Abstract
The emergence of highly pathogenic avian influenza H5N1 viruses has heightened global concern about the threat posed by pandemic influenza. To address the need for a highly effective universal influenza vaccine, we developed a novel M2-deficient single replication (M2SR) influenza vaccine virus and previously reported that it provided strong heterosubtypic protection against seasonal influenza viruses in mice. In the current study, we assessed M2SR induced protection against H5N1 influenza in mice and ferrets.
Mice were intranasally inoculated with M2SR viruses containing the HA and NA from A/Vietnam/1203/2004 (M2SR H5N1) or A/California/07/2009 (M2SR H1N1). All M2SR vaccinated mice survived lethal challenge with influenza A/Vietnam/1203/2004 (H5N1), whereas 40% of mice vaccinated with recombinant H5 HA and none of the naïve controls survived. M2SR H5N1 provided sterile immunity, whereas low levels of virus were detected in the lungs of some M2SR H1N1 vaccinated mice. In contrast, recombinant H5 HA vaccinated mice and naïve controls showed systemic infection.
M2SR H5N1 induced strong serum and mucosal antibody responses (IgG and IgA classes) against H5 HA, with high hemagglutination inhibition (HAI) titers. In contrast, while M2SR H1N1 elicited cross-reactive antibodies recognizing the H5 HA2 stalk region or the neuraminidase, no HAI activity against H5N1 virus was detected after M2SR H1N1 immunization.
Both M2SR H5N1 and H1N1 also protected ferrets against lethal challenge with A/Vietnam/1203/2004. A prime-boost regimen provided optimal protection with no virus detected in the respiratory tract or brain after challenge. As in the mouse model, only the M2SR H5N1 vaccine induced HAI antibodies against the challenge virus in ferrets, while the M2SR H1N1 was able to provide protection without the induction of HAI antibodies.
In summary, effective protection against highly pathogenic H5N1 influenza virus was provided by both homologous H5N1 M2SR and heterologous H1N1 M2SR demonstrating the cross-protective attributes of the M2SR platform.
Keywords: Universal influenza vaccine, M2-deficient, single replication, sterilizing immunity, heterosubtypic immunity, highly pathogenic H5N1 avian influenza, hemagglutination inhibition, HA2 stalk region, neuraminidase, long-lasting protection
INTRODUCTION
Highly pathogenic avian influenza A (H5N1) viruses are highly contagious in wild birds and poultry and cause high mortality in poultry flocks [1]. Human infections with these viruses are also associated with severe illness and high mortality [2–4]. Currently these viruses are not transmissible among humans via respiratory droplets and infection normally only occurs after close contact with infected birds, although rare human-to-human spread has been documented [5]. However, research in animal models, mathematical modeling and surveillance data suggests that as few as three to five amino acid substitutions in an A/H5N1 virus could enable the virus to be transmissible between mammals by respiratory droplets and hence have a high pandemic potential [6–8]. Effective vaccines are needed to counter this serious threat.
We previously described a novel M2-deficient single replication influenza vaccine virus (M2SR), which was able to provide strong cross-protective immunity against seasonal influenza A strains [9]. M2SR is able to infect cells, undergo a single round of replication and express all viral proteins except M2. The virus is produced in cell culture, using M2-complementing cells. This avoids many of the possible problems associated with influenza vaccine production in eggs, which, in the case of an avian influenza pandemic, might include a shortage of eggs due to decimation of poultry flocks. The vaccine induced strong systemic and mucosal antibody and virus-specific T cell responses. It also provided effective protection against both homologous and heterologous seasonal influenza A strains. In the current study, we investigated whether M2SR (homologous or heterologous) vaccines could also provide protection against H5N1 highly pathogenic avian influenza viruses.
MATERIALS AND METHODS
Cells and viruses
293T HEK (ATCC CRL-3261), MDCK (ATCC CCL-34 or Sigma) and M2CK (MDCK cells that stably express the influenza A M2 protein [10]) cells were maintained in DMEM (Thermo Fisher Scientific, Gibco, Waltham, MA, USA) for 293T and in MEM (Thermo Fisher Scientific, Gibco, Waltham, MA, USA) for MDCK and M2CK supplemented with 10% FCS (Omega Scientific, Tarzana, CA, USA). Cells were maintained at 37°C in 5% CO2
M2SR virus (an M2-deficient recombinant influenza virus) was generated using a plasmid rescue system described previously [9, 11]. The six internal genes of M2SR are derived from A/Puerto Rico/8/1934 (PR8, H1N1) [9]. M2SR H5N1 is an M2SR virus expressing the avirulent HA and NA of influenza A/Vietnam/1203/2004 (H5N1). The multibasic amino acids at the cleavage site of the HA of M2SR H5N1 were removed; providing an additional safety factor to the M2 deletion in M2SR. M2SR H1N1 is a M2SR virus expressing the HA and NA from influenza A/California/07/2009 (H1N1). M2SR viruses were prepared and titrated in M2CK cells.
Influenza A/Vietnam/1203/2004 (H5N1) (VN1203) (kindly provided by Richard Webby, St. Jude Children’s Research Hospital, Memphis, TN) was grown in embryonated eggs. Influenza A/California/07/2009 NYMC X-179A (H1N1) virus was obtained from Influenza Reagent Resource.
All experiments with wild-type VN1203 were conducted in enhanced BSL3 laboratories at the University of Wisconsin-Madison and the IIT Research Institute.
Animals
Seven to 8 week-old female BALB/c mice (Envigo, Madison, WI) and 5 month-old male ferrets (Triple F Farms, Inc., Sayre, PA, USA) were used. Ferret serum samples were tested to ensure that animals had not been exposed to influenza, prior to initiating the study. All animal study protocols were approved by the FluGen, University of Wisconsin-Madison, or IIT Research Institute Institutional Animal Care and Use Committees and all experiments were performed in accordance with the National Institute of Health guidelines for the care and use of laboratory animals.
Mouse infection and sampling
Groups of 11 BALB/c mice were immunized intranasally with either a single dose of 106 TCID50, two doses 28 days apart of 106 TCID50 M2SR H5N1 or two doses 28 days apart of 106 TCID50 M2SR H1N1. Additional groups were immunized with 2 doses of 1.5 ug/mouse H5 HA protein (Immune-Technology Corp., New York, NY, USA) intramuscularly or 2 doses intranasally of medium alone, as mock vaccination. Six weeks after boosting, serum and BAL were collected from three mice per group and anti-H5 HA IgG and IgA binding antibody titers and anti-H5N1 HA inhibition titers were determined by ELISA and by HAI assay, respectively, as previously described [9]. Twenty weeks after boosting, mice were challenged by lethal dose (20 MLD50) of VN1203 and body weight and survival were monitored for 16 days. On day 4 post-challenge, organs were collected from 3 mice per group and virus titers were determined by TCID50 assay in MDCK cells as previously described [12].
Ferret infection and sampling
Groups of 12 ferrets were inoculated intranasally with either a single dose of 108 TCID50 of M2SR (either H5N1 or H1N1) or 2 doses of 107 TCID50 M2SR (either H5N1 or H1N1) 28 days apart. Control groups of ferrets were mock-immunized with PBS intranasally. All ferrets were challenged with a 106 PFU of wild-type VN1203 virus on day 56 after priming or on day 28 after boosting. Ferret body weight, body temperature and clinical symptoms were monitored for 14 days after challenge. Nasal washes were collected from ferrets (9 per group) on days 1, 3, 5 and 7 after challenge. On Day 4 post-challenge, ferrets (3 animals per group) were euthanized and organs were collected for virus titer determination. Virus titers in nasal washes or organ homogenates were determined by TCID50 assay in MDCK cells as previously described [12].
Western Blotting
Recombinant HA1 (comprised of amino acids 1–345) and HA2 (comprised of amino acids 366–531) proteins of A/Vietnam/1203/2004 (H5N1) (Immune Technology Corp., New York, N.Y.) were separated by polyacrylamide gel electrophoresis, blotted on PVDF membrane and probed with sera from immunized or control mice, followed by HRP-conjugated anti-mouse IgG (Kirkegaard & Perr Laboratories Inc., Gaithersburg, MD). Bands were visualized using TMB membrane peroxidase substrate (KPL, Gaithersburg, MD).
RESULTS
M2SR vaccines elicit both humoral and mucosal antibody responses in mice
Mice were intranasally inoculated with one (prime only) or two doses (prime-boost) of M2SR H5N1, two doses of M2SR H1N1, two doses of recombinant H5 HA protein from VN1203, intramuscularly, or media control administered intranasally. Anti-H5 HA IgG and IgA were detected in both serum and BAL of all M2SR H5N1-vaccinated mice (Figure 1A), while M2SR H1N1 and recombinant H5 HA induced lower levels of antibody. The functional activity of these antibodies was determined using an HAI assay. M2SR H5N1 vaccination induced high H5 HAI titers, whereas recombinant H5 HA and M2SR H1N1 did not elicit HAI activity against H5 HA (Figure 1B). These results demonstrate that M2SR vaccines elicit both systemic and local mucosal immunity; including HAI antibodies, a surrogate for neutralizing antibodies.
Figure 1. M2SR vaccines elicits strong humoral and mucosal antibody responses.
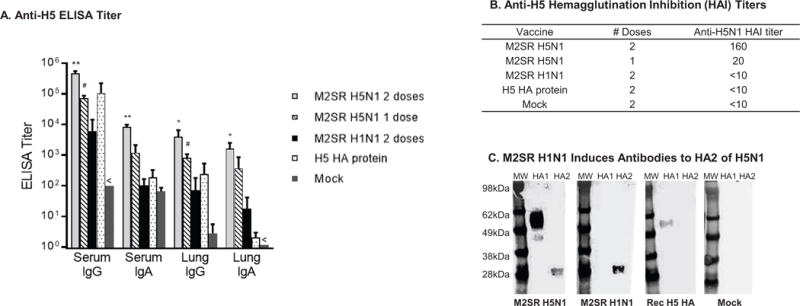
A: BALB/c mice were immunized as described in Materials and Methods and serum and trachea-lung washes were collected from 3 mice per group, 6 weeks after the last immunization. Anti-H5 HA immunoglobulin G (IgG) and IgA levels were measured by ELISA. Endpoint antibody titers (mean ± SD) are given as the reciprocal of the dilution determined by the least squares method giving an OD reading of 0.3 at 450 nm. Detection limits were 102 for serum IgG, 101 for serum IgA, and 100 for Lung IgG and IgA. The serum IgG and lung IgA titers for the mock group were below limit of detection (indicated by <). Asterisks indicate titers that are significantly different from those of control mice (Student’s t test): ** p<0.001; # p<0.01; * p<0.05. B: Serum samples were collected from immunized mice 3 weeks after the last dose. HAI titers against M2SR-H5N1 virus were determined for pooled RDE-treated sera per group. The detection limit was 10. C: Recombinant HA1 (amino acids 1–345) and HA2 (amino acids. 366–531) proteins of A/Vietnam/1203/2004 (H5N1) (Immune Technology Corp.) were blotted onto a PVDF membrane along with molecular weight markers (MW). Identical membranes were probed with sera from mice immunized with M2SR H5N1 2 doses, M2SR H1N1 2 doses, recombinant H5 HA protein 2 doses, or vehicle alone (mock) and detected using HRP-labeled anti-mouse IgG.
H1N1 M2SR elicits cross-reactive antibodies in mice that recognize the H5 HA2
Recently, antibodies to the highly conserved stalk region, HA2, of the HA have been demonstrated to have broad neutralizing ability and, therefore, ‘universal’ properties [13–17]. To determine whether M2SR induces antibodies to HA2, sera was collected 3 weeks after boosting from the mice immunized above, and were analyzed by Western analysis for reactivity against H5N1 HA1 or HA2. Recombinant H5 HA1 (head region) and HA2 (stalk region) were separated by gel electrophoresis and analyzed by Western blot for reactivity with sera from M2SR H5N1, M2SR H1N1, recombinant H5 HA or mock-immunized mice. As expected, the sera from mice vaccinated with the homologous M2SR H5N1 recognized both proteins, whereas sera from mice vaccinated with M2SR H1N1 did not recognize the H5 HA1 but did bind to the H5 HA2 protein (Figure 1C). In contrast, sera from recombinant H5 HA vaccinated mice did not react with HA2 supporting previous observations that inactivated or subunit vaccines are not strong inducers of stalk antibodies [18].
M2SR elicits cross-reactive neuraminidase antibody responses
Sera collected six weeks after the last immunization were analyzed for anti-N1 neuraminidase antibodies by ELISA. M2SR H1N1 viruses and M2SR H5N1 viruses elicited similar levels of IgG antibodies against the influenza N1 neuraminidase, whereas control mice showed no antibody to N1 (Supplementary Figure 1).
M2SR vaccines protect mice against lethal A/Vietnam/1203/2004 (H5N1) challenge
The protective efficacy of M2SR vaccine was evaluated by challenging the immunized mice with a lethal dose (20MLD50) of VN/1203 (H5N1) at 20 weeks post-vaccination. Mice were observed for 16 days and weighed daily. None of the mice immunized with M2SR H5N1 (one or two doses) lost any weight or displayed any clinical symptoms (Figure 2A). The mice vaccinated with M2SR H1N1 lost on average <5% body weight and did not exhibit any clinical symptoms. (Figure 2A). The group of mice vaccinated with recombinant H5 HA protein lost on average ~15% body weight, appeared extremely weakened and two mice exhibited neurological symptoms (Figure 2A and data not shown), while the control mock group lost more weight and were extremely weakened, resulting in euthanasia. All M2SR immunized mice, including single dose homologous and two dose heterologous, survived challenge (Figure 2B); thus vaccination with M2SR H1N1 provided complete protection against lethal H5N1 virus challenge. In contrast, all control mock-immunized mice, and more than half of the group immunized with recombinant H5 HA, succumbed to challenge.
Figure 2. M2SR vaccines protect mice against lethal A/Vietnam/1203/2004 (H5N1) challenge.
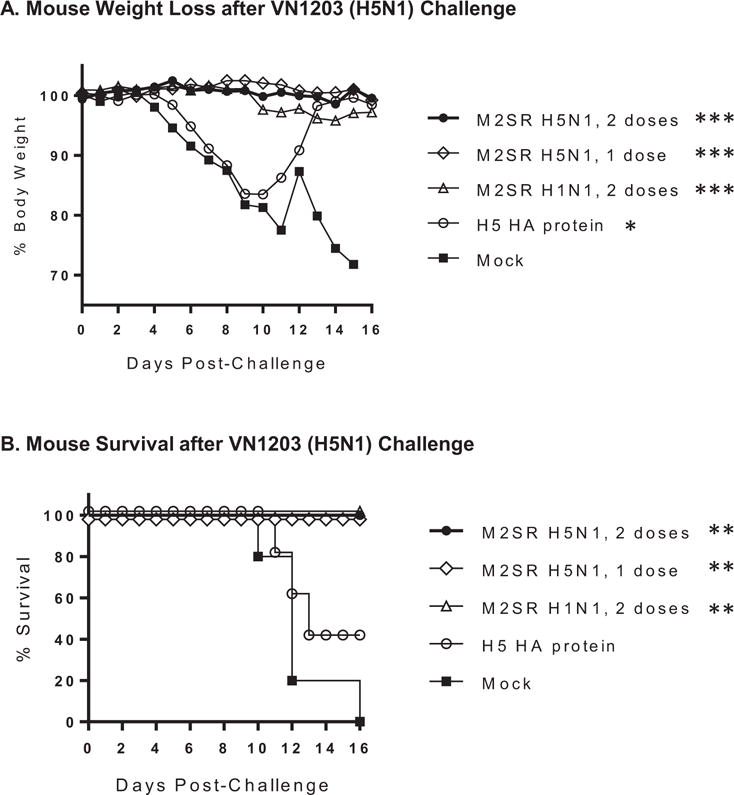
Mice were immunized as described in Materials and Methods. Twenty weeks after the final vaccination, animals were intranasally challenged with a lethal dose of influenza A/Vietnam/1203/2004 (H5N1) and weight loss (A) and survival (B) were monitored for 16 days. Asterisks indicate results that are significantly different from those of the mock control group: *** p<0.001; ** p<0.01; * p<0.05. Weight loss was analyzed using a paired t test and survival curves were compared using the Mantel-Cox Log-rank test.
In addition, M2SR H5N1 provided sterile immunity as demonstrated by the lack of virus recovered from mouse organs harvested on day 4 (Table 1) after challenge. Mice vaccinated with either one or two doses of M2SR H5N1 did not have any detectable virus in any organ, in contrast to the mock-immunized control mice, which showed extensive systemic spread of the virus. There was virus in the lungs of two mice vaccinated with the M2SR H1N1 (heterologous HA) vaccine while one mouse did not have any detectable virus in any organ. In contrast, in the comparator vaccine group, vaccination with recombinant H5 HA allowed for systemic infection, although less extensive than in the control group.
Table 1.
Virus titers in mouse organs after influenza A/Vietnam/1203/2004 challenge.
| Virus titersa in
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lungs | NT | Brain | Heart | Spleen | Liver | Kidney | Pancreas | Colon | |
| M2SR H5N1, 2 doses, IN | —b | — | — | — | — | — | — | — | — |
| M2SR H5N1, 1 dose, IN | — | — | — | — | — | — | — | — | — |
| M2SR H1N1, 2 doses, IN | 4.6, 5.1 | — | — | — | — | — | — | — | — |
| Recombinant H5 HA protein, 2 doses, IM | 5.9 ± 1.1 | — | — | 5.0, 3.9 | 3.7 | — | — | — | — |
| Mock, 2 doses, IN | 6.3 ± 0.5 | 4.5 | 2.6 | 4.8, 4.8 | 4.4 ± 1.1 | 2.4 | 2.9, 3.6 | — | — |
Organs were collected from 3 mice per group on day 4 post-challenge. Organs were homogenized in 0.5mL (lungs, nasal turbinates, heart, spleen, liver, kidneys, pancreas, and colon) or in 1mL (brain) of 0.3% BSA-MEM, and titrated in MDCK cells. Titers are shown in log PFU/g, average ± SD or individual organ titers if not all mice had titers.
—: Below detection limit, 1 PFU/100μL
M2SR vaccines protect ferrets against A/Vietnam/1203/2004 (H5N1) challenge
Ferrets were inoculated intranasally with either M2SR H5N1 or M2SR H1N1 vaccine in a prime-boost regimen (107 TCID50 dose) or a single dose of 108 TCID50. Control groups were mock-immunized with PBS alone. None of the animals exhibited any adverse clinical effects during the immunization period. On day 56, the ferrets were challenged with a lethal dose (106 PFU) of VN1203 and evaluated for body weight loss, clinical symptoms and fever for 14 days. Nasal washes were collected on days 1, 3, 5 and 7 post challenge from nine ferrets in each group for viral titer determination. Four days post challenge (Day 60), lungs, trachea, nasal turbinates, brain and olfactory bulb were collected for viral titers from three ferrets per group.
Weight loss after challenge was significantly reduced for all M2SR vaccinated groups compared to the control group (Figure 3A). Furthermore, clinical illness scores showed that the mock control group displayed the greatest morbidity while the M2SR H1N1 vaccinated groups had minimal and the H5N1 vaccinated groups had no illness scores (Supplementary Figure 2). All mock control ferrets exhibited inappetance, diarrhea, and lethargy after challenge (Supplementary Table 1). In contrast, the group of ferrets vaccinated with M2SR H5N1 (one or two doses) had fewer animals exhibiting inappetence, diarrhea or lethargy than the mock control group. Ferrets vaccinated with a single dose of M2SR H5N1 showed more clinical symptoms than the prime-boost regimen. Animals vaccinated with M2SR H1N1 showed more clinical symptoms than those vaccinated with M2SR H5N1 for both prime and prime-boost regimens, although all vaccine regimens were effective in preventing lethality (Figure 3B).
Figure 3. M2SR vaccines protect ferrets against A/Vietnam/1203/2004 (H5N1) challenge.
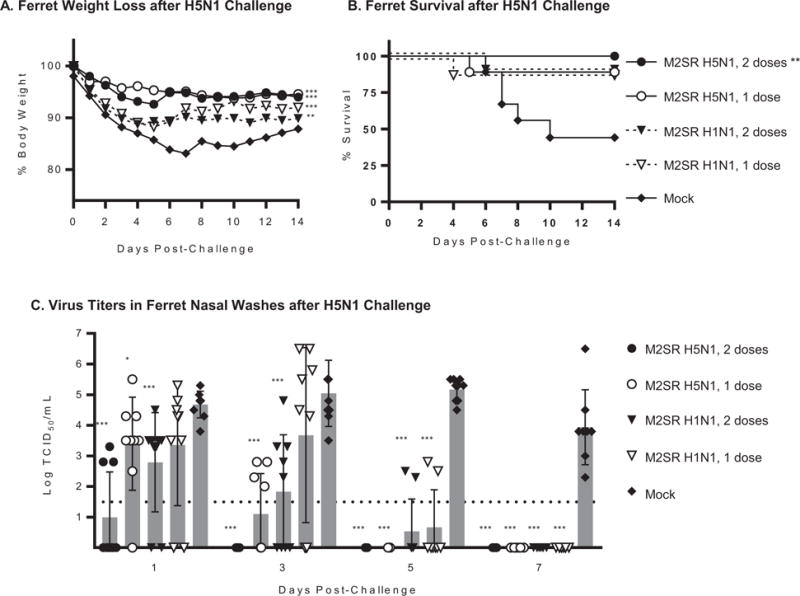
Ferrets were vaccinated as described in Materials and Methods and challenged with A/Vietnam/1203/2004 (H5N1) 4 weeks after the last immunization. A: Body weight changes for 14 days after challenge. B: Ferret survival for 14 days after challenge. C: Nasal washes were collected from 9 ferrets per group on days 1, 3, 5, and 7 post-challenge. Virus loads in the samples were determination by 50% tissue culture infection (TCID50) method in MDCK cells. Virus titers per mL in nasal washes from individual ferrets are plotted. Shadow bars are mean titers for group at indicated time-point and error bars are SD. The detection limit of the assay (horizontal dashed line) was 1.5 log10 TCID50/mL. Asterisks indicate results that are significantly different from those of the mock control group: *** p<0.001; ** p<0.01; * p<0.05. Weight loss was analyzed using a Paired t test, survival curves were compared using the Mantel Cox Log-rank test and virus titers in nasal washes were compared using a t test.
In addition, while all groups showed elevated temperatures on Day 1 after challenge, ferrets vaccinated with M2SR H5N1 vaccines had the lowest increases in body temperature (0.7°C and 0.9°C, respectively for two 107 TCID50 dose and single 108 TCID5o dose regimen), with body temperature in both groups returning to baseline by day 2 (Supplementary Table 2). M2SR H1N1-vaccinated ferrets had an increase in body temperature of 1.5°C (108 TCID50 dose) and 1.1°C (107 TCID50 dose) which remained elevated on day 2. In contrast, the control ferrets (mock) displayed the highest increase in body temperature (2.0°C) on day 1 which remained elevated on day 2 and did not return to baseline until day 8 post-challenge.
Following challenge, the prime-boost regimen with M2SR H5N1 (107 TCID50 dose) demonstrated 100% survival (Figure 3B), significantly reduced upper respiratory tract virus shedding (Figure 3C) and suppressed virus spread to organs (Table 2). The prime only regimen with M2SR H5N1 (108 dose TCID50) led to 88% survival (Figure 3B), with significantly reduced upper respiratory tract virus shedding (Figure 3C) and limited spread to organs (Table 2). The prime-boost regimen with M2SR H1N1 (107 dose) led to 88% survival and restricted virus replication in the upper respiratory tract and dissemination to the olfactory bulb. Viral shedding was also significantly reduced in this group. Survival of 88% was also observed in the single dose M2SR H1N1, 108 group (Figure 3B). However, virus was isolated from the lungs, trachea, nasal turbinates in this group (Table 2) but dissemination of virus to the olfactory bulbs was less than the mock controls. Viral shedding was significantly reduced from day 5 onwards in this group. In contrast, survival in the mock-immunized control group was 44%, with high virus titers in the lungs, trachea, nasal turbinates, olfactory bulb and brain at day 4. All surviving animals in the control group continued to shed virus at day 7 after challenge (Figure 3C), whereas viral shedding had ceased in all vaccinated groups by this time-point. None of the M2SR vaccinated animals had any virus detected in the brain in contrast to the mock-vaccinated animals.
Table 2.
Virus titers (log TCID50/g) in ferret organs after influenza A/Vietnam/1203/2004 challenge.
| Vaccine | Dose (TCID50/ferret) | # doses | Lungs
|
Trachea | Nasal Turbinates | Olfactory Bulb | Brain | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Left Caudal | Right Caudal | Left Cranial | Right Cranial | |||||||
| M2SR H5N1 | 107 | 2 | —a | — | — | — | — | — | — | — |
| M2SR H5N1 | 108 | 1 | — | — | — | — | — | — | 2.3 | — |
| M2SR H1N1 | 107 | 2 | — | — | — | — | — | 4.8, 3.8 | 2.3 | — |
| M2SR H1N1 | 108 | 1 | 4.8, 4.3 | 6.3 | 2.8 | — | 4.8, 2.3 | 5.1±1.3 | 2.3, 2.5 | — |
| Mock | N/A | 2 | 6.3, 4.8 | 3.0 | 4.5±0.9 | 3.8, 3.8 | 5.5±0.3 | 5.8±0.9 | 4.4±1.0 | 4.8, 3.5 |
—, Virus not detected
Ferret organs were harvested on day 4 post-challenge (N=3). The amount of infectious virus in organs was determined by TCID50 assay in MDCK cells. TCID50 titers were calculated using the method of Reed-Muench. Titers are shown in log TCID50/g, average ± SD or individual organ titers if not all ferret had titers.
M2SR induces HAI antibodies in ferrets
Sera from vaccinated and control ferrets were evaluated in an HAI assay using either H5N1 or H1N1 viruses. Sera from ferrets vaccinated with either 1 or 2 doses of M2SR H5N1 prevented hemagglutination by H5N1 virus, but not by H1N1 virus (Figure 4). Conversely, sera from ferrets vaccinated with 1 or 2 doses of M2SR H1N1 had high HAI titers against H1N1, but not against H5N1 influenza (Figure 4). HAI titers for sera from control ferrets were below the detection limit for both assays. These results show that M2SR H1N1 was able to confer protection against H5N1 virus in the absence of H5 HAI antibodies.
Figure 4. HAI titers in ferret serum after M2SR vaccination.
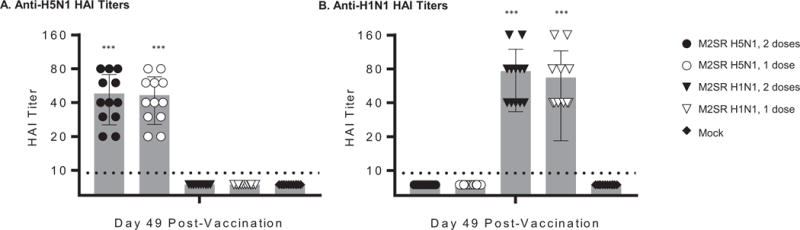
Serum samples were collected from immunized ferret 49 days after the first dose or 21 days after boost dose. HAI titers were determined for individual RDE-treated sera. Horse red blood cells and turkey red blood cells were used for M2SR H5N1 (A) and influenza A/California/07/2009 NYMC X-179A (H1N1) (B), respectively. The HAI assay detection limit was 10 indicated by a dashed line. Asterisks indicate results that are significantly different from those of the mock control group (Student’s t test) : *** p<0.001; ** p<0.01.
DISCUSSION
Due to the high pathogenicity of H5N1 influenza viruses and the possibility that they could mutate to forms with pandemic potential, there is an urgent need for an effective vaccine that could offer broad-spectrum immunity to these viruses. We previously described a novel influenza vaccine M2SR that could provide effective heterosubtypic protection against seasonal influenza strains. The data in the present study show that M2SR vaccines can also provide effective homo- and heterosubtypic immunity against highly pathogenic H5N1 avian influenza virus in both mice and ferrets. An additional advantage of the M2SR vaccine platform is that it can be rapidly produced in cell culture thus avoiding the many disadvantages of vaccine production in eggs, which could be in short supply during an avian influenza pandemic.
Vaccination with M2SR provided significant protection against H5N1 influenza in both mice and ferrets, even with a single dose, although a prime-boost regimen generally provided better protection in ferrets. Mice and ferrets vaccinated with M2SR H5N1 not only survived a lethal challenge with wildtype H5N1 virus, but also showed no, or significantly reduced, weight loss and other clinical symptoms compared to controls. Vaccination with both a single dose and a prime and boost with M2SR H5N1 also completely prevented viral spread to the organs in mice, as did the prime-boost regimen in ferrets. Importantly, both the level and duration of viral shedding after challenge was substantially reduced in M2SR-vaccinated ferrets and was completely ablated in some animals vaccinated with M2SR H5N1 using the prime-boost regimen. In addition to indicating better protection after vaccination, a reduction in viral shedding would limit spread of the virus to unvaccinated individuals, which could be critical in controlling a pandemic. Mice vaccinated with recombinant H5 HA protein, as a comparator, lost more body weight after challenge with H5N1 influenza virus and exhibited severe clinical symptoms and systemic infection (Figure 2A). Survival in the recombinant H5 HA group was only 40%, whereas in all M2SR vaccinated groups of mice survival was 100% (Figure 2B).
The M2SR vaccine induced substantial mucosal and systemic antibody responses in mice. Homologous protection and sterilizing immunity induced by the M2SR H5N1 vaccine probably resulted from the induction of neutralizing antibodies, correlating with high HAI titers that were observed in both mice and ferrets. In contrast, heterologous protection induced by M2SR H1N1 was in the absence of H5 HAI titers or sterilizing immunity, although full protection could be obtained in mouse and ferret models. However, cross-reactive antibodies to the conserved HA2 stalk region and the N1 neuraminidase were elicited (Figure 1C and Supplementary Figure 1) and it is likely that these contributed to cross-protection. Previous studies have also suggested that cross-reactive HA and NA antibodies between H5N1 and H1N1 influenza viruses contribute to heterologous protection [19–22]. In addition, our previous studies demonstrated that M2SR vaccine induced strong T cell responses, including CD8 T cells of an effector or effector/memory phenotype, armed with cytotoxic mediators such as granzyme B [9]. T cells with this phenotype are associated with protection against influenza in humans. It is possible that a similar T cell response was induced by the H1N1 M2SR vaccine in the current study, and could have contributed to protection against the H5N1 challenge virus.
Although both H1N1 and H5N1 M2SR vaccines protected against highly pathogenic H5N1 virus, there are practical advantages that are associated with the use of a seasonal vaccine that provides protection against a potential pandemic virus. While the homologous M2SR H5N1 provides better protection against H5N1 virus, with no virus recovered from organs and reduced illness and viral shedding, the M2SR H1N1 did provide a high degree of protection particularly when used in a prime-boost regimen. Use of a live H5 vaccine may raise concerns about possible reassortment, although these concerns may be misplaced for a single-replication virus such as M2SR. M2SR vaccines have been shown to be genetically stable over multiple passages [9]. Furthermore, when mice were concomitantly administered M2SR and live wildtype influenza viruses, no replication-competent viruses containing M2SR segments were observed (Y. Hatta & P. Bilsel, unpublished data). The potential safety and advantages of single replication cycle vaccines over traditional live attenuated vaccines have been discussed elsewhere [23, 24]. Our data suggest that the protective cross-reactive responses elicited by M2SR H1N1 may be important in boosting these responses to mitigate a pandemic infection as has been suggested previously [25–27].
In summary, both H5N1 and H1N1 M2SR vaccines provided effective protection against highly pathogenic H5N1 avian influenza limiting viral spread, viral shedding and illness. Protection was observed in both mouse and ferret models and was superior to that elicited by recombinant H5 HA protein alone.
Supplementary Material
Acknowledgments
The following reagent was obtained through BEI Resources, NIAID, NIH: N1 Neuraminidase (NA) Protein with N-Terminal Histidine Tag from Influenza Virus, A/California/04/2009 (H1N1pdm), Recombinant from Baculovirus, NR-19234.
This work was supported, in part, by National Institutes of Health grants AI 111451 and AI 109925 to S.S. and P.B.
ABBREVIATIONS
- av
avirulent
- BAL
bronchoalveolar lavage
- ELISA
enzyme-linked immunosorbent assay
- FCS
fetal calf serum
- HA
hemagglutinin
- HAI
hemagglutination inhibition
- HEK
human embryonic kidney
- M2SR
M2-deficient single replication vaccine virus
- M2CK
Madin-Darby canine kidney cells expressing M2 protein
- MDCK
Madin-Darby canine kidney
- MEM
minimal essential medium
- NA
neuraminidase
- OD
optical density
- PFU
plaque-forming unit
- RDE
receptor destroying enzyme
- TCID50
50% tissue culture infectious dose
- TMB
tetramethylbenzidine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statements: S.S. and D.B. have no conflicts of interest. G.N. and Y.K. are founders of FluGen. Y.H. and P.B. are employees of FluGen.
References
- 1.Shortridge KF, et al. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology. 1998;252(2):331–42. doi: 10.1006/viro.1998.9488. [DOI] [PubMed] [Google Scholar]
- 2.Claas EC, et al. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351(9101):472–7. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 3.Yuen KY, et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351(9101):467–71. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 4.Subbarao K, et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279(5349):393–6. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 5.Katz JM, et al. Antibody response in individuals infected with avian influenza A (H5N1) viruses and detection of anti-H5 antibody among household and social contacts. J Infect Dis. 1999;180(6):1763–70. doi: 10.1086/315137. [DOI] [PubMed] [Google Scholar]
- 6.Imai M, et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature. 2012;486(7403):420–8. doi: 10.1038/nature10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herfst S, et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science. 2012;336(6088):1534–41. doi: 10.1126/science.1213362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell CA, et al. The potential for respiratory droplet-transmissible A/H5N1 influenza virus to evolve in a mammalian host. Science. 2012;336(6088):1541–7. doi: 10.1126/science.1222526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarawar S, et al. M2SR, a novel live single replication influenza virus vaccine, provides effective heterosubtypic protection in mice. Vaccine. 2016;34(42):5090–8. doi: 10.1016/j.vaccine.2016.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwatsuki-Horimoto K, et al. The cytoplasmic tail of the influenza A virus M2 protein plays a role in viral assembly. J Virol. 2006;80(11):5233–40. doi: 10.1128/JVI.00049-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neumann G, et al. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci USA. 1999;96(16):9345–50. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe T, et al. Novel approach to the development of effective H5N1 influenza A virus vaccines: use of M2 cytoplasmic tail mutants. J Virol. 2008;82(5):2486–92. doi: 10.1128/JVI.01899-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sui J, et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat Struct Mol Biol. 2009;16(3):265–73. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steel J, et al. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. MBio. 2010;1(1) doi: 10.1128/mBio.00018-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekiert DC, et al. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324(5924):246–51. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashyap AK, et al. Combinatorial antibody libraries from survivors of the Turkish H5N1 avian influenza outbreak reveal virus neutralization strategies. Proc Natl Acad Sci USA. 2008;105(16):5986–91. doi: 10.1073/pnas.0801367105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okuno Y, et al. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J Virol. 1993;67(5):2552–8. doi: 10.1128/jvi.67.5.2552-2558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ledgerwood JE, et al. DNA priming and influenza vaccine immunogenicity: two phase 1 open label randomised clinical trials. Lancet Infect Dis. 2011;11(12):916–24. doi: 10.1016/S1473-3099(11)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suguitan AL, Jr, et al. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 2006;3(9):e360. doi: 10.1371/journal.pmed.0030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandbulte MR, et al. Cross-reactive neuraminidase antibodies afford partial protection against H5N1 in mice and are present in unexposed humans. PLoS Med. 2007;4(2):e59. doi: 10.1371/journal.pmed.0040059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia JM, et al. Heterosubtype neutralizing responses to influenza A (H5N1) viruses are mediated by antibodies to virus haemagglutinin. PLoS One. 2009;4(11):e7918. doi: 10.1371/journal.pone.0007918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahmood K, et al. H5N1 VLP vaccine induced protection in ferrets against lethal challenge with highly pathogenic H5N1 influenza viruses. Vaccine. 2008;26(42):5393–9. doi: 10.1016/j.vaccine.2008.07.084. [DOI] [PubMed] [Google Scholar]
- 23.Guo H, et al. Induction of CD8 T cell heterologous protection by a single dose of single-cycle infectious influenza virus. J Virol. 2014;88(20):12006–16. doi: 10.1128/JVI.01847-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baz M, et al. Nonreplicating influenza A virus vaccines confer broad protection against lethal challenge. MBio. 2015;6(5):e01487–15. doi: 10.1128/mBio.01487-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gioia C, et al. Cross-subtype immunity against avian influenza in persons recently vaccinated for influenza. Emerg Infect Dis. 2008;14(1):121–8. doi: 10.3201/eid1401.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Reeth K, et al. Prior infection with an H1N1 swine influenza virus partially protects pigs against a low pathogenic H5N1 avian influenza virus. Vaccine. 2009;27(45):6330–9. doi: 10.1016/j.vaccine.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 27.van Maurik A, et al. Seasonal influenza vaccine elicits heterosubtypic immunity against H5N1 that can be further boosted by H5N1 vaccination. Vaccine. 2010;28(7):1778–85. doi: 10.1016/j.vaccine.2009.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


