Abstract
Purpose
To assess the diagnostic power of the Corneal Visualization Scheimpflug Technology (Corvis ST) provided corneal biomechanical parameters in keratoconic corneas.
Methods
The following biomechanical parameters of 48 keratoconic eyes were compared with the corresponding ones in 50 normal eyes: time of the first applanation and time from start to the second applanation [applanation-1 time (A1T) and applanation-2 time (A2T)], time of the highest corneal displacement [highest concavity time (HCT)], magnitude of the displacement [highest concavity deformation amplitude (HCDA)], the length of the flattened segment in the applanations [first applanation length (A1L) and second applanation length (A2L)], velocity of corneal movement during applanations [applanation-1 velocity (A1V) and applanation-2 velocity (A2V)], distance between bending points of the cornea at the highest concavity [highest concavity peak distance (HCPD)], central concave curvature at the highest concavity [highest concavity radius (HCR)]. To assess the change of parameters by disease severity, the keratoconus group was divided into two subgroups, and their biomechanical parameters were compared with each other and with normal group. The parameters' predictive ability was assessed by receiver operating characteristic (ROC) curves. To control the effect of central corneal thickness (CCT) difference between the two groups, two subgroups with similar CCT were selected, and the analyses were repeated.
Results
Of the 10 parameters compared, the means of the 8 were significantly different between groups (P < 0.05). Means of the parameters did not show significant difference between keratoconus subgroups (P > 0.05). ROC curve analyses showed excellent distinguishing ability for A1T and HCR [area under the curve (AUC) > 0.9], and good distinguishing ability for A2T, A2V, and HCDA (0.9 > AUC > 0.7). A1T reading was able to correctly identify at least 93% of eyes with keratoconus (cut-off point 7.03). In two CCT matched subgroups, A1T showed an excellent distinguishing ability again.
Conclusions
The A1T seems a valuable parameter in the diagnosis of keratoconic eyes. It showed excellent diagnostic ability even when controlled for CCT. None of the parameters were reliable index for keratoconus staging.
Keywords: Keratoconus, Cornea, Corvis ST, Biomechanics
Introduction
Manifest keratoconus is easily detected by corneal topographers, but detection of its earlier stages and suspicious patients are major concerns for refractive surgeons.1 It is believed that the main cause of corneal ectasia after refractive surgery is activation of latent biomechanical instability in subclinical keratoconus by this operation.2, 3, 4 Moreover, it is now hypothesized that biomechanical destabilization of the cornea in keratoconic eyes may be present before topographical changes, and it may be detectable before the tomographic and clinical signs of the disease.1, 5, 6 Corneal biomechanical properties might have the ability to bridge the present gap in detection of early or marginal forms of keratoconus. A better understanding of these properties of cornea might help the detection of eyes at risk for developing ectasia after refractive surgery.7
Biomechanical properties of normal and keratoconic eyes have been investigated using ocular response analyzer (ORA: Reichert Ophthalmic Instruments, Buffalo, NY), the first instrument introduced for measuring corneal biomechanical properties in vivo in different studies. Most of them have indicated that the biomechanical properties of normal and keratoconic eyes were significantly different.2, 7, 8, 9, 10, 11 However, the exact correlation of ORA measured parameters to corneal mechanical properties should be known and investigated more.1, 6 Recently, the Corvis ST (CST: Corneal Visualization Scheimpflug Technology, Oculus; Wetzlar, Germany) has been introduced as a clinical tool for evaluating corneal biomechanical properties in vivo. By using the ultra-high-speed Scheimpflug camera, CST provides much more information than ORA.12 Both of them are noncontact tonometers that applanate cornea by air puff. The difference is that CST uses a high-speed Scheimpflug camera which takes cross-sectional images of the cornea during deformation process. The output parameters provided by the two instruments differ, and the results of the ORA and CST measurements cannot be compared directly.1 According to the related literature, CST has great potential for corneal biomechanics research.13 Assessing various corneal biomechanical parameters to identify the potentially discriminatory parameters could be an important step toward detecting early or marginal cases of keratoconus.
In this study, the ten CST provided biomechanical parameters of the eyes with mild to moderate keratoconus were compared with normal eyes. The same evaluations were done in two keratoconus subgroups to assess their change by the disease severity.
Methods
In this comparative study, two study groups were formed: the healthy control group and keratoconus group. The healthy control group comprised refractive surgery candidates. A detailed ophthalmologic examination confirmed their inclusion eligibility.
The diagnosis of keratoconus was made by a corneal specialist using microscopic signs and corneal imaging.14 In the keratoconus group, the first four stages of topographical keratoconus classification (TKC) provided by Pentacam (Oculus, Wetzlar, Germany) were included (i.e. 1, 1–2, 2, and 2–3). By this selection, they could be divided into two subgroups based on their TKC numbers: TKC 1 and 1–2 were grouped as stage I, and TKC 2 and 2–3 were grouped as stage II. Exclusion criteria in both groups were previous ocular surgery, corneal scars and opacities, history of glaucoma, pregnancy, and systemic disease affecting the eye. Data were collected from February 2014 to May 2014, and all participants consented.
This study included 98 eyes of 85 participants: 48 keratoconic eyes from 35 keratoconus patients (stage I: 22 eyes and stage II: 26 eyes) and 50 normal eyes from 50 controls. For the control group, one eye of each participant was selected randomly for measurements. In keratoconus groups, one or two eyes of the patients were included if they met the criteria.
Each subject underwent a comprehensive ophthalmologic examination, which included a medical history review, best corrected visual acuity, slit-lamp and fundoscopic examinations, Pentacam tomographic evaluation and CST measurements during the same visit. All measurements were taken between 8:00 AM and 1:00 PM. According to the literature, single measurements are not reliable for the 10 CST measured biomechanical parameters15; therefore, to increase accuracy of our results, two measurements were taken 10–15 min apart, and the results were averaged. The same operator performed all topographies using a Pentacam, and another experienced operator performed CST examination. The techniques of measurements by the above devices have already been well-described.16, 17
In this study, the following variables, measured by CST, were used for testing the biomechanical status of corneas: applanation-1 time (A1T: time of the first applanation), applanation-2 time (A2T: time from start to the second applanation), highest concavity time (HCT: time of the highest displacement of the corneal apex), highest concavity deformation amplitude (HCDA: magnitude of the highest displacement of the corneal apex), first applanation length (A1L: the length of the flattened segment in the first applanation), second applanation length (A2L: the length of the flattened segment in the second applanation), applanation-1 and applanation-2 velocity (A1V and A2V: corneal velocity of movement during two applanations), highest concavity peak distance (HCPD: distance between bending points of the cornea at the highest concavity), highest concavity radius (HCR: central concave curvature at the highest concavity).
The normality of data distribution was assessed with Kolmogorov–Smirnov test, and independent samples t-test or Mann–Whitney tests were applied to compare means of parametric or nonparametric data of the two study groups respectively. 1-way ANOVA or Kruskal–Wallis tests (for parametric and non-parametric data accordingly) were applied to compare means of CST parameters among the two keratoconic subgroups and normal groups followed by post-hoc analyses. The level of significance for each parameter was set at P < 0.05.
Receiver operating characteristic (ROC) curve analysis was employed to identify predictive accuracy of the CST parameters, as described by the area under the curve (AUC). ROC curves are obtained by plotting sensitivity versus 1 − specificity, which is calculated for each value observed. An area of 100% implies that the test perfectly discriminates between study groups. This method was also used to identify the cut-off points or studied parameters to maximize sensitivity and specificity in discriminating keratoconus corneas from normal ones.
To evaluate the effect of corneal thickness on CST parameters, two subgroups of normal and keratoconus participants whose corneal thicknesses did not show significant differences were formed, and the specificity and sensitivity of parameters were assessed by ROC curves. Central corneal thickness (CCT) provided by Pentacam was used for this part of the study. Statistical analyses and plots were performed using MedCalc software (v.12.1.0.0).
Results
The healthy control group consisted of 50 normal eyes, and the keratoconus group was comprised of 48 eyes with keratoconus. Age distribution, but not gender, was not significantly different between groups (P = 0.68). There were significantly more men in the keratoconus group and significantly more women in the normal group (P < 0.001). Table 1 compares demographic data of the two study groups.
Table 1.
Demographic characteristics of subjects.
| Characteristics | Normal | Keratoconus group |
|---|---|---|
| Patients, n | 50 | 35 (23 M, 12 F) |
| Eyes, n | 50 (10 M, 40 F) | 48 (30 M, 18 F) |
| Mean age (Y) ± SD | 27.46 ± 4.67 | 27.87 ± 5.25 |
| Male sex, n (%) | 20% | 62.5% |
SD: Standard deviation; Y: Year; M: Male; F: Female.
Of the ten biomechanical parameters A1T, A1V, A2T, A2V, HCDA, and HCR were normally distributed. Testing mean differences of biomechanical parameters showed that A1T, A1L, and HCR were significantly lower, and A1V, A2T, A2V, HCDA, and HCPD were significantly higher in keratoconus group (Table 2).
Table 2.
The means of Corvis parameters in two study groups and the results of their comparison.
| Variable | Normal group |
Keratoconus group |
P Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| A1T (ms) | 7.6 ± 0.51 | 6.72 ± 0.28 | <0.0001 |
| A1V (m/s) | 0.12 ± 0.03 | 0.14 ± 0.03 | <0.0006 |
| A2T (ms) | 20.88 ± 0.41 | 21.51 ± 0.33 | <0.0001 |
| A2V (m/s) | −0.31 ± 0.08 | −0.42 ± 0.08 | <0.0001 |
| HCDA (mm) | 0.88 ± 0.10 | 1.11 ± 0.15 | <0.0001 |
| HCR (mm) | 7.66 ± 0.68 |
5.72 ± 0.1 |
<0.0001 |
| Median |
Median |
||
| A1L (mm) | 1.78 | 1.68 | 0.0028 |
| A2L (mm) | 1.83 | 1.75 | 0.32 |
| HCPD (mm) | 4.49 | 4.82 | 0.0003 |
| HCT (ms) | 16.17 | 16.35 | 0.13 |
SD: Standard deviation; A1T: First applanation time; A1V: First applanation velocity; A2T: Second applanation time; A2V: Second applanation velocity; HCDA: Highest concavity deformation amplitude; HCR: Highest concavity radius; A1L: First applanation length; A2L: Second applanation length; HCPD: Highest concavity peak distance; HCT: Highest concavity time.
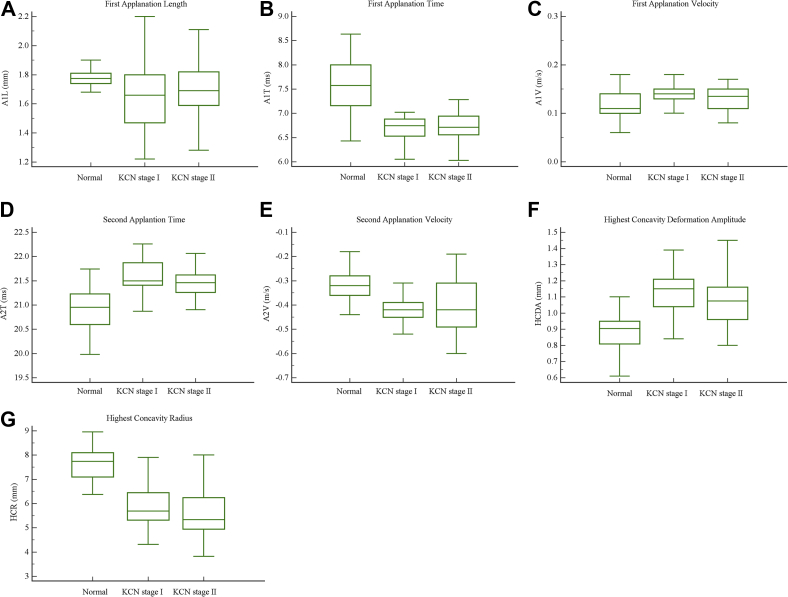
Comparison of parameters among normal group and two keratoconus subgroups indicated significant differences between normal group and each keratoconus subgroup in A1T, A1L, A1V, A2T, A2V, HCDA, and HCR. Moreover, means of the above mentioned parameters did not show a significant difference between the two subgroups (Fig. 1).
Fig. 1.
(A–G) Box-and-whisker plots of Corneal Visualization Scheimpflug Technology (Corvis ST) parameters for two keratoconus subgroups and normal group.
Mean of HCPD in stage I was significantly different from both stage II and the normal group, but its mean was not significantly different between stage II subjects and normal ones. The explanations for this unreasonable result could be that the device is not reliable in measuring HCPD. Because of showing such a result, this variable was not investigated anymore. As the previous part of the analyses, means of HCT and A2L were not significantly different among groups.
The biomechanical parameters that were significantly different between normal and keratoconus groups in previous analyses were assessed by ROC curve analysis for their distinguishing ability. Also, HCPD was excluded for the reason mentioned above. The results showed good overall predictive accuracy for tested parameters. Among biomechanical parameters A1T and HCR showed excellent ability in detecting keratoconic eyes (AUC = 0.955, P < 0.0001, and AUC = 0.936, P < 0.0001, respectively). Also, A2T, HCDA, and A2V showed good AUC. A1T showed cut-off point of ≤7.03 with 93.75% sensitivity and 92% specificity. Table 3 displays the results of ROC curve analyses and the best cut-offs of variables for optimizing sensitivity and specificity.
Table 3.
Area under curve (AUC) of Corneal Visualization Scheimpflug Technology (Corvis ST) parameters with their standard error (SE), 95% confidence interval (CI), and their best cut-offs for optimizing sensitivity and specificity to separate normal and keratoconic corneas.
| Variable | Cut-off point | Sensitivity (%) | Specificity (%) | AUC | SEa | 95% CIb |
|---|---|---|---|---|---|---|
| A1T (ms) | ≤7.03 | 93.75 | 92.00 | 0.955 | 0.0218 | 0.893–0.986 |
| A1L (mm) | ≤1.67 | 50.00 | 94.00 | 0.675 | 0.0594 | 0.573–0.766 |
| A1V (m/s) | >0.12 | 68.75 | 64.00 | 0.692 | 0.0533 | 0.591–0.782 |
| A2T (ms) | >21.4 | 68.75 | 98.00 | 0.898 | 0.0309 | 0.805–0.941 |
| A2V (m/s) | ≤−0.37 | 83.33 | 82.00 | 0.862 | 0.0385 | 0.777–0.923 |
| HCDA (mm) | >1 | 72.9 | 94.00 | 0.893 | 0.0328 | 0.814–0.946 |
| HCR (mm) | ≤6.35 | 77.08 | 100.00 | 0.936 | 0.0242 | 0.868–0.976 |
AUC: Area under curve; SE: Standard error; CI: Confidence interval; A1T: First applanation time; A1L: First applanation length; A1V: First applanation velocity; A2T: Second applanation time; A2V: Second applanation velocity; HCDA: Highest concavity deformation amplitude; HCR: Highest concavity radius.
DeLong et al., 1988.
Binomial exact.
To control the effect of CCT on CST parameters, two normal and keratoconus subgroups consisted of 20 normal and 20 keratoconic eyes, whose mean corneal thickness did not show significant difference (P = 0.35), were formed. The five CST parameters which had shown AUC of above 0.7 in the previous analyses were evaluated in these two subgroups. The results of independent samples t-test showed significant differences between normal and keratoconic subgroups in all tested variables (Table 4).
Table 4.
The means of Corneal Visualization Scheimpflug Technology (Corvis ST) parameters in central corneal thickness (CCT) controlled subgroups and the results of their comparison.
| Variable | Normal subgroup |
Keratoconus subgroup |
P Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| A1T time (ms) | 7.45 ± 0.55 | 6.8 ± 0.24 | <0.0001 |
| A2T (ms) | 20.1 ± 0.34 | 21.54 ± 0.37 | <0.0001 |
| A2V (m/s) | −0.34 ± 0.06 | −0.39 ± 0.06 | <0.0062 |
| HCDA (mm) | 0.92 ± 0.09 | 1.09 ± 0.16 | 0.0002 |
| HCR (mm) | 7.38 ± 0.84 | 6.21 ± 1.3 | 0.0017 |
SD: Standard deviation; A1T: First applanation time; A2T: Second applanation time; A2V: Second applanation velocity; HCDA: Highest concavity deformation amplitude; HCR: Highest concavity radius.
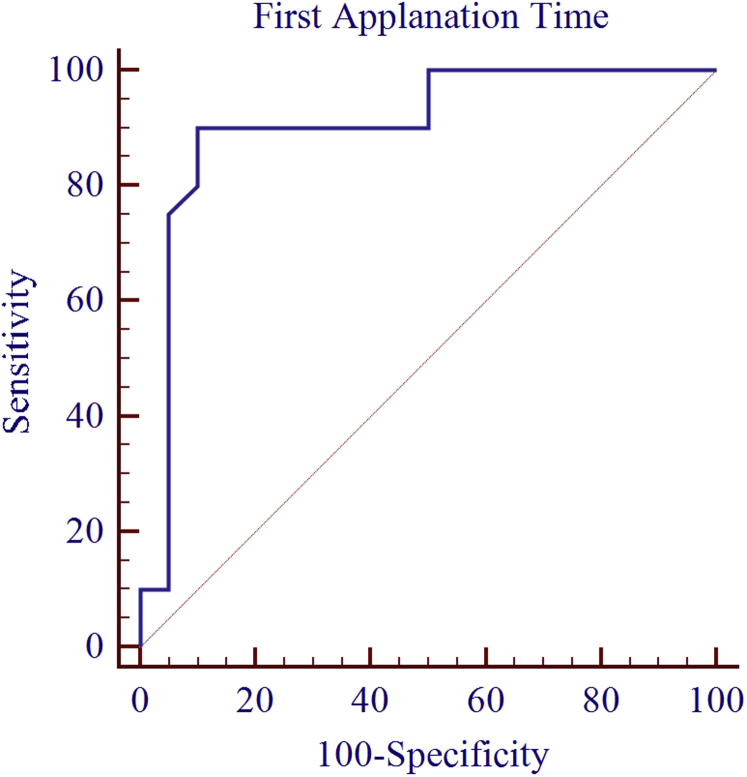
The results of ROC curve analysis showed the excellent distinguishing ability for A1T (AUC = 0.904, P < 0.0001). The four other parameters, i.e. A2T, HCDA, HCR, and A2V showed good AUC. The results of ROC curve analysis on CCT controlled subgroups have been summarized in Table 5. Fig. 2 shows ROC curve of A1T for distinguishing between keratoconus and normal eyes in CCT controlled subgroups.
Table 5.
Area under curve (AUC) of Corneal Visualization Scheimpflug Technology (Corvis ST) parameters with their standard error (SE), 95% confidence interval (CI), and their best cut-offs for optimizing sensitivity and specificity to separate normal and keratoconic corneas in two central corneal thickness (CCT) controlled subgroups.
| Variable | Cut-off point | Sensitivity (%) | Specificity (%) | AUC | SEa | 95% CIb |
|---|---|---|---|---|---|---|
| A1T (ms) | ≤7 | 90 | 90 | 0.904 | 0.0545 | 0.768–0.974 |
| A2T (ms) | >21.4 | 70 | 100 | 0.879 | 0.0538 | 0.737–0.960 |
| A2V (m/s) | ≤−0.37 | 75 | 75 | 0.737 | 0.0822 | 0.575–0.864 |
| HCDA (mm) | >1.1 | 55 | 100 | 0.794 | 0.0720 | 0.636–0.905 |
| HCR (mm) | ≤6.13 | 65 | 100 | 0.821 | 0.0703 | 0.668–0.924 |
AUC: Area under curve; SE: Standard error; CI: Confidence interval; A1T: First applanation time; A2T: Second applanation time; A2V: Second applanation velocity; HCDA: Highest concavity deformation amplitude; HCR: Highest concavity radius.
DeLong et al., 1988.
Binomial exact.
Fig. 2.
Receiver operating characteristic (ROC) curves of first applanation time (A1T) for distinguishing between keratoconus and normal eyes in central corneal thickness (CCT) controlled subgroups.
Discussion
Detecting keratoconus in its earlier stage is crucial for refractive surgery plans, but defining properties of its early and marginal forms has remained a clinical challenge.1 Corneal biomechanical changes have been mentioned as an effective factor in keratoconus formation, and it might be detectable before the tomographic and clinical signs of keratoconus become apparent.1, 5, 6, 8, 11 The ability to measure the biomechanical properties of the cornea could improve our understanding of corneal biomechanics in normal and pathological conditions. At present, there are two clinically available equipments which have made measuring biomechanical properties of cornea in vivo possible: ORA and CST. A few studies have evaluated biomechanical properties of cornea by ORA, but no cut-off point with high sensitivity and specificity for its parameters for the differentiation of keratoconus and healthy corneas has been established.7, 10, 18, 19 Evaluating other corneal biomechanical parameters to find some with more discriminative power than ORA provided parameters for manifest keratoconus could be an important step toward detecting early or marginal cases of it.
CST, a recently introduced instrument, is not widely used in clinics. A limited number of studies have tested its measured parameters ability to distinguish between keratoconic and normal eyes.5, 20, 21, 22, 23 In this study, CST was used to assess biomechanical properties of keratoconic and normal eyes. To reduce probable measurement error which corneal surface irregularity may induce the eyes with mild to moderate keratoconus were included. Also to increase reliability of the data15 two measurements were conducted and averaged. Of the ten biomechanical parameters measured by CST, eight were significantly different in normal and keratoconic eyes. A1T, A1L, and HCR were significantly lower in keratoconic eyes, and A1V, A2T, HCDA, A2V, and HCPD were higher in keratoconic eyes. Shetty and associates22 tested A1T, A2T, and HCDA in two groups and reported similar results. These results show that biomechanically normal and stiffer corneas take a longer time to applanate, and the velocity of applanation is lower. Normal structural and viscoelastic properties in normal eyes allow less deformation amplitude and as a result higher radius of curvature. In keratoconic eyes, distorting from normal structure and having abnormal viscoelastic properties could be the reason for easier deformation to a given force, lower resistance to deformation, and higher amplitude of deformation which results in lower radius of curvature at highest concavity. A2T could be an indicator of total viscoelasticity of the cornea, and it shows time element of viscoelastic response.5 This parameter had been increased in keratoconus, which might be an indicator of lower total viscoelasticity of cornea in this group. In the present study, HCT and A2L were not significantly different between the two groups. Previous studies5, 23 showed similar results for HCT between the two groups. They also showed that HCPD and A1L were not significantly different between the two groups. This part of their study is inconsistent with ours. However, in other parts of the present study, when we performed ROC curve analyses to assess distinguishing power of the parameters or when we assessed parameters differences among two keratoconus subgroups and normal group, we observed less distinguishing ability for these two parameters. The significant mean differences between two study groups in most of the CST provided parameters could be an indicator of impaired biomechanics and weakened strength in keratoconic eyes.
In the present study, the keratoconus group was divided into two subgroups according to their TKC grade in Pentacam, and CST measured parameters were compared among these two subgroups and normal group. The findings of these analyses did not show any significant differences in CST parameters of two keratoconus subgroups. These findings are mostly in agreement with Shetty and associates22 who compared some of CST parameters in normal eyes and different stages of keratoconus and reported no significant differences in A1T and HCDA between the two earlier stages of keratoconus. This finding in the present study does not support earlier studies which measured corneal biomechanical parameters by ORA and reported that they changed with keratoconus severity.7, 24 The reasons could be the different instruments used for measuring biomechanical properties, the different criteria measured, and the different methods used for subgrouping keratoconic eyes. The results of this part of the study could be helpful in understanding the changes occur in keratoconic eyes.
The results of ROC curve analyses showed excellent distinguishing value for A1T and HCR and good distinguishing value for A2T, A2V, and HCDA. In a previous study23 researchers reported good AUC for HCDA (0.882) that was close to the result we got for this parameter (0.893). However, the sensitivity they reported for this parameter was higher than the present study.
It is believed that the difference in mean CCT between normal and keratoconus groups (in the present study P was less than 0.0001) may cause a bias in findings of corneal biomechanical properties comparison.8, 11, 25 To control for the effect of CCT on CST provided biomechanical parameters, two 20-subject subgroups from normal and keratoconus groups whose CCT measures were not significantly different (P = 0.35) were selected. Distinguishing value of the parameters which had shown good or better distinguishing potential in previous analyses (i.e. A1T, A2T, A2V, HCDA, and HCR) was assessed in these two new subgroups. Although the AUCs of all 5 parameters were reduced compared to previous results, they were still above 0.7. In addition to CCT effect, part of the AUCs reductions in CCT compensated subgroups may be due to population size. Optimized cut-off values for A2T, HCDA, and HCR show specificity of 100%. For cut-off values with 70% specificity, sensitivity of tested parameters is increased (Table 6). This trade off in specificity (70%) with the hope of reduction in post-refractive surgery ectasia incidence might be applicable in clinical practice.
Table 6.
Corneal Visualization Scheimpflug Technology (Corvis ST) parameters cut-off values for specificity of 70% or above to separate normal and keratoconic corneas in two central corneal thickness (CCT) controlled subgroups.
| Variable | Cut-off point | Sensitivity | Specificity |
|---|---|---|---|
| A1T (ms) | ≤7 | 90 | 90 |
| A2T (ms) | >21.23 | 80 | 70 |
| A2V (m/s) | ≤−0.37 | 75 | 75 |
| HCDA (mm) | >0.96 | 70 | 70 |
| HCR (mm) | ≤6.8 | 75 | 70 |
A1T: First applanation time; A2T: Second applanation time; A2V: Second applanation velocity; HCDA: Highest concavity deformation amplitude; HCR: Highest concavity radius.
We concluded that although the thinner cornea was an effective factor in the obtained results in keratoconus, the viscoelastic properties of the cornea is a more determinant factor in the biomechanical responses that we got. This result is supported by the previous studies which reported significant differences in biomechanical properties of keratoconic and normal eyes after compensating for CCT difference or selecting keratoconic eyes with normal CCT as subjects.2, 8, 10, 20 Our study showed that A1T with AUC ROC of over than 0.9 and sensitivity and specificity of about 90% in both tests (with randomized groups and CCT matched subgroups) has the best distinguishing value among CST measured parameters, and its reduction could be a good marker of keratoconus.
In addition to CCT, IOP has been mentioned as a confounding factor in corneal biomechanical response, and it should be included as a parameter in the interpretation of the corneal deformation patterns measured in vivo.26, 27 In keratoconic eyes, corneal surface measurements and corneal biomechanical factors are deviated from normal condition which would result in lower reliability and error in IOP measurements.28, 29 To get reliable results from including IOP measurements in biomechanical studies, we should use IOP measurements compensated for corneal factors. During this study we had access to the first generation of Corvis ST Software (version 1.00r24) which does not measure corneal compensated IOP (IOPcc). So we could not control for IOP when comparing the biomechanical properties of normal and keratoconus groups. The effect of IOPcc on CST measured parameters should be controlled in future studies.
Different statistical analyses performed in this study showed none or less discriminating power for HCT, A1L, A2L, A1V, and HCPD. The other 5 CST measured corneal biomechanical parameters, especially A1T and HCDA which have shown high repeatability in previous studies5, 15, 16 and good discriminating ability in our study, deserve investigation with large CCT and IOPcc controlled groups to look for criteria for the detection of keratoconus especially in its earlier or marginal forms.
In conclusion, CST is a new instrument which measures corneal biomechanical parameters in vivo. Most of biomechanical parameters it measures were significantly different between normal and keratoconus group. A1T showed the best performance with AUC ROCs above 0.9 and sensitivity and specificity of 90% or above it in CCT controlled subgroups and randomized groups. It seems that A1T could provide an additional reference for discriminating keratoconic corneas from normal ones. Larger studies are required to investigate this parameter and four other potentially informative parameters, i.e. A2T, A2V, HCDA, and HCR. This study showed no significant difference in mean of CST measured biomechanical parameters between two stages of keratoconus. This finding could provide a better understanding of the corneal biomechanical response in keratoconic eyes. However, it should be investigated by using other keratoconus classification methods and including other stages of keratoconus.
Footnotes
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Vellara H.R., Patel D.V. Biomechanical properties of the keratoconic cornea: a review. Clin Exp Optom. 2015;98:31–38. doi: 10.1111/cxo.12211. [DOI] [PubMed] [Google Scholar]
- 2.Schweitzer C., Roberts C.J., Mahmoud A.M., Colin J., Maurice-Tison S., Kerautret J. Screening of forme fruste keratoconus with the ocular response analyzer. Invest Ophthalmol Vis Sci. 2010;51:2403–2410. doi: 10.1167/iovs.09-3689. [DOI] [PubMed] [Google Scholar]
- 3.O'Keefe M., Kirwan C. Laser epithelial keratomileusis in 2010 – a review. Clin Exp Ophthalmol. 2010;38:183–191. doi: 10.1111/j.1442-9071.2010.02198.x. [DOI] [PubMed] [Google Scholar]
- 4.Moshirfar M., Edmonds J.N., Behunin N.L., Christiansen S.M. Corneal biomechanics in iatrogenic ectasia and keratoconus: a review of the literature. Oman J Ophthalmol. 2013;6:12–17. doi: 10.4103/0974-620X.111895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali N.Q., Patel D.V., McGhee C.N. Biomechanical responses of healthy and keratoconic corneas measured using a noncontact Scheimpflug-based tonometer. Invest Ophthalmol Vis Sci. 2014;55:3651–3659. doi: 10.1167/iovs.13-13715. [DOI] [PubMed] [Google Scholar]
- 6.Roy A.S., Shetty R., Kummelil M.K. Keratoconus: a biomechanical perspective on loss of corneal stiffness. Indian J Ophthalmol. 2013;61:392–398. doi: 10.4103/0301-4738.116057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah S., Laiquzzaman M., Bhojwani R., Mantry S., Cunliffe I. Assessment of the biomechanical properties of the cornea with the ocular response analyzer in normal and keratoconic eyes. Invest Ophthalmol Vis Sci. 2007;48:3026–3031. doi: 10.1167/iovs.04-0694. [DOI] [PubMed] [Google Scholar]
- 8.Galletti J.G., Pfortner T., Bonthoux F.F. Improved keratoconus detection by ocular response analyzer testing after consideration of corneal thickness as a confounding factor. J Refract Surg. 2012;28:202–208. doi: 10.3928/1081597X-20120103-03. [DOI] [PubMed] [Google Scholar]
- 9.Labiris G., Giarmoukakis A., Gatzioufas Z., Sideroudi H., Kozobolis V., Seitz B. Diagnostic capacity of the keratoconus match index and keratoconus match probability in subclinical keratoconus. J Cataract Refract Surg. 2014;40:999–1005. doi: 10.1016/j.jcrs.2013.08.064. [DOI] [PubMed] [Google Scholar]
- 10.Fontes B.M., Ambrosio R., Jr., Velarde G.C., Nose W. Ocular response analyzer measurements in keratoconus with normal central corneal thickness compared with matched normal control eyes. J Refract Surg. 2011;27:209–215. doi: 10.3928/1081597X-20100415-02. [DOI] [PubMed] [Google Scholar]
- 11.Saad A., Lteif Y., Azan E., Gatinel D. Biomechanical properties of keratoconus suspect eyes. Invest Ophthalmol Vis Sci. 2010;51:2912–2916. doi: 10.1167/iovs.09-4304. [DOI] [PubMed] [Google Scholar]
- 12.Koprowski R. Open source software for the analysis of corneal deformation parameters on the images from the Corvis tonometer. Biomed Eng Online. 2015;14:31. doi: 10.1186/s12938-015-0027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rio-Cristobal A., Martin R. Corneal assessment technologies: current status. Surv Ophthalmol. 2014;59:599–614. doi: 10.1016/j.survophthal.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Rabinowitz Y. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 15.Nemeth G., Hassan Z., Csutak A., Szalai E., Berta A., Modis L., Jr. Repeatability of ocular biomechanical data measurements with a Scheimpflug-based noncontact device on normal corneas. J Refract Surg. 2013;29:558–563. doi: 10.3928/1081597X-20130719-06. [DOI] [PubMed] [Google Scholar]
- 16.Hon Y., Lam A.K. Corneal deformation measurement using Scheimpflug noncontact tonometry. Optom Vis Sci. 2013;90:e1–e8. doi: 10.1097/OPX.0b013e318279eb87. [DOI] [PubMed] [Google Scholar]
- 17.Steinberg J., Ahmadiyar M., Rost A. Anterior and posterior corneal changes after crosslinking for keratoconus. Optom Vis Sci. 2014;91:178–186. doi: 10.1097/OPX.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 18.Fontes B.M., Ambrosio J.R., Jardim D., Velarde G.C., Nose W. Ability of corneal biomechanical metrics and anterior segment data in the differentiation of keratoconus and healthy corneas. Arq Bras Oftalmol. 2010;73:333–337. doi: 10.1590/s0004-27492010000400006. [DOI] [PubMed] [Google Scholar]
- 19.Touboul D., Benard A., Mahmoud A.M., Gallois A., Colin J., Roberts C.J. Early biomechanical keratoconus pattern measured with an ocular response analyzer: curve analysis. J Cataract Refract Surg. 2011;37:2144–2150. doi: 10.1016/j.jcrs.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 20.Ambrosio R.J., Caldas D.L., Ramos I., Luz A. Diagnosis of keratoconus based on wavefront analysis. In: Barbara A., editor. Textbook on keratoconus new insights. Jaypee Brothers Medical Publishers; New Delhi: 2012. pp. 70–90. [Google Scholar]
- 21.Tian L., Wang D., Wu Y. Corneal biomechanical characteristics measured by the CorVis Scheimpflug technology in eyes with primary open-angle glaucoma and normal eyes. Acta Ophthalmol. 2016;94:e317–e324. doi: 10.1111/aos.12672. [DOI] [PubMed] [Google Scholar]
- 22.Shetty R., Nuijts R.M., Srivatsa P. Understanding the correlation between tomographic and biomechanical severity of keratoconic corneas. Biomed Res Int. 2015;2015 doi: 10.1155/2015/294197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian L., Huang Y.F., Wang L.Q. Corneal biomechanical assessment using corneal visualization Scheimpflug technology in keratoconic and normal eyes. J Ophthalmol. 2014;2014 doi: 10.1155/2014/147516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinero D.P., Alio J.L., Barraquer R.I., Michael R., Jimenez R. Corneal biomechanics, refraction, and corneal aberrometry in keratoconus: an integrated study. Invest Ophthalmol Vis Sci. 2010;51:1948–1955. doi: 10.1167/iovs.09-4177. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Porta N., Fernandes P., Queiros A., Salgado-Borges J., Parafita-Mato M., Gonzalez-Meijome J.M. Corneal biomechanical properties in different ocular conditions and new measurement techniques. ISRN Ophthalmol. 2014;2014 doi: 10.1155/2014/724546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kling S., Marcos S. Contributing factors to corneal deformation in air puff measurements. Invest Ophthalmol Vis Sci. 2013;54:5078–5085. doi: 10.1167/iovs.13-12509. [DOI] [PubMed] [Google Scholar]
- 27.Huseynova T., Waring G.O., Roberts C., Krueger R.R., Tomita M. Corneal biomechanics as a function of intraocular pressure and pachymetry by dynamic infrared signal and Scheimpflug imaging analysis in normal eyes. Am J Ophthalmol. 2014;157:885–893. doi: 10.1016/j.ajo.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 28.Liu J., Roberts C.J. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg. 2005;31:146–155. doi: 10.1016/j.jcrs.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 29.Touboul D., Roberts C., Kerautret J. Correlations between corneal hysteresis, intraocular pressure, and corneal central pachymetry. J Cataract Refract Surg. 2008;34:616–622. doi: 10.1016/j.jcrs.2007.11.051. [DOI] [PubMed] [Google Scholar]