Abstract
The direct asymmetric copper hydride (CuH)-catalyzed coupling of α,β-unsaturated carboxylic acids to aryl alkenes is reported to access chiral α-aryl dialkyl ketones. A variety of substrate substitution patterns, sensitive functional groups and heterocycles are tolerated in this reaction, which significantly expands the range of accessible products compared to existing hydroacylation methodology. Although mechanistic studies are ongoing, we propose that CuH-catalyzed silylation of unsaturated acids occurs to access a uniquely effective acyl electrophilic coupling partner.
Graphical Abstract
Chiral α-aryl ketones represent an important functional group due to their synthetic utility and common occurrence in molecules of broad interest.1 While a number of catalytic methods exist for preparing enantioenriched quaternary α-aryl ketones2, catalytic assembly of acyclic tertiary variants via C–C bond formation is made challenging by the acidic nature of the stereocenter.3,4 This has recently been addressed via transition metal-catalyzed asymmetric coupling reactions of α-bromo ketones to aryl organometallic reagents5a,b, benzylic bromides to acyl chlorides5c, and benzylic zinc reagents to thioesters5d; additionally, chiral Lewis acid-catalyzed insertion of aryldiazoalkanes into aldehyde C–H bonds has also been described.5e Despite this progress, complementary catalytic methods for preparing chiral tertiary α-aryl dialkyl ketones from abundant and stable functional groups remain in high demand.
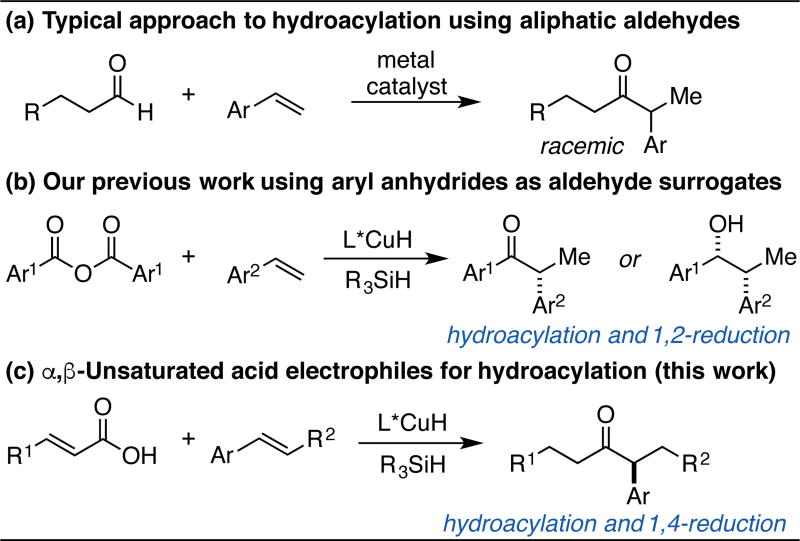
Hydroacylation, typically achieved by the addition of an aldehyde C–H bond across an alkene π-bond, is a streamlined approach for the construction of ketones from readily available functional groups.6 While numerous enantioselective intramolecular hydroacylation processes have been reported, intermolecular variants remain less developed.7 The primary challenge associated with this transformation is suppressing aldehyde decarbonylation, which often occurs readily for substrates without a coordinating substituent.8 Nonetheless, impressive examples of enantioselective intermolecular hydroacylation have been reported using substrates of broad value that feature a coordinating substituent (e.g. salicylaldehydes, 2-(methylthio)benzaldehydes and α-substituted acrylamides).9 Meanwhile, the branch-selective addition of simple aliphatic aldehydes to styrenes provides direct access to dialkyl ketones bearing an α-aryl stereocenter, although only racemic methods for this transformation have been reported (Scheme 1a).10 To avoid the problems associated with aldehyde decarbonylation and stereocenter epimerization, we reasoned that a complementary approach toward hydroacylation could potentially provide rapid access to enantioenriched tertiary α-aryl dialkyl ketones.
Scheme 1.
Previous work in hydroacylation using (a) aldehydes, (b) aryl anhydrides, and (c, this work) α,β-unsaturated carboxylic acids to access chiral α-aryl ketones.
As part of a broader program using chiral copper hydride (CuH) species as catalysts for enantioselective hydrofunctionalization reactions11, we sought to utilize acyl electrophiles as surrogates for aldehydes in order to address limitations of existing hydroacylation methodology. Inspired by prior work by Miura12a and Krische12b, we recently reported a CuH-catalyzed method for coupling styrenes to symmetrical aryl anhydrides to afford chiral α-aryl ketones or, after concomitant 1,2-reduction, chiral alcohols (Scheme 1b).13 In order to expand the synthetic utility of this hydroacylation strategy, we recognized the need to develop an improved method that avoids use of symmetrical anhydrides and, importantly, employs aliphatic aldehyde surrogates. We herein report the use of α,β-unsaturated carboxylic acids as direct coupling partners in a CuH-catalyzed dual hydroacylation and reduction process that provides access to highly enantioenriched tertiary α-aryl dialkyl ketones (Scheme 1c).
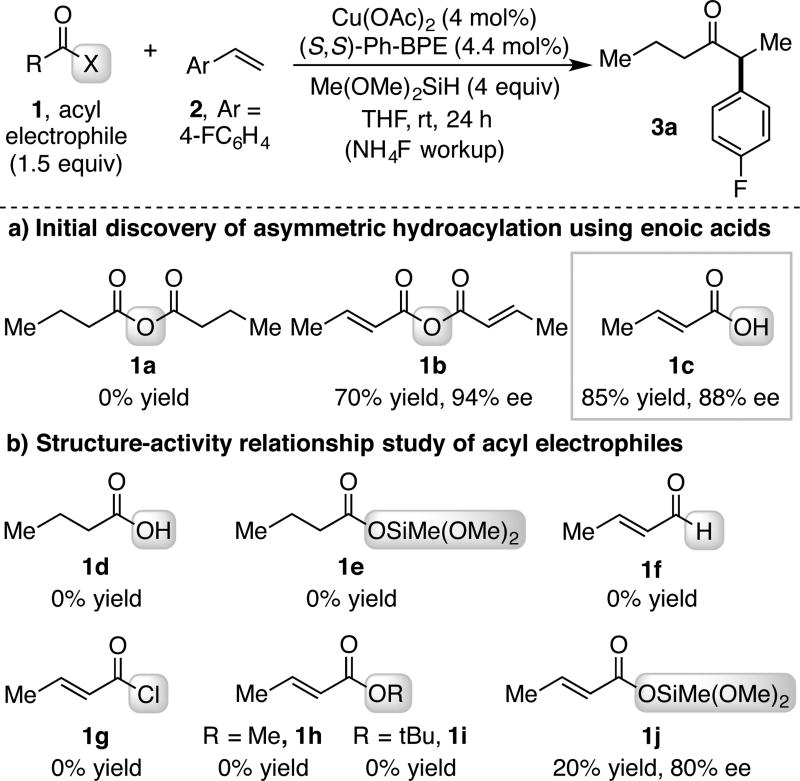
In CuH-catalyzed hydrofunctionalization reactions, chemoselective hydrocupration of an olefin in the presence of an electrophilic coupling partner is required in order to obtain high product yields.14 This requisite is realized when using aryl anhydride coupling partners (Scheme 1b); however, no hydroacylation of 4-fluorostyrene was seen when butyric anhydride, an aliphatic anhydride (1a), was subjected to the previously reported reaction conditions (Scheme 2a). Instead, only direct reduction of butyric anhydride to n-butanol was observed. We hypothesized that reactions of α,β-unsaturated anhydrides may display similar chemoselectivity as with aryl anhydrides to allow for styrenyl hydroacylation, and with concomitant 1,4-reduction of the unsaturated carbonyl, would produce chiral α-aryl dialkyl ketones. This hypothesis was validated when crotonic anhydride (1b) was subjected to the reaction conditions, which provided chiral ketone 3a in 70% yield and 94% ee after treatment with NH4F. In the course of searching for acyl electrophile alternatives to anhydrides, we discovered that α,β-unsaturated carboxylic acids directly engage in this hydroacylation process. Thus, the use of crotonic acid (1c) and 8 equiv of silane provided ketone 3a in 85% yield and 88% ee.
Scheme 2.
(a) Discovery and (b) SAR of CuH-catalyzed tandem hydroacylation and 1,4-reduction with unsaturated acyl electrophiles.a
a yields determined by 1H NMR analysis of crude reaction mixture, (S,S)-Ph-BPE = 1,2-Bis((2S,5S)-2,5-diphenylphospholano)ethane.
This finding prompted a structure-activity relationship (SAR) study of the electrophilic acyl species (Scheme 2b). First, no coupling was observed when butanoic acid (1d) or its silyl ester 1e were used, clearly indicating the requirement for α,β-unsaturation in this process. Crotonaldehyde (1f) also provided no product, which suggests reduction of the acid to an aldehyde does not occur prior to hydroacylation. Additionally, other crotonoyl-based electrophiles, such as crotonoyl chloride (1g) or alkyl crotonates (1h and 1i), provided no hydroacylation product. On the other hand, the use of methyl(dimethoxy)silyl crotonate (1j) led to 20% yield of ketone 3a in 80% ee, suggesting silylated crotonic acid may be an active intermediate in the direct coupling of acids.
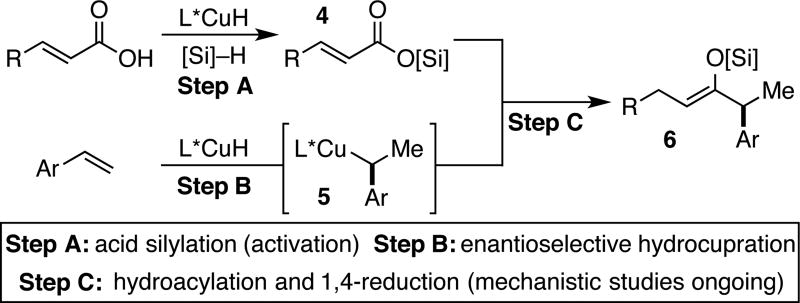
Based on the above observations and previous studies on CuH chemistry, we are currently able to propose the reaction pathway outlined in Scheme 3.15 First, carboxylic acid deprotonation and silylation is catalyzed by CuH in the presence of a hydrosilane, generating activated acyl electrophile 4 (Step A).16 Additionally, enantioselective hydrocupration of a styrene generates a chiral copper(I) benzylic intermediate (5, Step B), which represents the active nucleophilic partner. The reaction ultimately produces silyl enol ether 6, which is observed in high yield as judged by 1H NMR spectroscopy of the crude reaction mixture and allows access to chiral ketone 3 upon treatment with NH4F.17 At this time, the mechanism by which chiral benzyl copper 5 and the silylated acid 4 couple (Step C), and if other reaction intermediates are involved, is unknown and is the subject of ongoing investigations.18,19
Scheme 3.
Currently proposed pathway for hydroacylation.
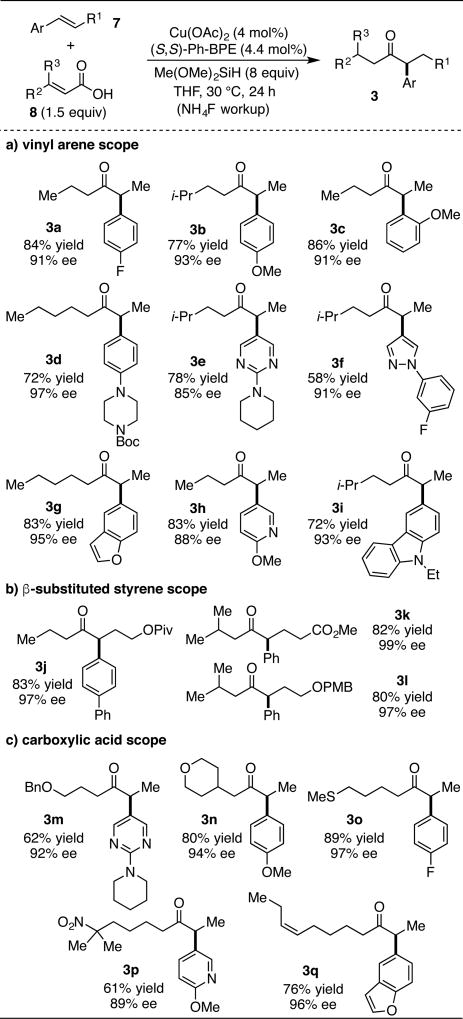
The current scope of accessible chiral α-aryl ketones through the CuH-catalyzed coupling of α,β-unsaturated acids to aryl alkenes is shown in Table 1.20a First, the vinyl arene scope was explored using simple unsaturated carboxylic acid coupling partners (Table 1a). Electron deficient and electron rich, as well as ortho-substituted styrenes, provide chiral ketones in excellent yield and enantioselectivity (3a–c). A variety of alkenes containing coordinating atoms or consisting of heterocycles, such as a piperizine, pyrimidine, pyrazole, pyridine, benzofuran and carbazole, engage in this hydroacylation process delivering ketones in high enantiopurity (3d–i). In contrast to our previous hydroacylation method utilizing aryl anhydride reagents, we found that β-substituted styrenes couple to unsaturated acids in both high yield and enantioselectivity (Table 1b).20b β-substituted styrenes containing functional groups that could potentially be reduced by reactive CuH intermediates, such as a t-butyl ester (3j) and methyl ester (3k), are well tolerated, as is a PMB-protected alcohol (3l). Carboxylic acid substrates containing ethers (3m and 3n), a thioether (3o) and potentially-reducible functional groups, such as a nitro group (3p) and a cis-alkene (3q), couple to styrenes in excellent yield and stereoselectivity (Table 1c).
Table 1.
Ketone substrate scope for the direct coupling of aryl alkenes to α,β-unsaturated carboxylic acids.a
All yields represent average isolated yields of two runs, performed with 1 mmol of alkene.
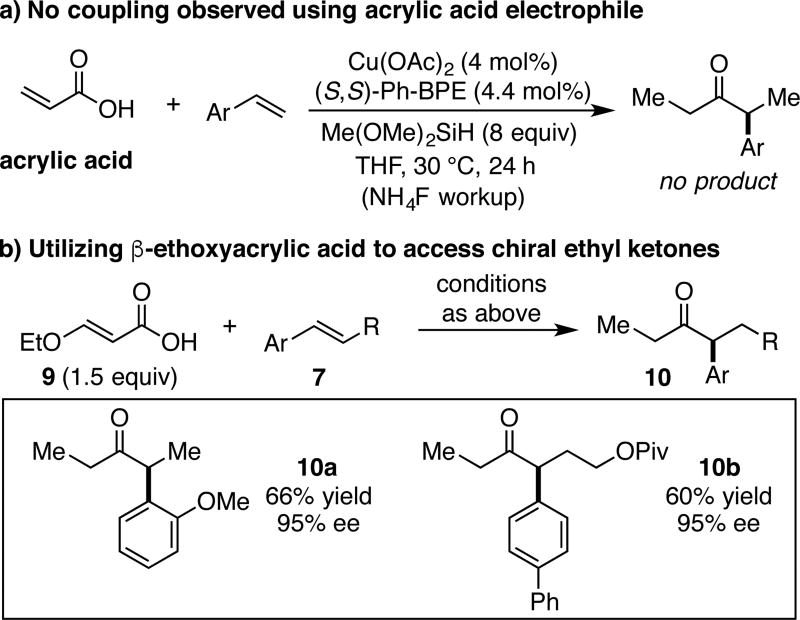
The substrates shown in Table 1 demonstrate that β-alkyl- and symmetrical β,β-dialkyl-substituted unsaturated carboxylic acids are competent hydroacylation coupling partners for a variety of styrene types.20c α-Alkyl substituted unsaturated acids, such as tiglic or angelic acid, do not engage in hydroacylation under the current reaction conditions. Similarly, acrylic acid, an unsubstituted unsaturated carboxylic acid that would provide access to ethyl ketones, does not participate in this transformation (Scheme 4a). To address this limitation, we found that β-ethoxyacrylic acid (9) acts as an acrylic acid surrogate to deliver chiral ethyl ketones in good yield and enantiopurity (Scheme 4b). This approach, which we propose proceeds via a 1,4-reduction/β-ethoxy elimination pathway, is general as shown for ortho-substituted substrate 10a and β-substituted styrene-derived ketone 10b.
Scheme 4.
The use of (a) acrylic acid and (b) β-ethoxyacrylic acid to access chiral ethyl ketones.a
a Yields represent average isolated yields of two runs, performed with 1 mmol of alkene.
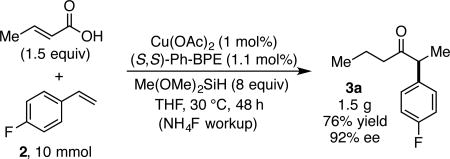
To demonstrate the scalability of this methodology, a 10 mmol scale reaction using just 1 mol% catalyst loading is shown in Eq 1 to provide 1.5 g of chiral ketone 3a with 92% ee. Given the ready accessibility of α,β-unsaturated acids and aryl alkenes, this method represents a highly practical approach to chiral α-aryl dialkyl ketones containing a range of functional groups and substitution patterns.21 Furthermore, by utilizing in situ activated acyl electrophiles as surrogates for aldehydes, this work addresses current substrate limitations of existing hydroacylation methodogy. The mechanism of this unprecedented coupling reaction is currently under investigation, as are additional CuH-catalyzed transformations involving unsaturated carboxylic acid substrates.
 |
(1) |
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institutes of Health (GM46059). We also thank the NIH for a supplemental grant for the purchase of supercritical fluid chromatography equipment (GM058160-17S1) and a postdoctoral fellowship for J. S. B. (GM112197). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Dr. Michael Pirnot for advice on this manuscript.
Footnotes
ASSOCIATED CONTENT
Supporting Information. The Supporting Information is available free of charge on the ACS Publications website. Experimental procedures and characterization data for all compounds (PDF).
The authors declare no competing financial interest.
References
- 1.Corey EJ, Kürti L. Enantioselective Chemical Synthesis: Methods, Logic and Practice. Direct Book Publishing; Dallas: 2010. [Google Scholar]
- 2.For general approaches toward quaternary α-aryl ketones, see reference 1. For reviews on and selected examples of catalytic asymmetric synthesis of quaternary α-aryl ketones: Bellina F, Rossi R. Chem. Rev. 2010;110:1082. doi: 10.1021/cr9000836.Johansson CCC, Colacot TJ. Angew. Chem., Int. Ed. 2010;49:676. doi: 10.1002/anie.200903424.Bella M, Gasperi T. Synthesis. 2009;10:1583.Hamada T, Chieffi A, Åhman J, Buchwald SL. J. Am. Chem. Soc. 2002;124:1261. doi: 10.1021/ja011122+.Lia X, Weng Z, Hartwig JF. J. Am. Chem. Soc. 2008;130:195. doi: 10.1021/ja074453g.Trost BM, Schroeder GM, Kristensen J. Angew. Chem., Int. Ed. 2002;41:3492. doi: 10.1002/1521-3773(20020916)41:18<3492::AID-ANIE3492>3.0.CO;2-P.Kano T, Hayashi Y, Maruoka K. J. Am. Chem. Soc. 2013;135:7134. doi: 10.1021/ja403340r.Xie X, Chen Y, Ma D. J. Am. Chem. Soc. 2006;128:16050. doi: 10.1021/ja066991j.
- 3.Bordwell FG, Harrelson JA., Jr Can. J. Chem. 1990;68:1714. [Google Scholar]
- 4.For reviews and selected examples of asymmetric protonation reactions to access chiral α-aryl ketones, see: Oudeyer S, Briere J-F, Levacher V. Eur. J. Org. Chem. 2014:6103.Mohr JT, Hong AY, Stoltz BM. Nat. Chem. 2009;1:359. doi: 10.1038/nchem.297.Cheon CH, Kanno O, Toste FD. J. Am. Chem. Soc. 2011;133:13248. doi: 10.1021/ja204331w.Cheon CH, Yamamoto H. J. Am. Chem. Soc. 2008;130:9246. doi: 10.1021/ja8041542.Yanagisawa A, Touge T, Arai T. Angew. Chem., Int. Ed. 2005;44:1546. doi: 10.1002/anie.200462325.
- 5.(a) Lundin PM, Esquivias J, Fu GC. Angew. Chem., Int. Ed. 2009;48:154. doi: 10.1002/anie.200804888. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lou S, Fu GC. J. Am. Chem. Soc. 2010;132:1264. doi: 10.1021/ja909689t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Cherney AH, Kadunce NT, Reisman SE. J. Am. Chem. Soc. 2013;135:7442. doi: 10.1021/ja402922w. [DOI] [PubMed] [Google Scholar]; (d) Oost R, Misale A, Maulide N. Angew. Chem., Int. Ed. 2016;55:4587. doi: 10.1002/anie.201600597. [DOI] [PubMed] [Google Scholar]; (e) Kang BC, Nam DG, Hwang G-S, Ryu DH. Org. Lett. 2015;17:4810. doi: 10.1021/acs.orglett.5b02370. [DOI] [PubMed] [Google Scholar]
- 6.For selected reviews on transition-metal catalyzed hydroacylation, see: Ghosh A, Johnson KF, Vickerman KL, Walker JA, Jr, Stanley LM. Org. Chem. Front. 2016;3:639.Yang L, Huang H. Chem. Rev. 2015;115:3468. doi: 10.1021/cr500610p.Willis MC. Chem. Rev. 2010;110:725. doi: 10.1021/cr900096x.Park YJ, Park J-W, Jun C-H. Acc. Chem. Res. 2008;41:222. doi: 10.1021/ar700133y.
- 7.Selected examples of enantioselective intramolecular alkene hydroacylation: Barnhart RW, Wang X, Noheda P, Bergens SH, Whelan J, Bosnich B. J. Am. Chem. Soc. 1994;116:1821.Kundu K, McCullagh JV, Morehead AT., Jr J. Am. Chem. Soc. 2005;127:16042. doi: 10.1021/ja0564416.Coulter MM, Dornan PK, Dong VM. J. Am. Chem. Soc. 2009;131:6932. doi: 10.1021/ja901915u.Janssen-Müller D, Schedler M, Fleige M, Daniliuc CG, Glorius F. Angew. Chem., Int. Ed. 2015;54:12492. doi: 10.1002/anie.201412302.Yang J, Yoshikai N. J. Am. Chem. Soc. 2014;136:16748. doi: 10.1021/ja509919x.Yang G, Rérat A, Lim YJ, Gosmini C, Yoshikai N. Angew. Chem., Int. Ed. 2017;56:2449. doi: 10.1002/anie.201611518.Du X-W, Ghosh A, Stanley LM. Org. Lett. 2014;16:4036. doi: 10.1021/ol501869s.Rastelli EJ, Truong NT, Coltart DM. Org. Lett. 2016;18:5588. doi: 10.1021/acs.orglett.6b02825.Hoffman TJ, Carreira EM. Angew. Chem., Int. Ed. 2011;50:10670. doi: 10.1002/anie.201104595.
- 8.Leung JC, Krische MJ. Chem. Sci. 2012;3:2202. [Google Scholar]
- 9.Phan DHT, Kou KGM, Dong VM. J. Am. Chem. Soc. 2010;132:16254.Coulter MM, Fou KGM, Galligan B, Dong VM. J. Am. Chem. Soc. 2010;132:16330. doi: 10.1021/ja107198e.Osborne JD, Randell-Sly HE, Currie GS, Cowley AR, Willis MC. J. Am. Chem. Soc. 2008;130:17232. doi: 10.1021/ja8069133.Shibata Y, Tanaka K. J. Am. Chem. Soc. 2009;131:12552. doi: 10.1021/ja905908z.(e) For a review on additional examples of Rh-catalyzed asymmetric hydroacylation: Murphy SK, Dong VM. Chem. Commun. 2014;50:13645. doi: 10.1039/c4cc02276a.Liu F, Bugaut X, Schedler M, Frohlich R, Glorius F. Angew. Chem., Int. Ed. 2011;50:12626. doi: 10.1002/anie.201106155.
- 10.(a) Murphy SK, Bruch A, Dong VM. Angew. Chem., Int. Ed. 2014;53:2455. doi: 10.1002/anie.201309987. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Murphy SK, Bruch A, Dong VM. Chem. Sci. 2015;6:174. doi: 10.1039/c4sc02026j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Xiao L-J, Fu X-N, Zhou M-J, Xie J-H, Wang L-X, Xu X-F, Zhou Q-L. J. Am. Chem. Soc. 2016;138:2957. doi: 10.1021/jacs.6b00024. [DOI] [PubMed] [Google Scholar]; (d) Kim J, Yi CS. ACS Catal. 2016;6:3336. doi: 10.1021/acscatal.6b00856. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Schedler M, Wang D-S, Glorius F. Angew. Chem., Int. Ed. 2013;52:2585. doi: 10.1002/anie.201209291. [DOI] [PubMed] [Google Scholar]
- 11.For reviews and selected examples, see: Pirnot MT, Wang Y-M, Buchwald SL. Angew. Chem., Int. Ed. 2016;55:48. doi: 10.1002/anie.201507594.Mohr J, Oestreich M. Angew. Chem., Int. Ed. 2016;55:12148. doi: 10.1002/anie.201606701.Nguyen KD, Park BY, Luong T, Sato H, Garza VJ, Krische MJ. Science. 2016;354:300. doi: 10.1126/science.aah5133.Gribble MW, Jr, Pirnot MT, Bandar JS, Liu RY, Buchwald SL. J. Am. Chem. Soc. 2017;139:2192. doi: 10.1021/jacs.6b13029.Friis SD, Pirnot MT, Buchwald SL. J. Am. Chem. Soc. 2016;138:8372. doi: 10.1021/jacs.6b04566.Yang Y, Shi S-L, Niu D, Liu P, Buchwald SL. Science. 2015;349:62. doi: 10.1126/science.aab3753.
- 12.(a) Kokubo K, Miura M, Nomura M. Organometallics. 1995;14:4521. [Google Scholar]; (b) Hong Y-T, Barchuk A, Krische MJ. Angew. Chem., Int. Ed. 2006;45:6885. doi: 10.1002/anie.200602377. [DOI] [PubMed] [Google Scholar]
- 13.Bandar JS, Ascic E, Buchwald SL. J. Am. Chem. Soc. 2016;138:5821. doi: 10.1021/jacs.6b03086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.For selected discussions on the chemoselectivity required in other CuH-catalyzed hydrofunctionalization reactions see: Niu D, Buchwald SL. J. Am. Chem. Soc. 2015;137:9716. doi: 10.1021/jacs.5b05446.Yang Y, Perry IB, Lu G, Liu P, Buchwald SL. Science. 2016;353:144. doi: 10.1126/science.aaf7720.Yang Y, Perry IB, Buchwald SL. J. Am. Chem. Soc. 2016;138:9787. doi: 10.1021/jacs.6b06299.
- 15.(a) Deutsch C, Krause N, Lipshutz BH. Chem. Rev. 2008;108:2916. doi: 10.1021/cr0684321. [DOI] [PubMed] [Google Scholar]; (b) Bandar JS, Pirnot MT, Buchwald SL. J. Am. Chem. Soc. 2015;137:14812. doi: 10.1021/jacs.5b10219. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Issenhuth J-T, Notter F-P, Dagorne S, Dedieu A, Bellemin-Laponnaz S. Eur. J. Inorg. Chem. 2010;2010:529. [Google Scholar]; (d) Tobisch S. Chem. Eur. J. 2016;22:8290. doi: 10.1002/chem.201600230. [DOI] [PubMed] [Google Scholar]; (e) Dang L, Zhao H, Lin Z, Marder TB. Organometallics. 2007;26:2824. [Google Scholar]
- 16.Hydrogen evolution (bubbling) is observed when a solution of LCuH is added to a reaction vial containing unsaturated carboxylic acid and alkene (see Supporting Information).
- 17.Upon prolonged reaction time or at higher reaction temperatures, no further reduction of 6 is observed.
- 18.It is possible that reduction of an α,β-unsaturated carbonyl intermediate occurs either before or after hydroacylation occurs.
- 19.When the commercially available Ph2SiD2 was used as the silane, 1H NMR analysis of semi-purified product showed deuterium incorporated into the ketone product at positions derived from styrene hydrocupration and 1,4-reduction of crotonic acid, indicating the dual role of the CuH catalyst. We thank a reviewer for suggesting this experiment.
- 20.(a) We found that for 1 mmol scale reactions heating the reaction solution to 30 °C is optimal for achieving high yield and selectivity. (b) β,β-Disubstituted styrenes do not engage in hydroacylation under the current reaction conditions. (c) β-Aryl unsaturated acids (e.g. cinnamic acid) do not engage in hydroacylation under the current reaction conditions.
- 21.(a) Hayama N, Azuma T, Kobayashi Y, Takemoto Y. Chem. Pharm. Bull. 2016;64:704. doi: 10.1248/cpb.c15-00983. [DOI] [PubMed] [Google Scholar]; (b) Gooßen LJ, Rodríguez N, Gooßen K. Angew. Chem., Int. Ed. 2008;47:3100. doi: 10.1002/anie.200704782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.