Abstract
Chemical analysis of exhaled breath metabolites is an emerging alternative to traditional clinical testing for many physiological conditions. The main advantage of breath analysis is its inherent noninvasive nature and ease of sample collection. Therefore, there exists a great interest in further development of this method for both humans and animals. The physiology of cetaceans is exceptionally well suited for breath analysis due to their explosive breathing behavior and respiratory tract morphology. At the present time, breath analysis in cetaceans has very limited practical applications, in large part due to lack of widely adopted sampling device(s) and methodologies that are well-standardized. Here, we present an optimized design and the operating principles of a portable apparatus for reproducible collection of exhaled breath condensate from small cetaceans, such as bottlenose dolphins (Tursiops truncatus). The device design is optimized to meet two criteria: standardized collection and preservation of information-rich metabolomic content of the biological sample, and animal comfort and ease of breath sample collection. The intent is to furnish a fully-benchmarked technology that can be widely adopted by researchers and conservationists to spur further developments of breath analysis applications for marine mammal health assessments.
Keywords: breath analysis, breath sampling, exhaled breath condensate (EBC), volatile organic compounds (VOCs), Bottlenose dolphins (Tursiops truncatus), cetaceans
Graphical abstract

INTRODUCTION
Exhaled breath analysis is an emerging field with tremendous promise to advance forward non-invasive diagnostics in both human medicine [1] and animal veterinary practices [2]. During respiration, mammals expire a mixture of compounds including CO2, NO and other inorganic compounds such as ammonia, as well as volatile organic compounds (VOCs) [3], and nonvolatile compounds trapped in aerosols [4]. Abundances of some of the compounds in this exhaled mixture may reflect the concentration at the alveolar-blood interface [5] or otherwise correlate with various systemic or local metabolic processes. Some compounds are likely inhaled from the surroundings and then exhaled in breath. These compounds may reflect the exogenous “exposome” that the organism has been experiencing [3, 6]. Other breath constituents are likely produced endogenously by the body during normal physiological processes, and their distribution shifts can reflect disease or disorder processes [7, 8]. Finally, microorganisms in the respiratory tract proliferate and can themselves produce metabolites including VOCs [9–12], and metabolite signatures can be unique to the pathogen species [13–15]. This signature is likely complimented by a host-response-to-infection signature that is equally as unique as immune system signaling responds to the specific pathogen [16, 17]. By capturing and analyzing all of these constituents in exhaled breath, we can potentially gain decision informative insight into health status [18–20]. Currently, breath samples are usually analyzed for metabolite content using commercial bench-top mass spectrometers. Alternatively, certain compounds of interest could be detected and measured using immunoassays such as enzyme-linked immunosorbent assays (ELISAs) [21–24]. Ideally, breath testing could be used directly at the point-of-care as new mobile detection technologies are developed. Ultimately, we envision mobile and routine breath diagnostics with potential applications in medical and veterinary disciplines. It is important to note that the future of this testing depends on optimized and reproducible breath sampling.
In humans, two variables have emerged as critical factors in breath sampling: exhalation flow rates for single chemical species gas analysis [25], and sampling temperatures for exhaled breath condensate analysis [26]. These are important to control to minimize variability of metabolites collection. For example, nitric oxide (NO) has been known for over a decade to be an important clinical respiratory marker of airway inflammation, but routine measurement of this metabolite was not wide spread until after 2011 when the American Thoracic Society (ATS) released guidelines for NO sampling that included standardized flow rates [27]. The ATS standard was based on data showing that NO measurements are flow dependent [28], which had previously led to great confusion in the literature. By standardizing the recommended sampling flow rate, NO metabolite measures for the first time could be compared and evaluated across different clinical settings and patient cohorts [8, 28–30]. As a result, use of the NO biomarker subsequently increased in the clinic. The importance of sampling has also been demonstrated for exhaled breath condensate (EBC) sampling, although this sampling mode has not yet been standardized due to lack of theoretical and experimental studies to support appropriate parameter choices. Similarly to gaseous exhaled breath, the chemical composition of EBC is known to depend on the temperature control of the sampling device, which is indirectly affected by heat transfer and respiratory flow rates through the sampler [26, 31].
Although tremendous progress has been made in breath research, the challenges of sample collection optimization and standardization still persist [32]. A few commercial devices are available for human exhaled breath condensate collection such as the RTube™ and Turbo DECCS™. They are inexpensive and easy to use, but their designs are known to possess certain performance drawbacks; the choice of condenser surface coating material (plastic) and not constant condenser temperature (shifting significantly above zero) during breath sampling affect measurement of biomarkers in exhaled breath condensate [26, 33, 34]. Breath analysis is also in development for veterinary applications [35]; this approach to health assessment would be very beneficial, especially for the animals that are difficult to monitor in the wild, such as large cetaceans (e.g. whales) [15, 36]. In such cases, it would be nearly impossible to collect conventional matrices for health assessment (e.g. blood plasma) or perform other assessments (e.g. ultrasound imaging), and breath collection may be a feasible alternative. Unfortunately, there is limited knowledge of the breath metabolome for cetaceans and no knowledge of biomarkers of specific disorders that can be practically utilized at this time. In order to gain such knowledge, the field of breath analysis must further mature in both cetacean-specific studies and adoption of better-developed methods from human breath analysis. One major impediment to advancing cetacean breath analysis is lack of an available and standardized breath sampling device and methodology.
Present efforts to comprehensively monitor and assess health of cetacean populations are ongoing around the world, for example the Sarasota Dolphin Research Program (SDRP) in the United States [37] or the Byron Bay Dolphin Project in Australia [38]. These programs study wild animals, establish factors that threaten their populations, and help better understand how to protect them. Incorporating breath assessments into these programs, as well as routine monitoring of animals under human care, would allow for increased knowledge of cetacean breath metabolomic content. Availability of a sampling device that is appropriately characterized and benchmarked is a critical necessity for these advancements.
Our group previously designed and tested a proof-of-concept prototype of an EBC sampler for small cetaceans, such as bottlenose dolphins (Tursiops truncatus) [7]. Given the potential benefits for use of EBC sampling for marine mammals, we sought to further develop a device design specifically optimized for use with small cetaceans. Here we describe both the theoretical and experimental validation for this next generation EBC sampler design that enables control of confounding factors for breath sampling from small cetaceans. The designed device is specifically intended for wide adoption and use. The engineering solutions are made to make the device simple, inexpensive to manufacture, portable and user-friendly. Metabolomic content of the EBC collected with this device is characterized using a variety of mass spectrometry (MS) analytical approaches.
2. MATERIALS AND METHODS
2.1. Exhaled Breath Condensate (EBC) Sampler Construction
Figure 1 shows an optimized dolphin breath sampler (DBS) that is significantly enhanced from our initial earlier prototype version [7]. An operator works with the preassembled device and uses only four main components: an insulated housing, a borosilicate glass condenser tube, a blowhole mask assembly, and a one-way exhale valve (figure 1b). The blowhole mask assembly and exhale valve are press fit on the inlet and outlet ends of the condenser tube, respectively. Then, the glass condenser tube assembly is centered in the insulated housing and locked in place with housing end plugs (figure 1c). The insulated pocket space is filled with cooling material, such as dry ice pellets.
Figure 1.
Dolphin breath sampler, CAD design - main components: (a) Exploded view: 1. Insulated housing with condenser tube guiding, door, and latches. 2. Housing end plugs to hold the tube and dry ice pellets in place. 3. Mask threaded connector. 4. Blowhole mask with soft cushion on the edge. 5. Inhale valves - mask connector (threaded end for mask and press fit on glass tube). 6. Membrane for one-way inhalation valve. 7. Inhalation valve. 8. Condenser borosilicate glass tube. 9. One-way exhalation flap valve, (b) Preassembled DBS; the components that an operator works with, (c) Device ready for dry ice loading; dry ice pellets are poured in; the door is latched tight, (d) Sample retrieval press: main components. 10. PTFE plunger with stainless steel pole. 11. Base to support the condenser tube in place. 12. PTFE threaded vial holder press fits over condenser tube. 13. Condenser tube with frozen EBC sample inside.
The outer casing is constructed from polycarbonate tubing (101.6 mm OD × 95.25 mm ID × 3.17 mm Wall) insulated with polyethylene foam pipe insulation (12.7 mm thick); the inner condenser tube center guides are machined from 6.35 mm thick PVC plate. The insulated housing is closed with rubber draw t-handle latches (McMaster-Carr®, part #1685A36). Borosilicate glass tubing with 31.7 mm OD × 23.7 mm ID × 4 mm wall (Wale Apparatus Co., Inc., Hellertown, PA) was used for the condenser element. The initial length of the tube (91 cm) was optimized later to 61 cm as adequate sampler performance could be maintained for the reduced size device, while improving the ease to operate the device. High-flow, one-way, swing valve, NSF/ANSI certified for use with drinking water (PVC body, EPDM seal) is used with custom modifications for exhale valve at the outlet end of the device. An assembly of custom-made blowhole mask and two one-way flap valves (25.4 mm ID openings, silicone rubber circular membranes) for the inhalation maneuver are installed at the inlet end of the device. The total weight of the apparatus charged with dry ice is approximately eight kilograms. The breath sampling apparatus was designed and fabricated in house at the Engineering Fabrication Laboratory at UC Davis.
2.2. Breath Flow and Thermodynamics
In order to develop an optimal breath sampler design, several key engineering parameters need to be considered. The flow development and regime, pressure resistance, heat transfer, and condensation rate are estimated with analytical and numerical solutions.
The pressure drop in the condenser tube is estimated with the Darcy friction factor using average fluid properties evaluated from the inlet and outlet conditions [39].
| (Eq. 1) |
where ρ is the fluid density, g is the acceleration of gravity, f is the Darcy friction factor, V is the flow velocity, and d is the tube inner diameter. Change due to gravity, Δz, is equal to the glass tube length, L, since the device operates in the vertical position.
The fluid properties of breath at the inlet are approximated with the properties of saturated air at the animal body temperature, 36 °C [40]. The fluid properties at the outlet are approximated with the properties of saturated air at the outlet condition. The temperature at the outlet of the device, To, is evaluated with the average convection heat transfer coefficient, h̄, assuming constant surface temperature, Ts, steady state, and negligible pressure gradient to treat the fluid as incompressible.
| (Eq. 2) |
where, Ti is the inlet temperature, P is the tube inner perimeter, ṁ is the mass flow rate, and Cp is the specific heat of breath (evaluated as saturated moist air). Turbulent flow regime is desired for effective heat exchange and results in higher condensation rate.
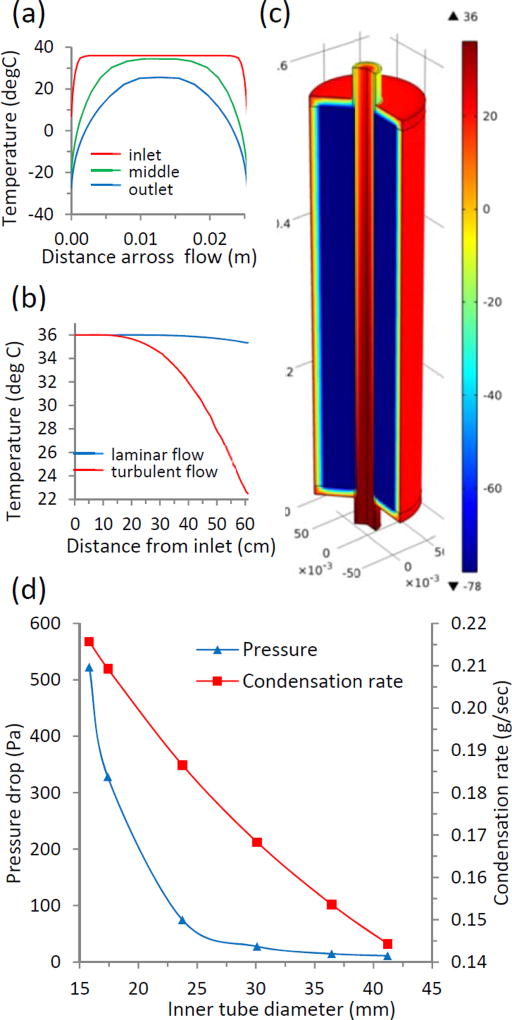
The constant surface temperature assumption is consistent with the estimated relative magnitude of the possible amount of heat conducted through the glass tube wall to the heat absorbed from the fluid during cooling, condensation, and freezing. Under the most conservative conditions, at minimum temperature difference and highest breath flow rate, the estimated amount of conducted heat exceeds the heat flux provided by the fluid [41]. The analytical solution results for pressure and temperature distribution closely correlate with a numerical simulation of non-isothermal turbulent flow in COMSOL® (see results, figure 5).
Figure 5.
Heat transfer optimization and condenser tube dimensions optimization for efficient device performance. (a) Temperature profiles at different length fractions of the flow in the condenser tube. (b) Total temperature drop in the condenser tube (61 cm) for laminar and turbulent flow. (c) Numerical model (COMSOL®) of non-isothermal flow confirms analytical solution results in terms of pressure and temperature drop across the condenser tube. (d) The optimal diameter of the condenser tube (for given animal breath flow rate, tube length, and condenser temperature) can be chosen from commercially available sizes. The condensation rate shows linear dependence; while pressure drop significantly decreases for tube diameters above 25 mm.
The rate of heat transfer, Q̇, between the moist breath stream and the chilled surface is evaluated with mass and energy rate balances. The mass of dry air is preserved, while water vapor partially condenses. The impact of other metabolites is disregarded as their concentrations are exceedingly low compared to water content in exhaled breath. Assuming steady state and saturated fluid properties at the inlet and outlet conditions, the condensation rate can be evaluated from
| (Eq. 3) |
where, ṁa is mass flow rate of dry air, ha is the specific enthalpy of dry air, hg is the specific enthalpy of water vapor, and hf is the specific enthalpy of the condensate, ω is the humidity ratio at the inlet and outlet, respectively.
2.3. Study Animals and EBC Sample Collection
Exhaled breath collection was performed under an animal care and use protocol reviewed and approved by the Navy Marine Mammal Program Institutional Animal Care and Use Committee and the Navy Bureau of Medicine (IACUC Protocol #106–2013 approved on September 24, 2013). The animals in the study were a managed population of bottlenose dolphins (Tursiops truncatus) housed in San Diego Bay, CA.
Total of 24 EBC samples were collected from 4 animals, 6 samples from each animal, during two months period. Animals varied in age and body size (168–253 kg) but had the same diet and living conditions. The sampling period was limited to 30 exhalation/inhalation cycles for all animals. The breathing pattern and sampling duration were not controlled; animals were allowed to breathe at their comfort rate. The time period (sec) was recorded for each sampling procedure. The EBC collection in this study was performed with the animal in seawater, positioned such that its blowhole was above the water level (abstract figure and figure 4a). During collection, the frozen exhaled breath sample covered the lumen surface area of the tube with a fine powdery snow-like layer. The original EBC samples were immediately transferred into a clean borosilicate vial using a custom-built sample extraction press (figure 1d, 4b), cap sealed, and stored in a −80 °C freezer until specific metabolite analysis was performed. No protease or peptidase inhibitors were added to the collected EBC before storage.
Figure 4.
Dolphin breath sampler in use. (a) Animal veterinarians are taking breath sample from a dolphin, (b) A researcher is retrieving frozen EBC sample from the condenser tube into a vial. The vial is sealed right after retrieval and is kept frozen until analysis. The metabolomic content of the sample is analyzed with GC-MS and LC-MS methods.
The mass collection efficiency of the device was evaluated based on these 24 EBC sampling procedures. The metabolomic content of the collected EBC samples was evaluated with GC/MS, LC/MS-RP, and LC/MS-HILIC analyses for 3 EBC samples collected from the same animal. Each of these 3 sampling procedures included steps from device cleaning to sample transfer for storage and metabolomics analysis. The primarily intention of this analysis was not to fully assess the metabolomic content but to develop sampling hardware and methodology for efficient and repetitive EBC sampling.
All parts of the device and sample retrieval press were thoroughly cleaned before and after each breath sampling. The cleaning protocol included three rinses: deionized (DI) water rinse, followed by 70% ethanol disinfectant rinse, followed by DI water rinse. All parts were dried prior to use.
2.4. Gas Chromatography / Mass Spectrometry (GC/MS) Analysis
GC/MS biochemical analysis of the samples was conducted with the sample extraction methodology known to provide comprehensive metabolite coverage for breath samples: liquid phase sample extraction with a polyacrylate (PA) df 85 Lim SPME fiber, which targets polar and semi-volatile compounds (MW 80–300) [42]. Varian 3800 GC (VF-5ms 5% phenol/95% PDMS column; Varian, Walnut Creek, CA), 4000 Ion Trap MS (Varian) equipped with Electron Ionization source (EI) was employed for all analyses. The instrument performance was verified before each analysis by injecting a standard Grob DA 280 Column Test Mix (Restek, Bellefonte, PA).
For the analysis, the EBC samples were aliquoted in cap-sealed borosilicate vials and transferred from the −80 °C freezer onto a 3 °C chilled tray of the GC/MS instrument and allowed to thaw until liquid. The polyacrylate (PA) SPME (Supelco, Bellefonte, PA) tip was automatically inserted by the sampling robot into the liquid fraction of EBC sample, and the sample was agitated for 30 min. Upon sample extraction, the SPME was inserted into the GC inlet maintained at 250 °C, and the extracted species were desorbed. The GC protocol for PA SPME analysis was as follows: cryofocusing on the head of the column at −10 °C for 1 min; 50 °C/min oven ramp to 40 °C, 20 °C/min oven ramp to 100 °C, 5 °C/min oven ramp to 180 °C, 10 °C/min oven ramp to 250 °C, 20 °C/min oven ramp to 280 °C, and a 5 min hold to purge the column for a complete run time of 35 min. The helium carrier gas was set to constant 1 mL/min flow. The m/z range scanned was 35–250 Th. The DI water blank, sea water blank, and empty vial blanks were included into the analysis. Quality controls of aqueous d8 naphthalene solution were run along with the samples.
2.5. Liquid Chromatography / Mass Spectrometry (LC/MS) Analysis
For liquid chromatography / mass spectrometry (LC/MS) analysis, each sample (0.5 mL) was lyophilized directly in the vial and then re-dissolved in 60 µL 90% acetonitrile in water with sonication. A total of 3 µL re-suspended sample was injected for analysis. The CUDA (12-[[(cyclohexylamino)carbonyl]amino]-dodecanoic acid) in methanol: toluene, 9:1 v/v internal standard was used for quality control and to assess reproducibility. Samples were housed in an autosampler maintained at 4 °C; the chromatography was performed using Waters ACQUITY UPLC BEH Amide 130 Å, 1.7 pm, 2.1 mm × 100 mm column (Waters, Milford, MA), held at 40 °C during analysis. Mobile phase A consisted of ultrapure water with 10 mM ammonium formate + 0.125% formic acid, pH 3. Mobile phase B was 95:5 v/v acetonitrile:ultrapure water with 10 mM ammonium formate + 0.125% formic acid, pH 3. The solvent gradient table was as follows: 0 min 100% (B), 0–2 min 100% (B), 2–7 min 70% (B), 7.7–9 min 40% (B), 9.5–10.25 min 30% (B), 10.25–12.75 min 100% (B), 16.75 min 100% (B). The flow rate was held at 0.4 mL/min over this time. LC eluents were analyzed with an Agilent 6530 QTOF MS (Agilent Technologies, Santa Clara, CA) in positive and negative ionization modes. The mass range was set to 60–1200 Th (m/z). ESI capillary voltage was set at +4.5 kV for ESI (+). The fragmentation was carried out with collision energy of +45 eV for ESI (+). Untargeted analysis of molecular data was carried out using data-independent acquisition (DIA) as described previously [43]. The device blanks, milliQ water rinse of the clean device inner surface that comes into contact with the sample and sea water blank, were included along the collected breath samples.
An additional experiment was conducted on an Agilent 1260 high-performance liquid chromatography (HPLC) system with the reverse phase UHPLC Waters Acquity CSH C18 1.7 pm, (2.1 × 100mm) (Milford, MA USA) column. The entire sample was dried, and then re-suspended in 100 µL 9:1 methanol: toluene. 3 µL of resuspended sample was injected for analysis. The CUDA (12-[[(cyclohexylamino)carbonyl]amino]-dodecanoic acid) in methanol: toluene, 9:1 v/v internal standard was used for quality control and to assess reproducibility. The samples were separated on the column held at 65 °C during analysis. Mobile phase A consisted of 60% acetonitrile in water. Mobile phase B was 10% acetonitrile in isopropanol. Formic acid and ammonium formate were added to make the final concentration of each mobile phase 10 mM for both formic acid and ammonium formate. Flow rate was 0.6 mL/min. The solvent gradient table was set as follows: 0 min 15% (B), 0–2 min 30% (B), 2–2.5 min 48% (B), 2.5–11 min 82% (B), 11–11.5 min 99% (B), 11.5–12 min 99% (B), 12–12.1 min 15% (B), 12.1–15 min 15% (B). Automatic valve switching after each injection to reduce sample carryover for highly lipophilic compounds was employed using a dual solvent wash: first with a water/acetonitrile mixture (1:1, v/v) and subsequently with a 100% isopropanol. Samples were analyzed with MS using an Agilent 6530 QTOF mass spectrometer in positive and Agilent 6550 QTOF mass spectrometer in negative ionization modes. The device blanks and sea water blanks were included along with the collected breath samples. The device blanks were milliQ water rinse of clean device inner surface that comes into contact with the breath.
2.6. Data Analysis
The GC/MS raw data files were first processed in AMDIS software in order to deconvolve each peak. The deconvolved chromatograms were then exported to .cef data format, and the peaks were aligned using Mass Profiler Professional 13.1 software. The alignment window was set at 1 min.
The LC/MS HILIC data were processed by the mzMine 2.0 free software. Alternatively, selected peaks could be collated and constrained into Agilent's MassHunter quantification method on the accurate mass precursor ion level, using the MS/MS information and the NIST14 / Metlin / MassBank libraries to identify metabolites with manual confirmation of adduct ions and spectral scoring accuracy.
For the RP analysis, raw data were processed in an untargeted (qualitative) manner by Agilent's software MassHunter Qual to find peaks in all chromatograms. Peak features were then imported into Mass Profiler Professional for peak alignments to seek which peaks were present in multiple chromatograms, using exclusion criteria by the minimum percentage of chromatograms in which these peaks were positively detected with 30% as a minimum criterion. These peaks were collated and constrained into a MassHunter quantification method on the accurate mass precursor ion level, using the MS/MS information and the LipidBlast library to identify lipids with manual confirmation of adduct ions and spectral scoring accuracy. MassHunter enabled back-filling of quantifications for peaks that were missed in the primary peak finding process, hence yielding data sets without missing values.
The global peak tables were collated for each method (LC/MS HILIC and RP) by removing low-abundance spurious peaks with a threshold for peak abundance set to 1000 a.u.. Furthermore, any peaks that appear in the blanks were removed from the sample peaks if the blank peak abundance exceeded 10% threshold of abundance of the corresponding peak in the sample.
3. RESULTS AND DISCUSSION
In order to develop a robust diagnostics methodology, three aspects were addressed: designing hardware for efficient sample collection, developing appropriate chemical methods to analyze the content of the biological sample, and performing reliable data interpretation to make conclusions about animal health status (figure 2). The requirement for the breath collection device was to capture and preserve a wide variety of breath metabolites and minimize variability due to sampling procedure. The collection and transfer of biological sample was designed to be done with minimum contamination and alteration. Any alterations of the collected frozen sample (e.g. thawing) that might lead to sample degradation needed to be avoided [31]. To attain comprehensive metabolite coverage, both volatile and non-volatile fractions (using GC/MS and LC/MS analyses, respectively) were conducted concomitantly on all collected EBC samples.
Figure 2.
Main steps for the methodology development for animal health diagnostics with breath analysis.
3.1. Breath Sampler Hardware Design Optimization
Compared to the previously reported prototype of the device for breath collection in cetaceans [7], a number of key improvements were made in the present design with the intention to make this device amenable for routine practical applications. The following design features were implemented: use of chemically inert materials in device construction, fluid flow and heat transfer optimization for minimum back pressure and efficient condensation, ergonomic sampler design for easy and quick workflow.
Collection of labile biological samples required the use of chemically inert materials that did not introduce any extraneous contaminants or deplete metabolite content. All inert materials were used for parts that were in contact with breath to minimize any potential chemical transformations in the collected sample [34, 44]: Polytetrafluoroethylene (PTFE) for connectors and parts in contact with the breath sample, borosilicate glass tube for condenser, chemical-resistant polyvinyl chloride (PVC) for valve connectors, Nalgene® plastic for blowhole mask, silicone rubber for valve membranes, and stainless steel for sample retrieval plunger press.
Evaluation of the fluid flow and heat transfer to optimize animal breath sampler resulted in effective capture of volatile and nonvolatile fractions of breath metabolites, minimal pressure drop for animal comfort, and sufficient condensation rate (larger sample amount) for easy on-site sampling.
Inhalation of chilled air might be uncomfortable or harmful to animals and was avoided. A pair of inhale and exhale valves was designed to establish unidirectional flow to allow for only exhaled breath to pass through the chilled condenser tube. This eliminated exposure of animals to extreme temperature differences in inhaled air during the inspiration maneuver as well as minimized condensation from the ambient air.
The blowhole mask had a soft cushion on the edge for better animal comfort and provided a good seal to minimize exhalant loss. Contact with the area closest to the animal's blowhole could be uncomfortable for the animal and might elicit a negative reaction. Thus, the size of the mask was chosen to allow for sufficient radial clearance around the blowhole. The shape of the mask accommodated the round body profile of the animal and small deepening behind the blowhole area. Dolphins under human care were trained and comfortably accepted the sampling device. During collection, the animals showed no signs of discomfort and readily complied with trainers' signals to breathe through the device. Some wild dolphins were less likely to tolerate any objects around the blowhole area. In this case, the exhale valve was removed from the device and the EBC sample was collected with the device positioned immediately (a few centimeters) above the animal blowhole without contact. The blowhole mask helped to route the exhaled breath into the chilled condenser tube.
When extracting the frozen, snow like, EBC from the collection device, efficient and quick sample transfer with no physical phase change from frozen ice into liquid was crucial for metabolite content preservation [31], which minimized contamination and maximized sampling throughput. To retrieve the collected sample from the condenser tube into a vial, the dry ice pellets were removed from the insulated housing, and condenser tube was placed into the sample retrieval press (figure 1d). The frozen EBC sample was removed from the inner lumen of the glass tube with a PTFE plunger. The two sharp notches, similar to end mill tool, on the front end of the plunger cleared the ice out with a clockwise rotational movement (abstract figure and figure 4b). Two PTFE gaskets in the middle part of the plunger were slightly larger than the plunger body and scraped remaining sample from the tube walls.
In case of a significant time lag between apparatus charging with the dry ice and breath sampling or an unexpected time lag between sampling and retrieval, the blowhole mask and exhale valve could be removed and the glass tube could be sealed with PTFE end caps to prevent condensation and potential contamination from the ambient air, or sample thawing and leakage. The insulated housing kept dry ice from rapid evaporation and allowed up to four hours for operation, though long time delays were not desirable.
3.2. Flow Optimization and Heat Transfer in the Sampler
Low airflow resistance and high condensation rate are the two desired requirements. Uncomfortable back pressure has been a significant problem for breath sampling apparatuses for humans [31]; also, deviation from normal respiration is known to affect the metabolomic content of breath [45]. A high condensation rate is desired for rapid on-site sampling since it allows collection of sufficient amount of breath sample (ice-frozen condensate) for analysis with a minimum number of breaths from animals.
Marine mammal breathing behavior is such that exhalation occurs in short powerful bursts. Although there exists no documented threshold pressure for animal comfort, human respiratory devices are rated at ~98 Pa [46]. We assume the pressure threshold for bottlenose dolphins to be twice this value because their lungs are about twice as large as human lungs and can sustain high pressure when diving to depth [45]. The lung volume of bottlenose dolphins is about 10–14 L, with tidal volumes of 6.5 L exhaled in about 600 msec [40, 45]. We considered a range of commercially available glass tubes with inner diameters from 15 to 40 mm to optimize the pressure drop and condensation rate. For this diameter range, the estimated pressure drop ranges from 519.75 to 10.78 Pa and condensation rate from 0.22 to 0.14 g/sec respectively (figure 5d). Increasing the condenser tube diameter reduces the pressure drop but changes the flow mode from turbulent to laminar which reduces the heat exchange and results in lower condensation rate. The smallest standard tube diameter that satisfied both requirements for turbulent flow regime and for pressure drop was 23.75 mm ID. The corresponding pressure drop was 80.41 Pa and the estimated condensation rate was 0.22 g/sec at the peak flow.
Although breath condensation is a multiphase process with unsteady flow (vapor cooling, phase change from vapor to liquid, liquid cooling, phase change from liquid to solid, and solid cooling), the experimental results confirmed that it was well estimated with the proposed analytical solution. The estimated mass of collected sample from thirty breaths of an individual dolphin was 2.55 for 91 and 1.64 mg for 61 cm long condenser tube assuming 30 exhalation cycles with tidal volume of 6.2 L and exhalation duration of 600 msec. Since all sampling procedures were based on the 30 breathing cycles, the actual sampling time varied greatly from animal to animal (90–600 sec) throughout the study. The actual average amount of frozen condensate in experiments was 2.1 mg (4 mg maximum, 0.4 mg minimum, standard deviation 0.9 mg) for 91 cm long device and 1.6 mg (2.5 mg maximum, 0.7 mg minimum, standard deviation 0.6 mg) for 61 cm long device. The amount of condensed sample was confirmed with the difference in mass of the tube with frozen EBC after sampling and the mass of the dry tube measured before sampling. There was a sample loss during sample transfer from the condenser tube into a storage vial. To evaluate that sample loss, the mass of the dry vial with cap and label was subtracted from the mass of the vial with EBC sample in it. The amount of the frozen EBC transferred into a sealed vial was about 1 mg for both lengths of the device. Eccentricity defects in longer condenser glass tube resulted in greater sample loss during sample retrieval step. The icy pads of frozen sample were left behind the plunger on the tube surface. Longer condenser tube also complicated the ease of device operation and sample retrieval procedure. The use of glass tube with lower eccentricity and bow tolerance would help reduce this sample loss.
Breath sampling was limited to 30 breathing cycles (inhalation/exhalation) for all animals but some animals completed 30 cycles very rapidly (~90 sec) while others completed 30 cycles slower (~600 sec). No standardization in breathing pattern or time duration was encouraged. Animals were allowed to breathe at their comfort rate which brought great variability in the mass of collected EBC samples. Later attempts to correlate mass of the collected samples to the animal body weight or sampling duration did not lead to any conclusive results. Therefore, we conclude that the breathing pattern and the total volume of exhaled breath have strong effects on the amount of condensed sample. We recommend using a spirometer (flow meter or volume meter) at the outlet of the device to integrate the flow rate into a total exhaled volume and standardize the sampling procedure based on the total exhaled volume rather than time period or the number of breathing cycles [35]. This may reduce sampling variability due to physiological differences of the animals and their breathing patterns.
3.3. Gas Chromatography/Mass Spectrometry Analysis of EBC Volatile Organic Compounds
The chemical content of the volatile fraction of EBC samples was assessed using gas chromatography mass spectrometry (GC/MS). A representative GC/MS chromatogram is shown in Figure 6(a). Over 300 individual compounds were identified in these samples. Approximately 35% of these peaks were found to be present in blanks (sea water and vial blanks) and may potentially be extraneous, though many peaks found in sea water were at significantly lower abundances. The classes of compounds that were detected by GC/MS included hydrocarbons, both branched and normal, mostly long-chain alcohols and aldehydes, carbonic acids, as well as some non-biogenic exogenous compounds, such as homosalate. The detected compounds were reported earlier both for humans [3] and dolphins [7]. Some of the non-biogenic compounds were reported to potentially originate from the environment. For example, homosalate is a component of sunscreen used to protect managed animals who spend greater time in proximity to the water surface, compared to their wild counterparts [7].
Figure 6.
a. A representative GCMS chromatogram for dolphin EBC obtained as described in the Materials and Methods section. The inset (14–20 minutes) shows high information content of the chromatogram.
b. GC/MS chromatograms of sea water blank and three replicate breath samples demonstrates excellent sample-to-sample reproducibility of abundances of metabolites in the volatile fraction across the ranges of volatility. The individual profiles are offset by 50,000 a.u. for clarity.
The number of dolphin EBC volatile metabolites detected in the present work exceeds the number reported in the earlier study of the dolphin breath metabolome [7]. Previously, approximately 230 distinct metabolites (including potential extraneous compounds) were identified using GC/MS without chemical derivatization. The direct side-by-side comparison of the metabolomic content observed in the present work and in previous reports would not be meaningful because the sampling, analysis procedures and number of studied animals differ in each case, thus altering metabolite coverage between studies. In the previous volatile analysis of dolphin EBC, a gas-phase headspace sampling technique was employed, while in the present work we used liquid phase extraction as described in the Materials and Methods section. Nevertheless, it is apparent that better capture and retention of volatiles is achieved with the optimized collection device in addition to improved ease of collection, sample handling, and animal comfort.
One important factor that enhances volatile capture and retention is the improved hardware design – specifically the optimized work flow. The sample collection protocol ensures the EBC sample remains frozen at all times, which minimizes alterations of sample chemical composition and sample loss. This is especially critical during the sample retrieval and transfer steps. Also, the stability of the temperature level in the collection device is intended to mitigate the impact of environmental factors and further reduce variability in the metabolomic content of the sample. Previous studies found that changes in condenser temperature had an effect on the metabolomic content of the EBC sample [26, 47].
In the present study, we observed excellent sample-to-sample reproducibility of abundances of metabolites in the volatile fraction across the ranges of volatility. Figure 6(b) shows a GC/MS chromatogram for sea water blank and overlaid chromatograms for three replicate samples; the individual profiles are offset by 50,000 a.u. for clarity. For different samples, we observed noticeable differences for some peaks, but for majority of peaks there were no differences in abundances across replicate samples. For the metabolites detected in all of the samples, the calculated coefficient of variation (relative standard deviations, RSD) for the peak abundance values varied from ~4% to ~101%, with the average value of RSD of ~37%. The value of RSD does not appear to correlate with the retention time, but tends to be greater for lower abundance metabolites. Spurious fluctuations in trace metabolite quantification can due to instrumental noise, which can affect this measure of variability. The reported variations encompass differences in sampling, sample transfer, and analysis. Continuing studies across a variety of collection conditions will allow future variability assessment and further expand the detectable metabolite content.
3.4. Liquid Chromatography/Mass Spectrometry of EBC Non-volatile Organic Compounds
The non-volatile chemical content of EBC samples was analyzed using liquid chromatography/mass spectrometry (LC/MS) in both reverse phase (RP) and hydrophilic (HILIC) chromatography. This allowed better metabolite coverage for lipophilic, polar and very polar compounds present in the sample. In the HILIC analysis, approximately 2,400 metabolites were found in the positive ion mode and 19 in the negative ion mode (Tables 1A, 1B; Supplemental Material). The identified metabolites, similarly to the GC/MS analysis, represent classes of compounds that are expected to be present in dolphin breath based on the previous data and comparison to the human data: carbonic acids, glycans, amino acids/peptides, lipids (including steroids), various nitrogen-containing compounds, especially amines. In the RP analysis, approximately 2,500 metabolites were found in the positive ion mode and ~800 in the negative ion mode (Tables 2A, 2B; Supplemental Material). As expected, the compounds detected in this case were lipids such as acylglycerols and sphingolipid. In the negative ion mode, long chain fatty acids were identified. Fewer compounds could be reliably identified in the RP analysis than in the HILIC analysis.
Similarly to the GC/MS analysis, the LC/MS analysis indicated that the metabolomic content of the sample from the improved device exceeded that of the previously reported [7]. As in the analysis of the volatile fraction, it was not feasible to directly compare the number of metabolites across studies because different methodologies were used. For example, compared to the previous study of dolphin EBC [7], the use of different instrumentation platforms and data- independent acquisition (DIA) [43] are expected to alter the number of detected compounds significantly. Also, even minor changes in deconvolution and alignment settings may greatly affect the number of indexed metabolites. Nevertheless, for the analysis of the non-volatile fraction, it is also apparent that broad metabolite coverage was achieved with the current improved sampling device, and the sum result of the methodology choices employed in the present work lead to detection of comparable or greater number of indexed metabolites. Thousands of distinct metabolites were detected across HILIC and RP analysis in both positive and negative ion modes (Tables 1, 2; Supplemental Material). The detected compounds span great range of sizes and polarities and represent all the key classes of biogenic compounds that may serve as potential biomarkers: small polar (e.g. heterocyclic amines) and non-polar (e.g. fatty acids) molecules, peptides/proteins, glycans and lipids/sterols. Due to the exceedingly complex nature of these samples, full comprehensive analysis of the metabolome is challenging; however, future advances in breath analysis research may allow further confirmation of the detected metabolites as well as characterization of others. Ultimately, the comprehensive description of the breath metabolome and its variations for cetaceans and other species may be attained for diagnostic purposes and research.
4. CONCLUSIONS
In the present work, we described a dolphin breath sampling device and workflow methodology for collection and analysis of exhaled breath condensate from small cetaceans. Although the device dimensions were optimized and tested for bottlenose dolphins (Tursiops truncatus), the design could be modified to accommodate cetaceans of larger and smaller sizes with the use of a dedicated EBC collection mask. The intent was to provide a simple, inexpensive and easy to use device that could be amenable to a wide range of users, including veterinarians, researchers, and conservationists, to promote advancement in breath analysis research and application for cetaceans and other species.
We demonstrated efficient collection of EBC samples from trained dolphins within a reasonably short time window (3–10 min). These samples were found to contain richer metabolomic content than in our previous study, both in the volatile and non-volatile fractions. The identified compounds were representative of typical biogenic metabolites expected in mammal breath and possible extraneous compounds from the environment. These compounds overlapped with the previously reported ranges of metabolites both in dolphin and human breath.
Future improvements of the hardware may include addition of a spirometer or a flow meter at the outlet end of the device to measure lung function parameters such as peak flow and total expired volume. This improvement may be an additional parameter for standardization of breath sample collection. The variability due to physiological animal differences and breathing patterns may be reduced by sampling over a time period defined with pre-determined volume of expired air rather than a set time period or number of breaths [25, 31]. Comparison of peak flow rate profiles and total expired volume per expiration across a number of cycles may also be an important parameter for the device operator during a sampling procedure. Measured and recorded values may be useful for data standardization during analysis stage and for comparison across different experimental studies.
It is our hope that this non-invasive methodology would be adopted for routine applications in population health studies or veterinary health assessments. As the breath analysis field matures, the knowledge of targeted metabolites of interest may guide specific engineering solutions for further improvements of the collection device design. Further research on the effect of certain environmental exposures on health may link the presence and abundance levels of specific breath biomarkers to the exposure level. Continued contribution of information of the metabolome, understanding metabolite origin, and comparison across species will also aid in understanding of underlying metabolic processes.
Supplementary Material
Figure 3.
A set of three one-way valves controls the direction of the air flow during inhalation and exhalation maneuvers. During inspiration, the two valves installed in the proximity to the blowhole mask provide restriction free air flow; the valve on the top is closed and allows no air flow through the device. During exhalation, the two lower valves are closed and the top exhalation valve is open; the plume of exhaled breath is routed through the condenser tube.
Acknowledgments
Materials and methods described in this paper are part of a pending U.S. patent submitted by the U.S. Navy (U.S. Patent Serial Number 14/859,612). This study was supported by the Office of Naval Research (ONR) grant #N-00014-13-1-0580 [CED, SVW, BCW], under an animal care and use protocol reviewed and approved by the Navy Marine Mammal Program Institutional Animal Care and Use Committee and the Navy Bureau of Medicine (#106–2013)[SVW]. Partial support was also provided by: The Hartwell Foundation [CED], the NIH National Center for Advancing Translational Sciences (NCATS) through grant#UL1 TR000002 [CED, JPD], NIH award U01 EB0220003-01 [CED, JPD], and NIH award 1P30ES023513-01A1 [CED]. Student support was provided by NIH award T32 HL07013 [KOZ] and NIH award # P42ES004699 [KOZ]. We thank management and animal care staff at the U.S. Navy Marine Mammal Program for their assistance with this project. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies.
References
- 1.Amann A, Smith D. Volatile Biomarkers Non-Invasive Diagnosis in Physiology and Medicine Foreword. Volatile Biomarkers: Non-Invasive Diagnosis in Physiology and Medicine. 2013:Xxvii–Xxix. [Google Scholar]
- 2.Reinhold P, Knobloch H. Exhaled breath condensate: lessons learned from veterinary medicine. Journal of Breath Research. 2010;4(1) doi: 10.1088/1752-7155/4/1/017001. [DOI] [PubMed] [Google Scholar]
- 3.de LCB, et al. A review of the volatiles from the healthy human body. J Breath Res. 2014;8:014001. doi: 10.1088/1752-7155/8/1/014001. (Copyright (C) 2014 U.S. National Library of Medicine.) [DOI] [PubMed] [Google Scholar]
- 4.Kuban P, Foret F. Exhaled breath condensate: Determination of non-volatile compounds and their potential for clinical diagnosis and monitoring. A review. Analytica Chimica Acta. 2013;805:1–18. doi: 10.1016/j.aca.2013.07.049. [DOI] [PubMed] [Google Scholar]
- 5.Phillips M, et al. Detection of an Extended Human Volatome with Comprehensive Two-Dimensional Gas Chromatography Time-of-Flight Mass Spectrometry. Plos One. 2013;8(9) doi: 10.1371/journal.pone.0075274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spanel P, Dryahina K, Smith D. A quantitative study of the influence of inhaled compounds on their concentrations in exhaled breath. Journal of Breath Research. 2013;7(1) doi: 10.1088/1752-7155/7/1/017106. [DOI] [PubMed] [Google Scholar]
- 7.Aksenov AA, et al. Metabolite Content Profiling of Bottlenose Dolphin Exhaled Breath. Analytical Chemistry. 2014;86(21):10616–10624. doi: 10.1021/ac5024217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yeates LC, et al. Nitric oxide in the breath of bottlenose dolphins: Effects of breath hold duration, feeding, and lung disease. Marine Mammal Science. 2014;30(1):272–281. [Google Scholar]
- 9.Zhu JJ, et al. Detecting bacterial lung infections: in vivo evaluation of in vitro volatile fingerprints. Journal of Breath Research. 2013;7(1) doi: 10.1088/1752-7155/7/1/016003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bean HD, et al. Identifying methicillin-resistant Staphylococcus aureus (MRSA) lung infections in mice via breath analysis using secondary electrospray ionization-mass spectrometry (SESI-MS) Journal of Breath Research. 2014;8(4) doi: 10.1088/1752-7155/8/4/041001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergmann A, et al. In Vivo Volatile Organic Compound Signatures of Mycobacterium avium subsp. paratuberculosis. Plos One. 2015;10(4) doi: 10.1371/journal.pone.0123980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trefz P, et al. Volatile Emissions from Mycobacterium avium subsp paratuberculosis Mirror Bacterial Growth and Enable Distinction of Different Strains. Plos One. 2013;8(10) doi: 10.1371/journal.pone.0076868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shnayderman M, et al. Species-specific bacteria identification using differential mobility spectrometry and bioinformatics pattern recognition. Analytical Chemistry. 2005;77(18):5930–5937. doi: 10.1021/ac050348i. [DOI] [PubMed] [Google Scholar]
- 14.Pete Schroeder J, Erin Zabek SR, Cameron Caroline E, Eshghi Azad, Bain David, Wood Robert, Rhodes Linda, Hanson Brad. Proceedings of the 2009 Puget Sound Georgia Basin Ecosystem Conference. Seattle, WA USA: 2009. Investigation into the Microbial Culture and Molecular Screening of exhaled breaths of Endangered Southern Resident Killer Whales (SRKW) and Pathogen Screening of the Sea Surface Microlayer (SML) in Puget Sound. [Google Scholar]
- 15.Acevedo-Whitehouse K, Rocha-Gosselin A, Gendron D. A novel non-invasive tool for disease surveillance of free-ranging whales and its relevance to conservation programs. Animal Conservation. 2010;13(2):217–225. [Google Scholar]
- 16.Filipiak W, et al. Breath analysis for in vivo detection of pathogens related to ventilator-associated pneumonia in intensive care patients: a prospective pilot study. Journal of Breath Research. 2015;9(1) doi: 10.1088/1752-7155/9/1/016004. [DOI] [PubMed] [Google Scholar]
- 17.Schnabel R, et al. Analysis of volatile organic compounds in exhaled breath to diagnose ventilator-associated pneumonia. Scientific Reports. 2015;5 doi: 10.1038/srep17179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bean HD, et al. Breathprints of model murine bacterial lung infections are linked with immune response. European Respiratory Journal. 2015;45(1):181–190. doi: 10.1183/09031936.00015814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bean HD, et al. Breath Biomarkers of Infection and Inflammation in Lung Infections. Pediatric Pulmonology. 2013;48:321-321. [Google Scholar]
- 20.Reinhold P, et al. An experimentally induced Chlamydia suis infection in pigs results in severe lung function disorders and pulmonary inflammation. Veterinary Research. 2008;39(3) doi: 10.1051/vetres:2008012. [DOI] [PubMed] [Google Scholar]
- 21.Bach J-P, et al. Measuring compounds in exhaled air to detect alzheimer's disease and parkinson's disease. PLoS One. 2015;10(7):e0132227/1–e0132227/13. doi: 10.1371/journal.pone.0132227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nunez-Naveira L, et al. Determination of ELISA reproducibility to detect protein markers in exhaled breath condensate. J. Breath Res. 2012;6(4):046003, 9. doi: 10.1088/1752-7155/6/4/046003. [DOI] [PubMed] [Google Scholar]
- 23.Bayley DL, et al. Validation of assays for inflammatory mediators in exhaled breath condensate. Eur. Respir. J. 2008;31(5):943–948. doi: 10.1183/09031936.00081707. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Reche LM, et al. Validity Assessment for the Results of Three Inflammatory Markers in Exhaled Breath Condensate: A Pilot Study. Chromatographia. 2009;70(9/10):1387–1392. [Google Scholar]
- 25.Bikov A, et al. Standardised exhaled breath collection for the measurement of exhaled volatile organic compounds by proton transfer reaction mass spectrometry. Bmc Pulmonary Medicine. 2013;13 doi: 10.1186/1471-2466-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loyola BR, et al. Temperature changes in exhaled breath condensate collection devices affect observed acetone concentrations. Journal of Breath Research. 2008;2(3) doi: 10.1088/1752-7155/2/3/037005. [DOI] [PubMed] [Google Scholar]
- 27.Dweik RA, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–15. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kapande KM, et al. Comparative repeatability of two handheld fractional exhaled nitric oxide monitors. Pediatr Pulmonol. 2011 doi: 10.1002/ppul.21591. [DOI] [PubMed] [Google Scholar]
- 29.Sayao LB, et al. Exhaled nitric oxide as a diagnostic tool for wheezing in preschool children: A diagnostic accuracy study. Respiratory Medicine. 2016;113:15–21. doi: 10.1016/j.rmed.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Thornadtsson A, et al. Extended nitric oxide analysis may improve personalized anti-inflammatory treatment in asthmatic children with intermediate FENO50. Journal of Breath Research. 2015;9(4) doi: 10.1088/1752-7155/9/4/047114. [DOI] [PubMed] [Google Scholar]
- 31.Horvath I, et al. Exhaled breath condensate: methodological recommendations and unresolved questions. European Respiratory Journal. 2005;26(3):523–548. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 32.Davis MD, Montpetit A, Hunt J. Exhaled Breath Condensate An overview. Immunology and Allergy Clinics of North America. 2012;32(3):363-+. doi: 10.1016/j.iac.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soyer OU, et al. Comparison of two methods for exhaled breath condensate collection. Allergy. 2006;61(8):1016–8. doi: 10.1111/j.1398-9995.2006.01064.x. [DOI] [PubMed] [Google Scholar]
- 34.Rosias PP, et al. Breath condenser coatings affect measurement of biomarkers in exhaled breath condensate. European Respiratory Journal. 2006;28(5):1036–1041. doi: 10.1183/09031936.06.00110305. [DOI] [PubMed] [Google Scholar]
- 35.Reinhold P, Jaeger J, Schroeder C. Evaluation of methodological and biological influences on the collection and composition of exhaled breath condensate. Biomarkers. 2006;11(2):118–142. doi: 10.1080/13547500600572764. [DOI] [PubMed] [Google Scholar]
- 36.Cumeras R, et al. Chemical analysis of whale breath volatiles: a case study for non-invasive field health diagnostics of marine mammals. Metabolites. 2014;4(3):790–806. doi: 10.3390/metabo4030790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wells RS. Dolphin social complexity: Lessons from long-term study and life history. In: DeWaal FBM, Tyack PL, editors. Animal Social Complexity: Intelligence, Culture, and Individualized Societies. Cambridge: Harvard Univ Press; 2003. p. 32-+. [Google Scholar]
- 38.Hawkins ER. Geographic variations in the whistles of bottlenose dolphins (Tursiops aduncus) along the east and west coasts of Australia. The Journal of the Acoustical Society of America. 2010;128(2):924–935. doi: 10.1121/1.3459837. [DOI] [PubMed] [Google Scholar]
- 39.Colebrook CF. Turbulent flow in pipes, with particular reference to the transition region between the smooth and rough pipe laws. Journal of the ICE. 1939;11(2):133–156. [Google Scholar]
- 40.Perrin WF, Wursig B, Thewissen JGM. Encyclopedia of MARINE MAMMALS Second Edition PREFACE. Encyclopedia of Marine Mammals. (2) 2009:152–156. [Google Scholar]
- 41.Burgstahler AW, Bricker CE. Measuring the Heat of Sublimation of Dry Ice with a Polystyrene Foam Cup Calorimeter. Journal of Chemical Education. 1991;68(4):332–333. [Google Scholar]
- 42.Sigma-aldrich/supelco. SPME Applications Guide. 2001 [Google Scholar]
- 43.Tsugawa H, et al. MS-DIAL: data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nature Methods. 2015;12(6):523-+. doi: 10.1038/nmeth.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosias PP, et al. Biomarker reproducibility in exhaled breath condensate collected with different condensers. European Respiratory Journal. 2008;31(5):934–942. doi: 10.1183/09031936.00073207. [DOI] [PubMed] [Google Scholar]
- 45.Ponganis PJ. Diving mammals. Compr Physiol. 2011;1(1):447–65. doi: 10.1002/cphy.c091003. [DOI] [PubMed] [Google Scholar]
- 46.Smyth RJ, Chapman KR, Rebuck AS. Maximal Inspiratory and Expiratory Pressures in Adolescents - Normal Values. Chest. 1984;86(4):568–572. doi: 10.1378/chest.86.4.568. [DOI] [PubMed] [Google Scholar]
- 47.Effros RM, et al. Comment on 'The effect of temperature on exhaled breath condensate collection'. J. Breath Res. 2012;6:048001, 2. doi: 10.1088/1752-7155/6/4/048001. (Copyright (C) 2014 American Chemical Society (ACS). All Rights Reserved.) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.