Abstract
Rhizoctonia solani Kühn infects most plant families and can cause significant agricultural yield losses worldwide; however, plant resistance to this disease is rare and short-lived, and therefore poorly understood, resulting in the use of chemical pesticides for its control. Understanding the functional responses of this pathogen during host infection can help elucidate the molecular mechanisms that are necessary for successful host invasion. Using the pathosystem model soybean-R. solani anastomosis group AG1-IA, we examined the global transcriptional responses of R. solani during early and late infection stages of soybean by applying an RNA-seq approach. Approximately, 148 million clean paired-end reads, representing 93% of R. solani AG1-IA genes, were obtained from the sequenced libraries. Analysis of R. solani AG1-IA transcripts during soybean invasion revealed that most genes were similarly expressed during early and late infection stages, and only 11% and 15% of the expressed genes were differentially expressed during early and late infection stages, respectively. Analyses of the differentially expressed genes (DEGs) revealed shifts in molecular pathways involved in antibiotics biosynthesis, amino acid and carbohydrate metabolism, as well as pathways involved in antioxidant production. Furthermore, several KEGG pathways were unique to each time point, particularly the up-regulation of genes related to toxin degradation (e.g., nicotinate and nicotinamid metabolism) at onset of necrosis, and those linked to synthesis of anti-microbial compounds and pyridoxine (vitamin B6) biosynthesis 24 h.p.o. of necrosis. These results suggest that particular genes or pathways are required for either invasion or disease development. Overall, this study provides the first insights into R. solani AG1-IA transcriptome responses to soybean invasion providing beneficial information for future targeted control methods of this successful pathogen.
Introduction
Rhizoctonia foliar blight (RFB) or aerial blight first appeared in Louisiana, U.S.A. in the 1950s on soybean (Glycine max (L.) Merr.) [1]. Recent outbreaks of the disease in Brazil and in the southern states of the U.S.A. have caused yield losses of 30–60% [2–4]. This diseases is caused by rain splashed soils containing sclerotia and mycelial fragments (asexual stage) that grow along the plant surface, eventually reaching the upper portion of the plant and spreading from leaf to leaf and plant to plant. RFB occurs in high humidity and temperate environments, and is most destructive following canopy closure and during seed pod development [5]. RFB of soybean is difficult to control as labeled fungicides and the use of less-susceptible cultivars has limited effectiveness [6–8]. Isolates of Rhizoctonia solani causing RFB have been characterized as anastomosis group (AG) 1 intraspecific group or subgroup IA [9] with populations that are associated with Fabaceous hosts (soybean), and others that are taxonomically related, yet genetically distinct and infect Poaceae hosts (e.g. rice) [4, 10].
The sheath blight disease (SBD) of rice caused by R. solani AG1-IA has been extensively studied due to its economic importance [11, 12]; however, studies examining its interactions with other economically important crops, such as soybean, have yet to be extensively studied. Recently, the draft genome sequence of AG1-IA rice isolate B275 was established and made publically available [12] enabling insights into the gene structure and functionality of the pathogen, and allowing for more comprehensive genetic studies of the AG1-IA rice pathogen. To date, only one study has utilized the genome to examine the global transcript responses of R. solani AG1-IA during root rot of turf grass (Zoysia japonica) [13].
Although certain effectors have been well studied in certain pathogens [14], studies examining the global omics fluctuations that occur following and during host invasion are few [15]. Little is known about the molecular components responsible for susceptibility or resistance of soybean to R. solani AG1-IA isolates. This information is crcuial because of the impact of RFB on agriculture. Understanding virulence of the pathogen and identifying genes during its interaction with soybean is not only undeniably vital for plant breeders, but would facilitate improved control strategies for this important pathogen.
Omics technologies allow for a more in-depth understanding of biological system responses to perturbations and are a promising tool for the examination of host-pathogen interactions. RNA sequencing (RNA-seq) provides a largely unbiased method to comprehensively and systematically define the transcript fluctuations of an organism in a manner that is significantly more sensitive than microarray hybridization approaches [16]. This technology has become available as a powerful tool to investigate the transcriptional profiles of microbes including plant pathogens [13, 17, 18]. RNA-seq is able to identify novel fungal pathogen transcripts such as pheromone receptors [12] and capsule formation genes [13], advocating its use as a promising tool for understanding plant-pathogen interactions, and in extent for development of alternative control methods and resistant cultivars.
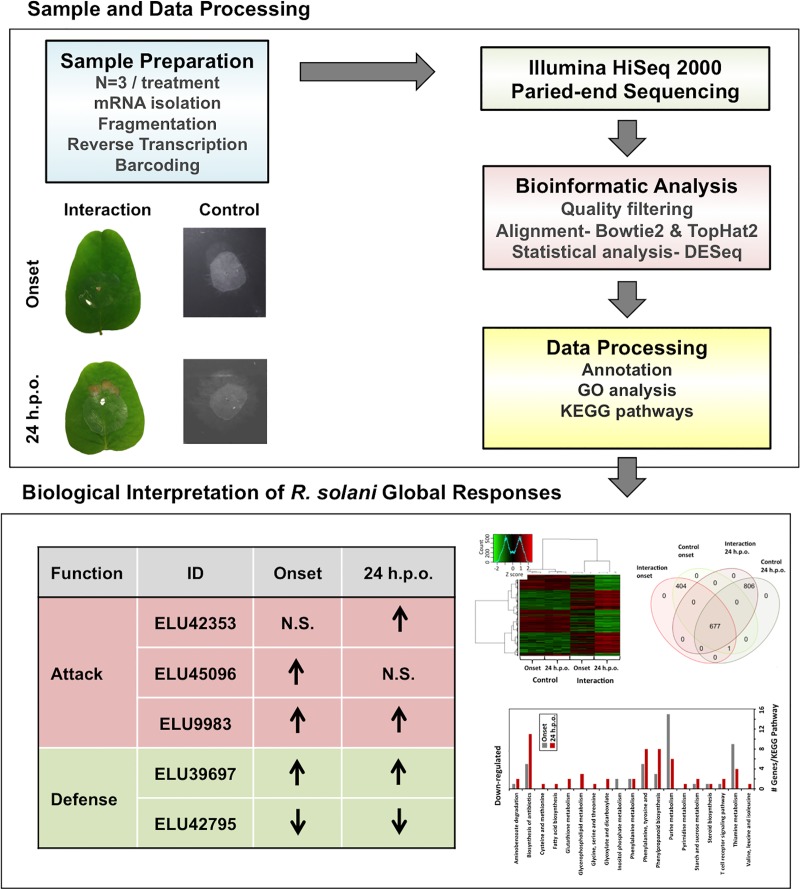
Here, we have taken advantage of high throughput RNA-seq to report a first comprehensive study on global transcriptome fluctuations of R. solani AG1-IA strain ROS-2A4 at onset and late necrosis stages of RFB disease development on soybean. The detailed in silico analysis (Fig 1) revealed modulation of multiple molecular pathways, and fluctuations in genes encoding virulence factors and stress responses whose expression analysis reflected many that exhibit differential gene expression during disease development.
Fig 1. Flowchart of steps taken for R. solani AG1-IA RNA-seq sample preparation and data analysis.
Hyphae of R. solani, grown on ¼ strength PDA (control) or on soybean unifoliate leaves were harvested and processed for RNA sequencing using the Illumina HiSeq 2000 platform. Data were analyzed using standard analytical pipelines for gene annotation and differential expression analysis. Data were further compared using heatmap analysis and gene ontology terms, and affected pathways examined using Kyoto encyclopedia of genes and genomes (KEGG).
Materials and methods
Growth and maintenance of Rhizoctonia solani AG1-IA
The highly virulent Rhizoctonia solani AG1-IA strain ROS-2A4 was obtained from P. Ceresini, University of São Paulo State (UNESP), Brazil. R. solani cultures were revived from stock cultures maintained at -80°C by placing mycelial plugs on fresh potato dextrose agar (PDA) for one week at 24°C in the dark. One-week-old cultures were sub-cultured onto fresh PDA until sclerotia initials began forming (approximately 10 days). Sclerotia initials were used for R. solani inoculation experiments.
Soybean growth and Rhizoctonia solani infection conditions
Soybean (Glycine max) cv. Williams 82 seeds were surface sterilized in 30% hydrogen peroxide for 7 minutes followed by 5 rinses in sterile water and imbibed on sterile filter paper moistened with 20 mL of water for 48 hours. Seeds with similar emergence rates were planted in Cone-tainers® containing 130 mL of Agro-Mix G10 (Fafard, Ltd., Saint-Bonaventure, Canada) and sand (1:1 v/v) and watered with 30 mL of sterile water every two days. To study R. solani disease progression, a detached leaf assay was conducted. These assays have been shown to have high correlations between field and greenhouse pathology trial assessments [19, 20]. To do so, 20 fully expanded soybean unifoliate leaves originating from 20 different plants were detached from 2 week-old plants. Leaves were then placed on a moistened filter paper in 15 cm Petri dishes such that each Petri dish contained 5 unifoliate leaves. A biological replicate consisted of 4 Petri dishes containing 20 pooled leaves. A total of 3 biological replicates were prepared for each time point.
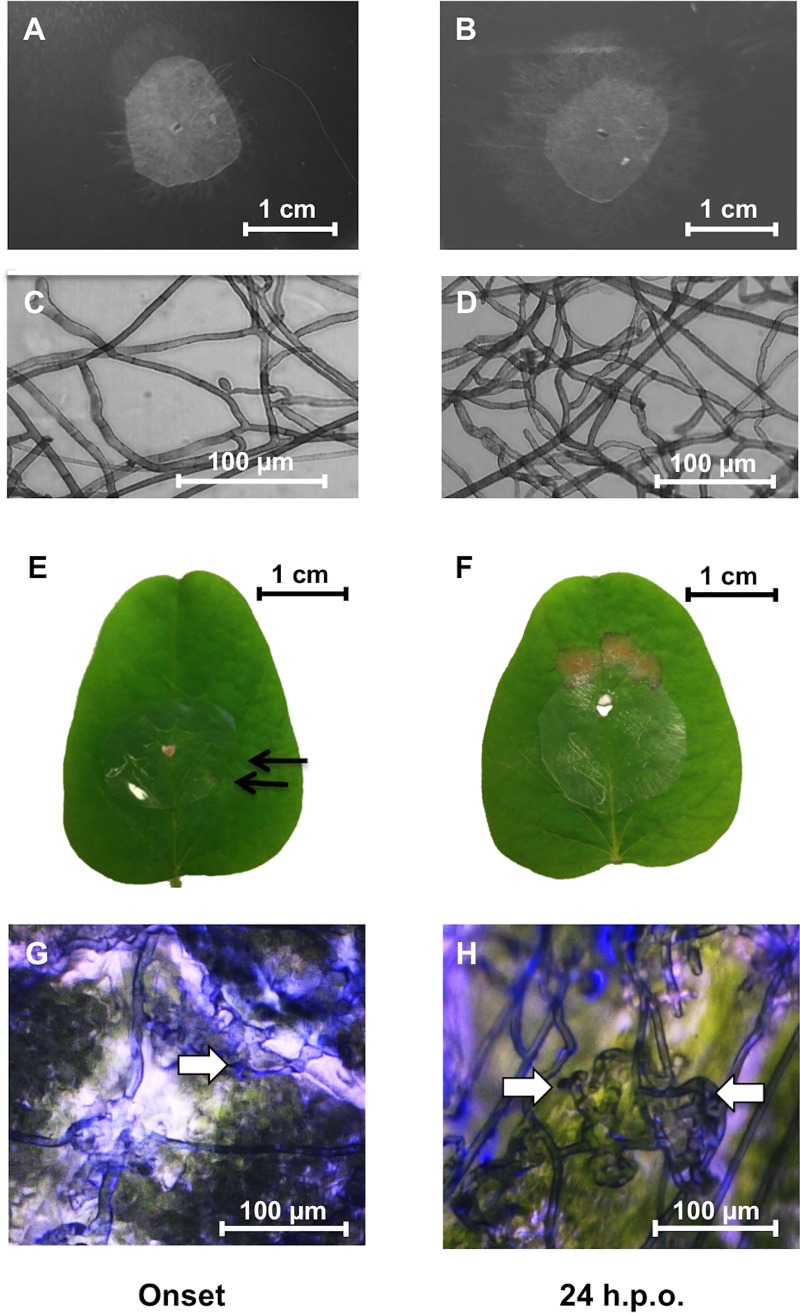
In order to obtain the maximum number of RNA-seq reads from R. solani, an infection system was developed enabling the collection of sufficient mycelia for RNA-seq library construction with minimal soybean RNA contamination. A sterile moistened nitrocellulose membrane (1 cm in diameter) was placed in the center of each unifoliate soybean leaf, and inoculated with a sclerotia initial of R. solani AG1-IA placed in the middle of the cellulose membrane (Fig 2). Control treatments consisted of sclerotia initials placed in the center of cellulose membranes (1 cm diameter) overlaid on ¼ strength PDA. The use of membranes in interaction and control treatments facilitates the harvesting of the mycelium while excluding unwanted plant tissue or PDA, respectively. The use of porous nitrocellulose membranes is a common practice when studying R. solani molecules during plant [21, 22] and microbe-microbe interactions [23]. The absence of infection structures and hyphal aggregates on membranes from samples grown on PDA (Fig 2) corroborates earlier findings that these structures are not governed by contact stimuli [22, 24]. Petri dishes were sealed with parafilm and placed in growth chambers at 24/22°C day/night with 12 h day/night cycles and humidity maintained at 65% throughout the day. Samples were harvested at onset and 24 h post-onset (h.p.o.) of necrosis (Fig 2). Onset of necrosis was determined by examining the leaves every hour until necrotic lesions (<1 mm), hyphal aggregation and infection cushion initials were visible on the leaf and the overlaid membrane (Fig 2G and 2H). The mycelia from control samples (PDA) and infected leaves were peeled from the membranes and immediately flash frozen in liquid nitrogen until further use. A total of 20 membrane discs were pooled together for each sample for a total of 3 biological replicates per treatment per time point.
Fig 2. Rhizoctonia solani-soybean interactions at onset and 24 h.p.o. of necrosis.
In vitro controls on PDA harvested at onset (A) and 24 h.p.o. (B) of necrosis. Microscope images of hyphae on nitrocellulose membranes in vitro at onset (C) and 24 h.p.o. (D) of necrosis showing normal growth and lack of hyphal aggregates and infection structures. Soybean leaf samples infected with R. solani AG1-IA at onset (E) and 24 h.p.o. (F) of necrosis. Arrows indicate the onset of necrotic lesions approximately 36 h post-inoculation. Microscope images of hyphae on nitrocellulose membranes from soybean leaves at onset (G) and 24 h.p.o. (H) of necrosis showing infection cushion structures (arrows). Note that hyphal aggregation and infection cushion initials occurred only in R. solani samples grown on membranes overlaid on leaves (G, H) and not on PDA (C, D).
RNA extraction and RNA-seq library preparation
Total RNA was extracted from mycelial samples using Trizol® (Diamed, Mississauga, Canada) following the manufacturer protocols. Briefly, 50 mg of hyphae per sample were ground to a fine powder in liquid nitrogen and 1 mL of Trizol added to each sample. Total RNA in the supernatant was purified using a chloroform wash, precipitated using isopropanol and dissolved in RNase-free water. Total RNA quantity and quality were measured using a NanoDrop ND-1000 Spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and denaturing formaldehyde gel electrophoresis, respectively. RNA-seq libraries were prepared for each replicate from 4 μg total RNA using the KAPA stranded mRNA-Seq kit (KAPA Biosystems, Inc., Wilmington, MA) with slight modifications. Briefly, mRNA was captured and purified by performing two purifications on the KAPA mRNA capture beads by mixing total RNA with 50 μL of beads, heating at 65°C for 5 minutes and cooling with shaking at 150 rpm at room temperature for 20 minutes. cDNA libraries with fragment lengths of 200–300 bp mRNA were constructed following the manufacturer protocols. The 12 libraries were barcoded using NEXTflex RNA-seq barcodes (BiooScientific, Toronto, Canada) 1 through 12 (v1-1-15-1), and amplified using the following conditions: initial denaturation at 98°C for 45 s, followed by 12 cycles of denaturation at 98°C for 15 s, annealing at 60°C for 30 s and elongation at 72°C for 30 s, and a final extension at 72°C for 5 minutes. Libraries were purified using NucleoMag NGS Clean-up and Size Selection beads (Machery-Nagel, Bethlehem, PA, USA) and quantified by a NanoDrop ND-1000 Spectrophotometer, and the size and quality were confirmed by agarose gel electrophoresis (2%). Libraries were pooled such that one final library contained equal amounts of each of the 12 barcoded libraries and the final quality and quantity were confirmed using a bioanalyzer at the Genome Quebec Research Centre (Montreal, Canada). The library was sequenced at the Genome Quebec Research Centre (Montreal, Canada) and sequenced in one lane using the Illumina HiSeq 2000 system with 100 bp paired-end sequencing.
Bioinformatics analysis
Illumina reads were separated by barcodes following the Genome Quebec pipeline (available at https://bitbucket.org/mugqic/mugqic_pipelines). Illumina adapters were removed and reads with average phred scores below 20 and lengths below 50 bp were removed using the software Trimmomatic version 0.35 [25]. Alignment of trimmed and clean reads was done for each library using TopHat2 v2.0.8b [26] with alignments to the R. solani AG1-IA genome and transcriptome Rhisol_AG1IA version 1.0 [12] available on NCBI (taxid: 983,506; BioProject Accession PRJNA51401), and also to the soybean genome v1.1 [27] available on the JGI Genome Portal (http://genome.jgi-psf.org/) using default settings. Reads aligning to the soybean genome were removed prior to alignment to the R. solani genome and annotation.
Transcript relative quantification and differential abundance analysis
Reads were counted using HTseq version 0.5.4p3 [28] and normalized using the estimateSizeFactors and variance stabilized using varianceStabilizingTransformation commands in the R statistical package DESeq version 1.14.0 [29]. First, differences between time points and detection of outliers were detected using PCA and Hotelling’s T2 confidence (95%) analysis on normalized and variance stabilized sequence counts using SIMCA-P+ v.13.0.3.0 software (Umetrics). Statistical analysis for differential gene expression was performed using the R software package DESeq version 1.14.0 using negative binomial comparisons between all treatments [29]. Differentially expressed genes (DEGs) were identified using the following criteria: 1) a fold change value >3 or <-3 and; 2) a Benjamini-Hochberg [30] false discovery rate (FDR) <0.01. Heatmap analysis using variance stabilized data of expressed genes and the command heatmap.2 from the R package gplots [31]. A Venn diagram to compare the differentially expressed genes within each treatment was constructed using the R packages VennDiagram [32] and limma [33].
Transcript annotation, functional annotation and pathway mapping
Sequence identification was done first by comparing reads to the publically available R. solani AG1-IA annotation fasta file available on the EnsemblFungi server (http://fungi.ensembl.org), and by using the BLASTx algorithm of the Blast2GO software version 3.2 [34, 35] at a statistical significance threshold of 1.0E-6. Functional categories were assigned using the Gene Ontology (GO) Slim terms using the Blast2GO software version 3.2 [34, 35]. Integration of InterPro Scan and ANNEX functions was done for improved functional annotation [34, 36, 37]. To determine if particular GO terms were over- or under-represented in the DEGs, enrichment analysis of the GO terms in all GO categories (biological process, molecular function and cellular component) was performed using a Fisher’s exact test and a FDR threshold <0.05. To determine to which metabolic pathways DEGs belonged, genes were assigned Kyoto Expedia of Genes and Genomes (KEGG) enzyme codes [38] using the Blast2GO GO-enzyme code mapping function [34] (S1 and S2 Tables).
Quantitative real-time PCR validation
Differentially expressed genes identified from sequencing data were confirmed by qRT-PCR analyses on 13 genes (S3 Table). To do so, samples were harvested from a second trial conducted under identical conditions and similar time points as previously described. Total RNA was extracted from the second trial samples using the Trizol method described above and 1 μg was converted to cDNA using the QuantiText Reverse Transcription Kit (Qiagen, Toronto, Canada) following the manufacturer protocols. Each 15 uL qRT-PCR reaction contained 1X SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories Ltd.), 0.175–0.25 μM each primer (S3 Table), and 500 ng cDNA. The thermocycling profile used an initial denaturation at 95°C for 3 min, followed by 35 or 40 cycles of denaturation at 95°C for 30 s, annealing for 30 s at the appropriate primer temperature (S3 Table) and extension at 72°C for 40 s, followed by a dissociation curve analysis. Gene expression was analyzed using the method of [39] with normalization over the housekeeping gene histone 3 (ELU43810). Transcript levels were quantified in three biological replicates per treatment and significant differences determined using analysis of variance (ANOVA) and student’s t test at the 95% significance level using R statistics software. Fold change differences ≤ -1.5 or ≥ 1.5 with P<0.05 were used for qRT-PCR rather than ≤ -3 or ≥ 3 due to the differences in methods of normalization between RNA-seq and qRT-PCR [40, 41].
Availability of supporting data
All data discussed in this publication have been deposited in the NCBI Sequence Read Archive (SRA) under the BioProject PRJNA369092.
Results
Transcriptomic analysis
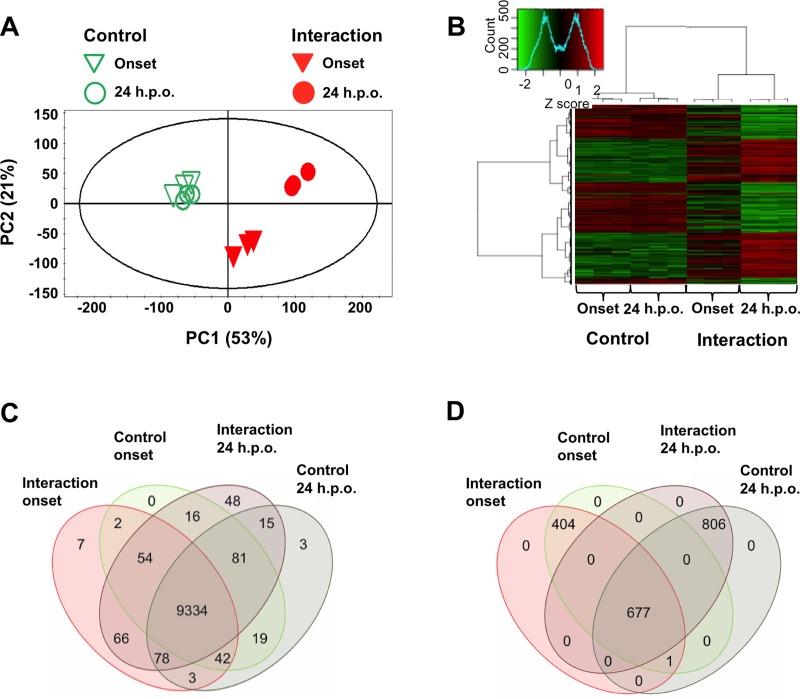
Onset of necrosis occurred approximately 36 hours post-inoculation (Fig 2), whereas full-blown necrosis was evident by approximately 60 hours post-inoculation (Fig 2). Libraries of twelve samples were sequenced on a single Illumina HiSeq 2000 sequencing lane resulting in high quality reads ranging from 11 to 13 million reads per sample (S4 Table). Alignment with TopHat2 showed that less than 1% of reads aligned to the soybean genome, indicating that the membrane method developed for leaf infection was effective and sufficient for extracting highly purified R. solani RNA during biological interactions. Principal component analysis (PCA) revealed tight clustering of all control samples and no outliers, irrespective of time of harvest, and clear separation of the soybean-R. solani interaction samples (Fig 3A). This was further supported by hierarchical cluster analysis (HCA) and heatmap analysis of the top 40% differentially expressed genes (Fig 3B). A clear separation was seen between fungal control and fungal interaction samples, with strong differential gene expression at 24 h.p.o. of necrosis, whereas DEG of fungal control samples were grouped together (Fig 3A and 3B). The differential expression data of onset of necrosis were grouped closer with those of both the control samples indicating that differential gene regulation was just commencing (Fig 3A and 3B).
Fig 3. Overview of analysis of Rhizoctonia solani differentially expressed genes at onset and 24 h.p.o. of necrosis in soybean.
(A) PCA score plot of R. solani-soybean interactions (solid inverted triangles and circles) and controls (open inverted triangles and circles) at onset of necrosis (inverted triangles) and 24 h.p.o. of necrosis (circles). (B) Heatmap and hierarchical cluster analysis of R. solani-soybean interactions at onset and 24 h.p.o. of necrosis. Dendrograms were constructed using the Ward method [42]. (C) Venn diagram of transcriptionally active genes (at least 2 reads per sample in 2/3 biological replicates for any treatment) and the treatments in which they were detected (n = 3 per treatment). (D) Venn diagram of the 1,888 differentially expressed genes and the treatments in which they were detected (n = 3 per treatment). A number of zero indicates that no genes were found to be unique to the specific comparison.
Genes were considered transcriptionally active if there were at least two reads per sample in two out of three biological replicates of any of the treatments. A total of 9768 genes (93.1%) out of the 10,489 currently identified R. solani AG1-IA coding sequences were transcriptionally active across the two time points (Fig 3C) [12]. A total of 11% and 15% of the expressed transcriptome was differentially expressed representing a total of 1082 and 1484 differentially expressed genes (DEGs) with fold-change values of +/- 3 at onset of necrosis and 24 h.p.o. of necrosis compared to their controls, respectively (S5 and S6 Tables). Of the DEGs, 678 were common between the two time points (Fig 3D). Comparison between the two time points in infected leaves resulted in 727 DEGs (S7 Table).
Annotation and gene ontology analysis of differentially expressed transcripts
Transcripts were annotated using the publically available annotated fasta file and BLASTx, followed by annotation enhancement using the InterproScan database [37] and the ANNEX augmentation procedure [36], resulting in a total of 1204 annotated genes or 63.8% of the DEGs. The top 20 annotated differentially up- and down-regulated genes at each time point are presented in S8 and S9 Tables respectively.
Enrichment analysis of the DEGs with the reference genome revealed significantly under- and over-represented gene ontology (GO) slim terms [43] at both time points (Table 1). Fewer GO slim terms were affected at onset compared to 24 h.p.o. of necrosis, with GO terms being under-represented at onset of necrosis. A total of 3 GO slim terms were under-represented at onset, whereas no over-representation was observed at this time point. Four under-represented and 23 over-represented GO slims were identified 24 h.p.o. (Table 1). Of these, none were common between the two time points.
Table 1. Gene ontology slim terms over- and under-represented in Rhizoctonia solani during soybean infection.a.
| GO Slim ID | Term | Classificationb | |
|---|---|---|---|
| Onset, Under-represented | GO:0005623 | cell | C |
| GO:0044464 | cell part | C | |
| GO:0005622 | intracellular | C | |
| 24 h.p.o., Over-represented | GO:1990904 | ribonucleoprotein complex | C |
| GO:0030529 | intracellular ribonucleoprotein complex | C | |
| GO:0005840 | ribosome | C | |
| GO:0043228 | non-membrane-bounded organelle | C | |
| GO:0043232 | intracellular non-membrane-bounded organelle | C | |
| GO:0003735 | structural constituent of ribosome | F | |
| GO:0005198 | structural molecule activity | F | |
| GO:0006518 | peptide metabolic process | P | |
| GO:0006412 | translation | P | |
| GO:1901566 | organonitrogen compound biosynthetic process | P | |
| GO:0034645 | cellular macromolecule biosynthetic process | P | |
| GO:1901576 | organic substance biosynthetic process | P | |
| GO:0044249 | cellular biosynthetic process | P | |
| GO:0043043 | peptide biosynthetic process | P | |
| GO:0043604 | amide biosynthetic process | P | |
| GO:0043603 | cellular amide metabolic process | P | |
| GO:0009059 | macromolecule biosynthetic process | P | |
| GO:0044271 | cellular nitrogen compound biosynthetic process | P | |
| GO:1901564 | organonitrogen compound metabolic process | P | |
| GO:0042254 | ribosome biogenesis | P | |
| GO:0010467 | gene expression | P | |
| GO:0022613 | ribonucleoprotein complex biogenesis | P | |
| GO:0044085 | cellular component biogenesis | P | |
| 24 h.p.o., Under-represented | GO:0043227 | membrane-bounded organelle | C |
| GO:0043231 | intracellular membrane-bounded organelle | C | |
| GO:0005634 | nucleus | C | |
| GO:0016740 | transferase activity | F |
aEnrichment analysis of the GO terms in all GO categories (biological process, molecular function and cellular component) was performed using a Fisher’s exact test and an false discovery rate threshold <0.05.
bClassification based on cellular component (C), molecular function (F) or biological process (P).
Metabolic classification of Rhizoctonia solani differentially expressed genes
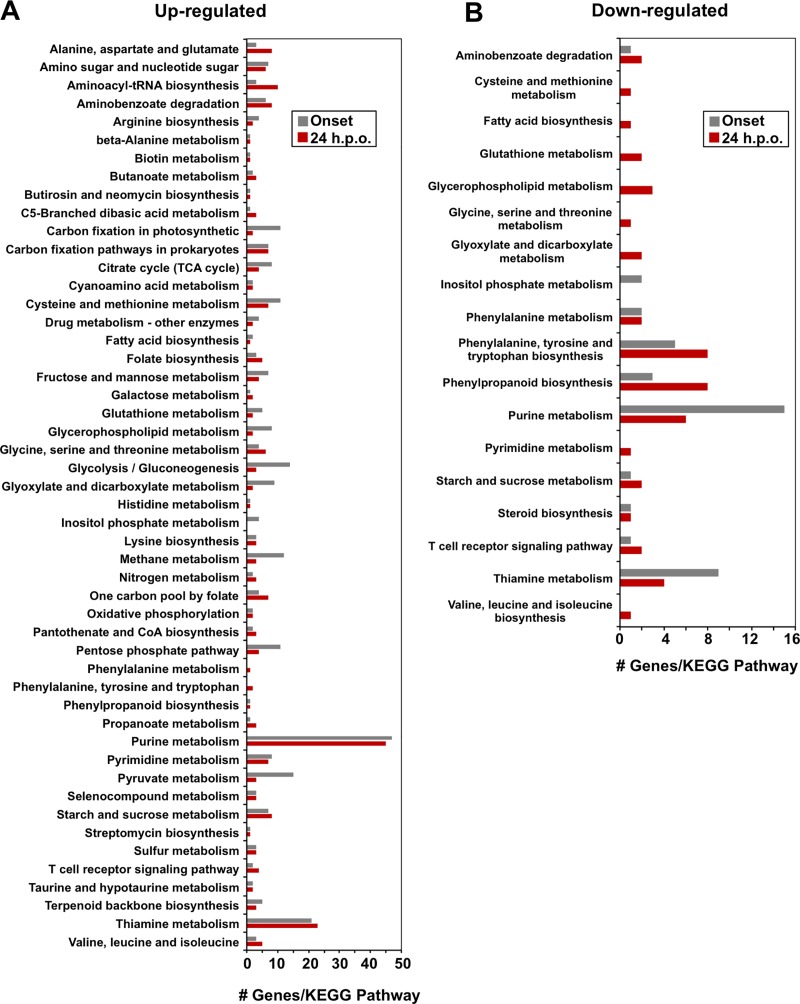
KEGG pathway analysis indicated that DEGs were implicated in a number of pathways during R. solani-soybean interactions (Fig 4 and S1 Fig). Mapping of annotated DEGs to KEGG pathways resulted in 116 DEGs mapping to 61 pathways and 151 DEGs mapping to 64 pathways at onset and 24 h.p.o. of necrosis, respectively, for a total of 52 KEGG pathways containing DEGs affected at both time points (Fig 4). The highest representation among genes that were up-regulated vs down-regulated at onset and 24 h.p.o. of necrosis were involved in purine metabolism (47 and 45 genes vs. 15 and 6, respectively) and thiamine metabolism (21 and 23 vs 9 and 4, respectively).
Fig 4. KEGG pathway annotations common between Rhizoctonia solani-soybean interaction time points.
KEGG pathway annotations common between R. solani-soybean interactions at onset (grey) and 24 hours-post onset (red) of necrosis for differentially expressed genes. (A) KEGG pathways commonly containing up-regulated DEGs, (B) KEGG pathways commonly containing down-regulated DEGs (n = 3 per treatment). Numbers represent the number of differentially expressed genes with fold change values +/- 3 detected for each KEGG pathway.
In some cases, very few DEGs mapped to particular KEGG pathways, including those that are up-regulated and associated to toxin degradation (nicotinate and nicotinamid metabolism), the synthesis of anti-microbial compounds (aflatoxin biosynthesis, novobiocin biosynthesis, and penicillin and cephalosporin biosynthesis) and pyridoxine (vitamin B6) biosynthesis at either onset or 24 h.p.o of necrosis (S1 Fig). Similarly, very few down-regulated genes were mapped to KEGG pathways involved in the degradation of xenobiotics as well as ascorbate and aldarate metabolism (S1 Fig).
Validation of transcripts by real-time PCR
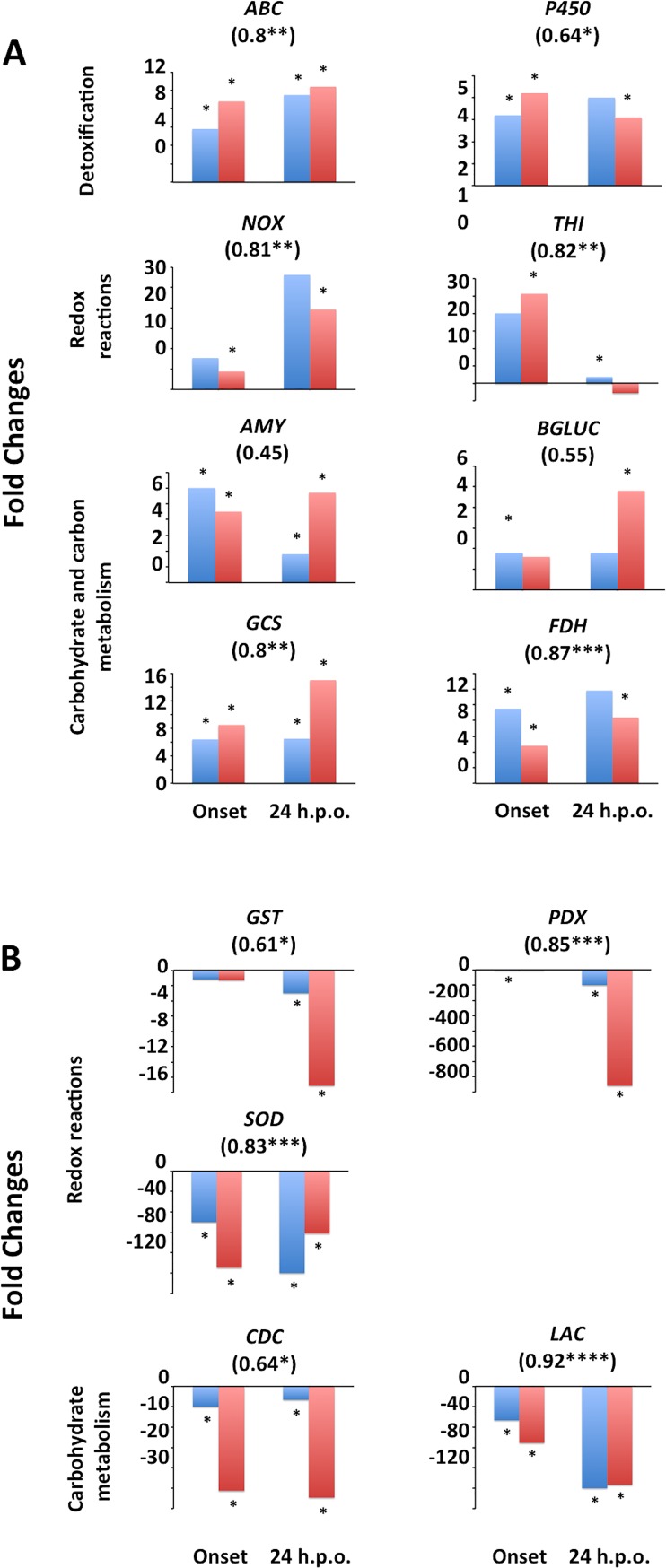
Thirteen genes, including 5 (NOX, THI, GST, PDX, SOD) linked with oxidative stress function, 6 (CDC, LAC, AMY, BGLUC, GCS, FDH) with carbohydrate and carbon metabolism, and 2 (ABC, P450) with detoxification or degradation of toxins were validated to be differentially expressed at the two time points using qRT-PCR (Fig 5). Examination of transcript abundance between RNA-seq and qRT-PCR transcript abundances showed similar trends between the two methods (Fig 5). Correlation analysis was performed between RNA-seq and qRT-PCR relative abundances for each gene. Among the 13 genes, 8 (ABC, NOX, THI, GCS, FDH, PDX, SOD and LAC) were highly correlated (r ≥0.75), 3 (P450, GST and CDC) were moderately correlated (r ≥0.5 and <0.75) and 2 (AMY and BGLUC) had poor correlations (r <0.5) (P<0.05). Although variations in correlations were observed as samples originated from different trials, and the two methods use different calculations for normalization of transcripts, strong correlations and similar trends in transcript abundances were generally observed confirming the observed trends in changes in transcript abundances.
Fig 5. Rhizoctonia solani transcript abundance fold changes in response to infection of soybean.
Transcript abundances were quantified using qRT-PCR (blue) or RNA-seq (red) in R. solani cultures infecting soybean compared to controls in vitro at onset of necrosis or 24 h.p.o. of necrosis. (A) Up-regulation of transcripts during infection of soybean. (B) Down-regulation of transcripts during infection of soybean. Stars represent fold changes that were statistically (P<0.01 for RNA-seq or P<0.05 for qRT-PCR) and biologically (fold change ≤ -3 or ≥ 3 for RNA-seq or ≤ -1.5 or ≥ 1.5 for qRT-PCR) significant (n = 3 per treatment). Numbers below gene names represent Spearman’s correlation coefficients with stars representing significance thresholds of: *P ≤0.05, **P ≤0.01, ***P ≤0.001 or ****P ≤0.0001. ABC, ABC transporter; AMY, ALPHA-AMYLASE; BGLUC, BETA-GLUCOSIDASE; CDC, CHITIN DEACETYLASE; FDH, FORMATE DEHYDROGENASE; GCS, GLYCOGEN SYNTHASE; GST, GLUTATHIONE-S-TRANSFERASE; LAC, LACCASE PRECURSOR; NOX, NADH OXIDASE; P450, CYTOCHROME P450 MONOXYGENASE PC-3; PDX, PYRIDOXAL-DEPENDENT DECARBOXYLASE; SOD, Cu/Zn SUPEROXIDE DISMUTASE; THI, THIAMINE BIOSYNTHESIS.
Discussion
The use of RNA-seq has provided substantial insights into plant-pathogen interactions; however, only recently have studies begun to examine the global transcriptome responses of phytopathogens such as Rhizoctonia solani while infecting host plants [12, 13]. Although RNA-seq can provide information on both the host and pathogen, previous studies have reported that such studies provide few reads and therefore little information on the pathogen responses [41, 44, 45]. As such, a method using nitrocellulose membranes to obtain an in-depth and thus highly informative study of R. solani responses during soybean infection was developed, similar to studies examining R. solani during plant [21, 22] and microbe-microbe [23] interactions. This method allowed for the vast majority of sequences to belong to R. solani, rather than its soybean host. Still, however, a large amount of reads (±30%) did not align to the R. solani reference genome. We speculate that this may be the result of: sequencing errors, quality thresholds for passing alignments, an incomplete reference genome are all legitimate reasons that could explain the low alignment. Additionally, the transcriptome used for alignment belonged to a divergent R. solani AG1-IA strain than that used in our study resulting in strain specific transcripts or genomic regions. Similar results have been reported for other phytopathogen RNA-seq studies [45].
The results presented in this study demonstrate that when R. solani infects soybean leaves, it regulates expression of genes associated with defence and attack. Functional annotation of these genes based on GO terms revealed that several of them encode important cellular, molecular and biological functions involved in defence through the synthesis of antioxidants for ROS quenching, manipulation of the surrounding environment, and attack via the synthesis of toxins, cell wall degrading enzymes and the use of alternative carbon sources. Significant differences in genes involved in certain KEGG metabolic pathways and individual genes involved in attack and defence during early (i.e., onset of necrosis) and late infection (i.e., 24 h.p.o. of necrosis) stages of R. solani were evident.
Defense
Necrotrophs such as R. solani require that the tissue be dead and externally digested prior to utilizing the plant nutrients. This destructive form of nutrient acquisition results in aggressive plant defense and attack mechanisms to limit the damage done by the pathogen. Our results indicate that R. solani employed two mechanisms to combat against soybean defenses, 1) ROS quenching, and 2) manipulation of intra- and extra-cellular environments.
1) ROS quenching
Synthesis of reactive oxygen species (ROS) generally occurs during the initial defense responses of plants acting as both defense compounds and signalling molecules [46]. As such, antioxidant synthesis in phytopathogens is commonly observed throughout the infection process. Fluctuations in Rhizoctonia genes resulting in synthesis of antioxidants and ROS quenching proteins were observed at onset and 24 h.p.o. of necrosis during R. solani-soybean interactions (S5 and S6 Tables), with decreases at 24 h.p.o. compared to onset (S7 Table). OXIDOREDUCTASE and OXIDASE genes provide means of inhibiting or diminishing the potency of ROS by converting them to less reactive forms. At early infection stages, R. solani penetrates its host cells and must defend itself against the sudden increase in ROS during the plant hypersensitive response (HR) [46]. The likelihood that protection of R. solani cells against ROS damage is reduced or stopped by the action of antioxidants was established by the up-regulation of 5 OXIDOREDUCTASE and 10 OXIDASE genes at onset of necrosis, including 3 NADH OXIDASE encoding genes, 2 GMC OXIDOREDUCTASE genes, and a gene encoding GLUTATHIONE-S-TRANSFERASE. Fold changes of these genes ranged from 3.1 to over 32.9 during infection (S5 and S6 Tables) and transcript abundances were significantly higher at onset compared to 24 h.p.o. of necrosis (S7 Table). Congruent with these results is the induction of transcripts related to antioxidants in R. solani when challenged with antagonistic bacteria Serratia proteamaculans and S. plymuthica [47], and with the mycoparasite Stachybotrys elegans [23], or during R. solani-wheat interactions [21, 48]. During late infection of soybean leaves, however, a general trend of down-regulation of OXIDOREDUCTASE and OXIDASE genes, as well as GLUTATHIONE-S-TRANSFERASE was observed suggesting that at this stage the plant had most likely succumbed to the pathogen as evidenced by the high levels of necrosis.
Genes related to several antioxidant pathways, such as thiamine (vitamin B1), riboflavin (vitamin B2), ascorbate (vitamin C) and pyridoxine (vitamin B6) were induced 3.9 to 25.6 fold at onset of necrosis, whereas only genes involved in thiamine and pyridoxine biosynthesis were up-regulated 24 h.p.o. of necrosis with fold changes of 4.6 and 3.3, respectively (S5 and S6 Tables). When comparing fold changes between onset and 24 h.p.o., genes involved in thiamine and pyridoxine biosynthesis were up-regulated 17.3 and 162.5 fold, respectively (S7 Table). Pyridoxine and thiamine biosynthetic genes are known to act as antioxidants to relieve ROS stress during abiotic and biotic stress in fungi. It has been shown that thiamine biosynthesis is down-regulated when R. solani is challenged with the mycoparasite Stachybotrys elegans [23], while pyridoxine biosynthesis pathway was induced during abiotic stresses [49] suggesting that these pathways have roles in relieving oxidative stress in R. solani. This is the first report of the involvement of thiamine and pyridoxine biosynthetic genes during interaction of R. solani with its host.
Although the induction of gene expression holds true for the majority of genes involved in ROS quenching and antioxidant synthesis, a few notable exceptions were observed. Two genes encoding COPPER-ZINC SUPEROXIDE DISMUTASE (SOD) (ELU42795 and ELU42796; S5 and S6 Tables) were down-regulated at both time points with fold changes ranging from 0.1 to 0.17, a finding similar to that reported in R. solani during invasion of wheat [48], suggesting that antioxidants may have highly specific roles during particular interactions and/or stages of interactions.
2) Manipulation of intra- and extra-cellular environment
Some fungi are capable of altering their environment to favour their survival by secreting enzymes such as OXALATE DECARBOXYLASE [50, 51]. High levels of oxalate in advance of a developing fungus may render plant tissue more susceptible to invasion as a result of calcium precipitation from the middle lamella of cell walls [52, 53]. In our study, two OXALATE DECARBOXYLASE genes of R. solani were up-regulated at both time points (S5 and S6 Tables), with similar amounts between the two time points. Similar overexpression of oxalate decarboxylase was also observed when an R. solani strain belonging to AG3 was challenged with two species of Serratia bacteria [47] and when rice was infected with a strain of R. solani AG1-IA [54]. The reason for the up-regulation is not clear, but it may imply that R. solani attempts to recycle the remaining oxalate, or that intra- or extra-cellular oxalate levels are high enough to pose a threat to the pathogen, a notion that remains open for speculation.
During host-pathogen interactions toxin synthesis by both partners is unavoidable. To minimize the intracellular concentration of toxins and secondary metabolites produced during pathogenesis, phytopathogens must be able to export these compounds from the target sites. Several genes encoding transporters, including several ABC TRANSPORTER, MULTIDRUG TRANSPORTER and CHROMATE ION TRANSPORTER, were differentially expressed during R. solani interactions with soybean (S5 and S6 Tables) suggesting a putative role in the protection of R. solani from soybean metabolites. Up-regulation of ABC TRANSPORTER has also been reported for R. solani in the presence of Serratia bacteria [47], and during invasion of the lawn grass Zoysia japonica [13] and rice [54].
The pigment, melanin is required to protect fungi and enhance their survival during adverse conditions, and it is also involved in sclerotial development [55]. One possible way for fungi to yield melanin is by catalyzing the oxidation of tyrosine via the action of tyrosinases [56]. In our study, we have identified 5 putative TYROSINASE genes that were differentially expressed at both time points (S5 and S6 Tables) and between time points (S7 Table) during infection of soybean leaves by R. solani AG1-IA. Consistent with this result, the EST dataset of R. solani AG1-IB (isolate 7/3/14), another subgroup of AG1 causing bottom rot of lettuce, also contained two TYROSINASE genes during exposure to lettuce exudates [57]. Generally, melanin is not known to have a direct role in fungal pathogens; however, some phytopathogenic fungi produce melanized infective structures for efficient pathogenicity [58]. The fact that 4 out of 5 R. solani TYROSINASE genes were down-regulated in our isolate at post-necrotic stages of soybean leaves suggests that tyrosinases may have a role during host penetration although this warrants further investigation.
Some members of the phylum Basidiomycota produce a polysaccharide capsule, comprised of xylose, mannose and gluconic acid, that protect them against environmental perturbations and are also reported to be involved in fungal virulence [59]. The EST dataset of R. solani subgroup AG1-IB includes sequences of key enzymes necessary for synthesis of a polysaccharide capsule: PHOSPHOMANNOSE ISOMERASE (PMI), PHOSPHOMANNOMUTASE (PMM) and GDP MANNOSE PYROPHOSPHORYLASE (GMPP) [57]. Formation of a polysaccharide capsule may protect the fungus against plant chitin degrading enzymes such as 1,3-beta-glucosidases. In our study, RNA-seq data of R. solani AG1-IA revealed the overexpression of a gene encoding PMI (ELU39697) at onset and 24 h.p.o. of necrosis (S5 and S6 Tables), with no differences between time points, suggesting an increased defense response to strengthen the cell wall and diminish the effects of plant chitin-degrading enzymes. Another putative polysaccharide capsule formation gene, GDP-MANNOSE 4,6-DEHYDRATASE gene (GMD; ELU42839), which has an important role in virulence of human pathogenic bacteria [60], was substantially over-expressed in R. solani 24 h.p.o. of necrosis, supporting the theory that polysaccharide capsules may have a role in R. solani virulence and defense. Interestingly, increases of polysaccharide capsule formation genes coincided with decreases in expression of CHITIN SYNTHASE D (CSD) and 3 CHITIN DEACETYLASE (CDC) genes at both time points compared to in vitro controls (S5 and S6 Tables), although higher CDC was observed at 24 h.p.o. compared to onset in samples infecting soybean (S7 Table). Acting primarily at hyphal tips, CSD aids in elongation of hyphae, while CDC converts chitin to chitosan making the hyphae more flexible for growth [61]. Reductions in these genes suggest that hyphal growth was decreased in R. solani during interaction with soybean compared to that growing on PDA, but was higher 24 h.p.o. compared to onset from soybean infecting samples. This, in conjunction with up-regulation of some polysaccharide capsule forming genes, strongly implies that R. solani has the ability to restructure its cell walls in order to protect itself from plant secondary compounds and fungal cell wall degrading enzymes. Similar results were observed when R. solani was challenged with species of the antagonistic bacteria Serratia [47].
Attack
Necrotrophic fungi must not only overcome plant defenses to survive, but must have innovative attack mechanisms that will kill their host but not themselves. Large fluctuations of attack responses were observed in R. solani AG1-IA during soybean infection, including: 1) toxin synthesis; 2) synthesis of plant cell wall and carbohydrate degrading enzymes; and 3) use of alternative carbon sources.
1) Toxin synthesis
Several ricin-type beta-trefoil lectin domain-containing proteins were temporally affected throughout the study (S5 and S6 Tables) with higher expression levels at onset of necrosis compared to 24 hp.o. (S7 Table). Rhizoctonia solani lectins have been associated with insecticidal activity [62], carbohydrate storage [63], fungi-fungi interactions [64] and cell surface and extracellular environment recognition [64]. Up-regulation of RICIN-TYPE BETA-TREFOIL LECTIN genes and proteins was observed during R. solani AG3 confrontation with Serratia bacteria [47] and confrontation of R. solani AG8 with wheat [21], respectively, suggesting an important role during biotic stress and plant invasion (this study). Interestingly, ricin-like lectins are involved in the production of the legume-specific toxin ricin and the up-regulation of ricin-like lectins observed here and in other studies [21, 47] suggest that they may have a role in R. solani toxin production; however the ricin-A domain, which is required for proper ricin protein function in plants, was not observed. Taken together, these results suggest that these domain-containing genes merit investigation for their role in R. solani adhesion, successful host penetration, attack and defense, and growth to determine the exact role of the ricin-B domain in R. solani.
The RNA-seq transcripts analysed in this study comprised of different genes involved in secondary metabolite biosynthesis, such as VELVET genes. The velvet (VeA) family of fungal regulatory proteins is linked to coordination of secondary metabolism and development [65]. In Aspergillus nidulans, mutants lacking the ability to produce VELVET proteins are incapable of producing the aflatoxin precursor sterigmatocystin and cannot produce fruiting bodies [66]. VELVET proteins also appear to have roles in Trichoderma virens secondary metabolite synthesis and mycoparasitism of R. solani [67], and isotigs featuring homology to aflatoxins have been identified in the R. solani AG1-IB genome [57]. In our study, a VELVET DOMAIN-CONTAINING gene was up-regulated 24 h.p.o. of necrosis compared to in vitro controls and onset of necrosis in samples infecting soybean (S6 and S7 Tables). Generally, these results suggest a variety of roles for these proteins in toxin and secondary metabolite synthesis, and possibly during plant invasion.
2) Synthesis of plant cell wall and carbohydrate degrading enzymes
Phytopathogens produce plant cell wall degrading enzymes that are essential for host penetration and invasion. R. solani AG1-IA has an expanded set of putative genes encoding cell wall degrading enzymes (e.g., pectinases, cellulases and ligninases). Some, such as those encoding PECTATE LYASE (PL) and CARBOHYDRATE ESTASE (CE), were highly expressed at onset of necrosis (S5 Table), while others related to the degradation of cutin, cellulose, and carbohydrates were up-regulated 24 h.p.o. of necrosis (S6 Table). Currently, 29 fungal glycoside hydrolase (GH) families are known to be involved in plant biomass degradation [68]. In our study, 18 genes encoding GHs were differentially expressed during R. solani-soybean interactions (S5 and S6 Tables), with some up- or down-regulated at both time points, and others at only one time point. Of these, one gene (ELU41238) belonged to GH family 35, which is known to be involved in plant cell wall degradation [68], was down-regulated and unaffected at onset and 24 h.p.o. of necrosis, respectively. Similar expression patterns have been reported during the interaction of different anastomosis groups of R. solani with their respective hosts including wheat [21], rice [12] and turf grass [13], as well as when grown on media amended with host-derived root exudates [57]. Collectively, these results indicate the existence of a universal mechanism underlying plant cell wall degradation by phytopathogenic fungi. The remaining GHs belonged either to GH families involved in fungal cell wall degradation (typically down-regulated at both time points) or energy storage and recovery (typically up-regulated at both time points) [68]. Interestingly, when comparing the two time points of samples infecting soybean, higher levels of GH abundances were observed 24 h.p.o. compared to onset of necrosis (S7 Table). This, along with the previously discussed expression patterns of CHITIN DEACETYLASE and CHITIN SYNTHASE, suggests a general growth and excess of energy available to the fungus grown on plants compared to when grown in vitro.
Laccase enzymes are key enzymes involved in lignin degradation and two LACCASE genes, belonging to the multicopper oxidase group, were differentially expressed throughout the study: one LACCASE (LAC) was up-regulated at onset of necrosis (S5 Table) and the other was down-regulated 24 h.p.o. of necrosis (S6 Table), and up-regulated at onset compared to 24 h.p.o. (S7 Table). Laccases oxidize molecules, such as lignin, phenols and aromatic amines, although fungal laccases appear to be substrate non-specific [69]. Despite their potential diverse roles, it is generally found that fungal laccases are activated in high carbon to nitrogen environments or during low sugar (glucose or sucrose) scenarios [69]. LACCASE gene expression is commonly reported as up-regulated during stress as in the case of interaction of different R. solani AGs with Serratia species [47], lettuce exudates [57], and potato and lupin [70]. Therefore, the up-regulation of R. solani LACCASE reported here, suggests that there is a lack of readily available nutrients or high stress at onset of necrosis, while its down-regulation at the later stage may signify an excess of readily available nutrients and/or reduced stress due to the high level of plant necrosis.
3) Use of alternative carbon sources
During attack, phytopathogens must be capable of responding to a rapidly changing environment in order to survive. One such strategy is their ability to utilize alternative carbon sources for energy. Two key enzymes in the glyoxylate pathway, ISOCITRATE LYASE (ICL) and MALATE SYNTHASE (MLS), aid in host penetration and disease development of several plant pathogenic fungi by relying on the catabolic products of lipids such as fatty acids and of carboxylic acids such as acetate as energy sources [71–74]. The up-regulation of ICL and MLS at onset of necrosis (S5 and S7 Tables) in this study implies that degradation of lipids and carboxylic acids can potentially be used as carbon sources for R. solani prior to onset of necrosis when nutrients are limited. These genes were also induced in R. solani in the presence of Serratia species [47], demonstrating their importance in stress tolerance and defense or attack during interaction with biotic stresses.
The γ-aminobutyric acid (GABA) shunt pathway can be used by fungi to obtain alternative carbon and nitrogen sources [75] and is derived by conversion of glutamate to GABA via the enzyme glutamate decarboxylase. A putative gene encoding GLUTAMATE DECARBOXYLASE (GAD; ELU39983) was highly up-regulated at onset and 24 h.p.o. of necrosis (S5 and S6 Tables), with no differences between the two time points in samples infecting soybean. Genes involved in the GABA shunt were also differentially expressed when a R. solani strain belonging to AG1-IB was grown in the presence of lettuce root exudates [57], suggesting that GABA utilization may have a strong role in the invasion and establishment of R. solani in its host. The possibility that fungi are able to utilize plant-derived GABA as a carbon and nitrogen source for pathogenicity during plant host invasion was suggested in the case of tomato-Cladosporium fulvum [76]. Interestingly, decreases in the abundance soybean-derived GABA during the interaction between R. solani AG1-IA and soybean compared to uninfected controls was reported [77]. Together these findings indicate that pathogenic fungi may alter plant leaf physiology in order to gain access to nitrogen and carbon sources. GABA in fungi can also act as a signalling molecule for the induction of plant cell wall degrading enzymes [78, 79]. The large increase in GLUTAMATE DECARBOXYLASE at onset of necrosis suggests that GABA may also act as a signalling molecular in R. solani.
Conclusions
We have conducted the first comprehensive high throughput RNA sequencing study of R. solani AG1-IA (strain ROS-2A4), the causal agent of Rhizoctonia foliar blight, at two different infection stages of soybean. The differential expression of R. solani AG1-IA transcripts provided us with insights on the shifts in gene expression of major primary and secondary metabolic processes and the activation of defence and attack mechanisms. The list of candidate genes associated with defence and attack identified in this study might provide a basis for future identification of fungal pathogenicity genes, as well as provide a foundation for targeted control methods and novel strategies for the development of RFB resistant soybean lines.
Supporting information
(XLSX)
(XLSX)
(DOCX)
(DOCX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
Financial support was provided as a Discovery grants to S. Jabaji (RGPIN137135-201 and RGPIN-2016-04805) and R. Duggavathi (RGPIN-371850) by the National Sciences and Engineering Research Council of Canada. We thank P. Ceresini for his technical advise on inoculation experiments of detached soybean leaves. A special thanks goes to R. Chamoun for his help in setting up the R. solani detached leaf assay.
Data Availability
All data discussed in this publication have been deposited in the NCBI Sequence Read Archive (SRA) under the BioProject PRJNA369092.
Funding Statement
This work was supported by a Discovery grant (RGPIN137135-201 and RGPIN-2016-04805) to S. Jabaji and R. Duggavathi (RGPIN-371850) provided by the Natural Sciences and Engineering Research Council of Canada (NSERC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Atkins J Jr, Lewis W. Rhizoctonia aerial blight of soybean in Louisiana. Phytopathology. 1954;44(1): 215–218. [Google Scholar]
- 2.Fenille RC, De Souza NL, Kuramae EE. Characterization of Rhizoctonia solani associated with soybean in Brazil. Eur J Plant Pathol. 2002;108(8): 783–792. doi: 10.1023/A:1020811019189 [Google Scholar]
- 3.Stetina K, Stetina S, Russin J. Comparison of severity assessment methods for predicting yield loss to Rhizoctonia foliar blight in soybean. Plant Dis. 2006;90(1): 39–43. doi: 10.1094/PD-90-0039 [DOI] [PubMed] [Google Scholar]
- 4.Ciampi M, Meyer M, Costa M, Zala M, McDonald B, Ceresini P. Genetic structure of populations of Rhizoctonia solani anastomosis group-1 IA from soybean in Brazil. Phytopathology. 2008;98(8): 932–941. doi: 10.1094/PHYTO-98-8-0932 [DOI] [PubMed] [Google Scholar]
- 5.Yang X, Berggren G, Snow J. Effects of free moisture and soybean growth stage on focus expansion of Rhizoctonia aerial blight. Phytopathology. 1990;80(5): 497–503. doi: 10.1094/Phyto-80-497 [Google Scholar]
- 6.Huang X, Zhang N, Yong X, Yang X, Shen Q. Biocontrol of Rhizoctonia solani damping-off disease in cucumber with Bacillus pumilus AQR-N43. Microbiol Res. 2012;167: 135–143. doi: 10.1016/j.micres.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 7.Kataria H, Grover R. Some factors affecting the control of Rhizoctonia solani by systemic and non‐systemic fungicides. Ann Appl Biol. 1976;82(2): 267–278. doi: 10.1111/j.1744-7348.1976.tb00562.x [Google Scholar]
- 8.Kataria H, Verma P, Gisi U. Variability in the sensitivity of Rhizoctonia solani anastomosis groups to fungicides. J Phytopathol. 1991;133(2): 121–133. doi: 10.1111/j.1439-0434.1991.tb00145.x [Google Scholar]
- 9.de Assis JB, Peyer P, Rush MC, Zala M, McDonald BA, Ceresini PC. Divergence between sympatric rice-and soybean-infecting populations of Rhizoctonia solani anastomosis group-1 IA. Phytopathology. 2008;98(12): 1326–1333. doi: 10.1094/PHYTO-98-12-1326 [DOI] [PubMed] [Google Scholar]
- 10.González García V, Portal Onco MA, Rubio S. Review. Biology and systematics of the form genus Rhizoctonia. Spanish Journal of Agricultural Research. 1999;4: 55–79. [Google Scholar]
- 11.Tan W-Z, Zhang W, Ou Z-Q, Li W, Zhou G-J, Wang Z-K, et al. Analyses of the temporal development and yield losses due to sheath blight of rice (Rhizoctonia solani AG1. 1a). Agricultural Sciences in China. 2007;6(9): 1074–1081. doi: 10.1016/S1671-2927(07)60149-7 [Google Scholar]
- 12.Zheng A, Lin R, Zhang D, Qin P, Xu L, Ai P, et al. The evolution and pathogenic mechanisms of the rice sheath blight pathogen. Nature Communications. 2013;4: Article 1424. doi: 10.1038/ncomms2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu C, Ai L, Wang L, Yin P, Liu C, Li S, et al. De novo transcriptome analysis of Rhizoctonia solani AG1 IA strain early invasion in Zoysia japonica root. Frontiers in Microbiology. 2016;7: Article 708. doi: 10.3389/fmicb.2016.00708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dou D, Zhou J-M. Phytopathogen effectors subverting host immunity: different foes, similar battleground. Cell Host & Microbe. 2012;12(4): 484–495. doi: 10.1016/j.chom.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 15.Jacob S, Yemelin A. Stress Biology in Fungi and “Omic” Approaches as Suitable Tools for Analyzing Plant–Microbe Interactions In: Unden G, Thines E, Schüffler A, editors. Host—Pathogen Interaction: Microbial Metabolism, Pathogenicity and Antiinfectives; doi: 10.1002/9783527682386.ch9 Weinheim, Germany: Wiley-VCH Verlag GmbH & Co.; 2016. p. 153–178. [Google Scholar]
- 16.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nature Reviews Genetics. 2009;10(1): 57–63. doi: 10.1038/nrg2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong W, Chen N, Liu T, Zhu J, Wang J, He X, et al. Large-Scale Transcriptome Analysis of Cucumber and Botrytis cinerea during Infection. PloS One. 2015;10(11): e0142221 doi: 10.1371/journal.pone.0142221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orshinsky AM, Hu J, Opiyo SO, Reddyvari-Channarayappa V, Mitchell TK, Boehm MJ. RNA-Seq analysis of the Sclerotinia homoeocarpa–creeping bentgrass pathosystem. PloS One. 2012;7(8): e41150 doi: 10.1371/journal.pone.0041150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Browne R, Cooke B. Development and evaluation of an in vitro detached leaf assay forc pre-screening resistance to Fusarium head blight in wheat. Eur J Plant Pathol. 2004;110(1): 91–102. doi: 10.1023/B:EJPP.0000010143.20226.21 [Google Scholar]
- 20.Vleeshouwers VG, van Dooijeweert W, Keizer LP, Sijpkes L, Govers F, Colon LT. A laboratory assay for Phytophthora infestans resistance in various Solanum species reflects the field situation. Eur J Plant Pathol. 1999;105(3): 241–250. doi: 10.1023/A:1008710700363 [Google Scholar]
- 21.Anderson JP, Hane JK, Stoll T, Pain N, Hastie ML, Kaur P, et al. Proteomic analysis of Rhizoctonia solani identifies infection-specific, redox associated proteins and insight into adaptation to different plant hosts. Molecular & Cellular Proteomics. 2016;15(4): 1188–1203. doi: 10.1074/mcp.M115.054502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keijer J. The initial steps of the infection process in Rhizoctonia solani. Rhizoctonia species: Taxonomy, molecular biology, ecology, pathology and disease control: Springer; 1996. p. 149–162. [Google Scholar]
- 23.Chamoun R, Jabaji S. Expression of genes of Rhizoctonia solani and the biocontrol Stachybotrys elegans during mycoparasitism of hyphae and sclerotia. Mycologia. 2011;103(3): 483–493. doi: 10.3852/10-235 [DOI] [PubMed] [Google Scholar]
- 24.Marshall D, Rush M. Infection cushion formation on rice sheaths by Rhizoctonia solani. Phytopathology. 1980;70(10): 947–950. doi: 10.1094/Phyto-70-947 [Google Scholar]
- 25.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30: 2114–2120. doi: 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4): Article R36. doi: 10.1186/gb-2013-14-4-r36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, et al. Genome sequence of the palaeopolyploid soybean. Nature. 2010;463(7278): 178–183. doi: 10.1038/nature08670 [DOI] [PubMed] [Google Scholar]
- 28.Anders S, Pyl PT, Huber W. HTSeq–A Python framework to work with high-throughput sequencing data. Bioinformatics. 2014;31(2): 166–169. doi: 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anders S, Huber W. Differential expression analysis for sequence count data. Genome biology. 2010;11(10): Article R106. doi: 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;Series B (Methodological): 289–300. doi: 10.2307/2346101 [Google Scholar]
- 31.Wanrnes G, Bolker B, Bonebakker L, Gentleman R, Huber W, Liaw A, et al. gplots: Various R programming tools for plotting data. R package version 3.0.1. ed2016.
- 32.Chen H, Boutros PC. VennDiagram: a package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinformatics. 2011;12(1): Article 35. doi: 10.1186/1471-2105-12-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7): e47 doi: 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conesa A, Götz S. Blast2GO: A comprehensive suite for functional analysis in plant genomics. International Journal of Plant Genomics. 2008;2008: Article 619832. doi: 10.1155/2008/619832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21(18): 3674–3676. doi: 10.1093/bioinformatics/bti610 [DOI] [PubMed] [Google Scholar]
- 36.Myhre S, Tveit H, Mollestad T, Lægreid A. Additional gene ontology structure for improved biological reasoning. Bioinformatics. 2006;22(16): 2020–2027. doi: 10.1093/bioinformatics/btl334 [DOI] [PubMed] [Google Scholar]
- 37.Labarga A, Valentin F, Anderson M, Lopez R. Web services at the European bioinformatics institute. Nucleic Acids Res. 2007;35(Suppl 2): W6–W11. doi: 10.1093/nar/gkm291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1): 27–30. doi: 10.1093/nar/28.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. J Comput Biol. 2005;12(8): 1047–1064. doi: 10.1089/cmb.2005.12.1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beane J, Vick J, Schembri F, Anderlind C, Gower A, Campbell J, et al. Characterizing the impact of smoking and lung cancer on the airway transcriptome using RNA-Seq. Cancer prevention research. 2011;4(6): 803–817. doi: 10.1158/1940-6207.CAPR-11-0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Copley TR, Aliferis KA, Kliebenstein DJ, Jabaji SH. An integrated RNAseq-1H NMR metabolomics approach to understand soybean primary metabolism regulation in response to Rhizoctonia foliar blight disease. BMC Plant Biol. 2017;17(1): Article 84. doi: 10.1186/s12870-017-1020-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward JH Jr. Hierarchical grouping to optimize an objective function. Journal of the American Statistical Association. 1963;58(301): 236–244. doi: 10.1080/01621459.1963.10500845 [Google Scholar]
- 43.G.O.Consortium. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 2004;32(suppl 1): D258–D261. doi: 10.1093/nar/gkh036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayden KJ, Garbelotto M, Knaus BJ, Cronn RC, Rai H, Wright JW. Dual RNA-seq of the plant pathogen Phytophthora ramorum and its tanoak host. Tree Genet Genom. 2014;10(3): 489–502. doi: 10.1007/s11295-014-0698-0 [Google Scholar]
- 45.Kawahara Y, Oono Y, Kanamori H, Matsumoto T, Itoh T, Minami E. Simultaneous RNA-seq analysis of a mixed transcriptome of rice and blast fungus interaction. PloS One. 2012;7(11): e49423 doi: 10.1371/journal.pone.0049423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shetty NP, Jørgensen HJL, Jensen JD, Collinge DB, Shetty HS. Roles of reactive oxygen species in interactions between plants and pathogens. Eur J Plant Pathol. 2008;121(3): 267–280. doi: 10.1007/s10658-008-9302-5 [Google Scholar]
- 47.Gkarmiri K, Finlay RD, Alström S, Thomas E, Cubeta MA, Högberg N. Transcriptomic changes in the plant pathogenic fungus Rhizoctonia solani AG-3 in response to the antagonistic bacteria Serratia proteamaculans and Serratia plymuthica. BMC Genomics. 2015;16(1): Article 630. doi: 10.1186/s12864-015-1758-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foley RC, Kidd BN, Hane JK, Anderson JP, Singh KB. Reactive Oxygen Species Play a Role in the Infection of the Necrotrophic Fungi, Rhizoctonia solani in Wheat. PloS One. 2016;11(3): e0152548 doi: 10.1371/journal.pone.0152548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samsatly J, Chamoun R, Gluck-Thaler E, Jabaji S. Genes of the de novo and Salvage Biosynthesis Pathways of Vitamin B6 are Regulated under Oxidative Stress in the Plant Pathogen Rhizoctonia solani. Frontiers in Microbiology. 2015;6: Article 429. doi: 10.3389/fmicb.2015.01429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mäkelä M, Galkin S, Hatakka A, Lundell T. Production of organic acids and oxalate decarboxylase in lignin-degrading white rot fungi. Enzyme Microb Technol. 2002;30(4): 542–549. doi: 10.1016/S0141-0229(02)00012-1 [Google Scholar]
- 51.Kesarwani M, Azam M, Natarajan K, Mehta A, Datta A. Oxalate decarboxylase from Collybia velutipes molecular cloning and its overexpression to confer resistance to fungal infection in transgenic tobacco and tomato. J Biol Chem. 2000;275(10): 7230–7238. doi: 10.1074/jbc.275.10.7230 [DOI] [PubMed] [Google Scholar]
- 52.Nagarajkumar M, Jayaraj J, Muthukrishnan S, Bhaskaran R, Velazhahan R. Detoxification of oxalic acid by Pseudomonas fluorescens strain PfMDU2: Implications for the biological control of rice sheath blight caused by Rhizoctonia solani. Microbiol Res. 2005;160(3): 291–298. doi: 10.1016/j.micres.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 53.Godoy G, Steadman J, Dickman M, Dam R. Use of mutants to demonstrate the role of oxalic acid in pathogenicity of Sclerotinia sclerotiorum on Phaseolus vulgaris. Physiol Mol Plant Pathol. 1990;37(3): 179–191. doi: 10.1016/0885-5765(90)90010-U [Google Scholar]
- 54.Ghosh S, Gupta SK, Jha G. Identification and functional analysis of AG1-IA specific genes of Rhizoctonia solani. Curr Genet. 2014;60(4): 327–341. doi: 10.1007/s00294-014-0438-x [DOI] [PubMed] [Google Scholar]
- 55.Chen J, Wang C, Shu C, Zhu M, Zhou E. Isolation and characterization of a melanin from Rhizoctonia solani, the causal agent of rice sheath blight. Eur J Plant Pathol. 2015;142(2): 281–290. doi: 10.1007/s10658-015-0612-0 [Google Scholar]
- 56.Bell AA, Wheeler MH. Biosynthesis and functions of fungal melanins. Annu Rev Phytopathol. 1986;24(1): 411–451. doi: 10.1146/annurev.py.24.090186.002211 [Google Scholar]
- 57.Wibberg D, Jelonek L, Rupp O, Kröber M, Goesmann A, Grosch R, et al. Transcriptome analysis of the phytopathogenic fungus Rhizoctonia solani AG1-IB 7/3/14 applying high-throughput sequencing of expressed sequence tags (ESTs). Fungal Biology. 2014;118(9): 800–813. doi: 10.1016/j.funbio.2014.06.007 [DOI] [PubMed] [Google Scholar]
- 58.Henson JM, Butler MJ, Day AW. The dark side of the mycelium: melanins of phytopathogenic fungi. Annu Rev Phytopathol. 1999;37(1): 447–471. doi: 10.1146/annurev.phyto.37.1.447 [DOI] [PubMed] [Google Scholar]
- 59.Wills EA, Roberts IS, Del Poeta M, Rivera J, Casadevall A, Cox GM, et al. Identification and characterization of the Cryptococcus neoformans phosphomannose isomerase‐encoding gene, MAN1, and its impact on pathogenicity. Mol Microbiol. 2001;40(3): 610–620. doi: 10.1046/j.1365-2958.2001.02401.x [DOI] [PubMed] [Google Scholar]
- 60.Cottrell TR, Griffith CL, Liu H, Nenninger AA, Doering TL. The pathogenic fungus Cryptococcus neoformans expresses two functional GDP-mannose transporters with distinct expression patterns and roles in capsule synthesis. Eukaryot Cell. 2007;6(5): 776–785. doi: 10.1128/EC.00015-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y, Park R-D, Muzzarelli RA. Chitin deacetylases: properties and applications. Mar Drugs. 2010;8(1): 24–46. doi: 10.3390/md8010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamshou M, Van Damme E, Smagghe G. Entomotoxic effects of fungal lectin from Rhizoctonia solani towards Spodoptera littoralis. Fungal biology. 2010;114(1): 34–40. doi: 10.1016/j.mycres.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 63.Kellens JT, Peumans WJ. Developmental accumulation of lectin in Rhizoctonia solani: a potential role as a storage protein. Microbiology. 1990;136(12): 2489–2495. doi: 10.1099/00221287-136-12-2489 [Google Scholar]
- 64.Varrot A, Basheer SM, Imberty A. Fungal lectins: structure, function and potential applications. Curr Opin Struct Biol. 2013;23(5): 678–685. doi: 10.1016/j.sbi.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 65.Bayram Ö, Braus GH. Coordination of secondarymetabolism and development in fungi: the velvet familyof regulatory proteins. FEMS Microbiol Rev. 2012;36(1): 1–24. doi: 10.1111/j.1574-6976.2011.00285.x [DOI] [PubMed] [Google Scholar]
- 66.Kato N, Brooks W, Calvo AM. The expression of sterigmatocystin and penicillin genes in Aspergillus nidulans is controlled by veA, a gene required for sexual development. Eukaryot Cell. 2003;2(6): 1178–1186. doi: 10.1128/EC.2.6.1178-1186.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mukherjee PK, Kenerley CM. Regulation of morphogenesis and biocontrol properties in Trichoderma virens by a VELVET protein, Vel1. Appl Environ Microbiol. 2010;76(7): 2345–2352. doi: 10.1128/AEM.02391-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Z, Liu H, Wang C, Xu J-R. Comparative analysis of fungal genomes reveals different plant cell wall degrading capacity in fungi. BMC Genomics. 2013;14(1): Article 274. doi: 10.1186/1471-2164-14-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kunamneni A, Ballesteros A, Plou FJ, Alcalde M. Fungal laccase—a versatile enzyme for biotechnological applications In: Mendez-Vilas A, editor. Communicating current research and educational topics and trends in applied microbiology. 1 Badajoz, Spain: Formex; 2007. p. 233–245. [Google Scholar]
- 70.Bora P, Hardy GSJ, O’Brien P. Laccase activity and maceration of lupin tissue by Rhizoctonia solani is inhibited by arginine. Australasian Plant Pathology. 2005;34(4): 591–594. doi: 10.1071/AP05077 [Google Scholar]
- 71.Wang ZY, Thornton CR, Kershaw MJ, Debao L, Talbot NJ. The glyoxylate cycle is required for temporal regulation of virulence by the plant pathogenic fungus Magnaporthe grisea. Mol Microbiol. 2003;47(6): 1601–1612. doi: 10.1046/j.1365-2958.2003.03412.x [DOI] [PubMed] [Google Scholar]
- 72.Idnurm A, Howlett BJ. Isocitrate lyase is essential for pathogenicity of the fungus Leptosphaeria maculans to canola (Brassica napus). Eukaryot Cell. 2002;1(5): 719–724. doi: 10.1128/EC.1.5.719-724.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Asakura M, Okuno T, Takano Y. Multiple contributions of peroxisomal metabolic function to fungal pathogenicity in Colletotrichum lagenarium. Appl Environ Microbiol. 2006;72(9): 6345–6354. doi: 10.1128/AEM.00988-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Solomon PS, Lee RC, Wilson T, Oliver RP. Pathogenicity of Stagonospora nodorum requires malate synthase. Mol Microbiol. 2004;53(4): 1065–1073. doi: 10.1111/j.1365-2958.2004.04178.x [DOI] [PubMed] [Google Scholar]
- 75.Divon HH, Fluhr R. Nutrition acquisition strategies during fungal infection of plants. FEMS Microbiol Lett. 2007;266(1): 65–74. doi: 10.1111/j.1574-6968.2006.00504.x [DOI] [PubMed] [Google Scholar]
- 76.Solomon PS, Oliver RP. Evidence that γ-aminobutyric acid is a major nitrogen source during Cladosporium fulvum infection of tomato. Planta. 2002;214(3): 414–420. doi: 10.1007/s004250100632 [DOI] [PubMed] [Google Scholar]
- 77.Copley T, Aliferis KA, Kliebenstein DJ, Jabaji S, editors. Integrated transcriptomics and metabolomics decipher differences in the defense response of soybean leaves to Rhizoctonia foliar blight. Northeast American Society of Plant Biologist Conference; 2015 2015; Boston, MA2015.
- 78.Carapito R, Hatsch D, Vorwerk S, Petkovski E, Jeltsch J-M, Phalip V. Gene expression in Fusarium graminearum grown on plant cell wall. Fungal Genet Biol. 2008;45(5): 738–748. doi: 10.1016/j.fgb.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 79.Shelp BJ, Bown AW, Faure D. Extracellular γ-aminobutyrate mediates communication between plants and other organisms. Plant Physiol. 2006;142(4): 1350–1352. doi: 10.1104/pp.106.088955 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
(DOCX)
(DOCX)
(XLSX)
(XLSX)
(XLSX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All data discussed in this publication have been deposited in the NCBI Sequence Read Archive (SRA) under the BioProject PRJNA369092.