Abstract
Purpose
To investigate the short-term outcomes after intravitreal injection of ziv-aflibercept in the treatment of choroidal and retinal vascular diseases.
Methods
Thirty-four eyes of 29 patients with age-related macular degeneration (AMD), diabetic retinopathy, and retinal vein occlusion (RVO) received a single dose intravitreal injection of 0.05 ml ziv-aflibercept (1.25 mg). Visual acuity, spectral domain optical coherence tomography (SD-OCT) activity, and possible side effects were assessed before and at 1 week and 1 month after the intervention.
Results
At 1 month after treatment, mean central macular thickness (CMT) significantly decreased from 531.09 μm to 339.5 μm (P < 0.001), and no signs of side effects were observed in any subject. All patients responded to treatment in terms of reduction in CMT. The improvement in visual acuity was statistically non-significant.
Conclusion
Our findings suggest that a single dose intravitreal injection of ziv-aflibercept may have acceptable relative safety and efficacy in the treatment of patients with intraocular vascular disease.
The trial was registered in the Iranian Registry of Clinical Trials (IRCT2015081723651N1).
Keywords: Ziv-aflibercept, Age-related macular degeneration, Diabetic retinopathy, Anti-vascular endothelial growth factor, Retinal vein occlusion
Introduction
Nowadays, many anti-vascular endothelial growth factor (anti-VEGF) agents are used as the first line in the treatment of intraocular vascular diseases.1 Until recently, the choice of drugs was limited to bevacizumab (Avastin) and ranibizumab (Lucentis), the former being more popular on account of its lower cost.2, 3 Aflibercept (Eylea) is a new addition to the group, which may offer better efficiency and a longer effect.4
Ziv-aflibercept, an anti-VEGF anticancer drug, has the same structure and exerts the same function as aflibercept, but the latter undergoes a different purification process and contains different buffer solutions that are less irritating when injected intravitreally and has a lower osmolarity.5 However, from a commercial perspective, ziv-aflibercept is a much cheaper recombinant fusion protein. Mansour et al4 have used evidence from in vitro and in vivo studies6, 7 to address certain concerns in relation to safety in its intraocular use such as osmolality differences and the risk of inducing changes to retinal morphology. Regarding the little available information on the safety and efficacy of ziv-aflibercept in patients with intraocular vascular diseases, we performed this study.
Methods
In this prospective interventional case series, patients with age-related macular degeneration (AMD), diabetic macular edema (DME), or retinal vein occlusion (RVO) who had no sign of eye infection and no history of myocardial infarction or cerebrovascular accident were included in this study.
The Ethics Committee of Tehran University of Medical Sciences approved this study; it adhered to the tenets of the Declaration of Helsinki, and a written informed consent was obtained from all participants.
First, 0.05 ml of aqueous humor was extracted through the limbus area with a 29 gauge needle. Then 0.05 ml (1.25 mg) of Ziv-aflibercept (Zaltrap, Sanofi and Regeneron Pharmaceuticals, Inc.) was injected into the intravitreal space through the pars plana using a 31 gauge needle.
In addition to intraocular pressure (IOP) monitoring and an ophthalmic exam, the patients were observed for signs of any progression in lens opacity, intraocular inflammation, and change in retinal structure using the spectral domain optical coherence tomography (SD-OCT) before, 1 week, and 1 month after the injections. In this study, “efficacy” referred to reduction in central macular thickness (CMT). Statistical analysis was performed using paired-test or McNemar's test.
Results
Thirty-four eyes of 29 consecutive patients with a mean age 66.6 ± 11.0 years were enrolled. Five diabetic patients received ziv-aflibercept injections bilaterally. The diagnosis was DME in 20 eyes (58.8%), AMD in 8 (23.5%), and RVO in 6 eyes (17.7%). The baseline characteristics of 3 diagnostic groups are presented in Table 1.
Table 1.
Patient demographics and baseline characteristics.
| Characteristic | AMD | RVO | DME | |
|---|---|---|---|---|
| N (full set analysis): | 8 eyes in 8 patients | 6 eyes in 6 patients | 20 eyes in 15 patients | |
| Age (Mean ± SD) | 77.4 ± 3.1 | 66.0 ± 11.7 | 63.3 ± 10.6 | |
| Gender: n (%) | Male | 4 (50.0%) | 3 (50.0%) | 7 (46.7%) |
| Female | 4 (50.0%) | 3 (50.0%) | 8 (53.3%) | |
| Prior therapies, n (%) | ||||
| Laser therapy: n (%) | 1 (12.5%) | 1 (16.7%) | 13 (65.0%) | |
| Avastin injection: n (%) | 6 (75.0%) | 1 (16.7%) | 16 (80.0%) | |
| Average number of treatments prior to study entry | Mean ± SD: 7.17 ± 3.3 Range: 4–13 injections |
6 times | Mean ± SD: 3.7 ± 2.6 Range: 1–12 injections |
|
| Time since last injection prior to study enrollment | Median (IQR): 2.0 months (2.25 months) Range: 1–4 months |
4 months ago | Median (IQR): 4.5 months (7 months) Range: 1–42 months |
|
N: number, AMD: Age-related macular degeneration, DME: Diabetic macular edema RVO: Retinal vein occlusion, SD: Standard deviation, IQR: Inter quartile range.
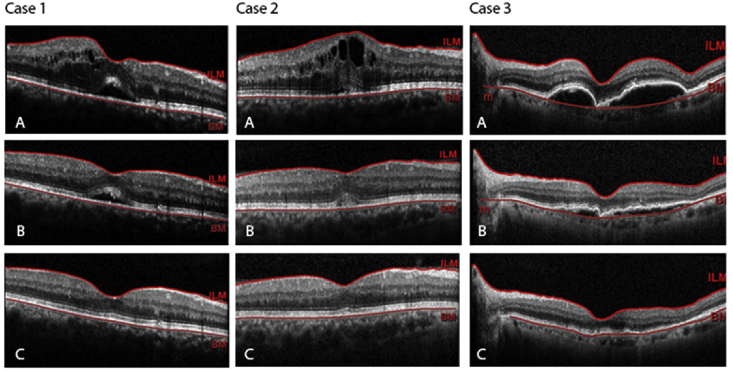
At 1 month after the intervention, the overall mean corrected distance visual acuity (CDVA) had improved compared to baseline, although the change was not statistically significant (0.75 ± 0.51 vs. 0.68 ± 0.48 logMAR; P = 0.09). Mean IOP was unchanged (14.88 ± 1.65 vs. 14.76 ± 1.54 mm Hg, P = 0.72). Mean CMT improved from 531.09 ± 185.7 μm (range, 230–948 μm) to 339.5 ± 129.1 μm (range, 153–695 μm) (P < 0.001). The rate of cystoid macular edema (CME) decreased from 61.8% (n = 21) to 14.7% (n = 5) (P < 0.001). Subretinal fluid was observed in 19 cases (55.9%) at baseline which reduced to 6 cases (17.6%). CMT decreased in all cases, but visual acuity did not improve in 8 patients (Fig. 1).
Fig. 1.
Spectral domain optical coherence tomography (SD-OCT) scans of 3 eyes (Case 1: DME, Case 2: RVO and Case 3: AMD), which underwent intravitreal ziv-aflibercept, at baseline (A), 1 week (B) and at 1-month (C) follow-up, showing disappearance of subretinal fluid (Case 1), intraretinal fluid (Case 1 and Case 2), CME (Case 2), and PED (Case 3). DME: Diabetic macular edema; RVO: Retinal vein occlusion; AMD: Age-related macular degeneration; CME: Cystoid Macular Edema; PED: Pigment epithelial detachment.
No signs of inflammation or new cataract formation were observed, and none of the patients developed infection, retinal detachment, or any other post-injection complication. Table 2 presents these results in the 3 groups of patients with DME, AMD, and RVO.
Table 2.
Efficacy and safety of ziv-aflibercept injection in eyes with age-related macular degeneration (AMD).
| Diagnosis | Variables | Baseline | 1 week after injection | 1 month after injection | P Value |
|---|---|---|---|---|---|
| AMD (n = 8) | CMT (Mean (SD)) | 511.1 (220.1) | 355.9 (162.5) | 342.8 (162.8) | 0.02 |
| PED (Mean (SD)), N = 5 | 269.8 (84.3) | 199.4 (75.1) | 184.2 (82.2) | 0.10 | |
| CDVA in logMAR (Mean (SD)) | 0.83 (0.50) | 0.74 (0.39) | 0.73 (0.34) | 0.12 | |
| IOP (Mean (SD)) | 14.4 (2.0) | 14.1 (2.2) | 15.0 (1.4) | 0.28 | |
| CME: n (%) | 3 (37.5%) | 1 (12.5%) | 0 (0.0%) | – | |
| Sub retinal fluid: n (%) | 5 (62.5%) | 2 (25.0%) | 1 (12.5%) | – | |
| Intra retinal fluid: n (%) | 5 (62.5%) | 4 (50.0%) | 1 (12.5%) | – | |
| RVO (n = 6) | CMT (Mean (SD)) | 607.0 (163.5) | 325.5 (99.3) | 272.3 (60.6) | 0.007 |
| CDVA in logMAR (Mean (SD)) | 0.86 (0.65) | 0.83 (0.68) | 0.80 (0.63) | 0.76 | |
| IOP (Mean (SD)) | 14.7 (2.0) | 14.6 (1.5) | 14.7 (1.9) | 1.00 | |
| CME: n (%) | 5 (83.3%) | 1 (16.6%) | 0 (0.0%) | – | |
| Sub retinal fluid: n (%) | 4 (66.7%) | 4 (66.7%) | 1 (16.6%) | – | |
| Intra retinal fluid: n (%) | 6 (100.0%) | 6 (100.0%) | 5 (83.3%) | – | |
| DME (n = 20) | CMT (Mean (SD)) | 521.4 (174.2) | 386.5 (154.4) | 358.6 (127.6) | <0.001 |
| CDVA in logMAR (Mean (SD)) | 0.68 (0.51) | 0.62 (0.47) | 0.60 (0.49) | 0.26 | |
| IOP (Mean (SD)) | 15.3 (1.4) | 14.8 (1.3) | 14.7 (1.7) | 0.32 | |
| CME: n (%) | 13 (65.0%) | 7 (35.0%) | 5 (25.0%) | – | |
| Sub retinal fluid: n (%) | 10 (50.0%) | 8 (40.0%) | 5 (25.0%) | – | |
| Intra retinal fluid: n (%) | 20 (100.0%) | 20 (100.0%) | 18 (90.0%) | – |
AMD: Age-related macular degeneration, DME: Diabetic macular edema RVO: Retinal vein occlusion, CMT: Central macular thickness, PED: Pigment epithelial detachment, CDVA: Corrected distance visual acuity, IOP: Intraocular Pressure, CME: cystoid macular edema, SD: Standard deviation.
Discussion
At 1 month after injection, mean CMT and the rate of CME had significantly declined compared to baseline, which is in accordance with previous case studies.4, 8, 9, 10, 11, 12, 13, 14 Avastin is currently the most cost-effective therapy for the management of choroidal and retinal vascular diseases.4 In this study, although all ziv-aflibercept injections were prepared from a multi-dose vial, no case of intraocular infection was observed. The possibility to use a multi-dose vial for several patients, in addition to administering an 8-cycle rather than a 12-cycle regimen in the first year contribute to better cost-effectiveness of ziv-aflibercept compared to Avastin.4
Additionally, at 1 month after injection, there was no case of serious side effects. Following in vitro and in vivo studies which established that the intraocular application of the ziv-aflibercept was devoid of toxic effects on the retinal pigment epithelium cells,6, 7, 14, 15 the first report concerning the intraocular use of ziv-aflibercept in humans was published by Rafael et al in 2015.14 Their study was conducted on a patient with exudative AMD receiving intravitreal injection of ziv-aflibercept (0.07 ml, 25 mg/ml, total 1.75 mg) and no side effects or ocular toxicity was observed after a one-month follow-up. The latter studies also noted the relative safety of ziv-aflibercept after intravitreal administration for intraocular vascular disease.4, 8, 13, 16 However, complications may occur with frequent use and higher doses (up to 2 mg) of the drug.
The limitations of this study include the small sample size, the diverse diagnoses, and lack of a control group. Also, the possible adverse effect of the drug on electrophysiologic testing was not evaluated. Studies with longer follow-up of cases receiving multiple doses are needed to assess the risk of intraocular inflammation and other side effects.
Footnotes
Conflict of interest: The authors have no financial or proprietary interest in a product, method, or material described herein.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Brand C.S. Management of retinal vascular diseases: a patient-centric approach. Eye. 2012;26:1–16. doi: 10.1038/eye.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raftery J., Clegg A., Jones J., Tan S.C., Lotery A. Ranibizumab (Lucentis) versus bevacizumab (Avastin): modelling cost effectiveness. Br J Ophthalmol. 2007;91(9):1244–1246. doi: 10.1136/bjo.2007.116616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein J.D., Newman-Casey P.A., Mrinalini T., Lee P.P., Hutton D.W. Cost-effectiveness of bevacizumab and ranibizumab for newly diagnosed neovascular macular degeneration. Ophthalmology. 2014;121(4):936–945. doi: 10.1016/j.ophtha.2013.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mansour A.M., Al-Ghadban S.I., Yunis M.H., El-Sabban M.E. Ziv-aflibercept in macular disease. Br J Ophthalmol. 2015;99:1055–1059. doi: 10.1136/bjophthalmol-2014-306319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trichonas G., Kaiser P.K. Aflibercept for the treatment of age-related macular degeneration. Ophthalmol Ther. 2013;2(2):89–98. doi: 10.1007/s40123-013-0015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik D., Tarek M., del Carpio J.C. Safety profiles of anti-VEGF drugs: bevacizumab, ranibizumab, aflibercept and ziv-aflibercept on human retinal pigment epithelium cells in culture. Br J Ophthalmol. 2014;98(suppl 1):11–16. doi: 10.1136/bjophthalmol-2014-305302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marmor M.F., Martin L., Tharpe S. Osmotically induced retinal detachment in the rabbit and primate. Electron microscopy of the pigment epithelium. Invest Ophthalmol Vis Sci. 1980;19(9):1016–1029. [PubMed] [Google Scholar]
- 8.Chhablani J. Intravitreal ziv-aflibercept for recurrent macular edema secondary to central retinal venous occlusion. Indian J Ophthalmol. 2015;63(5):469. doi: 10.4103/0301-4738.159909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chhablani J., Narayanan R., Mathai A., Yogi R., Stewart M. Short-term safety profile of intravitreal ziv-aflibercept. Retina. 2016;36(6):1126–1131. doi: 10.1097/IAE.0000000000000913. [DOI] [PubMed] [Google Scholar]
- 10.Andrade G.C., Dias J.R., Maia A., Farah M.E., Meyer C.H., Rodrigues E.B. Intravitreal injections of ziv-aflibercept for diabetic macular edema. Retina. 2016;36:1640–1645. doi: 10.1097/IAE.0000000000001000. [DOI] [PubMed] [Google Scholar]
- 11.de Oliveira Dias J.R., Xavier C.O., Maia A. Intravitreal injection of ziv-aflibercept in patient with refractory age-related macular degeneration. Ophthalmic Surg Lasers Imaging Retin. 2015;46(1):91–94. doi: 10.3928/23258160-20150101-17. [DOI] [PubMed] [Google Scholar]
- 12.Mansour A.M., Chhablani J., Antonios R.S. Three-month outcome of ziv-aflibercept for exudative age-related macular degeneration. Br J Ophthalmol. 2016;100(12):1629–1633. doi: 10.1136/bjophthalmol-2015-308319. [DOI] [PubMed] [Google Scholar]
- 13.Mansour A.M., Dedhia C., Chhablani J. Three-month outcome of intravitreal ziv-aflibercept in eyes with diabetic macular oedema. Br J Ophthalmol. 2016 doi: 10.1136/bjophthalmol-2016-308679. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.de Oliveira Dias J.R., Badaró E., Novais E.A. Preclinical investigations of intravitreal ziv-aflibercept. Ophthalmic Surg Lasers Imaging Retin. 2014;45(6):577–584. doi: 10.3928/23258160-20141118-15. [DOI] [PubMed] [Google Scholar]
- 15.Klettner A., Tahmaz N., Dithmer M., Richert E., Roider J. Effects of aflibercept on primary RPE cells: toxicity, wound healing, uptake and phagocytosis. Br J Ophthalmol. 2014;98:1448–1452. doi: 10.1136/bjophthalmol-2014-305105. [DOI] [PubMed] [Google Scholar]
- 16.Baghi A., Jabbarpoor Bonyadi M.H., Ramezani A. Two doses of intravitreal ziv-aflibercept versus bevacizumab in treatment of diabetic macular edema: a three-armed, double-blind randomized trial. Ophthalmol Retina. 2017;1(2):103–110. doi: 10.1016/j.oret.2016.08.007. [DOI] [PubMed] [Google Scholar]