Abstract
Objective
To estimate the future risk and time trends of venous thromboembolism (VTE) in individuals with newly diagnosed primary Sjögren’s syndrome (SjS) in the general population.
Methods
Using a population database that includes all residents of British Columbia, Canada we created a study cohort of all patients with incident SjS and up to 10 age-, sex-, and entry-time-matched controls from the general population. We compared incidence rates (IRs) of pulmonary embolism (PE), deep vein thrombosis (DVT) and VTE between the two groups according to SjS disease duration. We calculated hazards ratios (HR), adjusting for confounders.
Results
Among 1,175 incident primary SjS cases (mean age 56.7 years, 87.6% female), the IRs of PE, DVT, and VTE were 3.9, 2.8, and 5.2 per 1000 person-years, respectively; the corresponding rates in the comparison cohort were 0.9, 0.8 and 1.4 per 1000 person-years. Compared with non-SjS individuals, the multivariable HRs for PE, DVT, and VTE among SjS cases were 4.07 (95% CI, 2.04–8.09), 2.80 (95% CI, 1.27–6.17), and 2.92 (95% CI, 1.66–5.16), respectively. The age-, sex-, and entry time-matched HRs for VTE, PE and DVT were highest during the first year after SjS diagnosis (8.29 [95% CI, 2.57–26.77], 4.72 [95% CI, 1.13–19.73], and 7.34 [95% CI, 2.80–19.25], respectively).
Conclusion
These findings provide population-based evidence that patients with primary SjS have a substantially increased risk of VTE, especially within the first year after SjS diagnosis. Further research into the role of monitoring and prevention of VTE in SjS may be warranted.
Keywords: Sjögren’s syndrome, Thrombosis, Embolism, Cardiovascular diseases, Risk
1. INTRODUCTION
Sjögren’s syndrome (SjS) is an inflammatory autoimmune disease characterized by lymphocytic infiltration of the exocrine glands and subsequent gland dysfunction. Dysfunction of the lacrimal and salivary glands produces dry eyes and dry mouth, respectively, and this constellation of symptoms (sicca complex) is the hallmark of SjS. SjS is seen both as a primary entity in isolation, as well as a secondary entity in the context of other established autoimmune diseases—typically lupus, rheumatoid arthritis, or systemic sclerosis. In addition to the classic sicca complex, the disease can also have a wide variety of extra-glandular manifestations potentially affecting almost any body system [1].
Venous thromboembolism (VTE), which comprises both deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common disease associated with significant morbidity and mortality [2,3]. Classic risk factors for VTE include trauma, surgery, prolonged immobilization, and malignancy; however, a wide range of medications, lifestyle factors, inherited factors, and medical comorbidities have also been associated with increased rates of VTE [4].
It is well documented that many inflammatory autoimmune diseases are associated with increased rates of VTE, including inflammatory bowel disease, rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis, polymyositis/dermatomyositis, and giant cell arteritis [5–11]. Research suggests that inflammation may play a role in the inappropriate activation of the coagulation cascade, suggesting a plausible mechanism for the risk of VTE in the above inflammatory disorders [12,13]. Although other inflammatory conditions have been well studied, the data on VTE in SjS is scarce, particularly at the general population level [7,8,14].
Given the clinical importance of VTE and the emergence of evidence to suggest an increased risk in patients with SjS, it is important to fully understand and quantify this effect. In order to address some of the limitations of previous studies, we evaluated the risk of VTE in a cohort of patients with incident primary SjS compared to randomly selected matched controls from the general population, including an analysis of the time trends after SjS diagnosis.
2. MATERIALS AND METHODS
2.1 Data source
We used Population Data BC, a province-wide database generated from the British Columbia (BC) healthcare system, which includes approximately 4.5 million people. Population Data BC identifies population-based administrative data including linkable data files on all provincially funded healthcare professional visits [15], hospital admissions and discharges [16], interventions [15], investigations [15], demographic data [17], cancer registry [18], and vital statistics since 1990 [19]. Furthermore, Population Data BC encompasses the comprehensive outpatient prescription drug database, PharmaNet, with data since 1996 [20]. Numerous general population-based studies have been successfully conducted using these databases [10,11,21–24].
2.2 Study design
We conducted cohort analyses of incident VTE among individuals with incident primary SjS (SjS cohort) as compared with individuals without SjS randomly selected from the general population using Population Data BC (control cohort) and matched for age-, sex-, and entry-time. We created an incident SjS cohort with cases diagnosed for the first time between January 1996 and December 2010, defined as either of the following: ≥ 2 ICD-CM-9 codes (710.2) for SjS at least two months apart but within a 2-year period by non-rheumatologists; or ≥ 1 ICD-CM-9 for SjS by a rheumatologist or from hospitalization (ICD-CM-9 710.2 and ICD-10: M35). Additionally we required no ICD-CM-9 for SjS from January 1990 until cohort entry (to ensure incident SjS cases). A validation study of ICD-9 codes in a Canadian context found that the specificity and sensitivity of primary SjS diagnosis from administrative health data was 95.5% and 95.8%, respectively [25].
To further improve specificity for primary SjS, we excluded individuals with two or more visits at least to two months apart subsequent to the SjS diagnostic visit with diagnoses of other systemic autoimmune rheumatic diseases (rheumatoid arthritis, psoriatic arthritis, spondyloarthropathy, systemic lupus erythematosus, systemic sclerosis and adult systemic vasculitis) after the index date (date of diagnosis). For each cohort we matched up to 10 individuals, randomly selected from the general population, without SjS to each SjS case based on age, sex, and calendar year of study entry.
2.3 Ascertainment of PE and DVT
Incident PE and DVT cases were defined by a corresponding ICD code and prescription for anticoagulant therapy (heparin, warfarin sodium, or a similar agent) [26]. The codes used were as follows: PE (ICD-9-CM: 415.1, 673.2, 639.6; ICD-10-CM: O88.2, I26) and DVT (ICD-9-CM: 453; ICD-10-CM: I82.4, I82.9). Because VTE is a potentially fatal outcome, we also included patients with a fatal outcome. Patients with a recorded code of DVT or PE were included in the absence of recorded anticoagulant therapy (i.e., the patients died before discharge, and therefore never received a prescription for anticoagulant therapy from an outpatient service [such cases would not be identified by PharmaNet, which only identifies medication received outside of hospital]) if there was a fatal outcome within two months of diagnosis. These definitions have been successfully used in previous studies and found to have a positive predictive value of 94% in a general practice database [26].
2.4 Assessment of Covariates
Covariates included potential risk factors for VTE assessed during the year before the index date: surgery, trauma, fractures, and cancer (embedded in the Charlson’s comorbidity index). We also corrected for other relevant medical conditions (alcoholism, hypertension, varicose veins, inflammatory bowel disease, sepsis), medications (glucocorticoids, hormone replacement therapy, contraceptives, and COX-2 inhibitors), and comorbidity burden (healthcare use and Charlson comorbidity index) [27,28].
2.5 Cohort Follow-up
Our study cohorts spanned the period from January 1, 1996 to December 31, 2010. Individuals with SjS entered the case cohort after all inclusion criteria were met, and matched individuals entered the comparison cohort after a doctor’s visit or hospital admission in the same calendar year. Participants were followed until they experienced an outcome, died, un-enrolled from the health plan through leaving the province (~ 1%), or the follow-up period ended (December 31, 2010), whichever occurred first.
2.6 Statistical Analysis
We compared baseline characteristics between the SjS and comparison cohorts. We calculated the incidence rates (IRs) per 1,000 person-years (PY) for each outcome in the SjS and comparison cohorts. The associations between SjS and study outcomes are expressed as incidence rate ratios (IRR) with 95% CIs. We calculated and plotted the cumulative IRs of end-points for individuals with and without SjS, accounting for the competing risk of death [29].
We used Cox proportional hazard regression models to assess the risk of PE, DVT, and VTE associated with SjS after adjusting for the covariates listed in. Table 1 [30]. We entered confounders one at a time into the Cox models in a forward selection according to each confounder’s effect on the hazard ratio (HR) of SjS, relative to the HR in the model selected in the previous step. Cut-off for the minimum important relative effect at each step was set to 5% [31]. To evaluate the time-trend of VTE risk according to the time since SjS diagnosis, we estimated HRs yearly for the first five years. We assessed the proportional hazards assumption by plotting log–log S(t) versus log t, stratified by each covariate. If lines were not close to parallel, time interaction terms were included and tested at alpha=0.05.
Table 1.
Characteristics of SjS and Comparison Cohorts at Baseline. Values are N (%) unless otherwise specified.
| Variable | SjS | Non-SjS | p-value |
|---|---|---|---|
| N=1,175 | N=11,947 | ||
| Age (mean years) | 56.73 (15.27) | 56.67 (15.2) | 0.902 |
| Female | 1029 (87.6) | 10441 (87.4) | 0.890 |
| Alcoholism with liver disease | 10 (0.9) | 86 (0.7) | 0.589 |
| Hypertension | 303 (25.8) | 2778 (23.3) | 0.052 |
| Sepsis | 13 (1.1) | 18 (0.2) | <0.001 |
| Varicose veins | 27 (2.3) | 121 (1) | <0.001 |
| Inflammatory bowel disease | 4 (0.3) | 32 (0.3) | 0.560 |
| Trauma | 1 (0.1) | 25 (0.2) | 0.726 |
| Fractures | 28 (2.4) | 166 (1.4) | 0.011 |
| Surgery | 13 (1.1) | 84 (0.7) | 0.149 |
| Charlson’s Comorbidity Index , Mean (SD) | 1.06 (1.54) | 0.36 (1.07) | <0.001 |
| Glucocorticoids | 239 (20.3) | 510 (4.3) | <0.001 |
| Hormone replacement therapy | 139 (11.8) | 823 (6.9) | <0.001 |
| Oral contraceptives | 44 (3.7) | 439 (3.7) | 0.871 |
| Cox-2 Inhibitors | 94 (8) | 356 (3) | <0.001 |
| Hospitalized | 364 (31) | 1898 (15.9) | <0.001 |
| Number of outpatient visits, mean (SD) | 20.68 (17.95) | 9.09 (10.85) | <0.001 |
SjS: Sjögren syndrome; COX: cyclooxyg enase.
2.7 Sensitivity Analyses
We performed two sensitivity analyses to test the robustness of our results. First, we estimated the cumulative incidence of each event accounting for the competing risk of death according to Lau et al. [32], and expressed the results as sub-distribution HRs with a 95% CI. Second, to assesses how a hypothetical unmeasured confounder might have affected our estimates of the association between SjS and VTE, we simulated unmeasured confounders with prevalences ranging from 10% to 20% in both the SjS and control cohorts, and odds ratios ranging from 1.3 to 3.0 for the associations between the unmeasured confounder, VTE, and SjS [33]. For reference, the respective odds ratios for VTE and smoking and obesity are 1.2 and 2.3 [34], respectively, and the prevalences are about 17% and 13%, respectively, based on Canadian census data [35,36].
SAS V.9.3 (SAS Institute, Cary, North Carolina, USA) was used for all analyses. For all IRRs and HRs, we calculated 95% CIs. All p values are two-sided.
2.8 Role of the Funding Source
The funding sources were not involved in the design, conduct, or reporting of the study, or the decision to submit the manuscript for publication.
2.9 Ethical Approval
No personal identifying information was made available as part of this study. Procedures used were in compliance with British Columbia's Freedom of Information and Privacy Protection Act. Ethical approval was obtained from the University of British Columbia’s Behavioural Research Ethics Board (H12-01530). .
3. RESULTS
3.1 Baseline Characteristics
Our primary analysis included 1,175 incident primary SjS cases (mean age 56.7 years, 87.6% women) and, in the comparison cohort, 11,947individuals matched for age-, sex-, and entry-time. Table 1 summarizes the baseline characteristics of the SjS and comparison cohorts. Compared with the control group, the SjS cases were treated with significantly more glucocorticoids, COX-2 inhibitors, and hormone replacement therapy, had more fractures, sepsis, and varicose veins, had higher Charlson’s comorbidity index scores, and had more hospitalizations and outpatient visits during the twelvemonths prior to diagnosis.
3.2 Association between a Diagnosis of Primary SjS and Incident VTE
Overall, SjS was significantly associated with an increased incidence of VTE, as well as individual DVT and PE (Table 2, Figure 1). Among individuals with SjS, the IRs for PE, DVT and VTE were 3.85, 2.75, and 5.24 per 1000 PY versus 0.89, 0.79 and 1.44 per 1,000 PY in the comparison cohort, respectively. The corresponding HR matched for age-, sex-and entry time were 5.57 (95% CI, 2.90–10.68), 4.04 (95% CI, 1.95–8.40), and 4.24 (95% CI, 2.48–7.26), respectively. In a multivariable proportional hazards analysis the following covariates were found to be significant confounders: for PE, number of outpatient visits, glucocorticoids, COX-2 inhibitors, hypertension; for DVT, number of outpatient visits, glucocorticoids, Charlson comorbidity index; for VTE, number of outpatient visits, glucocorticoids, COX-2 inhibitors, Charlson comorbidity index. After adjustment for these confounders the HRs remained significant at 4.07 (95% CI, 2.04–8.09), 2.80 (95% CI, 1.27–6.17), and 2.92 (95% CI, 1.66–5.16) for PE, DVT, and VTE, respectively (Table 2). When we evaluated the effect of follow-up time after SjS diagnosis, the adjusted HRs for PE, DVT, and VTE events were significantly elevated for five years following SjS diagnosis, and substantially higher in the first year (Table 3)
Table 2.
Relative Risk of Incident PE and DVT According to SjS Status
| SjS | Non-SjS | |
|---|---|---|
| N=1,175 | N=11,947 | |
| PE | ||
| Cases, N | 14 | 36 |
| Incidence Rate/1000 Person-Years | 3.85 | 0.89 |
| Age-, Sex-, Entry Time-Matched Cox HR (95% CI) | 5.57 (2.90, 10.68) | 1.00 |
| * Fully-Adjusted Age-, Sex-, Entry Time-Matched Cox HR (95% CI) | 4.07 (2.04, 8.09) | 1.00 |
| DVT | ||
| Cases, N | 10 | 32 |
| Incidence Rate/1000 Person-Years | 2.75 | 0.79 |
| Age-, Sex-, Entry Time-Matched Cox HR (95% CI) | 4.04 (1.95, 8.40) | 1.00 |
| * Fully-Adjusted Age-, Sex-, Entry Time-Matched Cox HR (95% CI) | 2.80 (1.27, 6.17) | 1.00 |
| VTE | ||
| Cases, N | 19 | 58 |
| Incidence Rate/1000 Person-Years | 5.24 | 1.44 |
| Age-, Sex-, Entry Time-Matched Cox HR (95% CI) | 4.24 (2.48, 7.26) | 1.00 |
| * Fully-Adjusted Age-, Sex-, Entry Time-Matched Cox HR (95% CI) | 2.92 (1.66, 5.16) | 1.00 |
Fully adjusted models include the following selected covariates: for PE, hormone replacement therapy, no. outpatient visits and glucocorticoids; DVT, glucocorticoids and no. outpatient visits; PE or DVT, glucocorticoids and no. outpatient visits. PE: pulmonary embolism; DVT: deep vein thrombosis; SjS: Sjögren syndrome; VTE: venous thromboembolism.
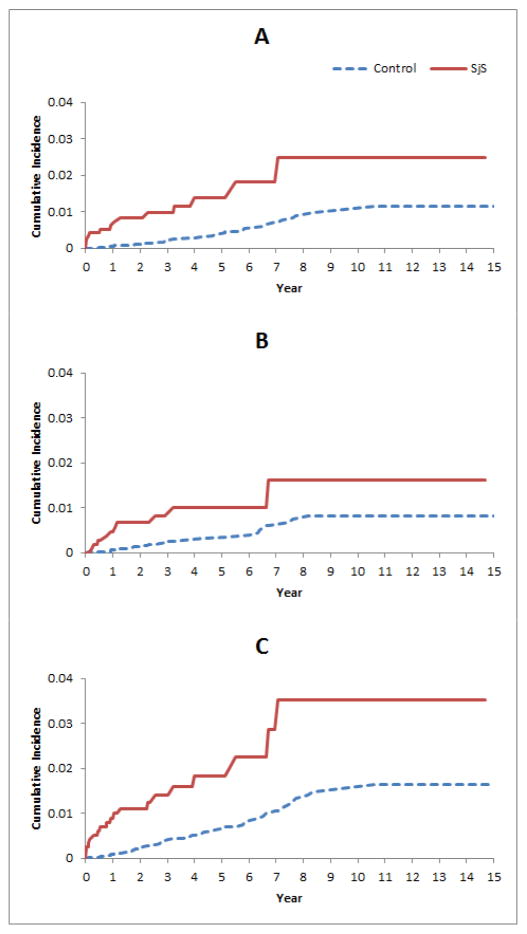
Figure 1.
Cumulative incidence of (A) PE, (B) DVT, and (C) VTE in the 1,175 cases with incident primary SjS compared with controls randomly selected from the general population and matched for age-, sex-, and entry time. PE: pulmonary embolism; DVT: deep vein thrombosis; VTE: venous thromboembolism; SjS: Sjögren’s syndrome.
Table 3.
Age and sex adjusted Cox HR for PE, DVT, and VTE in Sjögren syndrome according to follow-up period. Values are HR (95% CI).
| Time after diagnosis | PE | DVT | VTE |
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| <1 year | 8.29 (2.57, 26.77) | 4.72 (1.13, 19.73) | 7.34 (2.80, 19.25) |
| <2 years | 5.64 (2.19, 14.49) | 4.06 (1.52, 10.86) | 4.50 (2.09, 9.68) |
| <3 years | 4.38 (1.88, 10.25) | 3.24 (1.30, 8.03) | 3.59 (1.82, 7.07) |
| <4 years | 4.65 (2.15, 10.05) | 3.08 (1.31, 7.27) | 3.35 (1.79, 6.28) |
| <5 years | 4.15 (1.93, 8.93) | 3.01 (1.29, 7.04) | 2.96 (1.59, 5.51) |
| Total follow-up | 4.07 (2.04, 8.09) | 2.80 (1.27, 6.17) | 2.92 (1.66, 5.16) |
PE: pulmonary embolism; DVT: deep vein thrombosis; VTE: venous thromboembolism.
3.3 Sensitivity Analysis
The adjusted HRs were attenuated but remained statistically significant when we tested for both the competing risk of death and a potential unmeasured confounder, even at extreme values for the prevalence and magnitude of the confounder (Table 4).
Table 4.
Primary sensitivity analysis is the fully adjusted model. Subdistribution model is also adjusted for competing risk of early death (age and sex). Simulated confounder models included additional covariates. Values are HR (95% CI).
| Outcome | Primary analysis | Sub-distribution Cox model (competing risk of death) | Simulated confounder 10%/OR=1.3 | Simulated confounder 20%/OR=3.0 |
|---|---|---|---|---|
| PE | 4.07 (2.04, 8.09) | 3.29 (1.71, 6.32) | 3.83 (1.92, 7.62) | 3.29 (1.61, 6.72) |
| DVT | 2.80 (1.27, 6.17) | 2.73 (1.39, 5.38) | 2.65 (1.20, 5.87) | 2.24 (0.99, 5.09) |
| VTE | 2.92 (1.66, 5.16) | 2.70 (1.58, 4.61) | 2.78 (1.57, 4.90) | 2.37 (1.31, 4.27) |
PE: pulmonary embolism; DVT: deep vein thrombosis; VTE: venous thromboembolism
4. DISCUSSION
Our large, general population-based observational study demonstrates a significantly elevated risk of VTE in patients with primary SjS as compared with matched controls from the general population. The fully adjusted HRs for PE, DVT, and VTE were 4.07 (95% CI, 2.04–8.09), 2.80 (95% CI, 1.27–6.17), and 2.92 (95% CI, 1.66–5.16), respectively. This effect was particularly pronounced in the first year after SjS diagnosis, and was found to persist for at least five years.
Our results confirm that primary SjS is an independent risk factor for PE, DVT, and VTE in a general population context. Although our observational study cannot provide information pertaining to causation or a mechanism for the association, previous research suggests that inflammation can contribute to the development of VTE because it initiates clotting, decreases the activity of natural anticoagulant mechanisms, and impairs the fibrinolytic system [12,13]. This would be consistent with our finding that the risk is highest during the period where the disease is most active and the inflammation least controlled: immediately following diagnosis.
Our results are somewhat higher, but largely consistent with previous findings. Four previous studies have investigated the association between SjS and VTE: three registry-based cohort studies of SjS populations and one case-control study of VTE events [7–9,14]. Ramagopalan et al. [7] analyzed an English registry and compared the rates of VTE in patients hospitalized for various rheumatologic diseases with controls hospitalized for minor medical/surgical issues. They found a risk ratio (RR) of 2.02 (95% CI, 1.80–2.26) for VTE in patients with SjS [7]. Zoller et al. [8] analyzed a Swedish registry, restricting their analysis to PE in hospitalized patients. They found an IRR of 2.19 (95% CI, 1.78–2.66) for PE in patients with SjS overall, and an IRR of 7.40 (95% CI, 5.09–10.40) during the first year after diagnosis [8]. Chung et al. [14] analyzed a Taiwanese registry with a methodology similar to that of Zoller et al., but broadened their analysis to include PE and DVT, as well as a distinction between primary and secondary SjS. They found HRs for SjS of 3.29 (95% CI, 2.03–5.31) and 1.83 (95% CI, 1.16–2.89) for PE and DVT, respectively [14]. An alternative explanation to the early increased risk of VTE in SjS may be related to the depletion of susceptible individuals over time. Regardless, our incident case analyses demonstrated that the induction time of SjS effect on the risk of VTE was relatively short (i.e., within months).
Unfortunately, these results may not be generalizable to North American and European populations because of previously described differences in rates of VTE between races [37]. Additionally, none of these studies included prescription drug usage or healthcare resource use, limiting their ability to adjust pre-existing differences on comorbidity as well as limiting the generalizability of their results. . Finally, Johannesdottir et al. [9] analyzed a Danish registry that incorporated both medication usage and health resource use data. They used a case-control design to analyze VTE cases and determined that the HR for SjS was 3.3 (95% CI, 2.1–5.0). However, once adjusted for confounders, their result became non-significant at 1.6 (95% CI, 0.9–2.7) [9].
We acknowledge some limitations of our study. Our results are subject to the accuracy of ICD codes in administrative data; however, the validity of SjS diagnosis based on administrative data in this context has been previously documented [25]. Because the SjS cohort was identified using an algorithm based on diagnostic codes rather than verification of individual medical records, we cannot exclude the possibility that some of these cases were falsely identified. However, this will be a conservative bias where the association would favor the null hypothesis.
Additionally, we are limited by the possibility of unknown or unmeasured confounders, including, but not limited to, undocumented lifestyle factors such as smoking status, physical activity, and body mass index. This was addressed through a simulated confounder analysis; our results remained significant, suggesting that our findings are robust. Moreover, our data did not include laboratory data, such as anti-phospholipid antibodies, or biopsy data.
The strengths of our study include the large sample size, adjustment for pre-existing comorbidities, inclusion of prescription medications for both cases and controls, and adjustment for the competing risk of death and unmeasured confounders in a general population context. Moreover, we used a cohort of incident cases to avoid the biases associated with prevalent cohorts (e.g. survivors’ bias).
5. CONCLUSION
We have demonstrated that SjS is associated with a statistically significant increased risk of VTE in a general population context. Further, early after disease onset there is a particularly elevated risk, with an 8-fold risk of PE, 4-fold risk of DVT, and 7-fold risk of VTE in the first year of disease diagnosis compared with matched controls. This finding remains significant after correction for confounding comorbidities, medications, and demographic variables, as well as the inclusion of potential unmeasured confounders. Considering this, additional research into the involvement of surveillance and prevention of VTE in SjS through early treatment may be warranted.
Acknowledgments
We want to thank Kathryn Reimer and Lindsay Belvedere for their editorial assistance in the preparation of this manuscript. The study funders were not involved in study design, collection, analysis or interpretation of data, or the writing and publishing of this article. All inferences, opinions, and conclusions drawn in this paper are those of the authors, and do not reflect the opinions or policies of the Data Stewards.
Funding: The study was funded by the Canadian Arthritis Network, The Arthritis Society of Canada, the British Columbia Lupus Society (Grant 10-SRP-IJD-01) and the Canadian Institutes for Health Research (Grants MOP-125960 and THC-135235).
Footnotes
COMPETING INTERESTS
None.
References
- 1.Fox RI. Sjögren's syndrome. Lancet. 2005;366:321–31. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 2.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107:4I–8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 3.Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 4.Anderson FA, Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107:I9–16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 5.Choi HK, Rho Y-H, Zhu Y, et al. The risk of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a UK population-based outpatient cohort study. Ann Rheum Dis. 2013;72:1182–7. doi: 10.1136/annrheumdis-2012-201669. [DOI] [PubMed] [Google Scholar]
- 6.Lee JJ, Pope JE. A meta-analysis of the risk of venous thromboembolism in inflammatory rheumatic diseases. Arthritis Res Ther. 2014;16:435. doi: 10.1186/s13075-014-0435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramagopalan SV, Wotton CJ, Handel AE, et al. Risk of venous thromboembolism in people admitted to hospital with selected immune-mediated diseases: record-linkage study. BMC Med. 2011;9:1. doi: 10.1186/1741-7015-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zöller B, Li X, Sundquist J, et al. Risk of pulmonary embolism in patients with autoimmune disorders: a nationwide follow-up study from Sweden. Lancet. 2012;379:244–9. doi: 10.1016/S0140-6736(11)61306-8. [DOI] [PubMed] [Google Scholar]
- 9.Johannesdottir SA, Schmidt HJ, Horváth-Puhó E, et al. Autoimmune skin and connective tissue diseases and risk of venous thromboembolism: a population-based case-control study. J Thromb Haemost. 2012;10:815–21. doi: 10.1111/j.1538-7836.2012.04666.x. [DOI] [PubMed] [Google Scholar]
- 10.Aviña-Zubieta JA, Bhole VM, Amiri N, et al. The risk of deep venous thrombosis and pulmonary embolism in giant cell arteritis: a general population-based study. Ann Rheum Dis. doi: 10.1136/annrheumdis-2014-205665. Published Online First: 29 September 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carruthers EC, Choi HK, Sayre EC, et al. Risk of deep venous thrombosis and pulmonary embolism in individuals with polymyositis and dermatomyositis: a general population-based study. Ann Rheum Dis. doi: 10.1136/annrheumdis-2014-205800. Published Online First: 5 September 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131:417–30. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Lupu F, Esmon CT. Inflammation, innate immunity and blood coagulation. Hamostaseologie. 2010;30:5–6. 8–9. [PubMed] [Google Scholar]
- 14.Chung WS, Lin CL, Sung FC, et al. Increased risks of deep vein thrombosis and pulmonary embolism in Sjogren syndrome: a nationwide cohort study. J Rheumatol. 2014;41:909–15. doi: 10.3899/jrheum.131345. [DOI] [PubMed] [Google Scholar]
- 15.British Columbia Ministry of Health [creator] Data Extract. MOH; 2015. (2013): Medical Services Plan (MSP) Payment Information File. V2. Population Data BC [publisher] http://www.popdata.bc.ca/data. [Google Scholar]
- 16.British Columbia Ministry of Health [creator] Data Extract. MOH; 2015. (2013): Discharge Abstract Database (Hospital Separations). V2. Population Data BC [publisher] http://www.popdata.bc.ca/data. [Google Scholar]
- 17.British Columbia Ministry of Health [creator] Data Extract. MOH; 2015. (2013): Consolidation File (MSP Registration & Premium Billing). V2. Population Data BC [publisher] http://www.popdata.bc.ca/data. [Google Scholar]
- 18.BC Cancer Agency Registry Data. Data Extract. BC Cancer Agency; 2015. (2013): V2. Population Data BC [publisher] http://www.popdata.bc.ca/data. [Google Scholar]
- 19.BC Vital Statistics Agency [creator] Data Extract. BC Vital Statistics Agency; 2015. (2013): Vital Statistics. V2. Population Data BC [publisher] http://www.popdata.bc.ca/data. [Google Scholar]
- 20.BC Ministry of Health [creator] Data Extract. Data Stewardship Committee; 2015. (2013): PharmaNet. V2. BC Ministry of Health [publisher] http://www.popdata.bc.ca/data. [Google Scholar]
- 21.Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525–31. doi: 10.1001/jama.2011.878. [DOI] [PubMed] [Google Scholar]
- 22.Avina-Zubieta JA, Sayre EC, Lacaille D. The risk of deep vein thrombosis and pulmonary embolism in systemic lupus erythematosus: a population-based cohort study. Arthritis Rheum. 2013;65:S774. [Google Scholar]
- 23.Etminan M, Forooghian F, Maberley D. Inflammatory ocular adverse events with the use of oral bisphosphonates: a retrospective cohort study. CMAJ. 2012;184:E431–4. doi: 10.1503/cmaj.111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aviña-Zubieta JA, Abrahamowicz M, Choi HK, et al. Risk of cerebrovascular disease associated with the use of glucocorticoids in patients with incident rheumatoid arthritis: a population-based study. Ann Rheum Dis. 2011;70:990–5. doi: 10.1136/ard.2010.140210. [DOI] [PubMed] [Google Scholar]
- 25.Bernatsky S, Linehan T, Hanly JG. The accuracy of administrative data diagnoses of systemic autoimmune rheumatic diseases. J Rheumatol. 2011;38:1612–6. doi: 10.3899/jrheum.101149. [DOI] [PubMed] [Google Scholar]
- 26.Huerta C, Johansson S, Wallander M-A, et al. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med. 2007;167:935–43. doi: 10.1001/archinte.167.9.935. [DOI] [PubMed] [Google Scholar]
- 27.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46:1075–9. doi: 10.1016/0895-4356(93)90103-8. discussion1081–90. [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 30.Cox DR. Regression and life tables. J R Stat Soc Series B. 1972;34:187–220. [Google Scholar]
- 31.Bursac Z, Gauss CH, Williams DK, et al. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–56. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303. doi: 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- 34.Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117:93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]
- 35.Statistics Canada. Table 104-0007 - Body mass index (BMI), by age group and sex, household population aged 18 and over excluding pregnant women, Canada and provinces, every 2 years. CANSIM (database) [Google Scholar]
- 36.Statistics Canada. Table 104-0027 - Smoking status, by age group and sex, household population aged 12 and over, Canada and provinces, every 2 years. CANSIM (database) [Google Scholar]
- 37.White RH, Keenan CR. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb Res. 2009;123(Suppl 4):S11–7. doi: 10.1016/S0049-3848(09)70136-7. [DOI] [PubMed] [Google Scholar]