Abstract
Entamoeba histolytica (Eh) is the causative agent of amebiasis, one of the major causes of dysentery-related morbidity worldwide. Recent studies have underlined the importance of the intercellular junction between Eh and host cells as a determinant in the pathogenesis of amebiasis. Despite the fact that direct contact and ligation between Eh surface Gal-lectin and EhCP-A5 with macrophage α5β1 integrin are absolute requirements for NLRP3 inflammasome activation and IL-1β release, many other undefined molecular events and downstream signaling occur at the interface of Eh and macrophage. In this study, we investigated the molecular events at the intercellular junction that lead to recognition of Eh through modulation of the macrophage cytoskeleton. Upon Eh contact with macrophages key cytoskeletal-associated proteins were rapidly post-translationally modified only with live Eh but not with soluble Eh proteins or fragments. Eh ligation with macrophages rapidly activated caspase-6 dependent cleavage of the cytoskeletal proteins talin, Pyk2 and paxillin and caused robust release of the pro-inflammatory cytokine, IL-1β. Macrophage cytoskeletal cleavages were dependent on Eh cysteine proteinases EhCP-A1 and EhCP-A4 but not EhCP-A5 based on pharmacological blockade of Eh enzyme inhibitors and EhCP-A5 deficient parasites. These results unravel a model where the intercellular junction between macrophages and Eh form an area of highly interacting proteins that implicate the macrophage cytoskeleton as a sensor for Eh contact that leads downstream to subsequent inflammatory immune responses.
Author summary
The protozoan parasite Entamoeba histolytica can establish an enteric infection in human hosts that leads to symptoms ranging from diarrhea to abscesses in the liver and the brain. Host susceptibility to amebic infection is in part determined by the quality and potency of the host immune response that occurs once the parasite overcomes the mucus bilayers and colonic epithelial barriers, and invades underlying tissues. At the cellular level, one of the key events that shape the inflammatory response occurs during direct parasite interaction with host macrophages via surface proteins. The ensuing cascades of intracellular signaling events have only partly been uncovered. Interestingly, only direct interaction between live parasites and macrophages, as opposed to soluble factors or dead parasites, is a prerequisite to the generation of a prompt raging pro-inflammatory response. We have sought to further elucidate the mechanisms by which macrophages distinguish live parasites and found that the macrophage cell skeleton undergoes rapid significant alteration upon Eh contact. Furthermore, we uncovered a previously unknown role for two Eh enzymes in triggering macrophage pro-inflammatory responses. Through this work, we gain a better understanding of the molecular interactions that occur at the macrophage-ameba interface that regulate host inflammatory responses.
Introduction
Amebiasis is caused by the protozoan parasite Entamoeba histolytica (Eh) and is estimated to affect approximately 50 million people worldwide [1]. It is a major cause of mortality and morbidity, particularly in children of developing countries. Eh carriers remain asymptomatic in most cases, but in approximately 10% of infected individuals, Eh invades the intestinal tissues and triggers symptoms such as diarrhea, dysentery and colitis [2]. Eh can also migrate to other soft tissue organs, notably the liver where it causes abscesses, and can lead to death. The reasons that lead to the development of symptomatic infection are not fully defined but host genetic makeup, quality of the immune response and Eh expression of virulence factors are thought to be contributing factors. For example, polymorphisms in the leptin gene, as well as expression of certain HLA class II alleles, have been associated with increased vulnerability to amebiasis [3–6]. At the parasite level, expression of many virulence factors by Eh have been shown to modulate the host inflammatory response as well as the ability to invade soft tissue organs outside the gut [7, 8]. Eh binding to the mucus layers and host cells is mediated by its major surface component, the Gal/GalNAc lectin (Gal-lectin) [9, 10]. This 170kDa cell surface molecule has been shown to trigger innate immune responses and is a major target of the adaptive immune response [11–16]. Through homology search of the Eh genome, at least 50 genes encoding for cysteine peptidases (CP) have been identified [17, 18]. Of these, EhCP-A1, EhCP-A2 and EhCP-A5 are the most highly expressed in axenically cultured Eh isolate HM-1:IMSS and account for the majority of cysteine proteinase activity in vitro [17, 19]. EhCP-A5 is expressed on the cell surface, EhCP-A2 localizes to the internal and external cell membrane and EhCP-A1 localizes to intracellular vesicles [20–22]. These CPs have been shown to play a role in the pathogenesis of amebiasis [20, 23–25]. Moreover, as these CPs are required for Eh life cycle and are key virulence factors, they represent attractive pharmaceutical targets [26].
The immune response is also a major determinant of the capacity of Eh to cause disease [2, 7, 8]. Upon invasion of underlying gut tissue, Eh are met by gut resident macrophages. These cells have been shown to secrete high amounts of pro-inflammatory cytokines such as TNF-α and IFN-γ and possess amebicidal activity through the release of nitric oxide [11, 27]. TNF-α secretion has been associated with increased diarrheal disease, whereas nitric oxide and IFN-γ are protective [28]. We have shown that macrophages only secrete IL-1β upon direct contact with Eh [16, 29] that was dependent on initial adhesion by Eh Gal-lectin and engagement of macrophage α5β1 integrin by Eh cysteine protease 5 (EhCP-A5) RGD sequences that activated the NLRP3 inflammasome. Thus, there is growing evidence that signaling events initiated at the intercellular junction between Eh and macrophages shape the ensuing immune response that are central in determining host susceptibility. We hypothesize that the macrophage cytoskeleton acts as a sensor that can distinguish the threat posed by invasion of live pathogens within the underlying gut tissue as opposed to soluble factors that bind to host immune cell receptors.
In this study, we sought to uncover the molecular mechanisms that allow macrophage recognition of invasive Eh. We found that live Eh in contact with macrophages induces caspase-6-dependent cleavage of cytoskeletal proteins triggered by EhCP-A1 and EhCP-A4 and culminates in IL-1β secretion by macrophages. These studies demonstrate a novel macrophage cellular pathway, as well as a novel function for parasite CPs in triggering IL-1β secretion upon contact with Eh. Moreover, this study further strengthens the hypothesis that direct contact between macrophages and Eh is a key event that initiates multiple cellular mechanisms at the intercellular junction that culminates in a potent pro-inflammatory response.
Results
Macrophage cytoskeleton undergoes rearrangement upon contact with Eh
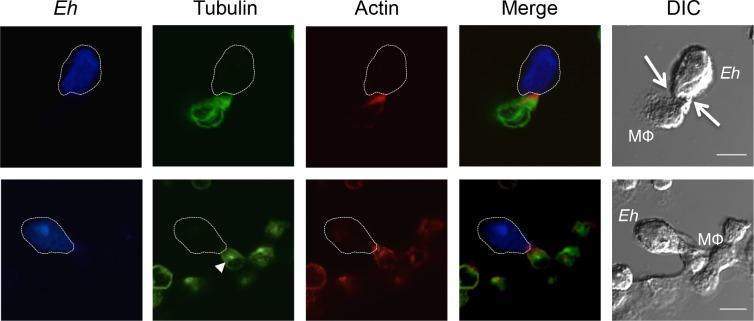
Recent studies [16, 29, 30] have established that direct contact between host cells and Eh mediated through the Gal-lectin and engagement of host cell integrins by EhCP-A5 at the intercellular junction is a critical initiation step in eliciting a robust pro-inflammatory response. These events require an intact actin cytoskeleton for recruitment of the NLRP3 inflammasome to contact sites as well as caspase-1 activation and IL-1β secretion [29]. Eh have been shown to distinguish between live and dead cells and adapt their capacity to interact with the host membrane, as underlined by the fact that the former triggers trogocytosis preferentially whereas the latter induces phagocytosis of host cells [31, 32]. These findings suggest that dynamic remodeling of the host cell cytoskeleton is probable upon direct contact with Eh and may have functional implications in terms of host cell, as well as parasite responses. To establish whether Eh induces cytoskeletal remodeling in macrophages upon direct contact, macrophages were incubated with Eh for 10 min, and the cytoskeletal proteins actin and tubulin were visualized by confocal microscopy. As predicted, both tubulin and actin were heavily concentrated at the macrophage-Eh contact site (Fig 1). Furthermore, microtubules were rapidly polarized towards the Eh contact site at the macrophage microtubule-organizing center. These results show that the cytoskeleton dynamically responded to Eh attachment at the macrophage intercellular junction.
Fig 1. Cytoskeletal modulation in macrophages upon contact with Eh.
Confocal microscopy of THP-1 macrophages incubated with Eh for 10 min. Eh were stained with Cell Tracker Blue prior to incubation. Cells were then fixed, permeabilized and stained with Alexa-488-conjugated anti-tubulin, or isotype control, and with Alexa-633-conjugated phalloidin. Images are representative of three independent experiments. The dotted white line indicates the position of Eh and the arrows in the DIC images shows the point of contact with the macrophage (MΦ). The arrowhead (tubulin frame in lower panel) shows the macrophage microtubule-organizing center. Scale bar 10μm.
Cytoskeletal-associated proteins undergo cleavage upon contact with Eh
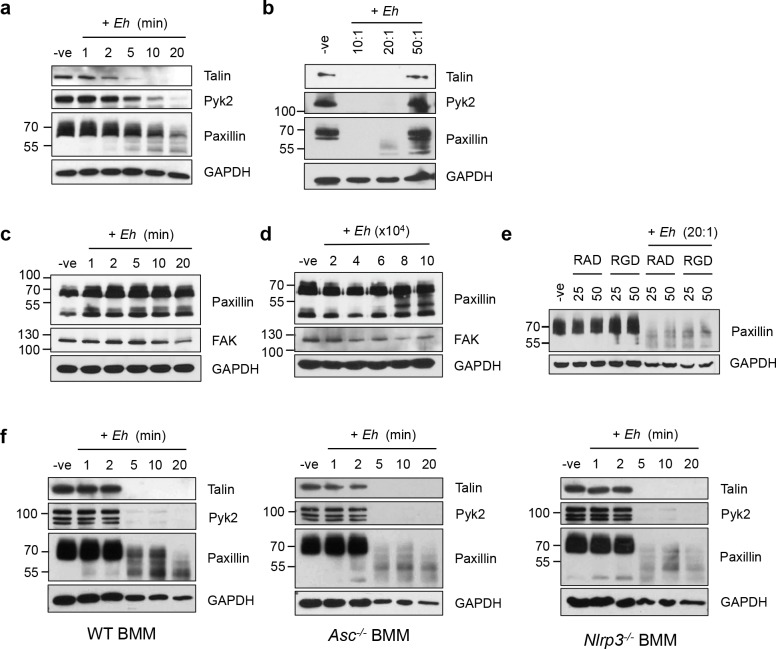
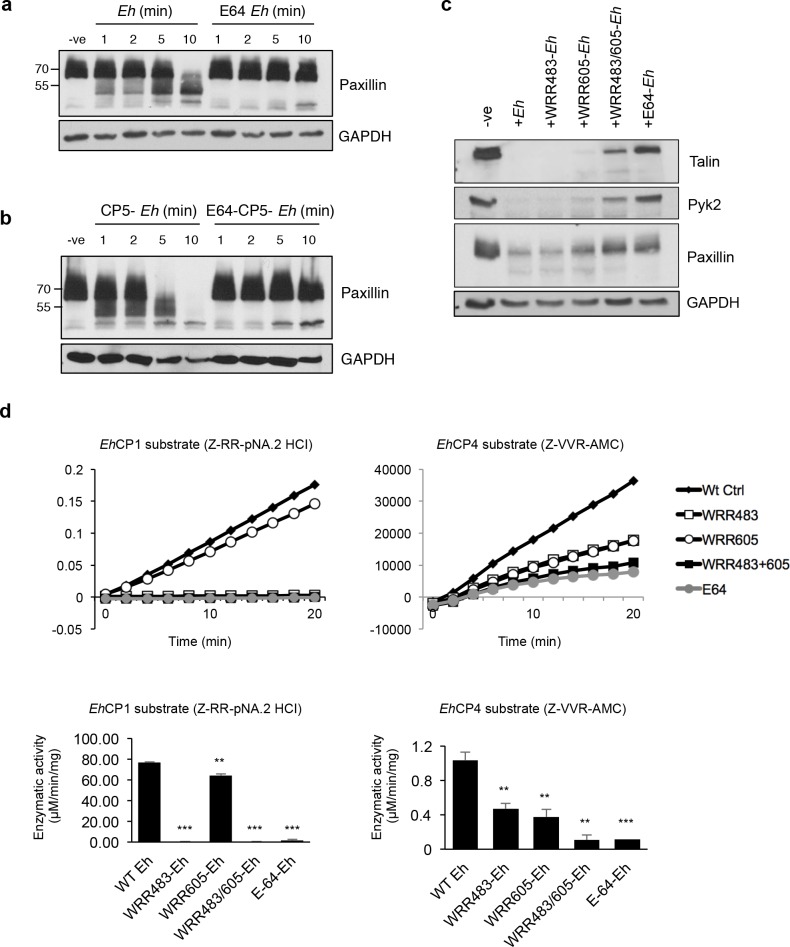
Cytoskeletal-associated proteins are intricately and extensively regulated by post-translational modification to regulate cytoskeletal dynamics during many cellular processes such as adhesion, cell death as well as cell-specific functions such as cytokine secretion. Previous studies have shown that Eh in contact with liver sinuisoidal endothelial cells triggers the breakdown of actin stress fibers, and relocalization of cytoskeletal-associated proteins [33, 34]. In the setting of macrophage-Eh interactions, phosphorylated paxillin and α5β1 integrin have been shown to concentrate at sites of contact between macrophages and Eh [29]. We investigated whether other macrophage cytoskeletal proteins were subjected to post-translational modifications and found that upon Eh contact with macrophages the degradation of at least three key cytoskeletal-associated proteins, talin, Pyk2 and paxillin occurred in a time- and dose-dependent manner (Fig 2A and 2B). Pyk2 is a signaling kinase mostly expressed in immune cells that triggers signaling downstream of integrins [35]. Paxillin and talin both act as scaffold proteins downstream of integrin signaling and play a key role in cytoskeletal remodeling [36, 37]. Appearance on western blot of a 53-kDa paxillin cleavage product occurred within 2 min suggesting that this is an early event during macrophage interaction with Eh. Although we originally postulated that paxillin would be phosphorylated upon contact with Eh, we were unable to assess the phosphorylation status of paxillin by western blot, as it was rapidly degraded. Interestingly, although Eh also engage integrins on the surface of T84 human colonic cells cytoskeletal cleavage was minimal, as appearance of paxillin cleavage products (~55 kDa) were only observed with high concentrations of Eh (Fig 2C and 2D). The 53-kDa and lower bands on the other hand, are similar to the main cleavage product observed in macrophages, but are only present with high concentrations of Eh (Fig 2D). As Pyk2 is not expressed at detectable levels in T84 colonic cells cleavage of its ortholog Focal Adhesion Kinase (FAK) was assessed and it was not degraded.
Fig 2. Degradation of cytoskeletal-associated proteins upon contact with Eh is observed in macrophages but not in colonic epithelial cells and is independent of inflammasome activation downstream of EhCP-A5 ligation to the α5β1 integrin.
THP-1 macrophages were incubated for (a) increasing times with Eh (20:1 ratio), or (b) for 5 min with the indicated macrophage-to-Eh ratios. After incubation, cells were washed and lysed. Equal amounts of lysates were loaded onto SDS-PAGE gel and immunoblotted with the indicated antibodies. T84 colonic epithelial cells were grown to confluency in wells of 12-well plates and (c) incubated for increasing times with 4 x 105 Eh, or (d) for 5 min with increasing amounts of Eh. (e) THP-1 macrophages were incubated for 5 min with the RGD small inhibitory peptide, or RAD as control, prior to stimulation with 20:1 Eh for 5 min, or left untreated (-ve). Bone marrow macrophages (BMM) derived from WT, Asc-/- or Nlrp3-/- mice were incubated with Eh (10:1) for the indicated amount of time prior to cell lysis (f). Lysates were loaded onto SDS-PAGE and immunoblotted with anti-Talin, anti-Pyk2, anti-paxillin and anti-GAPDH. Results are representative of more than three (a, b, e, f), or two independent experiments (c, d).
We have previously shown that Eh engages the α5β1 integrin at the surface of macrophages through EhCP-A5 RGD sequences to promote NLRP3 inflammasome activation. Although many of the cytoskeletal proteins cleaved upon contact with Eh were found downstream of integrins, pre-treatment of macrophages with the RGD integrin α5β1 inhibitory peptide did not inhibit cleavage of paxillin using high concentrations of Eh (Fig 2E compared to Fig 2B). Furthermore, cleavage of macrophage cytoskeletal proteins was similar in WT BMM as well as in Asc-/- BMM that lack the Nlrp3 adapter ASC, and in Nlrp3-/- BMMs (Fig 2F). These results show that macrophage interaction with Eh triggers rapid and potent cleavage of cytoskeletal-associated proteins that is independent of the previously characterized EhCP-A5/α5β1 integrin interaction [29].
Degradation of cytoskeletal-associated proteins upon Eh contact is mediated by caspase-6
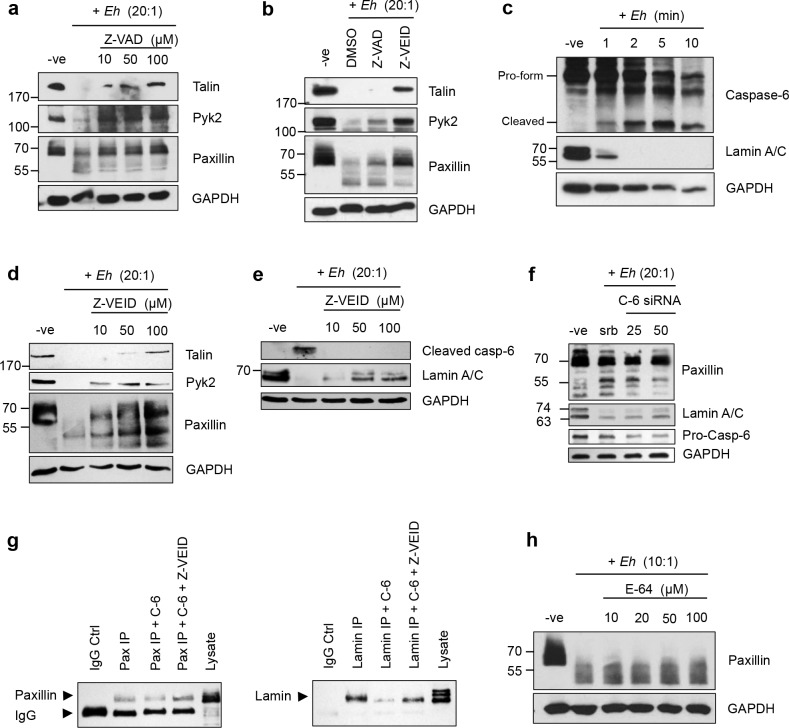
Paxillin has been shown to be susceptible to proteolytic cleavage by calpains and caspase-3 in the context of adhesion regulation and apoptosis [38–40]. Similarly, calpain-mediated cleavage of talin and Pyk2 is necessary for formation and disassembly of focal adhesions in osteoclasts [41]. Furthermore, both talin and paxillin have been shown to be cleaved by calpains in Jurkat T-cells incubated with Eh [42]. Accordingly, we next investigated whether cleavage of these cytoskeletal proteins were mediated by proteolytic cleavage by calpains or caspases. To do this, macrophages were pre-treatment with the pan-caspase inhibitor Z-VAD-fmk and in response to Eh, inhibited the cleavage of talin, Pyk2 and paxillin in a dose-dependent manner (Fig 3A). Through the use of a caspase inhibitor panel, we next identified the specific caspase responsible for cleavage of these proteins as caspase-6 (Fig 3B). None of the other inhibitors specific for caspases-1, -2, -3, -5, -8, and -9 inhibited Eh-induced cleavage of cytoskeletal-associated proteins. Consistent with these findings we found that caspase-6 was rapidly activated in Eh contacted macrophages, as shown by the appearance of the cleaved form of caspase-6 and degradation of one of its substrates, lamin (Fig 3C). Predictably, cleavage of macrophage cytoskeletal proteins was inhibited in a dose-dependent manner by the caspase-6-specific inhibitor Z-VEID (Fig 3D). Similarly, cleavage and activation of caspase-6 and its substrate lamin were also inhibited with increasing concentrations of Z-VEID upon macrophage stimulation with Eh (Fig 3E). To confirm specificity for caspase-6 involvement in paxillin degradation, we silenced caspase-6 by siRNA in THP-1 cells and used scramble siRNA as a control, followed by incubation with Eh for 2 min. As predicted, silencing caspase-6 inhibited the degradation of paxillin cleavage products (proteins >55 kDa) with increasing concentration of caspase-6 siRNA (Fig 3F). Cleavage of lamin was used as a positive control that was also inhibited from degradation with increasing concentration of caspase-6 siRNA as compared to the negative and scrambled siRNA controls. This data, together with the inhibition of paxillin by treatment with Z-VEID, suggests that Eh-induced cytoskeletal cleavage in macrophages was dependent on caspase-6 activation. To determine whether paxillin is a direct substrate of caspase-6, we assessed whether caspase-6 was able to cleave paxillin in vitro by incubating paxillin immunoprecipitated from THP-1 cells with recombinant human caspase-6. We found that recombinant caspase-6 was able to cleave immunoprecipitated paxillin, and that this cleavage was inhibited by treatment with Z-VEID (Fig 3G). The bottom band corresponds to mouse IgG from the immunoprecipitate, which cross-reacts with the secondary antibody used for western blot and served as a negative control for caspase-6 cleavage. A similar pattern was observed with the known caspase-6 substrate lamin (Fig 3G). To assess whether calpains also participated in the cleavage of the cytoskeletal protein paxillin, macrophages were pre-treated with increasing concentrations of E-64 that inhibits cysteine proteases as well as calpains, and it did not inhibit cleavage upon incubation with Eh (Fig 3H).
Fig 3. Degradation of macrophage cytoskeletal-associated proteins upon contact with Eh is mediated by caspase-6.
(a) THP-1 macrophages were pre-treated with increasing amounts of the pan-caspase inhibitor Z-VAD-fmk or DMSO as control, for 1 h prior to incubation with Eh (20:1 ratio) for 5 min. Cleavage of the indicated cytoskeletal proteins was assessed by Western blot. (b) THP-1 macrophages were pre-treated with 50μM Z-VAD-fmk, 50μM of the caspase-6 inhibitor Z-VEID-fmk or DMSO as a control, followed by incubation with Eh (20:1) for 5 min. (c) Immunoblot of caspase-6 and its lamin A/C in macrophages incubated with Eh (20:1) for the increasing incubation times. Dose-dependent inhibition of cleavage of the indicated cytoskeletal proteins (d) or caspase-6 and its substrate (e) by Z-VEID. To inhibit calpain activation in macrophages, cells were pretreated with E-64 prior to incubation with Eh (10:1) and cell lysis (f). Lysates were loaded onto SDS-PAGE and immunoblotted with the indicated antibodies. (g) THP-1 cells were transfected with 25 and 50nM of caspase-6 siRNA, or scramble siRNA. After 48 h, transfected cells were stimulated for 2 min with Eh, followed by Western blot with the indicated antibodies. (h) Direct cleavage of paxillin was assessed by incubated immunoprecipitated paxillin with recombinant caspase-6 (C-6), with or without Z-VEID. Immunoprecipitated lamin was used as a positive control.
Cytoskeletal remodeling is dependent on contact with live Eh
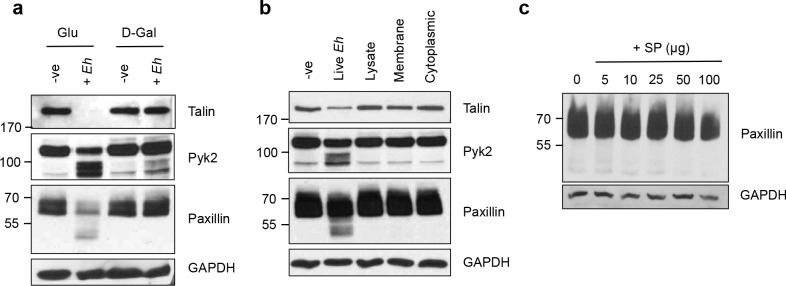
We next investigated the Eh requirements for triggering cleavage of the cytoskeletal-associated proteins. Gal-lectin is the major surface adhesin expressed by Eh and is necessary for binding macrophages [10, 43]. Predictably, inhibited Eh binding to macrophages with galactose completely abrogated cleavage of the cytoskeletal proteins talin, Pyk2 and paxillin whereas glucose as an osmotic control had no inhibitory effect (Fig 4A). We next evaluated whether stimulation of macrophages with subcellular fractions of Eh was capable of triggering cleavage of the cytoskeletal-associated proteins, as to further assess and identify the Eh virulence factors responsible for this mechanism. Cleavage of talin, Pyk2 and paxillin was only observed upon stimulation of macrophages with live Eh but not with fresh lysates of whole Eh, the membrane fraction or the cytoplasmic fraction derived from equivalent numbers of Eh (Fig 4B), or with increasing concentrations of Eh excreted/secreted soluble proteins (SP) (Fig 4C). These results indicate that contact with live Eh to macrophages was critical and necessary to initiate cytoskeletal cleavage. This could be due to the necessity of the dynamic engagement of multiple receptors during contact between Eh and macrophages, as supported by our observations for EhCP-A5 and Gal-lectin [16, 29]. Similarly, the process of trogocytosis by Eh has been shown to preferentially occur upon contact with live host cells [31, 32].
Fig 4. Macrophage cytoskeleton remodeling is dependent on direct contact with live Eh.
(a) Eh were pre-treated for 5 min with 55mM D-galactose, or glucose as an osmotic control prior to incubation with macrophages for 5 min at a 20:1 ratio. After incubation, cells were washed, lysed and equal amounts of lysates were loaded onto SDS-PAGE gel and immunoblotted with the indicated antibodies. (b) THP-1 cells were stimulated for 5 min with live Eh at a 20:1 ratio, whole lysates of Eh, or with membrane or cytoplasmic fractions of an equal amount of Eh. Lysates were analyzed as in (a). (c) Increasing amounts of Eh secreted/excreted proteins (SP) were incubated with macrophages for 15 min. Equal amounts of lysates were loaded onto SDS-PAGE and analyzed by Western blot with anti-paxillin and anti-GAPDH.
EhCP-A1 and EhCP-A4 mediate cleavage of cytoskeletal-associated proteins
To assess whether EhCP activity was necessary for cleavage of macrophage proteins, Eh were pre-treated overnight with the CP inhibitor E-64. Not surprisingly, E-64 treated Eh completely inhibited cleavage of cytoskeletal-associated proteins, indicating a role for Eh cysteine proteinase(s) in triggering caspase-6 activation and cleavage of paxillin (Fig 5A). To determine whether EhCP-A5, a cell-surface CP that has been shown to directly bind integrins on the surface of epithelial cells and macrophages [29, 30], plys a role in this process we exposed macrophages to EhCP-A5 deficient Eh and they were efficient at triggering the cleavage of paxillin that was inhibited in E64 treated EhCP-A5 parasites (Fig 5B). This mechanism thus appears to be independent of the previously identified interaction between EhCP-A5 and α5β1.
Fig 5. Inhibition of both EhCP-A1 and EhCP-A4, but not EhCP-A5 prevents cleavage of macrophage cytoskeletal proteins.
(a) Macrophages were incubated with wild-type Eh or E64-treated Eh (20:1) for the indicated amount of time. Cleavage of paxillin was assessed by Western blot. (b) Macrophages were incubated with CP-A5-deficient Eh or E64-treated CP-A5-deficient Eh (20:1) for the indicated amount of time. (c) Macrophages were stimulated with Eh pre-incubated 30 min with an inhibitor to EhCP-A1 (WRR483), EhCP-A4 (WRR605), both, or with E-64 at a 20:1 ratio for 5 min. (d) Enzymatic specificity of WRR inhibitors was evaluated by incubating inhibitor-treated Eh with EhCP-A1 or EhCP-A4 substrates for 20 min. Enzymatic activity for each substrate in the presence of inhibitors was calculated in μM/min/mg and shown in the bottom panel. Data are from three independent experiments and statistical significance was calculated with Student’s t-test (***p<0.0001, **p<0.005).
Based on these results, we next set out to identify the Eh cysteine proteases responsible for activating caspase-6 and subsequent cleavage of macrophage cytoskeletal proteins. Inhibitors to EhCP-A1 and EhCP-A4 (WRR483 and WRR605, respectively) have been carefully designed and synthetized to specifically inhibit their activity both in vitro and in vivo [20, 44]. EhCP-A1, along with EhCP-A5, is unique as they are not found in non-invasive E. dispar [19]. EhCP-A1 has been shown to be highly transcribed in vitro and released by cultured trophozoites [17, 23, 45]. Inhibition of EhCP-A1 with the WRR483 inhibitor has been shown to reduce Eh invasion in human intestinal xenografts in SCID mice [20]. EhCP-A4 transcription levels, on the other hand, are low in vitro, however it has been shown to be one of the most up-regulated CPs during Eh cecal infection in mice [17, 46]. Fittingly, administration of WRR605 to Eh-infected mice has been shown to significantly reduce Eh burden and decrease inflammation [44], however the underlying molecular mechanisms remain unknown. To determine whether EhCP-A1, EhCP-A4, or both, were involved in mediating cleavage of macrophage cytoskeletal-associated proteins, we pre-treated Eh with either one of the synthetic inhibitors WRR483 and WRR605, or both, prior to incubation with macrophages. Pre-treatment of Eh with these inhibitors individually partially inhibited cleavage of talin, Pyk2 and paxillin in macrophages (Fig 5C). Whereas WRR483 did not inhibit the cleavage of any cytoskeletal protein, WRR605 partially rescued the cleavage of talin and Pyk2 and modestly paxillin. Interestingly, pre-treatment with both inhibitors had an additive effect and was similar to E64 treated Eh indicating that both EhCP-A1 and EhCP-A4 participated in cytoskeletal cleavage.
To confirm the specificity of the WRR483 and WRR483 inhibitors, we treated Eh trophozoites with either WRR483, WRR605, both, or E-64 as a control with known substrates to EhCP-A1 (Z-RR) and EhCP-A4 (Z-VVR). Degradation of Z-RR occurred in a linear fashion over time (Fig 5D, top panel). Enzymatic activity in the presence of the inhibitors is displayed in the bottom panel (Fig 5D). Differences in the units in the velocity and specific activity graphs are due to differences in the fluorogenic nature of the substrates used. As expected, the WRR483 inhibitor completely inhibited the degradation of the Z-RR substrate, and to a level similar to E-64, whereas WRR605 has a minimal effect (Fig 5D). Degradation of the known EhCP-A4 substrate (Z-VVR), on the other hand, was inhibited by both WRR483 and WRR605 (Fig 5D). Together, they had an additive effect that was similar to the E-64 control. Whether this represents inhibition of EhCP-A4 by WRR483, or whether EhCP-A1 has specificity for the Z-VVR substrate remains to be determined, although the additive effect of both inhibitors suggest the later. Nevertheless, given that complete inhibition of paxillin cleavage is not seen with either inhibitor alone, but rather in an additive fashion when both inhibitors are present, suggest that both cysteine proteases are involved in triggering macrophage cytoskeletal-associated protein cleavage. The relative contribution of each cysteine protease remains to be further investigated with the use of recombinant proteins.
EhCP-A1 and EhCP-A4 activate caspase-6 at the Eh-macrophage intercellular junction
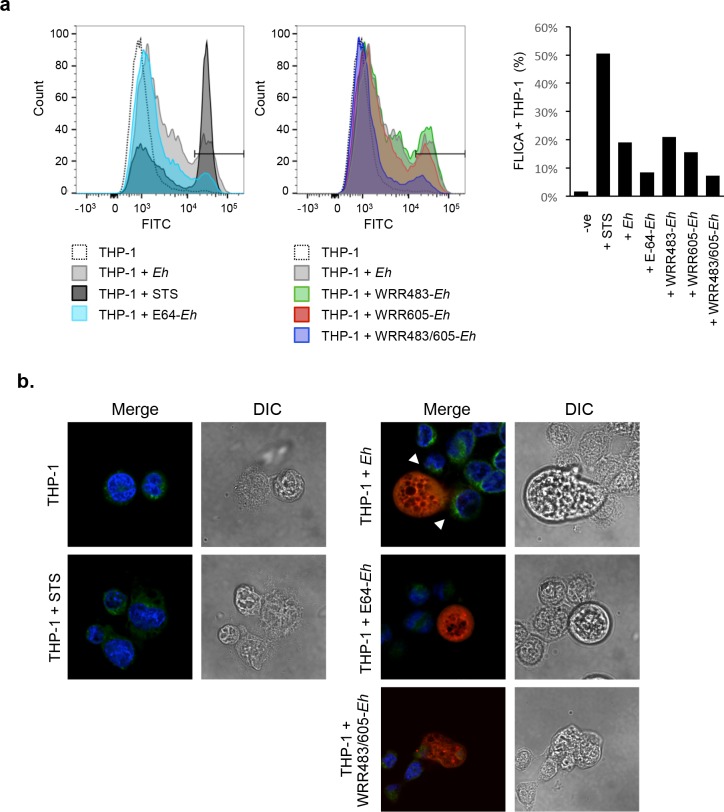
To quantify the involvement of EhCP-A1 and EhCP-A4 upstream of caspase-6 activation, we assessed caspase-6 activation by flow cytometry using a caspase-6-FLICA probe, which detects active caspase-6. To do this THP-1 macrophages were either left untreated or exposed to Eh, or treated with various EhCP inhibitors (E-64, WRR483, WRR605, or both WRR483 and WRR605), or with staurosporine (STS) as a positive control for caspase-6 activation. Inhibition with E64 or with EhCP-A1 and EhCP-A4 in combination had the greatest effect on preventing caspase-6 activation (Fig 6A). We also assessed localization of caspase-6 with the FLICA probe by confocal microscopy. As envisaged, activated caspase-6 was strongly localized to regions within macrophages that were polarized towards Eh (Fig 6B). This polarization was disrupted in interactions with WRR483/605-treated Eh. STS was included as a positive control that shows diffuse caspase-6 activation within the macrophage cytoplasm (Fig 6B).
Fig 6. Eh-triggered caspase-6 activation is dependent on EhCP-A1 and EhCP-A4 and is localized at sites of contact.
(a) Caspase-6 activation was measured by flow cytometry with the FITC-labeled caspase-6-FLICA probe in THP-1 macrophages incubated with 10:1 Eh, E64-treated Eh, CP-A5-deficient Eh, WRR483 pre-treated Eh, WRR605 pre-treated Eh, Eh pre-treated with both WRR483 and WRR605, left untreated, or with STS as a positive control. Eh were excluded from analysis based on forward and side scatter. Gating of percent positive cells was established according to the STS-positive population and percent positive cells for each treatment are indicated in the accompanying graph. (b) Caspase-6 activation for each treatment was also visualized by confocal microscopy using caspase-6-FLICA (green), DAPI (blue). Eh were pre-treated with Cell Tracker Orange (red) prior to incubation with THP-1 macrophages. STS was used as a positive control. Arrowheads show areas of polarization of caspase-6 towards the Eh-macrophage interface. The dotted white line indicates the position of Eh and the point of contact with the macrophage (MΦ). Results are representative of three independent experiments.
EhCP-A1 and EhCP-A4 polarize to the macrophage-Eh interface
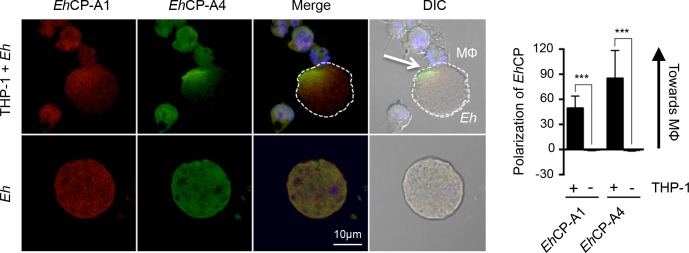
Previous studies have shown that EhCP-A1 is contained in cytoplasmic vesicles of trophozoites, whereas EhCP-A4 localizes to intracellular vesicles, the nucleus and perinuclear endosplasmic reticulum [20, 44]. EhCP-A1 is released upon stimulation of trophozoites with mucin whereas EhCP-A4 is released upon contact of target cells [44, 47]. We sought to determine whether these cysteine proteinases are expressed on the Eh cell surface and whether they polarized to the macrophage-Eh interface. Cell-surface localization of these two amebic cysteine proteinases upon contact with macrophages was assessed by staining non-permeabilized cells. We found that in the absence of macrophages, EhCP-A1 is diffusely distributed in a punctate-like pattern on the surface of Eh. Upon contact with macrophages, however, EhCP-A1 polarized to the macrophage contact point (Fig 7). EhCP-A4 displays a similar diffuse pattern in the absence of macrophages, and similarly polarizes to the macrophage junction upon contact. Quantification of the polarization of EhCP-A1 and EhCP-A4 is included on the right-hand side (Fig 7). Positive values indicate asymmetrical staining in the axis towards the macrophages, whereas null values are indicative of a symmetrical distribution of the staining patterns throughout Eh. Therefore, EhCP-A1 and EhCP-A4 staining shows polarization towards the macrophage contact interface.
Fig 7. Cell surface EhCP-A1 and EhCP-A4 polarize to the macrophage contact site.
To assess EhCP-A1 and EhCP-A4 localization, Eh were incubated with DAPI-treated THP-1 cells (top panels) or not (bottom panel). Slides were then stained with EhCP-A1 and EhCP-A4, and the appropriate secondary antibodies. EhCP-A1 is shown in red, EhCP-A4 is shown in green. Both EhCP1 and EhCP4 polarized on the Eh surface towards the macrophage whereas no polarization was observed in Eh basally (bottom panel). The dotted white line indicates the position of Eh and the arrow in the DIC images shows the point of contact with the macrophage (MΦ). Polarization of EhCPs was quantified by comparing density of EhCP staining at the leading half facing the macrophage in comparison to the tailing half. ***p<0.001 as determined by Student’s t-test.
Eh-triggered caspase-6 activation triggers IL-1β secretion by macrophages
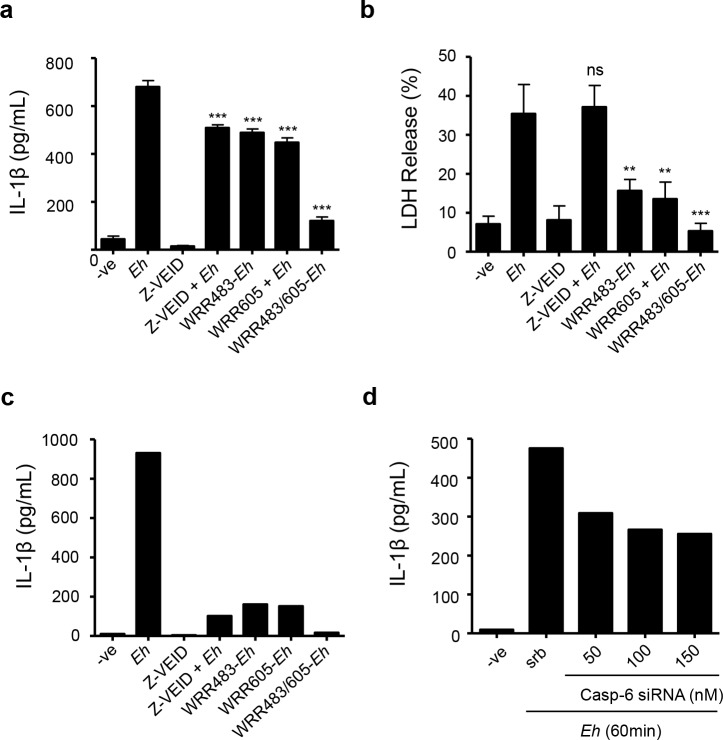
As caspase-6-mediated cleavage of cytoskeletal proteins of macrophages is an early event upon contact with Eh, we next determined whether it impacted pro-inflammatory cytokine secretion. We hypothesized that caspase-6-mediated cleavage of cytoskeletal proteins may participate to the macrophage inflammatory response upon contact with Eh. To determine this, we first measured IL-1β pro-inflammatory cytokine secretion in macrophages pre-treated with the caspase-6 inhibitor, or DMSO control, for 60 min followed by incubation with Eh for 60 min. Pre-treatment of macrophages with Z-VEID-fmk significantly (p<0.0001) inhibited IL-1β release upon contact with Eh (Fig 8A). To evaluate the contribution of cell death in active secretion of IL-1β as opposed to passive release following cell death, we also measured LDH levels in media as an indicator of cell death. LDH levels corresponded to up to (35%) of LDH levels from the positive lysis control, indicating that a portion of macrophages was killed by Eh following incubation though the majority was still intact (Fig 8B). Z-VEID pre-treatment of macrophages prior to Eh incubation did not significantly alter LDH release (Fig 8B). Therefore, caspase-6 activation does not seem to significantly contribute to cell death within the first 60 min. To quantify the contribution and upstream involvement of EhCP-A1 and EhCP-A4 to IL-1β secretion, we incubated macrophages with Eh pre-treated with WRR483, WRR605, or both. IL-1β secretion by macrophages was significantly decreased when stimulated with either inhibitor (p<0.0001), and more so when used in combination (Fig 8A). LDH release was significantly inhibited by WRR483 and WRR605 (p<0.003), or both (p<0.0002) indicating these CPs may contribute to macrophage cell death, although this may be mediated through a mechanism independent of caspase-6 (Fig 8B). With the use of a highly sensitive Luminex human focused 13-plex-discovery assay we determined that no other cytokine other than IL-1β was secreted at detectable levels, or dependent on caspase-6 (Fig 8C). IL-2, IL-4, IL-6, IL-12 and IL-13 secretion was undetectable by Luminex upon stimulation of THP-1 by Eh trophozoites. IL-5, GM-CSF, IFN-γ, MCP-1 and TNF-α were secreted at very low levels (less than 20pg/ml). We further confirmed a role for caspase-6 in THP-1-mediated secretion of IL-1β upon stimulation by Eh. Consistent with results obtained with the Z-VEID inhibitor, we found that silencing of caspase-6 by siRNA decreased IL-1β secretion in a concentration-dependent manner (Fig 8D). Taken together, these data show that EhCP-A1, EhCP-A4, and caspase-6 participate in the pro-inflammatory response of macrophages to Eh.
Fig 8. Inhibition of caspase-6 or EhCP-A1 and EhCP-A4 reduces IL-1β secretion by macrophages in response to Eh.
Caspase-6 was inhibited by pre-treatment of macrophages with 50mM of Z-VEID, or DMSO as control, prior to incubation with Eh (10:1 ratio) for 60 min. EhCP-A1 and EhCP-A4 were inhibited by pre-treatment of Eh with 20 μM of WRR483, or WRR605, or both, for 30 minutes prior to incubation with macrophages for 60 min. Both IL-1β secretion (a) and LDH release (b) were measured in media by ELISA. Results are representative of three independent experiments. Statistical significance was assessed by Student’s t-test (**p<0.003, ***p<0.0002). (c) Samples were also analyzed by Luminex addressable laser bead-based immunoassay (Human Focused 13-Plex Discovery Assay). (d) Caspase-6 involvement in IL-1β secretion by THP-1 cells was also assessed by knockdown with the indicated concentrations of caspase-6 siRNA. Scramble siRNA was used as a negative control. IL-1β levels shown are representative of two independent experiments (c, d).
Discussion
In this study, we determined the molecular events that take place at the intercellular junction between macrophages and Eh. We observed cytoskeletal rearrangement consistent with Eh engagement of cell-surface receptors, where microtubules reorient towards the target Eh, as well as actin accumulation at the contact point. This is reminiscent of many immune cell interactions such as the immune synapse and the phagocytic cup where receptor engagement, including integrins, induces microtubule polarization towards the target as well as actin accumulation at the site of contact [48, 49]. This polarization could be a means to deliver key signaling complexes at the contact site, including the NLRP3 inflammasome which has been shown to co-localize with actin at the contact site [29]. The α5β1 integrin as well as phosphorylated paxillin was also shown to localize at the contact site indicating downstream signaling occurred upon interaction [29]. We thus sought to further investigate post-translational regulation of key cytoskeletal-associated proteins upon macrophage contact with Eh. While we hypothesized that these proteins would be subjected to phosphorylation, what we observed was that these proteins were rapidly subjected to cleavage. Cleavage of talin, Pyk2 and paxillin by calpains or caspase-3 has been reported in the context of adhesion disassembly and apoptosis [40, 41, 50–52]. We cannot, however, preclude the fact that phosphorylation may precede cleavage and actually make these proteins susceptible to cleavage by inducing conformational change [38, 53]. This is supported by previous studies showing that serine phosphorylation of paxillin promotes its cleavage during cell migration [54]. Interference with the host cell cytoskeleton may be a mechanism by which Eh ingest host cells, either by trogocytosis or by phagocytosis. Trogocytosis is observed rapidly upon contact with live host cells that exhibit cell membrane deformability [31, 32]. Modification of the host cell cytoskeleton upon contact may represent the early molecular mechanisms allowing for cell deformability and subsequent ingestion of membrane portions by Eh. The process of trogocytosis, however, has not yet been reported in macrophages.
Our data unveil a new role for caspase-6 in the alteration of cytoskeletal-associated proteins, as well as in the macrophage inflammatory response to Eh. We show that cleavage of talin, Pyk2 and paxillin upon contact with Eh was mediated by caspase-6 activation as inhibition of caspase-6 by Z-VEID prevented their cleavage. In the latter, caspase-6 was shown to concentrate proximally to points of contact with Eh. Although caspase-6 is classified as an effector caspase along with caspase-3 and caspase-7, there is increasing evidence that its role extends beyond apoptosis [55]. A role for caspase-6 in B-cell activation and differentiation, and more recently in macrophage activation, has been shown [56, 57]. Moreover, caspase-6 is ubiquitously expressed and the highest levels of pro-caspase-6 are observed in fetal and adult colon [58]. Therefore, further studies in the role of caspase-6 in gut inflammatory responses are warranted to assess whether this mechanism is unique to amebiasis or whether caspase-6 plays a broader role in triggering cytokine secretion and cytoskeletal remodeling.
The caspase-6-mediated cleavage of cytoskeletal-associated proteins was only observed when macrophages were incubated with live Eh. Similar results were observed with NLRP3 inflammasome activation upon ligation of EhCP-A5 and Gal-lectin. Soluble EhCP-A5 or Gal-lectin alone was not sufficient to induce NLRP3 and caspase-1 activation and subsequent IL-1β release [29]. This likely reflects the importance that Eh factors to be delivered to the intercellular junction in a directed manner allowing for saturation of macrophage receptors to trigger downstream signaling cascades. We propose that this mechanism allows macrophages to distinguish between interactions of live invading Eh in comparison to sampling of soluble components of the gut lumen. The escalating pro-inflammatory response ensuing detection of live pathogens thus reflects the tailoring of the reaction against tissue-invading pathogens. The requirement for direct cell contact may also reflect the necessity of Eh to attach to host cells to release intracellular contents at the intercellular junction. In fact, EhCP-A1 and EhCP-A4, which we show are necessary for triggering cleavage of cytoskeletal-associated proteins, localize to intracellular vesicles in Eh [20, 44]. EhCP-A1 localizes to large cytoplasmic vesicles whereas EhCP-A4 is contained in acidic vesicles in Eh, yet has been shown to be released in culture medium as well as in the microenvironment in vivo. EhCP-A4 undergoes autocatalytic activation at acidic pH, provided by acidic vesicles, yet its optimal proteolytic activity is observed at physiological pH [44]. By staining the surface of non-permeabilized cells, we showed that both Eh cysteine proteinases are found at the cell surface, and polarized to the macrophage contact site. This supports a model by which these cysteine proteinases are delivered at the junction site in a defined manner, and have a role in triggering the macrophage inflammatory response. Future studies on the dynamics of EhCP-A1- and EhCP-A4-containing vesicles upon contact with host cells are necessary to uncover the mechanism of release in the microenvironment.
Our data unveil new roles for both EhCP-A1 and EhCP-A4 in eliciting pro-inflammatory responses by macrophages. Interestingly, EhCP-A4 expression is significantly higher in HM-1:IMSS than in the less virulent Rahman strain, whereas EhCP-A1 and EhCP-A5 transcription levels are similar between those two strains [45]. Similarly, EhCP-A1 release in medium is significant only for HM-1:IMSS compared to E. dispar and the less virulent L6 Eh clone [23]. EhCP-A1 expression has been shown to increase almost twofold following invasion in a mouse model of amebic colitis, whereas EhCP-A5 expression was unchanged [46]. Contrary to our initial hypothesis that EhCP-A5 would have a central role in regulating key cytoskeletal-associated proteins as it binds to macrophage integrin, it was not necessary for triggering cleavage of talin, Pyk2 or paxillin. Rather, the finding of another mechanism that relies on direct contact between macrophage and Eh, and that culminates in IL-1β release further reinforces that the intercellular junction is a hot spot for molecular events that shape the subsequent pro-inflammatory response during amebiasis. We propose an integrated model (Fig 9) by which contact between live Eh and macrophages is first established by binding via the Gal-lectin at the Eh cell surface as well as EhCP-A5 binding to macrophage α5β1 integrin. We hypothesize that contact mediates the release of EhCP-A1 and EhCP-A4 from Eh in a polarized fashion at the intercellular junction. The EhCP-A5/integrin axis mediates NLRP3 inflammasome activation whereas EhCP-A1 and EhCP-A4 mediate caspase-6 activation and subsequent cytoskeletal-associated protein cleavage. Together, these pathways culminate in potent IL-1β release from macrophages. The molecular mechanisms linking some of these events remain to be addressed. For example, the requirements for EhCP-A1 and EhCP-A4 release and interaction with macrophages as well as the signaling events that culminate in caspase-6 activation remain unknown. The consequence of post-translational modification of cytoskeletal-associated proteins in remodeling of the macrophage cytoskeleton upon contact with Eh also needs to be examined. Nonetheless, this study underlines the fact that the intercellular junction is a hotspot for initiation of the inflammatory response to Eh and further investigation will unravel mechanistic pathways that will provide a better understanding of macrophage and amebic biology.
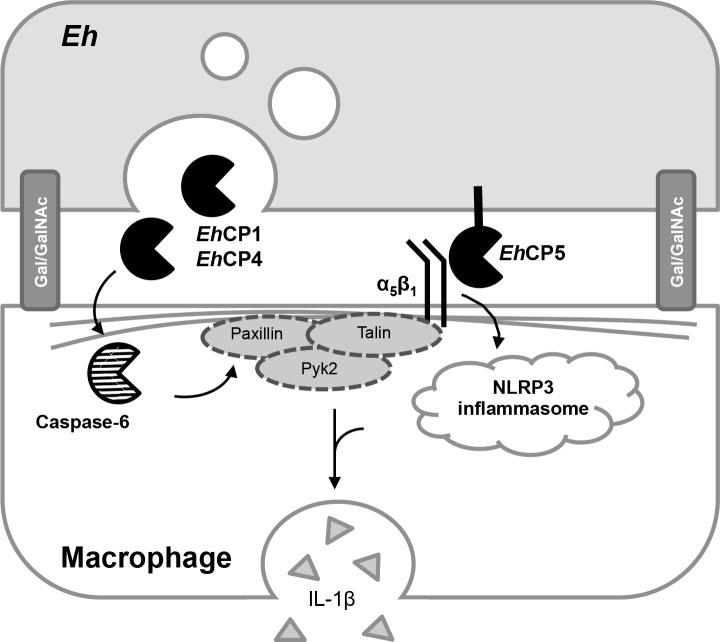
Fig 9. Schematic representation of the Eh-macrophage interacting proteins at the intercellular junction.
Contact between Eh and macrophages are initiated by high affinity binding with the Gal-lectin. EhCP-A5, which is expressed at the surface of Eh, has been shown to bind to the α5β1 integrin at the surface of macrophages through the RGD motif. This in turn activates the NLRP3 inflammasome and triggers IL-1β secretion by macrophages. In parallel, EhCP-A1 and EhCP-A4, which are localized in intracellular vesicles, are released at the intercellular junction. Through an unidentified mechanism, these cysteine proteinases trigger caspase-6 activation and subsequent cleavage of cytoskeletal-associated proteins paxillin, Pyk2 and talin. This arm of the Eh-macrophage interaction contributes to enhance IL-1β release.
Methods
Reagents
E-64 was obtained from Sigma-Aldrich. Caspase inhibitors were obtained from Enzo Life Sciences. Anti-paxillin (clone 349) was purchased from BD Biosciences. Anti-Pyk2 and anti-GAPDH were acquired from EMD Millipore. Anti-talin was obtained from Santa Cruz Biotechnologies. Anti-caspase-6 and anti-lamin were acquired from Cell Signaling Technology. Anti-mouse and anti-rabbit conjugated to HRP were from Jackson ImmunoResearch. Cell Tracker Blue, Cell Tracker Orange, A647-conjugated phalloidin and A488-conjugated anti-tubulin were obtained from Life Technologies. Recombinant human caspase-6 and the Z-VVR-AMC substrate were purchased from Enzo Life Sciences. The Z-Arg-Arg-pNA.2 HCl (Z-RR) substrate was purchased from Bachem. The FAM-FLICA caspase-6 assay kit was purchased from ImmunoChemistry Technologies. Transfection was carried out with the INTERFERin (Polypus) and caspase-6 siRNA (Santa Cruz Biotechnologies) or scramble siRNA (GE Dharmacon). Antibodies to EhCP1 and EhCP4, and WRR483 and WRR605 inhibitors were a gift from Dr. Sharon Reed, University of California, San Diego). Human IL-1β secretion in cell culture was quantified by ELISA (R&D Systems). LDH levels were measured using the CytoTox-ONE homogeneous membrane integrity assay (Promega).
E. histolytica culture
Eh trophozoites of the highly virulent HM-1:IMSS strain were grown axenically in TYI-S-33 medium supplemented with 100U/ml penicillin and 100μg/ml streptomycin sulfate as described previously [59]. EhCP-A5-deficient trophozoites were a gift from Dr. David Mirelman (Weizmann Institute of Science, Rehovot, Israel) and identically cultured. Virulence was maintained by regular sub passage in gerbil livers as previously described [60]. Trophozoites were harvested during log-phase growth by centrifugation at 200 x g at 4°C for 5 minutes and resuspended in RPMI.
Cell preparation and stimulation
The THP-1 monocytic cell line (ATCC, Manassas, VA) was cultured in RPMI with 10% FBS, 10mM HEPES, 50μM 2-mercaptoethanol, 100U/ml penicillin and 100μg/ml streptomycin sulfate in a humidified incubator with 5% CO2. THP-1 cells were differentiated into macrophages by seeding 8 x 105 cells per well in 12-well plates in complete medium supplemented with 50ng/ml phorbol-12-myristate-13-acetate (PMA) overnight. Bone marrow derived macrophages (BMM) were cultured from bone marrow cells of C57BL/6 mice and cultured for 6 days in complete medium supplemented with 30% L929-cell supernatant. Cells were then plated at 5 x 105 cells per well in 12-well plates in complete medium. On the day of experiment, BMDM were treated with 1μg/ml LPS for 3.5h prior to stimulation with Eh. The T84 epithelial cell line (ATCC, Manassas, VA) was cultured in DMEM/F-12 with 10% FBS, 10mM HEPES, 100U/ml penicillin and 100μg/ml streptomycin sulfate. For experiments, cells were seeded at 3 x 105 cells per well in 12-well plates and grown to a confluent monolayer.
For stimulation with Eh, cells were incubated at 37°C with in serum-free RPMI for the indicated times. Eh were washed off with cold PBS and cells were lysed in lysis buffer (1% Triton X-100, 20mM Tris, 100mM NaCl, 1mM EDTA, 200mM orthovanadate, sodium fluoride, 0.1% SDS, PMSF, leupeptin, aprotinin, and protease inhibitor cocktail). For inhibitor treatment of macrophages, PMA-differentiated THP-1 cells were pre-treated with inhibitors for 1h at 37°C in serum-free RPMI prior to incubation with Eh. For inhibition of adhesion, Eh were incubated with 55mM D-galactose, or glucose as control, for 5 min prior to incubation with THP-1 macrophages. For irreversible inhibition of total cysteine proteinase activity of Eh, Eh were incubated overnight in medium with 100μM of E-64, as previously described [61]. For inhibition of specific EhCP activity, Eh were incubated with 20μM of the EhCP-A1 inhibitor (WRR483), 20μM of the EhCP-A4 inhibitor (WRR605), or both, for 30 min and then washed prior to incubation with THP-1 macrophages.
In vitro enzymatic assays
To evaluate enzymatic specificity of the WRR483 and WRR605 inhibitors, known EhCP-A1 and EhCP-A4 substrates (Z-Val-Val-Arg and Z-Arg-Arg, respectively) were incubated for 0 to 20 minutes at 37°C with Eh trophozoites pre-treated with either WRR483, WRR605, both, or E-64 as described above. Cleavage of the Z-VVR-AMC fluorogenic substrate was detected at the 460nm wavelength, and cleavage of the chromogenic Z-RR-pNA.2 HCl substrate was detected at 405nm. For in vitro cleavage of proteins by caspase-6, THP-1 cells were lysed with lysis buffer as described above, and 10μg of lysate was incubated with anti-paxillin, anti-lamin or anti-mouse IgG for 15 min and 1.5 h with Protein A/G plus agarose. The immunoprecipitate was then incubated with 2 units of recombinant caspase-6 for 16 h at 37°C. Immunoprecipitates were then resuspended in reducing sample buffer and boiled prior to loading onto SDS-PAGE gel.
Caspase-6 siRNA
THP-1 cells were differentiated overnight with PMA prior to transfection as described above. Cells were transfected with caspase-6 siRNA, or scramble siRNA as a control, with the INTERFERin reagent, as per the manufacturer’s protocol. A total of 8uL of INTERFERin reagent was used for each transfection with caspase-6 siRNA, at the indicated concentrations, or scrambled siRNA as the control. Media was replaced with complete RPMI 24 h later. Eh stimulation was performed 48 h following siRNA transfection for 2 min for analysis by Western blot, or 60 min for assessment of IL-1β secretion.
Western blots
Equal amounts of lysate were loaded on SDS-PAGE gel followed by transfer onto polyvinylidene difluoride (PVDF) membrane and blocking in 5% skim milk. Western blots were done using the indicated primary antibody and appropriate HRP-conjugated secondary antibody, and visualized with either SuperSignal Chemiluminescence Reagents (Pierce Biotechnology) or ChemiLucent ECL detection (EMD Millipore). When sequential Western blots were performed, PVDF membranes were incubated with stripping buffer (25mM Glycine, 1% SDS, pH 2.0) for 30 min at room temperature, followed by extensive washing and blocking with 5% skim milk. None of the antibodies used cross-reacted with proteins in Eh lysate, as verified by Western blot.
Confocal microscopy
For visualization of Eh contact with THP-1, THP-1 cells were PMA-differentiated and seeded on glass coverslips overnight. Eh were stained with Cell Tracker Blue according to the manufacturer’s protocol prior to incubation with THP-1 cells for 15 min at 37°C. After incubation, cells were gently washed with PBS, fixed with 4% paraformaldehyde for 10 min, permeabilized with 0.1% NP-40 in PBS followed by staining with Alexa-488-conjugated anti-tubulin, or isotype control, and with Alexa-647-conjugated phalloidin. For visualization of caspase-6 activation in THP-1 cells following stimulation with Eh, Eh were stained with Cell Tracker Orange according to the manufacturer’s protocol prior to incubation with PMA-differentiated THP-1 cells for 15 min at 37°C. Slides were fixed and permeabilized as described above. Slides were stained with the FAM-FLICA caspase-6 probe as per the manufacturer’s protocol for 60 min, and with DAPI for 15 min for visualization of nuclei. Coverslips were then washed with the provided apoptosis washing buffer and mounted onto microscope slides with ProLong antifade (Thermofisher) and visualized using the Olympus IX81 FV1000 Fluoview Laser Scanning Confocal.
For staining of EhCP-A1 and EhCP-A4, THP-1 were seeded onto glass coverslips and incubated with Eh as described above. Slides were fixed in 4% paraformaldehyde for 15 min but not permeabilized to detect only extracellular surface-bound EhCPs. Following extensive washing post-fixation with 0.2% Tween 20 in PBS, slides were blocked with 5% normal donkey serum and subsequently incubated overnight with primary antibodies against EhCP-A1 and EhCP-A4 at 4°C. Slides were then washed and incubated for 60 min with the appropriate secondary antibodies (Donkey anti-Rabbit 1:250 and donkey anti-Chicken IgY 1:250) and DAPI at room temperature. Quantification of the abundance of activated caspase-6 at the THP-1/Eh interface was done in ImageJ as previously published30. Quantification of EhCP polarization was performed in ImageJ by measuring the integrated density of the front half of Eh and rear facing half, expressing this as a ratio adjusted for total area size. Figures for confocal images were created in Adobe Photoshop CS5.
Flow cytometry
PMA-differentiated THP-1 cells were harvested with TrypLE (ThermoFisher Scientific) for 5 min at 37°C followed by washing with serum-containing media. 106 THP-1 cells were incubated with Eh (10:1) and stained for 60 min with the FAM-FLICA caspase-6 reagent as per the manufacturer’s protocol. One sample of cells was also treated with STS for 3 h as a positive control for caspase-6 activation. A sample with Eh cells only was used to establish exclusion gates based on forward and side scatter and FAM-FLICA caspase-6-stained Eh showed no non-specific staining of trophozoites. Samples were washed with the washing buffer provided in the FAM-FLICA caspase-6 assay kit and fixed with 4% paraformaldehyde prior to acquisition with the BD FACSCanto Flow Cytometer. Data analysis was done with FlowJo.
Cytokine and LDH detection
PMA-differentiated THP-1 cells were incubated with Eh (10:1) in serum-free media for 60 min at 37°C. Supernatants were collected, centrifuged at 3000 x g for 5 min to spin out cells, and transferred to new eppendorf tubes. Quantification of IL-1β and LDH levels for each sample were measured by ELISA as per the manufacturer’s protocol. Samples were also analyzed by Luminex addressable laser bead-based immunoassay (Human Focused 13-Plex Discovery Assay; Eve Technologies, Calgary, AB).
Animals
C57BL/6 mice were obtained from Charles River. Asc-/- mice, bred onto a C57BL/6 background, were obtained from Drs. Muruve and Beck (University of Calgary). Femurs and tibia from these mice were used for the growth of bone-marrow-derived macrophages (BMM).
Ethics statement
The Health Sciences Animal Care Committee from the University of Calgary, have examined the animal care and treatment protocol (AC17-0017) and approved the experimental procedures proposed and certifies with the applicant that the care and treatment of animals used was in accordance with the principles outlined in the most recent policies on the “Guide to the Care and Use of Experimental Animals” by The Canadian Council on Animal Care.
Statistics
Experiments are representative of at least three independent experiments unless otherwise noted. Statistical significance between groups was assessed by Student’s t-test whereby p<0.01 was considered significant. For quantification of confocal images, a minimum of 6 images was used for each condition.
Acknowledgments
We are grateful to Dr. Sharon Reed for providing the WRR483 and WRR605 inhibitors, the EhCP-A1 and EhCP-A5 antibodies, and technical advice. We thank D. Muruve and P. Beck for the Asc-/- and Nlp3-/- mice. We thank Pina Colarusso and Jen Amon from the Snyder Institute live cell imaging facility for technical support.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by a Discovery Grant (RGPIN-2014-04023) from the Natural Sciences and Engineering Research Council of Canada awarded to KC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bercu T. E., Petri W. a & Behm J. W. Amebic colitis: new insights into pathogenesis and treatment. Curr. Gastroenterol. Rep. 9, 429–33 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Haque R., Huston C. D., Hughes M., Houpt E. & Petri W. A. Amebiasis. N. Engl. J. Med. 348, 1565–1573 (2003). doi: 10.1056/NEJMra022710 [DOI] [PubMed] [Google Scholar]
- 3.Guo X. et al. Leptin signaling in intestinal epithelium mediates resistance to enteric infection by Entamoeba histolytica. Mucosal Immunol. 4, 294–303 (2011). doi: 10.1038/mi.2010.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duggal P. et al. Influence of human leukocyte antigen class II alleles on susceptibility to Entamoeba histolytica infection in Bangladeshi children. J. Infect. Dis. 189, 520–6 (2004). doi: 10.1086/381272 [DOI] [PubMed] [Google Scholar]
- 5.Duggal P. et al. A mutation in the leptin receptor is associated with Entamoeba histolytica infection in children. J. Clin. Invest. 121, 1191–8 (2011). doi: 10.1172/JCI45294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marie C. S., Verkerke H. P., Paul S. N., Mackey A. J. & Petri W. A. Leptin protects host cells from Entamoeba histolytica cytotoxicity by a STAT3-dependent mechanism. Infect. Immun. 80, 1934–1943 (2012). doi: 10.1128/IAI.06140-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marie C. & Petri W. Jr a. Regulation of virulence of Entamoeba histolytica. Annu. Rev. Microbiol. 493–520 (2014). doi: 10.1146/annurev-micro-091313-103550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faust D. M. & Guillen N. Virulence and virulence factors in Entamoeba histolytica, the agent of human amoebiasis. Microbes Infect. 14, 1428–1441 (2012). doi: 10.1016/j.micinf.2012.05.013 [DOI] [PubMed] [Google Scholar]
- 9.Petri W. A., Smith R. D., Schlesinger P. H., Murphy C. F. & Ravdin J. I. Isolation of the galactose-binding lectin that mediates the in vitro adherence of Entamoeba histolytica. J. Clin. Invest. 80, 1238–1244 (1987). doi: 10.1172/JCI113198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chadee K., Petri W. A., Innes D. J. & Ravdin J. I. Rat and human colonic mucins bind to and inhibit adherence lectin of Entamoeba histolytica. J. Clin. Invest. 80, 1245–1254 (1987). doi: 10.1172/JCI113199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seguin R., Mann B. J., Keller K. & Chadee K. The galactose adherence lectin of Entamoeba histolytica activates primed macrophages for amebicidal activity mediated by nitric oxide. Arch Med Res 28 Spec No, 228–229 (1997). [PubMed] [Google Scholar]
- 12.Seguin R., Mann B. J., Keller K. & Chadee K. Identification of the galactose-adherence lectin epitopes of Entamoeba histolytica that stimulate tumor necrosis factor-alpha production by macrophages. Proc Natl Acad Sci U S A 92, 12175–12179 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivory C. P. A. & Chadee K. Activation of dendritic cells by the Gal-lectin of Entamoeba histolytica drives Th1 responsesin vitro andin vivo. Eur. J. Immunol. 37, 385–394 (2007). doi: 10.1002/eji.200636476 [DOI] [PubMed] [Google Scholar]
- 14.Houpt E. et al. Prevention of intestinal amebiasis by vaccination with the Entamoeba histolytica Gal/GalNac lectin. Vaccine 22, 611–617 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Haque R. et al. Amebiasis and mucosal IgA antibody against the Entamoeba histolytica adherence lectin in Bangladeshi children. J. Infect. Dis. 183, 1787–93 (2001). doi: 10.1086/320740 [DOI] [PubMed] [Google Scholar]
- 16.Mortimer L., Moreau F., Cornick S. & Chadee K. Gal-lectin-dependent contact activates the inflammasome by invasive Entamoeba histolytica. Mucosal Immunol. 7, 1–13 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Tillack M. et al. The Entamoeba histolytica genome: primary structure and expression of proteolytic enzymes. BMC Genomics 8, 170 (2007). doi: 10.1186/1471-2164-8-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark C. G. et al. Structure and content of the Entamoeba histolytica genome. Adv. Parasitol. 65, 51–190 (2007). doi: 10.1016/S0065-308X(07)65002-7 [DOI] [PubMed] [Google Scholar]
- 19.Bruchhaus I., Jacobs T., Leippe M. & Tannich E. Entamoeba histolytica and Entamoeba dispar: differences in numbers and expression of cysteine proteinase genes. Mol. Microbiol. 22, 255–263 (1996). [DOI] [PubMed] [Google Scholar]
- 20.Meléndez-López S. G. et al. Use of recombinant Entamoeba histolytica cysteine proteinase 1 to identify a potent inhibitor of amebic invasion in a human colonic model. Eukaryot. Cell 6, 1130–1136 (2007). doi: 10.1128/EC.00094-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Que X. et al. Cysteine proteinases from distinct cellular compartments are recruited to phagocytic vesicles by Entamoeba histolytica. Mol. Biochem. Parasitol. 119, 23–32 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Jacobs T., Bruchhaus I., Dandekar T., Tannich E. & Leippe M. Isolation and molecular characterization of a surface-bound proteinase of Entamoeba histolytica. Mol. Microbiol. 27, 269–276 (1998). [DOI] [PubMed] [Google Scholar]
- 23.Hirata K. K. et al. A phagocytosis mutant of Entamoeba histolytica is less virulent due to deficient proteinase expression and release. Exp. Parasitol. 115, 192–199 (2007). doi: 10.1016/j.exppara.2006.08.004 [DOI] [PubMed] [Google Scholar]
- 24.Tillack M. et al. Increased expression of the major cysteine proteinases by stable episomal transfection underlines the important role of EhCP5 for the pathogenicity of Entamoeba histolytica. Mol. Biochem. Parasitol. 149, 58–64 (2006). doi: 10.1016/j.molbiopara.2006.04.009 [DOI] [PubMed] [Google Scholar]
- 25.Ankri S., Stolarsky T., Bracha R., Padilla-Vaca F. & Mirelman D. Antisense inhibition of expression of cysteine proteinases affects Entamoeba histolytica-induced formation of liver abscess in hamsters. Infect. Immun. 67, 421–2 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kissoon-Singh V., Mortimer L. & Chadee K. Entamoeba histolytica cathepsin-like enzymes: Interactions with the host gut. Adv. Exp. Med. Biol. 712, 62–83 (2011). doi: 10.1007/978-1-4419-8414-2_5 [DOI] [PubMed] [Google Scholar]
- 27.Lin J. Y., Seguin R., Keller K. & Chadee K. Tumor necrosis factor alpha augments nitric oxide-dependent macrophage cytotoxicity against Entamoeba histolytica by enhanced expression of the nitric oxide synthase gene. Infect. Immun. 62, 1534–1541 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haque R. et al. Correlation of interferon-gamma production by peripheral blood mononuclear cells with childhood malnutrition and susceptibility to amebiasis. Am. J. Trop. Med. Hyg. 76, 340–4 (2007). [PubMed] [Google Scholar]
- 29.Mortimer L., Moreau F., Cornick S. & Chadee K. The NLRP3 inflammasome is a pathogen sensor for invasive Entamoeba histolytica via activation of alpha5beta1 integrin at the macrophage-amebae intercellular junction. PLoS Pathog. 11, e1004887 (2015). doi: 10.1371/journal.ppat.1004887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornick S. et al. Entamoeba histolytica cysteine proteinase 5 evokes mucin exocytosis from colonic goblet cells via αvβ3 integrin. PLOS Pathog. 12, e1005579 (2016). doi: 10.1371/journal.ppat.1005579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ralston K. S. Chew on this: Amoebic trogocytosis and host cell killing by Entamoeba histolytica. Trends in Parasitology 31, 442–452 (2015). doi: 10.1016/j.pt.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ralston K. S. et al. Trogocytosis by Entamoeba histolytica contributes to cell killing and tissue invasion. Nature 508, 526–30 (2014). doi: 10.1038/nature13242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faust D. M., Markiewicz J. M., Danckaert A., Soubigou G. & Guillen N. Human liver sinusoidal endothelial cells respond to interaction with Entamoeba histolytica by changes in morphology, integrin signalling and cell death. Cell. Microbiol. 13, 1091–1106 (2011). doi: 10.1111/j.1462-5822.2011.01604.x [DOI] [PubMed] [Google Scholar]
- 34.Faust D. M., Marquay Markiewicz J., Santi-Rocca J. & Guillen N. New insights into host-pathogen interactions during Entamoeba histolytica liver infection. Eur. J. Microbiol. Immunol. (Bp). 1, 10–8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avraham H., Park S.-Y., Schinkmann K. & Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell. Signal. 12, 123–133 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Brown M. C. & Turner C. E. Paxillin: Adapting to change. Physiol. Rev. 84, (2004). [DOI] [PubMed] [Google Scholar]
- 37.Kim C., Ye F. & Ginsberg M. H. Regulation of integrin activation. Annu. Rev. Cell Dev. Biol. 27, 321–345 (2011). doi: 10.1146/annurev-cellbio-100109-104104 [DOI] [PubMed] [Google Scholar]
- 38.St-Pierre J. & Ostergaard H. L. A Role for the protein tyrosine phosphatase CD45 in macrophage adhesion through the regulation of paxillin degradation. PLoS One 8, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chay K. O., Park S. S. & Frederic Mushinski J. Linkage of caspase-mediated degradation of paxillin to apoptosis in Ba/F3 murine pro-B lymphocytes. J. Biol. Chem. 277, 14521–14529 (2002). doi: 10.1074/jbc.M111639200 [DOI] [PubMed] [Google Scholar]
- 40.Shim S. R., Kook S., Kim J. Il & Song W. K. Degradation of focal adhesion proteins paxillin and p130cas by caspases or calpains in apoptotic rat-1 and L929 cells. Biochem. Biophys. Res. Commun. 286, 601–8 (2001). doi: 10.1006/bbrc.2001.5441 [DOI] [PubMed] [Google Scholar]
- 41.Marzia M. et al. Calpain is required for normal osteoclast function and is down-regulated by calcitonin. J. Biol. Chem. 281, 9745–9754 (2006). doi: 10.1074/jbc.M513516200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee Y. A., Kim K. A. & Shin M. H. Calpain mediates degradation of cytoskeletal proteins during Jurkat T-cell death induced by Entamoeba histolytica. Parasite Immunol. 33, 349–356 (2011). doi: 10.1111/j.1365-3024.2011.01290.x [DOI] [PubMed] [Google Scholar]
- 43.Mann B. J. et al. Neutralizing monoclonal antibody epitopes of the Entamoeba histolytica galactose adhesin map to the cysteine-rich extracellular domain of the 170-kilodalton subunit. Infect. Immun. 61, 1772–8 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He C. et al. A novel Entamoeba histolytica cysteine proteinase, EhCP4, is key for invasive amebiasis and a therapeutic target. J. Biol. Chem. 285, 18516–18527 (2010). doi: 10.1074/jbc.M109.086181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davis P. H., Schulze J. & Stanley S. L. Transcriptomic comparison of two Entamoeba histolytica strains with defined virulence phenotypes identifies new virulence factor candidates and key differences in the expression patterns of cysteine proteases, lectin light chains, and calmodulin. Mol. Biochem. Parasitol. 151, 118–128 (2007). doi: 10.1016/j.molbiopara.2006.10.014 [DOI] [PubMed] [Google Scholar]
- 46.Gilchrist C. A. et al. Impact of intestinal colonization and invasion on the Entamoeba histolytica transcriptome. Mol. Biochem. Parasitol. 147, 163–176 (2006). doi: 10.1016/j.molbiopara.2006.02.007 [DOI] [PubMed] [Google Scholar]
- 47.Debnath A., Tashker J. S., Sajid M. & McKerrow J. H. Transcriptional and secretory responses of Entamoeba histolytica to mucins, epithelial cells and bacteria. Int. J. Parasitol. 37, 897–906 (2007). doi: 10.1016/j.ijpara.2007.01.016 [DOI] [PubMed] [Google Scholar]
- 48.Niedergang F. Comparative anatomy of phagocytic and immunological synapses. 7, 1–9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freeman S. A. & Grinstein S. Phagocytosis: Receptors, signal integration, and the cytoskeleton. Immunol. Rev. 262, 193–215 (2014). doi: 10.1111/imr.12212 [DOI] [PubMed] [Google Scholar]
- 50.Franco S. J. et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat. Cell Biol. 6, 977–983 (2004). doi: 10.1038/ncb1175 [DOI] [PubMed] [Google Scholar]
- 51.Carragher N. O., Levkau B., Ross R. & Raines E. W. Degraded collagen fragments promote rapid disassembly of smooth muscle focal adhesions that correlates with cleavage of pp125 FAK, Paxillin, and Talin. J. Cell Biol. 147, 619–629 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glading A., Lauffenburger D. A. & Wells A. Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol. 12, 46–54 (2002). [DOI] [PubMed] [Google Scholar]
- 53.Li Y. et al. High stoichiometry phosphorylation of Talin at T144/T150 or S446 produces contrasting effects on calpain-mediated Talin cleavage and cell migration. J. Cancer 7, 1645–1652 (2016). doi: 10.7150/jca.14192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cortesio C. L., Boateng L. R., Piazza T. M., Bennin D. A. & Huttenlocher A. Calpain-mediated proteolysis of paxillin negatively regulates focal adhesion dynamics and cell migration * □. 286, 9998–10006 (2011). doi: 10.1074/jbc.M110.187294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Creagh E. M., Conroy H. & Martin S. J. Caspase-activation pathways in apoptosis and immunity. Immunol. Rev. 193, 10–21 (2003). [DOI] [PubMed] [Google Scholar]
- 56.Watanabe, C., Shu, G. L., Zheng, T. S., Flavell, R. A. & Clark, E. A. Caspase-6 regulates B cell activation and differentiation into plasma cells 1. [DOI] [PMC free article] [PubMed]
- 57.Yao Y. et al. Identification of Caspase-6 as a new regulator of alternatively activated macrophages. J. Biol. Chem. 291, 17450–17466 (2016). doi: 10.1074/jbc.M116.717868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Godefroy N., Foveau B., Albrecht S., Goodyer C. G. & LeBlanc A. C. Expression and activation of caspase-6 in human fetal and adult tissues. PLoS One 8, e79313 (2013). doi: 10.1371/journal.pone.0079313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diamond L. S., Harlow D. R. & Cunnick C. C. A new medium for the axenic cultivation of Entamoeba histolytica and other entamoeba. Trans. R. Soc. Trop. Med. Hyg. 72, 431–432 (1978). [DOI] [PubMed] [Google Scholar]
- 60.Denis M. & Chadee K. Cytokine activation of murine macrophages for in vitro killing of Entamoeba histolytica trophozoites. Infect. Immun. 57, 1750–1756 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hou Y., Mortimer L. & Chadee K. Entamoeba histolytica cysteine proteinase 5 binds integrin on colonic cells and stimulates NFκB-mediated pro-inflammatory responses. J. Biol. Chem. 285, 35497–35504 (2010). doi: 10.1074/jbc.M109.066035 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.