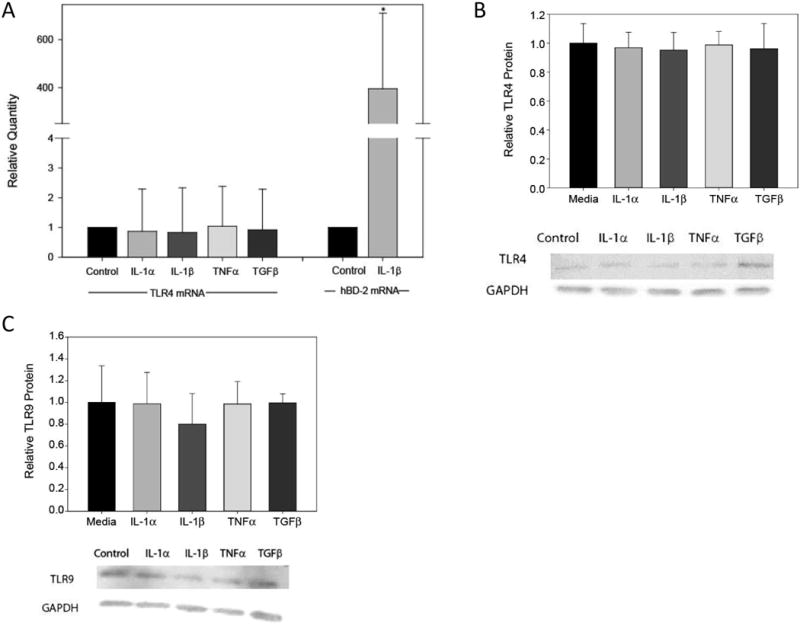
SV40 HCEC were cultured with DED associated cytokines, IL-1α, IL-1β, TNFα, and TGFβ (10 ng/ml for 24 hrs). Quantitative RT-PCR was performed to determine TLR4 or hBD-2 mRNA expression, n=3. IL-1α, IL-1β, TNFα, and TGFβ did not modulate the mRNA expression levels for TLR4 and TLR9 (data not shown) (A). In addition, western blotting was performed to determine TLR4 and TLR9 protein levels, n=3. Neither TLR4 nor TLR9 protein expression was affected by any of the DED associated cytokines (B). Data were analyzed using an unpaired Student's t-test where P ≤ 0.05 was considered to be statistically significant when compared to control (*).
Table 2. Relative Quantity (RQ) of TLR mRNA expression in response to IL-1β.
| 24 hours | TLR4 | TLR5 | TLR9 |
|---|---|---|---|
| (ng/ml)IL-1β | RQ ± std. dev. | RQ ± std. dev. | RQ ± std. dev. |
| 1 | 1.10 ± 0.22 | 1.46 ± 0.31 | 1.01 ± 0.07 |
| 10 | 1.66 ± 0.38 | 1.86 ± 0.26 | 1.12 ± 0.15 |
| 100 | 1.48 ± 0.25 | 1.62 ± 0.42 | 1.21 ± 0.18 |
| 1000 | 1.09 ± 0.03 | 1.93 ± 0.57 | 1.31 ± 0.20 |
|
| |||
| 10ng/ml IL-1β | TLR4 | TLR5 | TLR9 |
| (time) | RQ ± std. dev. | RQ ± std. dev. | RQ ± std. dev. |
|
| |||
| 3hr | 1.25 ± 1.24 | 1.24 ± 0.20 | 0.61 ± 0.75 |
| 9hr | 2.06 ± 0.74 | 0.94 ± 0.28 | 1.31 ± 023 |
| 12hr | 1.52 ± 0.71 | 1.15 ± 0.09 | 1.55 ± 0.54 |
3.3. Desiccation modulates TLR4, 5 and 9 expression in human corneal epithelial cells
TLR4 and TLR5 mRNA were up-regulated in HCEC harvested from the desiccation culture model by 4.81 fold and 2.51 fold respectively whereas TLR9 was down-regulated by 0.86 fold of the control, n=3 (Fig. 4A). Semi-quantitative western blotting demonstrated an up-regulation of TLR4 protein by approximately 10% (Fig. 4B) and a down-regulation of TLR9 protein by 20.47% (Fig.4C) in response to desiccation in two of the three samples tested compared to the submerged control, n=3. No significant changes were observed at protein level for TLR5 (data not shown).
Figure 4. Desiccation modulates the expression of TLR4, TLR5 and TLR9 in human corneal epithelial cells in an organ culture model.
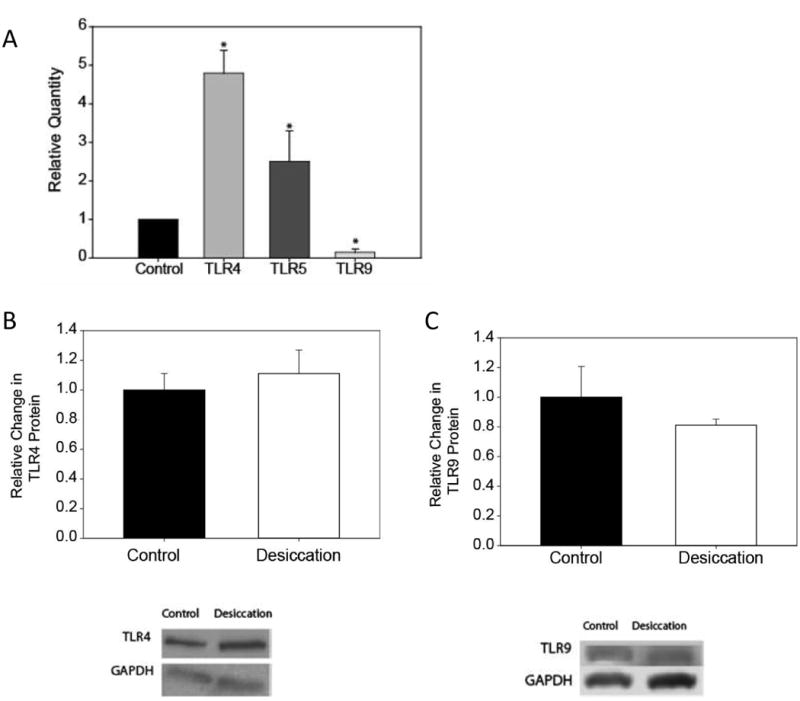
TLR mRNA expression by quantitative RT-PCR showed that TLR4 and TLR5 mRNA were up-regulated by 4.81 and 2.51 fold respectively; whereas TLR9 was down-regulated by 0.86 fold when compared to the control (A). Protein expression by western blotting was determined in epithelial cells harvested from human corneas in organ culture for 24 hrs. At the protein level, TLR4 was up-regulated by 10% (B) and TLR9 was down-regulated by 20.47% (C); TLR5 remained unaltered (data not shown). Data are representative of a minimum of three experiments and were analyzed using a one-way ANOVA where P ≤ 0.05 was considered to be statistically significant when compared to control (*).
3.4. TLR9 expression is down-regulated in conjunctival impression cytology samples from dry eye subjects compared to normal subjects
Thirty-two subjects (10 males, 22 females) enrolled in the study for mRNA analysis and of these, 8 DES subjects were excluded due to either low RNA yield from CIC samples or low tear volume collection. Clinical objective measurements for subjects that were included in this part of the study (n=24) are listed in Table 3. When comparing DES subjects to age and gender-matched normal subjects, there was a significant decrease in tear film stability (TBUT) and in tear production (phenol red thread test). DES subjects also had a significant increase in OSDI score, tear film osmolarity, corneal and conjunctival staining.
Table 3.
| Subject | Age (years) |
OSDI (score) |
Phenol Red (mm) |
TBUT (sec) |
Osmolality (mosM/kg) |
Corneal Staining (grade) |
Conjunctival Staining (grade) |
|---|---|---|---|---|---|---|---|
| Normal | 44.2 ± 13.3 | 5.32 ± 5.28 | 28.8 ± 6.45 | 13.2 ± 6.44 | 305.4 ± 2.40 | 1 ± 0 | 1 ± 0 |
| DES | 45.1 ± 15.6 | 47.23 ± 18.46 | 21.7 ± 5.43 | 5.54 ± 3.01 | 324.1 ± 19.0 | 1.98 ± 0.944 | 2.31 ± 0.833 |
| P-Value | NS | <0.0001 | <0.001 | <0.0001 | <0.05 | <0.05 | <0.005 |
Conjunctival impression cytology samples were collected after all the objective measurements were made to compare TLR4, TLR5 and TLR9 mRNA expression among DES subjects and normal age and gender-matched controls (Fig. 5A). TLR5 mRNA was down-regulated to 0.67 ± 0.53 fold of that of the normal subjects but this was not statistically significant. There was a significant (P≤ 0.005) down-regulation of TLR9 mRNA to 0.42 ± 0.51 fold or by almost 59.5% on average in the dry eye subjects compared to the normal subjects. TLR4 was up-regulated by 1.9 ± 3.2 fold, but as with TLR5, this change was not statistically significant. However, it is worth noting that in DES subjects, the greatest increase in TLR4 expression was in subjects with a high OSDI score (>65) as shown in the scatter plot in Fig. 5B, however across the entire data set the correlation was not statistically significant. To determine if TLR4 protein expression was modulated in DES subjects, CIC samples were analyzed to compare TLR4 intracellular and extracellular protein levels to those of normal subjects by flow cytometry analysis in an additional 19 subjects at the University of Genoa. As with the mRNA data, there was an increase in intracellular protein expression for TLR4 (8.2 ± 7.2%) in DES subjects when compared to normal subjects (1.7 ± 0.9%) however this did not reach statistically significance. As it is shown in Fig. 5C, there was very little TLR4 expressed on the cell surface and there was no significant difference between the DES and normal subjects (0.6 ± 0.8% vs 0.3 ± 0.2% respectively).
Figure 5. TLR mRNA expression in dry eye subjects.
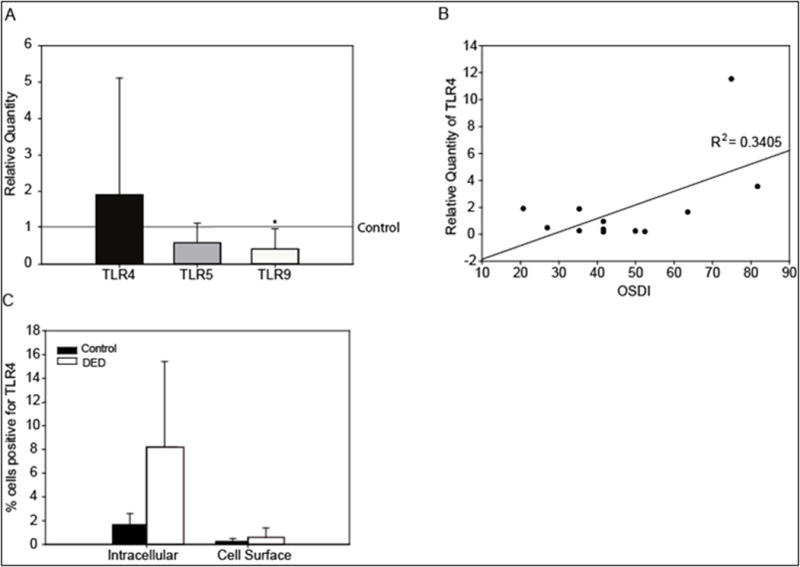
The relative quantity of TLR4, TLR5 and TLR9 mRNA expression in CIC samples from DED and age-and gender-matched controls was evaluated. Relative quantity data derived from the control group were normalized to one and it is represented by the control line. Bars rising above or below the control line represent an up-regulation or down-regulation in TLR expression respectively. There was a significant down-regulation of TLR9 mRNA to 0.42 ± 0.51 fold or by almost 59.5% on average in the dry eye subjects compared to the normal subjects (A). Correlation between TLR4 mRNA expression and increasing severity of dry eye as measured by the OSDI, n=12, R2=0.3405 (B). TLR4 protein intracellular and extracellular expression from CIC samples from dry eye and age and gender-matched normal control subjects. Protein expression for TLR4 was increased in DED subjects when compared to normal subjects at both intracellular and cell surface compartments, however the differences (8.2± 7.2% vs 1.7 ± 0.9%; and 0.6 ± 0.8% vs 0.3 ± 0.2% respectively) were not significant (C). Data were analyzed using an unpaired Student's t-test, where P ≤ 0.05 was considered to be statistically significant when compared to the age-and gender-matched control group (*).
4. Discussion
The results from this study suggest that TLRs are modulated in DES and dry eye associated conditions (HOS, cytokines, and desiccation), pointing out the possible role of TLRs in the pathogenesis of this complex disease. Overall it was found that dry eye conditions result in low protein levels of TLR4 and TLR9, while TLR5 was up-regulated or unchanged.
Hyperosmolar stress has been suggested to be an accurate test for diagnosing DES and is thought to be the best indicator for disease severity (Sullivan et al., 2010). The accepted range for normal tear film osmolarity is 296-308 mOsm/L (Gilbard et al., 1978) however in dry eye subjects, it has been documented to reach as high as 440 mOsm/L (Farris, 1994). Topical application of hyperosmolar solutions in the range 800-900 mOsm/kg has been found to induce ocular surface burning sensations similar to those reported for dry eye (Liu et al., 2009) leading to the suggestion that similar transient spikes in osmolarity may be responsible for some dry eye symptoms, although such spikes are currently difficult to detect. In the present study, HOS was induced by increasing the osmolarity of the culture media by 70-170 mOsm/kg, which is large compared to the increase in tear film osmolarity found in the DES subjects in this study, however this is consistent with the increase in the tear film osmolarity described in subjects with severe DES (Farris, 1994), as well as several in vitro (Li et al., 2006; Narayanan et al., 2006a) and in vivo (Luo et al., 2005) studies.
Under hyperosmotic conditions, TLR4 mRNA was increased while TLR4 protein expression was decreased. TLR9 mRNA and protein were both decreased and TLR5 remained unchanged. Potential differences between mRNA and protein translation and degradation rates, might explain why a correlation between TLR4 mRNA and protein levels was not observed. Further, mRNA levels often do not correlate well with protein expression levels and it is widely accepted that mRNA abundance serves as a poor indicator for protein abundance in most cases. This fact has been observed by the use of conventional SAGE (Gygi et al., 1999) and microarray (Chen et al., 2002) techniques as well as under the light of more recent technologies (e.g. RNA sequencing) (Maier et al., 2009). Furthermore, low protein expression of HLA-DR and high transcript expression and vice versa has been recently described in CIC samples obtained with a novel cell collection device (Kessal et al., 2014). Being HLA-DR a well-recognized marker of ocular surface inflammation, these results suggest that the relationship and correlation between proteins and transcripts of inflammatory ocular diseases deserve to be further explored. Besides the differences mentioned above, we might theorize that for TLR4, overproduction of TLR4 mRNA was a response to low concentrations of the expressed TLR4 protein, which seems to be caused by HOS.
Although this study attempted to isolate the effects of HOS, previous studies have shown that HOS increases the expression of several cytokines such as IL-1α, TNFα and IL-8 (Li et al., 2006). Little is reported in the literature regarding the role of cytokines in modulating TLR expression. TLR2 is up-regulated in cultured conjunctival epithelial cells in response to interferon gamma (IFNγ) (Cook et al., 2005). At longer time points than tested in this study, IFNγ and TNFα increased the expression of TLR2- TLR5, and TLR9 after 72 hrs of stimulation in keratinocytes (Begon et al., 2007). These studies suggest that cytokines could act synergistically with HOS and be responsible for the change in TLR expression found in this study. However, DES associated cytokines when tested individually, were not able to modulate HCEC expression of TLR4, TLR5, or TLR9 mRNA or TLR4 and TLR9 protein expression. TLR expression was also examined in response to desiccation using an organ culture model. This model is unique as it may most mimic the DES environment (i.e. HOS, desiccation and cytokines) that occurs in vivo. In the corneal epithelium of this model, there was an increase in TLR4 and TLR5 mRNA and a significant decrease in TLR9 mRNA and protein. The TLR4 protein increased under desiccation but was not significant when compared to the control.
A preliminary study showed an up-regulation in TLR2 mRNA but not protein in CIC samples from DES subjects (Barabino S, 2006), and here we have found that TLR9 was significantly down-regulated and there was no significant change in TLR5 and TLR4 mRNA or protein expression in DES subjects. It is unclear why there was no change in TLR4 and TLR5 expression in the CIC samples that correlated with the in vitro experiments in primary HCEC and why there was weak TLR4 expression in the CIC samples by flow cytometry since baseline TLR4 expression has been well documented in the conjunctival epithelium (Redfern et al., 2011). Although CIC has many advantages over more invasive techniques and is a well-established method to sample ocular surface cells, this technique is limited since it generally retrieves only the superficial layers of the conjunctival epithelium. A previous study reported that TLR1-5 expression in the conjunctival epithelium is more intense in basal epithelium than in the superficial epithelium (Li et al., 2007) therefore a reduced TLR4 expression in the superficial layers of the conjunctival epithelium or poor antibody recognition may be responsible for the weak expression of TLR4 by flow cytometry. Furthermore, since TLR4 and TLR5 are most intensely expressed on the basal conjunctival epithelium, it may be difficult to detect a change in mRNA expression between the control and DES subjects since CIC, as noted above, primarily retrieves superficial conjunctival epithelial cells. In addition to this, although one might expect to see a similar change in TLR expression in both in vitro experiments in primary HCEC and the conjunctival epithelial cells from CIC samples, it is difficult to make a direct comparison between these experiments since they are different cell types and experimental conditions. Interestingly, the fact that CIC samples from DES subjects showed higher levels of TLR4 protein in the intracellular compartment compared to the cell surface might also indicate that under HOS, TLR4 does not respond to LPS because it is poorly expressed on the cell surface.
It remains to be determined if a change in TLR expression is the cause or result of ocular surface inflammation. Two recent reviews have examined changes in TLR expression in various ocular surface inflammatory and infections which suggest their involvement in these conditions (Lambiase et al., 2011; Redfern and McDermott, 2010). Relevant to this study, TLR4 and TLR9 mRNA expression has been shown to be up-regulated in corneal infections such as herpes simplex keratitis (Jin et al., 2007), while others also report changes in TLR4 and TLR9 expression in other ocular surface inflammatory conditions, such as seasonal vernal keratoconjunctivitis (VKC) and atopic keratoconjunctivitis (AKC). In VKC subjects, there was an increase in TLR4 and a decrease in TLR9 (Bonini et al., 2005) which is in agreement with the findings in this study. Also TLR2 expression has been observed in CIC samples from human subjects with AKC and allergic conjunctivitis, but not in subjects without ocular allergies (Cook et al., 2005).
Of all the TLRs examined, TLR9 was consistently down-regulated in the corneal and conjunctival epithelial cells in all the conditions tested with the exception of exposure to individual cytokines, which did not modulate the expression of any of the TLRs tested. This suppression in TLR9 expression appears to be a distinct signature of noninfectious inflammation. TLR9 recognizes microbial DNA, which is characterized by an abundance of unmethylated CpG dinucleotides, which can induce a strong inflammatory response (Bauer et al., 2001; Hemmi et al., 2000). In the cornea, activation of TLR9 induces sight threatening keratitis (Johnson et al., 2005) while inhibiting TLR9 by siRNA in C57BL/6 mice infected with Pseudomonas aeruginosa decreases corneal inflammation (Huang et al., 2005). Thus down-regulation of TLR9 may have an anti-inflammatory effect on the ocular surface. A change in TLR expression pattern may have both beneficial and detrimental effects. Increased TLR expression may enhance pathogen recognition, but may also lead to inappropriate and exacerbated inflammatory responses, thereby contributing to disease processes such as ocular allergy and DES. Alternatively, reduced expression may lead to an inadequate recognition response and increased risk of infection. However, the latter may be compensated for by the fact that many pathogens are recognized in more than one way, including interactions with multiple TLRs and with other pattern recognition receptors. Although the current data are largely correlative and not causal, differences in the TLR pattern expression seen here in DES compared to other pathologies provides new avenues for functional and mechanistic studies in the future.
When looking at the responsiveness of TLR4 and TLR9, SV40 HCEC failed to elicit IL-8 secretion in the presence of TLR4 agonist: LPS or Pseudomonas aeruginosa DNA when compared to the media alone control under hyperosmolar stress. This fact correlates with the low protein expression found for TLR4 and TLR9 by the western blot analysis.
In conclusion our data point towards a reduction of TLR4 and TLR9 on the cell surface, which dampens their ability to elicit an inflammatory response when the ocular surface has succumbed to DES.
Acknowledgments
We are very grateful to Heartlands Eye Bank for supplying the donor corneas. The IOBA-NHC cells and SV40 HCEC were kindly donated by Drs. Diebold and Araki-Sasaki, respectively. We would also like to acknowledge Dr. Reza Dana at Harvard Medical School for his scientific consultation and assistance with this manuscript.
Abbreviations
- TLR
Toll-like receptor
- DES
Dry Eye Syndrome
- HCEC
Human corneal epithelial cells
- SS
Sjögren's syndrome
- HOS
Hyperosmolar stress
- TE
Tris-EDTA buffer
- PBS
Phosphate-buffered saline
- OD
Optical density
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- OSDI
ocular surface disease index
Footnotes
Author contributions: RLR, SB, JB and CL undertook experimental work. AMM, RLR, SB provided the facilities and supplies. RLR, SB and AMM provided intellectual input. All authors reviewed the manuscript.
Conflict of interest: The authors declare no competing financial interest or conflict of interest relevant to this manuscript.
References
- Adhikary G, Sun Y, Pearlman E. C-Jun NH2 terminal kinase (JNK) is an essential mediator of Toll-like receptor 2-induced corneal inflammation. J Leukoc Biol. 2008;83:991–997. doi: 10.1189/jlb.1107783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonso AA, Sobrin L, Monroy DC, Selzer M, Lokeshwar B, Pflugfelder SC. Tear fluid gelatinase B activity correlates with IL-1alpha concentration and fluorescein clearance in ocular rosacea. Invest Ophthalmol Vis Sci. 1999;40:2506–2512. [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Barabino S, Montaldo E, Solignani F, Valente C, Mingari MC, Rolando M. Immune response in the conjunctival epithelium of patients with dry eye. Exp Eye Res. 2010;91:524–529. doi: 10.1016/j.exer.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Barabino S, R M, Bonini S, Mingari v, Moretti S, Micera A, Dana R. Toll-Like Receptor (TLR) 2 and TLR4 Expression in Dry Eye. Invest Ophthalmol Vis Sci. 2006;47 ARVO e-abstract # 5594. [Google Scholar]
- Bauer S, Kirschning CJ, Hacker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci U S A. 2001;98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begon E, Michel L, Flageul B, Beaudoin I, Jean-Louis F, Bachelez H, Dubertret L, Musette P. Expression, subcellular localization and cytokinic modulation of Toll-like receptors (TLRs) in normal human keratinocytes: TLR2 up-regulation in psoriatic skin. Eur J Dermatol. 2007;17:497–506. doi: 10.1684/ejd.2007.0264. [DOI] [PubMed] [Google Scholar]
- Beutler B. TLR4 as the mammalian endotoxin sensor. Curr Top Microbiol Immunol. 2002;270:109–120. doi: 10.1007/978-3-642-59430-4_7. [DOI] [PubMed] [Google Scholar]
- Bonini S, Micera A, Iovieno A, Lambiase A. Expression of Toll-like receptors in healthy and allergic conjunctiva. Ophthalmology. 2005;112:1528. doi: 10.1016/j.ophtha.2005.04.009. discussion 1548-1529. [DOI] [PubMed] [Google Scholar]
- Bron AJ, Tiffany JM, Yokoi N, Gouveia SM. Using osmolarity to diagnose dry eye: a compartmental hypothesis and review of our assumptions. Adv Exp Med Biol. 2002;506:1087–1095. doi: 10.1007/978-1-4615-0717-8_153. [DOI] [PubMed] [Google Scholar]
- Chen G, Gharib TG, Huang CC, Taylor JM, Misek DE, Kardia SL, Giordano TJ, Iannettoni MD, Orringer MB, Hanash SM, Beer DG. Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics. 2002;1:304–313. doi: 10.1074/mcp.m200008-mcp200. [DOI] [PubMed] [Google Scholar]
- Christopherson PL, S J, Sosne G. Early Corneal and Lacrimal Gland Expression of Inflammatory Genes in a Murine model of Sjogren's Syndrome. Invest Ophthalmol Vis Sci. 2005;46 ARVO E-Abstract # 4462. [Google Scholar]
- Cook EB, Stahl JL, Esnault S, Barney NP, Graziano FM. Toll-like receptor 2 expression on human conjunctival epithelial cells: a pathway for Staphylococcus aureus involvement in chronic ocular proinflammatory responses. Ann Allergy Asthma Immunol. 2005;94:486–497. doi: 10.1016/S1081-1206(10)61120-9. [DOI] [PubMed] [Google Scholar]
- Diebold Y, Calonge M, Enriquez de Salamanca A, Callejo S, Corrales RM, Saez V, Siemasko KF, Stern ME. Characterization of a spontaneously immortalized cell line (IOBA-NHC) from normal human conjunctiva. Invest Ophthalmol Vis Sci. 2003;44:4263–4274. doi: 10.1167/iovs.03-0560. [DOI] [PubMed] [Google Scholar]
- Farris RL. Tear osmolarity--a new gold standard? Adv Exp Med Biol. 1994;350:495–503. doi: 10.1007/978-1-4615-2417-5_83. [DOI] [PubMed] [Google Scholar]
- Gariboldi S, Palazzo M, Zanobbio L, Selleri S, Sommariva M, Sfondrini L, Cavicchini S, Balsari A, Rumio C. Low molecular weight hyaluronic acid increases the self-defense of skin epithelium by induction of beta-defensin 2 via TLR2 and TLR4. J Immunol. 2008;181:2103–2110. doi: 10.4049/jimmunol.181.3.2103. [DOI] [PubMed] [Google Scholar]
- Giddabasappa A, Hamilton WR, Chaney S, Xiao W, Johnson JE, Mukherjee S, Fox DA. Low-level gestational lead exposure increases retinal progenitor cell proliferation and rod photoreceptor and bipolar cell neurogenesis in mice. Environ Health Perspect. 2011;119:71–77. doi: 10.1289/ehp.1002524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbard JP, Farris RL, Santamaria J., 2nd Osmolarity of tear microvolumes in keratoconjunctivitis sicca. Arch Ophthalmol. 1978;96:677–681. doi: 10.1001/archopht.1978.03910050373015. [DOI] [PubMed] [Google Scholar]
- Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Huang X, Barrett RP, McClellan SA, Hazlett LD. Silencing Toll-like receptor-9 in Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2005;46:4209–4216. doi: 10.1167/iovs.05-0185. [DOI] [PubMed] [Google Scholar]
- Jhanji V, Constantinou M, Taylor HR, Vajpayee RB. Microbiological and clinical profiles of patients with microbial keratitis residing in nursing homes. Br J Ophthalmol. 2009;93:1639–1642. doi: 10.1136/bjo.2008.154468. [DOI] [PubMed] [Google Scholar]
- Jin X, Qin Q, Chen W, Qu J. Expression of toll-like receptors in the healthy and herpes simplex virus-infected cornea. Cornea. 2007;26:847–852. doi: 10.1097/ICO.0b013e318093de1f. [DOI] [PubMed] [Google Scholar]
- Johnson AC, Heinzel FP, Diaconu E, Sun Y, Hise AG, Golenbock D, Lass JH, Pearlman E. Activation of toll-like receptor (TLR)2, TLR4, and TLR9 in the mammalian cornea induces MyD88-dependent corneal inflammation. Invest Ophthalmol Vis Sci. 2005;46:589–595. doi: 10.1167/iovs.04-1077. [DOI] [PubMed] [Google Scholar]
- Kessal K, Riancho L, Rabut G, Liang H, Boucher C, Baudouin C, Melik-Parsadaniantz S, Brignole-Baudouin F. Correlation between mRNA and protein expression profiles of HLA-DR in Conjunctival Impression Cytology using a new device for collecting epithelial cells. ARVO 2014 Annual Meeting; Orlando, FL, US. 2014. Poster Board Number: A0193. [Google Scholar]
- Kumar A, Zhang J, Yu FS. Toll-like receptor 3 agonist poly(I:C)-induced antiviral response in human corneal epithelial cells. Immunology. 2006;117:11–21. doi: 10.1111/j.1365-2567.2005.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase A, Micera A, Sacchetti M, Mantelli F, Bonini S. Toll-like receptors in ocular surface diseases: overview and new findings. Clin Sci (Lond) 2011;120:441–450. doi: 10.1042/CS20100425. [DOI] [PubMed] [Google Scholar]
- Li DQ, Luo L, Chen Z, Kim HS, Song XJ, Pflugfelder SC. JNK and ERK MAP kinases mediate induction of IL-1beta, TNF-alpha and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res. 2006;82:588–596. doi: 10.1016/j.exer.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Shen J, Beuerman RW. Expression of toll-like receptors in human limbal and conjunctival epithelial cells. Mol Vis. 2007;13:813–822. [PMC free article] [PubMed] [Google Scholar]
- Liu H, Begley C, Chen M, Bradley A, Bonanno J, McNamara NA, Nelson JD, Simpson T. A link between tear instability and hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci. 2009;50:3671–3679. doi: 10.1167/iovs.08-2689. [DOI] [PubMed] [Google Scholar]
- Luo L, Li DQ, Corrales RM, Pflugfelder SC. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye Contact Lens. 2005;31:186–193. doi: 10.1097/01.icl.0000162759.79740.46. [DOI] [PubMed] [Google Scholar]
- Maier T, Guell M, Serrano L. Correlation of mRNA and protein in complex biological samples. FEBS Lett. 2009;583:3966–3973. doi: 10.1016/j.febslet.2009.10.036. [DOI] [PubMed] [Google Scholar]
- McDermott AM, Redfern RL, Zhang B. Human beta-defensin 2 is up-regulated during re-epithelialization of the cornea. Curr Eye Res. 2001;22:64–67. doi: 10.1076/ceyr.22.1.64.6978. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Miljanovic B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143:409–415. doi: 10.1016/j.ajo.2006.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan S, Manning J, Proske R, McDermott AM. Effect of hyperosmolality on beta-defensin gene expression by human corneal epithelial cells. Cornea. 2006a;25:1063–1068. doi: 10.1097/01.ico.0000228785.84581.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan S, Miller WL, McDermott AM. Conjunctival cytokine expression in symptomatic moderate dry eye subjects. Invest Ophthalmol Vis Sci. 2006b;47:2445–2450. doi: 10.1167/iovs.05-1364. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren's syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999;19:201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- Redfern RL, McDermott AM. Toll-like receptors in ocular surface disease. Exp Eye Res. 2010;90:679–687. doi: 10.1016/j.exer.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern RL, Reins RY, McDermott AM. Toll-like receptor activation modulates antimicrobial peptide expression by ocular surface cells. Exp Eye Res. 2011;92:209–220. doi: 10.1016/j.exer.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118:615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci. 2001;42:2283–2292. [PubMed] [Google Scholar]
- Sullivan BD, Whitmer D, Nichols KK, Tomlinson A, Foulks GN, Geerling G, Pepose JS, Kosheleff V, Porreco A, Lemp MA. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci. 2010;51:6125–6130. doi: 10.1167/iovs.10-5390. [DOI] [PubMed] [Google Scholar]
- Sun Y, Pearlman E. Inhibition of corneal inflammation by the TLR4 antagonist Eritoran tetrasodium (E5564) Invest Ophthalmol Vis Sci. 2009;50:1247–1254. doi: 10.1167/iovs.08-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, Beutler B. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- Vivino FB, Minerva P, Huang CH, Orlin SE. Corneal melt as the initial presentation of primary Sjogren's syndrome. J Rheumatol. 2001;28:379–382. [PubMed] [Google Scholar]
- Zhang J, Xu K, Ambati B, Yu FS. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to Pseudomonas aeruginosa flagellin. Invest Ophthalmol Vis Sci. 2003;44:4247–4254. doi: 10.1167/iovs.03-0219. [DOI] [PubMed] [Google Scholar]
- Zheng L, Zhang Z, Yu C, Yang C. Expression of Toll-like receptors 7, 8, and 9 in primary Sjogren's syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:844–850. doi: 10.1016/j.tripleo.2010.01.006. [DOI] [PubMed] [Google Scholar]


